Abstract
Purpose
Translational research allowed us to hypothesize that endoscopic surgery performed with new generation 3D systems could improve surgeons’ performance, reducing the learning curve, and the perceived workload. However, there is currently a lack of evidence in randomized clinical trials considering advantages for the surgeon and the patient of using the new 3D systems. This systematic review of literature aims to understand what are the differences when performing an endoscopic surgery with new 3D or 2D systems when it comes to intra-operative, post-operative and surgeons perspective outcomes, and at the same time, understand what were the difficulties encountered when performing research about as different imaging systems for surgeons.
Methods
A systematic review of literature was conducted through an online search in databases MEDLINE ©/PubMed © to identify articles published in English, from 1st January 2014 to 31st May 2019, that compared clinical results of 2D and 3D third-generation video-assisted surgery.
Results
A total of 30 articles were included in the qualitative analyses. Of the 30 articles analyzed, 13 were articles in which patients were randomly selected, of which 7 were considered to be at “Low” risk of bias. From the 7 articles, 2 demonstrated an association between lower blood loss and 3D systems. In this selection of low risk randomized articles, no differences were observed in any of the studies when it comes to conversion to open surgery, intra-operative complications, morbidity, length of stay, and oncological outcomes.
Conclusion
In conclusion, this systematic review presents the current knowledge on clinical use of 3D systems for endoscopic surgery. Significant scientific evidence puts 3D technology with advantages in surgeon performance, learning curve, and fatigue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 30 years, laparoscopy became the gold standard surgical approach for the majority of digestive, thoracic, urological, and gynecological surgical conditions. Conventional laparoscopy systems experimented a huge development along the years, concerning definition and light delivery, which resulted in an increase in image quality for the surgeon [1].
Since the earliest years of the endoscopic era that the limitation arising from the two-dimensional view was noticed [2]. For the surgeon with stereo-normal-vision, the learning of indirect clues for depth perception is long, difficult, and tricky. Spatial orientation with a two-dimensional (2D) view is highly dependent on the distance of the scope and uses cumulative knowledge along years of experience of comparison of different structures, shadows, motion parallax effect, and acquainted images recognition. Actually, anatomical misperception can be a major cause of error, as illustrated in a study on biliary iatrogenic injury in laparoscopic cholecystectomy [3].
The lack of depth perception and spatial orientation led to technological efforts to overcome these limitations of 2D vision. The first generation of 3D systems were launched during 1990s. This equipment provide an artificial 3D image captured with a mono-channel optical system transmitted to shutter glasses. Image quality was poorly defined, lighting was scarce and caused many side effects on users, such as headache and dizziness. These limitations were the impediment to its widespread use [1].
Later on, a second generation of equipment offered bi-channel scopes to present different images to each eye. This was a big leap to provide a true 3D image that results from the disparity of retinal images. The necessity to wear a heavy head mounted display restraint its use by surgeons, with frequent complains of discomfort and secondary effects [1].
More recently, with polarized technology, a new set of tools arises in the surgeons’ arsenal. Images are captured with high definition double channel scopes and transmitted to a screen that displays simultaneously images to the right and left eyes. With the use of light polarized glasses, the surgeon can now easily obtain a three-dimensional high definition image. Special considerations in the position of the surgeon and monitor, as well as room lightning, should be optimized to improve depth perception and alleviate undesirable effects, such as blurred vision [1].
A correct selection of stereo impairments within 3D systems users is obligatory. Population studies have shown that about 30% of people have some kind of stereopsis limitation and at least 3% are actually stereo-blind. It is clear that these professionals cannot benefit from the advantages of a 3D display [1].
In the last 10 years, several laboratory studies comparing third-generation 3D vs. 2D results were published. Most of them used validated phantom exercises like E-BLUS and FLS models. The advantages found for new 3D systems were shorter learning curve for novices [4,5,6,7], faster performance [5, 7, 8], error reduction [4, 9, 10], surgeons preference [8, 10,11,12], and reduced strain [9].
Translational research allowed us to hypothesize that endoscopic surgery (laparoscopy, thoracoscopy, cervicoscopy, retroperitoneoscopy) performed with new generation 3D systems could improve surgeons’ performance, reducing the learning curve and the perceived workload. However, there is currently a lack of evidence in randomized clinical trials considering advantages for the surgeon and the patient of using the new 3D systems.
This systematic review of literature aims to understand what are the differences when performing an endoscopic surgery with new 3D or 2D systems, when it comes to intra-operative, post-operative, and surgeons’ perspective outcomes, and at the same time, understand what were the difficulties encountered when performing research about different imaging systems for surgeons.
Methods
A systematic review of literature was conducted through an online search in databases MEDLINE ©/PubMed © to identify articles published in English, from 1st January 2014 to 31st May 2019, that compared clinical results of 2D and 3D third-generation video-assisted surgery. A search using the terms 2-dimensional [All Fields] AND 3-dimensional [All Fields] AND (“laparoscopy” [MeSH Terms] OR “laparoscopy” [All Fields]) and (2D[All Fields] AND 3D[All Fields]) was performed. Filters were applied: “last 5 years,” “Review,” “Clinical trial,” and only prospective or randomized studies were included. All laboratorial, animal, or robotic studies were excluded from the analyzes. Additional articles were added to the analysis based on the references of the works included. Repeated articles were excluded from the review and all articles with a summary eligible according to the criteria described above were included and evaluated through the full text of the article. No direct contact with authors of the included articles was done. Articles excluded after evaluation of the full text had the exclusion reasons presented in the text. The articles to be included were considered for qualitative synthesis analysis. Quantitative synthesis analysis and meta-analysis was not performed. The articles were analyzed by two authors independently and when doubts arose regarding the inclusion of articles the decision was reached through a consensus between the two authors (Fig. 1).
Basic information and study design were collected from the included studies such as year, author, country, methodology, risk of bias, number of patients, surgeon’s experience, and stereoscopic capacity. Technical aspects were collected such as type of 3D and 2D video system used, description on the text of viewer condition, surgical technique, and type of minimally invasive approach. A four-grade procedure complexity grading system (Appendix Table 1) was used to classify the surgeries performed in each study (I—organ resection; II—plasty; III—resection and anastomoses; IV—complex multi-resection and anastomoses). Intra-operative factors such as operative time, blood loss, complications, conversion, and post-operative factors such as morbidity, length of stay, and oncological outcome were collected. References to side effects of 3D systems and surgeon’s perspectives when using 3D third generation systems were also collected.
Statistical measures were described, if possible, including risk measures, differences between means, and measures of association. A categorical analysis of risk of bias was performed for randomized studies using the Risk-of-Bias 2.0 Tool from Chocrane Collaboration. In this two-step tool, seven specific domains are addressed: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and “other bias.” The bias of each domain is evaluated on basis of their reporting in the RCTs and the overall risk of the domain is then categorized as low risk of bias, high risk of bias, or unclear risk of bias thus giving support for an overall judgment to be applied. As operative time was studied in all RCTs included, it allowed the authors to evaluate the risk of bias in a transversal way through all the studies comprised in this review.
Randomized studies with low risk of bias had their results grouped and analyzed separately. Studies describing financial support or conflicts of interest were described.
This study received no funding. The authors declare that they have no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
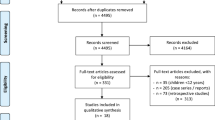
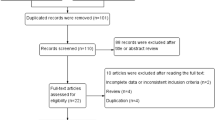
According to the search terms described above, authors identified 189 articles. From this, 10 articles were added through references. A total of 68 articles were ready for screening after duplicates removed and filters applied. After screening, 35 articles were excluded, and 33 full-text articles were assessed for eligibility. A total of 3 full-text articles were excluded, two for no clear 2D/3D comparison groups and one because it was an interim report of a paper already included in the analyses. In the end, a total of 30 articles were included in the qualitative synthesis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. (PRISMA flow diagram).
Characteristics of Included Studies
Of the 30 articles analyzed (Appendix Table 2), 13 were articles in which patients were randomly selected, of which 1 was considered to be at high risk of bias according to the RoB Score used, 5 as “Some Concerns” in their methodology, and 7 as “Low” risk of bias. Low risk of bias articles (Appendix Table 6) are presented separately in Appendix Table 3 and described below.
A total of 3513 patients were included in the 30 articles, 1933 (55%) of patients were operated with 2D systems, and 1580 (45%) had surgery with 3D systems. In 18 studies, every surgery was performed by the same surgeon or the same team; in 11 studies, two or more surgeons or teams operated the patients. A single article did not mention who performed the operations. Seven (23%) articles quantified surgeon expertise. The variability of what was considered expertise surgeon ranged from 1 to 500 surgical procedures performed by surgeon. Four (13%) articles mentioned a prior evaluation of the surgeon’s stereoscopic capacity.
Four (13%) articles [22, 30, 37, 41] did not detailed the surgical technique steps used, and in 3 (75%) of these, surgeries were performed by 2 or more surgeons. As shown in Appendix Table 2, in 60% of the articles, patients underwent a minimally invasive surgery requiring resection and surgical anastomosis (grade III). Just one study include high complexity surgeries [17]. In 23 (77%) studies the approach was laparoscopic, in 6 (20%) was thoracoscopic, and in 1(3%) was retroperitoneal.
Intra-operative Results
Operative time was substantially decreased in all the results (Appendix Table 4). In 22 (73%), an association with p < 0.05 between a decrease in operative time and the use of 3D systems was found; from these 22, two authors described this difference only when analyzing the group of novice surgeons and not when expert surgeons were performing the procedure.
Data on blood loss was not presented in 8 studies. In the other 22, there was an association between the use of 3D systems and a decrease of blood loss in 5 articles [14, 16, 27, 31, 41]. There were no data regarding a possible difference between expert surgeons and novices regarding blood loss. Considering only studies in which the degree of complexity of the intervention was equal to or greater than III, 5 (24%) did not mention blood loss, 12 (57%) obtained similar results with the 2D or 3D system, and 4 (19%) obtained a decrease in blood loss with p < 0.05 when using 3D systems.
There was no association between different visualization systems and the rate of conversion. Besides, there was no paper showing a significant association between different system used and operative complications or errors; a total of 30 complications were observed in the 2D group and 13 were observed in the 3D group (detailed in Appendix Table 4).
Post-operative Results
Concerning post-operative results (Appendix Table 5), from the 30 articles analyzed, only one article presented post-operative complications according to a validated classification, such as Clavien-Dindo. Three papers did not mention if there was any post-operative complication. From the other 27, in 6 (22.2%), authors stated that there were no complications with the patients involved in the study. Authors from two articles stated that complications within 2D vs. 3D were similar; however, they did not present them in the paper. Only one article demonstrated an association between the use of 3D systems and lower complication rate. In the paper by Padin [37], novice surgeons that were within their learning curve (< 100 procedures) for performing gastric bypass and sleeve gastrectomy had lower fistula rate using 3D systems when they were performing the procedures with 2D systems (0 vs. 6.9% p = 0.02).
Hospital length of stay (Los) was mentioned in 20 (67%) studies, both in days and in hours. 3D systems resulted in lower Los in 3 (15%) studies including the study mentioned above by Padin [37], in which 3D systems were both associated with lower LoS in experienced and novice surgeons. No randomized studies demonstrated an association with lower Los and lower post-operative complications and from the studies that observed this association two were retrospective analyses and one was a prospective single center study where data was compared to an historical cohort.
Fourteen studies presented data on oncological outcomes of the performed resection, which included resections for gastrointestinal, urologic (prostate, kidney, and bladder), and gynecologic tumors.
In this 14 studies, one presented data on R0 resection, one on a oncological and functional outcome composite called Pentafecta [42], and 12 on the number of lymph nodes harvested. A single retrospective analysis of a prospective database of consecutive patients performed by Yoon [31] demonstrated higher number of lymph nodes harvested with 3D systems (41.0 (32.0–51.5) 2D vs. 47.0 (37.5–60.0) 3D p = 0.001).
Surgeons Perspective Outcomes
No mention on side effects of 3D systems were done in 14 (46.7%) of the articles, and 8 articles mentioned that there were no symptoms felt by the surgeons using the new generation 3D systems. From the other eight studies, 4 used a validated score to assess side effects and/or symptoms. Kinoshita [15] used the Fatigue by Simulation sickness questionnaire (SSQ) and critical flicker fusion (CFF) test for surgeons and “scopists” separately. The scores consist of the Flicker test—a critical perceiving frequency according to reducing the frequency of red flicker light: % prolongation = [(postsurgical CFF − presurgical CFF)/presurgical CFF]*100, and a the SSQ a questionnaire of 16 questions for various symptoms. The choices for each question are based on the 4 point Likert scales, none (0), slightly (1), moderate (2), and severe (3). No difference in pre- and post-operative symptoms were found between 2D and 3D. Curro used the Surgical Strain Score in both studies for bariatric surgery and colectomy; similar results were presented. In both bariatric surgery and colectomy, lower neck strain and eye strain were associated with 3D systems (p = 0.024; p = 0.0006). Patankar [41] utilized the STAI score, a State-trait anxiety inventory for adults (short version), and demonstrated better symptoms with 3D systems (13 vs. 17, p < 0.0001). All articles that used validated scores were randomized studies.
The remaining 4 studies that mentioned side effects of 3D systems used non-validated scores to analyze symptoms such as nausea, dizziness, ocular fatigue, etc. Lui [30] demonstrated an association between nausea, dizziness, ocular fatigue, and blurring of vision when using 3D systems (5.3% 2D/45.9% 3D (p < 0.001) with a non-validated score in a randomized study. No differences were found in the other studies where a non-validated score was used.
Most of the works that presented results on the side effects of 3D systems also presented a surgeon’s perspective of using these systems compared to conventional 2D. The surgeon’s preference was analyzed through subjective questionnaires at the end of the operation in 11 studies. An association to 3D systems and a general satisfaction of the surgeon was observed by Kinoshita. Similarly, all the studies where a questionnaire was performed at the end of the operation reported better precision, enhanced surgical planes’ definition, improved depth perception, and reduced workload. Of the studies that evaluated the surgeon’s preference, seven (70%) had a single surgeon performing the operations and answering the questionnaire, three had two or more teams operating, and nine (90%) of them were randomized studies.
Randomized, Low Risk Studies
Seven randomized articles [13, 18,19,20, 26, 29, 34] had a low risk of bias after the Cochrane RoB score was applied. The results for intra-operative and post-operative factors of these articles are presented in Appendix Table 3. Only one article did not demonstrate a lower operative time with 3D systems. From those that did, two demonstrated lower operative time when a novice was performing the procedure [20, 26] and one study demonstrated lower operative time in one of the procedures performed (gastric bypass) [18]. From the seven articles, 2 demonstrated an association between lower blood loss and 3D systems [26, 29]. In this selection of low risk randomized articles, no differences were observed in any of the studies when it comes to conversion to open surgery, intra-operative complications, morbidity, length of stay, and oncological outcomes.
Discussion
3D systems for endoscopic surgery have three very important potential benefits: better performance, faster learning curve, and reduced workload. Since the appearance of the last generation of 3D equipment, characterized by bi-channeled scopes, simultaneous high definition display of the image for the right and left eye, and light polarized glasses for the surgeon and all the surgical team, many experimental studies addressed these advantages with quite evidence of superiority for 3D systems. However, clinical studies are scarce and with methodological limitations.
Performance: Time and Errors
The majority of studies included in this revision showed a reduction in total operative time, regardless of the surgeons’ expertise. Of particular interest is the fact that critical steps in specific complex operations benefit significantly with 3D systems. This was observed during different surgical steps between the articles included such as the time of warm ischemia and suturing during partial nephrectomy in the studies of Yuan[43] and Komatsuda [39], the performance on anastomotic and suturing in six of the presented articles [14, 18, 19, 27, 41, 42], or the lymphadenectomy procedure in three articles [25,26,27].
No clear benefit of decreased complications during surgery was observed. However, it is important to address that surgical procedures may have important variability of its own and that this variability was not addressed in most of the included articles, even though the procedure to be compared was the same. For instance, when comparing performance, surgeons could try and quantify the difficulty of the surgery performed on individual level; in this case, it would be possible, for instance to separate surgery performed for an acute cholecystitis with difficult dissection, necessity of biliar exploration, and significant adhesions and fibrosis than a linear, early onset cholecystitis with a less technical demanding procedure. This could be important in a near future when performing comparative studies to analyze different techniques or technologies. Even though, standard protocoled surgeries performed by well-experienced surgeons probably will reveal errors or significant intra-operative complication differences in larger series or in the presence of unexpected intra-operative scenarios. Anyway, when analyzing the studies that detail total blood loss account, in 23% of the studies [14, 21, 23, 34, 36], the total blood loss account was significantly less when 3D systems were used. In the group of controlled randomized trials with low risk of bias, 29% of the studies [18, 21] referred a significant reduction in the total blood loss account, when 3D technology was applied (Appendix Table 3).
Learning Curve
Although most participants had experience in 2D, the same was not true with 3D technology. Immediate use with clear improvements in performance, as well as no harm to patients with the use of 3D, shows that the application of these systems has no learning curve.
In what concerns to novices learning curve, several studies addressed this particular topic. In a prospective randomized clinical trial ran by Fanfani and colleagues [26], the operative time of pelvic lymphadenectomy performed by surgeons with less than 10 procedures done was significantly lower in the group using 3D technology. The authors state that 3D may help in the learning curve for novice surgeon for difficult steps. In the retrospective cohort study of Esther Padin and colleagues [37], 312 consecutive patients who underwent bariatric surgery were included. Of these, 141 were operated by three surgeons with less than 50 surgeries before the beginning of the study. A significant difference in terms of total time (p < 0.005) and complications (p = 0.034) favoring the use of 3D was observed. The authors suggest that the total number of procedures to be proficient in gastric bypass or sleeve gastrectomy could be significantly reduced with the availability of 3D systems in bariatric surgery training centers.
Another example of clear benefit of 3D systems for novices is well evident in the prospective randomized trial of Curro with a series of laparoscopic cholecystectomies [20]. Forty consecutive operations for uncomplicated gallstone disease were performed by a single novice surgeon (around 20 previous laparoscopic cholecystectomies) using either 2D or 3D systems. There was a significant difference favoring 3D, in the time for Calot’s triangle dissection (p = 0.03), gallbladder removal (p = 0.02), and complete procedure (p = 0.02). Moreover, the total time of all operations after the 9th case with 3D system was in the time range of an experienced surgeon that participated in the same study. On the contrary, all twenty procedures done with 2D system were above that range (Appendix Table 6).
Workload: Strain, Feasibility, and Preference
It is known that surgeon’s comfort while performing laparoscopic procedures is of utmost importance to reduce the rate of error, complications, and burnout. In the clinical studies compiled in this revision, single procedures are included and compared, but no data about cumulative strain after several laparoscopic surgeries is available. In fact, to avoid bias depending on surgeon’s warming-up and strain, the procedure selection for comparison is the first of the day in some studies [18]. Anyway, there is sufficient evidence that 3D technology can reduce fatigue and be preferred by most surgeons.
Different methodologies are used in the evaluation of surgical strain. Kinoshita [15] studied the use of 2D compared with 3D systems in a high-demand grade III procedure (radical prostatectomy). In this multicenter controlled randomized study with 122 patients, feasibility of basic tasks and fatigue of surgeons and scopists were evaluated. For feasibility measurement, questionnaires using 7-point Likert scale were used (from none or worst (0) to excellent (6)). This subjective evaluation showed 3D imaging was better, namely recognizing needle direction, precise position of the target tissue, and in the recognition of various fine structures. Regarding fatigue appraisal, two methods were used before and just after the procedure; one was the Simulation Sickness Questionnaire (SSQ) and the other the Critical Flicker Fusion test (CFF) for eye fatigue evaluation. These two tests were similar between groups, which means that at least, actual HD 3D image does not increase fatigue when compared to HD 2D systems.
Considering the controlled randomized trials with low risk bias included in the systematic review, six out of seven addressed the topic of preference, strain, and feasibility evaluation [13, 18,19,20, 29, 34]. All these 6 used non-validated questionnaires rating the answers on a scale of 3–5, and observed undeniable advantage for 3D systems. Better depth perception was found in all of them, but also better definition of planes[18, 19, 34], better precision[13, 18, 19], and better hand-eye coordination and image quality[13] was observed. Two studies of Curro and Navarra [18, 19] subjected surgeons to a 5-point questionnaire considering fatigue at the end of the operations. Significant better scores with 3D systems were observed for the evaluation of neck strain, and eye strain in sleeve gastrectomy, gastric bypass, and right colectomy. In this same paper, it was found that feasibility and fatigue advantages with 3D systems were noted particularly during longer periods of surgery.
In the publication of Pakantar and colleagues [41], a senior surgeon intervened on 108 patients subjected to urological procedures that were randomly assigned to either 2D or 3D laparoscopic surgeries. They used the well validated test, State-Trait Anxiety Inventory for Adults (STAI-6) Short version, to quantify emotional, physical, and cognitive aspects of stress experienced during each operative procedure. There was a significant STAI score difference favoring the use of 3D system (p < 0.0001) for the entire group, as well as for each of the patient subgroups (simple nephrectomy, pyeloplasty, and radical nephrectomy).
First- and second-generation 3D systems were criticized to have frequent adverse effects on their users. The seminal randomized study by George Hanna with grade I surgeries (cholecystectomies) used first-generation (shutter-glasses) systems and significant adverse symptoms like visual strain, headache, and facial discomfort were reported by surgeons [36]. Also complains of head-mounted systems were pointed out. These heavy systems were uncomfortable for surgeons that frequently referred headaches and dizziness, about everything when they need to peer through the side vents of the helmet to see the operating room. New generation equipment offers a much more wearable light polarized glasses with high-definition image. Although none of the 7 controlled randomized trials with low risk of bias included in the review referred increased undesirable effects with 3D systems, still some conflicting results appeared in some studies. The study of Lui and colleagues [30] include grade I complexity surgery (ovarian cystectomy) and no data were given concerning stereo-acuity selection of participants and conditions of visualization of the screen. As stated in the review of Scinichiro Sakata [9], “suboptimal viewing conditions caused by head tilt from display elevation and acute eccentric viewing angles increase crosstalk by incorrect orientation of glasses relative to the display”. Likewise, Sakata underlines that high levels of crosstalk reduces stereoacuity and raised the rate of fatigue, motion sickness, and other symptoms.
A considerable variety of studies were selected for this review. Ten of 30 (30%) papers are Chinese studies, 10 countries are represented, and 3 continents with just one study from an American country (Canada). Some general criticisms can be made of the selection of existing works. First, only 7 out of 30 (23%) are CRT with low risk of bias. Second, surgeon’s expertise in 2D laparoscopic surgery was quantified in a small number of studies (23%), and yet expertise criteria was widely variable. Third, more than half of the studies (60%) include operations performed by a single surgeon. Fourth, only a minority of studies select participants for their stereo acuity (13%) and standardized visualization conditions (20%). Five, no single study analyzes the rate of minor events during surgery.
Minor events like miss the target in needle puncture, or fail to grasp a specific structure, are associated with a higher incidence of major intra-operative complications. The study of these inconsequential incidents could be an important and definitive way to establish 3D technology as safer. At the same time, it is uncertain if minor events that may induce complications can have a significant impact capable of being observed in a prospective or randomized trial. As noted in our results, although no study could find significant less complications favoring 3D, a total number of morbidities was verified (2D:30 vs. 3D:13). Perhaps an increase in the sample size and power could show a small difference in complications when using different systems. On the other hand, only one of the included articles classified complications according to a validated score. Predictably, no difference was found on hospital length of stay, except scientific works with low level of evidence. As referred in same articles, an impediment to the implementation of 3D systems in public hospitals may be the differential in cost. A limitation of our article was that we did not performed a cost calculation; however, it is the opinion of the authors that this difference in cost may be diluted along the years, especially when the benefits of 3D systems are confirmed, with articles such as the one presented here.
This revision used only comparative studies published on the last 5 years. Regarding the difficulties encountered when performing research about the different imaging systems for surgeons, the feeling of the authors is that besides there being a significant amount of recent clinical studies evaluating the benefits of 3D endoscopic procedures, there is still an important outcome variability and lack of consensus when it comes to assessment scores used. Methodological refinements from correct stratification of participants according to their stereoscopic acuity and experience, conditions of display visualization, multiple surgeons and multiple centers involvement, performance fine evaluation (like interpretation of an unexpected scenario), feasibility and performance precision (like recorded instrument-movement evaluation and minor events), and validated strain and fatigue measurement after a single operation and at the end of the day are examples of measures that may help to improve randomized studies in the future. Therefore, one could more fully demonstrate the superiority and benefit of 3D systems.
In conclusion, this systematic review presents the current knowledge on clinical use of 3D systems for endoscopic surgery. Significant scientific evidence puts 3D technology with advantages in surgeon performance, learning curve, and fatigue. More well-designed and powerful studies are needed to assess the clinical impact of these benefits on surgical performance.
Data Availability
Not applicable.
References
Sakata S, Watson MO, Grove PM, Stevenson ARL. The conflicting evidence of three-dimensional displays in laparoscopy a review of systems old and new. Ann Surg. 2016;263:234–9. https://doi.org/10.1097/SLA.0000000000001504.
Durrani AF, Preminger GM. Three-dimensional video imaging for endoscopic surgery. Comput Biol Med. 1995;25:237–47. https://doi.org/10.1016/0010-4825(95)00001-K.
Stewart L, Way LW. The prevention of laparoscopic Bile duct injuries: An analysis of 300 cases of from a human factors and cognitive psychology perspective. Proc Hum Factors Ergon Soc. 2007;2:617–20. https://doi.org/10.1177/154193120705101103.
Cicione A, Autorino R, Breda A, De Sio M, Damiano R, Fusco F, et al. Three-dimensional vs standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills. Urology. 2013;82:1444–50. https://doi.org/10.1016/j.urology.2013.07.047.
Storz P, Buess GF, Kunert W, Kirschniak A. 3D HD versus 2D HD: Surgical task efficiency in standardised phantom tasks. Surg Endosc. 2012;26:1454–60. https://doi.org/10.1007/s00464-011-2055-9.
El Boghdady M, Ramakrishnan G, Tang B, Alijani A. A comparative study of generic visual components of two-dimensional versus three-dimensional laparoscopic images. World J Surg. 2018;42:688–94. https://doi.org/10.1007/s00268-017-4220-3.
Wagner OJ, Hagen M, Kurmann A, Horgan S, Candinas D, Vorburger SA. Three-dimensional vision enhances task performance independently of the surgical method. Surg Endosc. 2012;26:2961–8. https://doi.org/10.1007/s00464-012-2295-3.
Tanagho YS, Andriole GL, Paradis AG, Madison KM, Sandhu GS, Varela JE, et al. 2D versus 3D visualization: impact on laparoscopic proficiency using the fundamentals of laparoscopic surgery skill set. J Laparoendosc Adv Surg Tech. 2012;22:865–70. https://doi.org/10.1089/lap.2012.0220.
Sakata S, Grove PM, Hill A, Watson MO, Stevenson ARL. Impact of simulated three-dimensional perception on precision of depth judgements, technical performance and perceived workload in laparoscopy. Br J Surg. 2017;104:1097–106. https://doi.org/10.1002/bjs.10528.
Vilaça J, Leite M, Correia-Pinto J, Högemann G, Costa P, Leão P. The influence of 3D in single-port laparoscopy surgery: an experimental study. Surg Laparosc Endosc Percutaneous Tech. 2018;28:261–6. https://doi.org/10.1097/SLE.0000000000000536.
Honeck P, Wendt-Nordahl G, Rassweiler J, Knoll T. Three-dimensional laparoscopic imaging improves surgical performance on standardized ex-vivo laparoscopic tasks. J Endourol. 2012;26:1085–8. https://doi.org/10.1089/end.2011.0670.
Kong SH, Oh BM, Yoon H, Ahn HS, Lee HJ, Chung SG, et al. Comparison of two- and three-dimensional camera systems in laparoscopic performance: a novel 3D system with one camera. Surg Endosc. 2010;24:1131–43. https://doi.org/10.1007/s00464-009-0740-8.
Sahu D, Mathew MJ, Reddy PK. 3D laparoscopy—help or hype; initial experience of a tertiary health centre. J Clin Diagn Res. 2014;8:13–5. https://doi.org/10.7860/JCDR/2014/8234.4543.
Aykan S, Singhal P, Nguyen DP, Yigit A, Tuken M, Yakut E, et al. Perioperative, pathologic, and early continence outcomes comparing three-dimensional and two-dimensional display systems for laparoscopic radical prostatectomy-a retrospective, single-surgeon study. J Endourol. 2014;28:539–43. https://doi.org/10.1089/end.2013.0630.
Kinoshita H, Nakagawa K, Usui Y, Iwamura M, Ito A, Miyajima A, et al. High-definition resolution three-dimensional imaging systems in laparoscopic radical prostatectomy: randomized comparative study with high-definition resolution two-dimensional systems. Surg Endosc. 2015;29:2203–9. https://doi.org/10.1007/s00464-014-3925-8.
Li Z, Li JP, Qin X, B Bin X, Han YD, Da LS, et al. Three-dimensional vs two-dimensional video assisted thoracoscopic esophagectomy for patients with esophageal cancer. World J Gastroenterol. 2015;21:10675–82. https://doi.org/10.3748/wjg.v21.i37.10675.
Velayutham V, Fuks D, Nomi T, Kawaguchi Y, Gayet B. 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc. 2016;30:147–53. https://doi.org/10.1007/s00464-015-4174-1.
Currò G, La Malfa G, Caizzone A, Rampulla V, Navarra G. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic bariatric surgery: a Single-Surgeon Prospective Randomized Comparative Study. Obes Surg. 2015;25:2120–4. https://doi.org/10.1007/s11695-015-1674-y.
Currò G, Cogliandolo A, Bartolotta M, Navarra G. Three-dimensional versus two-dimensional laparoscopic right hemicolectomy. J Laparoendosc Adv Surg Tech. 2016;26:213–7. https://doi.org/10.1089/lap.2015.0557.
Currò G, La Malfa G, Lazzara S, Caizzone A, Fortugno A, Navarra G. Three-dimensional versus two-dimensional laparoscopic cholecystectomy: Is surgeon experience relevant? J Laparoendosc Adv Surg Tech. 2015;25:566–70. https://doi.org/10.1089/lap.2014.0641.
Tao K, Liu X, Deng M. Three-dimensional against 2-dimensional laparoscopic. 2016;26:324–7.
Lara-Domínguez MD, López-Jiménez A, Grabowski JP, Arjona-Berral JE, Zapardiel I. Prospective observational study comparing traditional laparoscopy and three-dimensional laparoscopy in gynecologic surgery. Int J Gynecol Obstet. 2017;136:320–4. https://doi.org/10.1002/ijgo.12078.
Raspagliesi F, Bogani G, Martnelli F, Signorelli M, Scaffa C, Sabatucci I, et al. 3D vision improves outcomes in early cervical cancer treated with laparoscopic type B radical hysterectomy and pelvic lymphadenectomy. Tumori. 2017;103:76–80. https://doi.org/10.5301/tj.5000572.
Agrusa A, di Buono G, Chianetta D, Sorce V, Citarrella R, Galia M, et al. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic adrenalectomy: A case-control study. Int J Surg. 2016;28:S114–7. https://doi.org/10.1016/j.ijsu.2015.12.055.
Tang FJ, Qi L, Jiang HC, Tong SY, Li Y. Comparison of the clinical effectiveness of 3D and 2D imaging systems for laparoscopic radical cystectomy with pelvic lymph node dissection. J Int Med Res. 2016;44:613–9. https://doi.org/10.1177/0300060515621445.
Fanfani F, Rossitto C, Restaino S, Ercoli A, Chiantera V, Monterossi G, et al. How technology can impact surgeon performance: a randomized trial comparing 3-dimensional versus 2-dimensional laparoscopy in gynecology oncology. J Minim Invasive Gynecol. 2016;23:810–7. https://doi.org/10.1016/j.jmig.2016.03.020.
Kanaji S, Suzuki S, Harada H, Nishi M, Yamamoto M, Matsuda T, et al. Comparison of two- and three-dimensional display for performance of laparoscopic total gastrectomy for gastric cancer. Langenbeck's Arch Surg. 2017;402:493–500. https://doi.org/10.1007/s00423-017-1574-9.
Leon P, Rivellini R, Giudici F, Sciuto A, Pirozzi F, Corcione F. 3D vision provides shorter operative time and more accurate intraoperative surgical performance in laparoscopic hiatal hernia repair compared with 2D vision. Surg Innov. 2017;24:155–61. https://doi.org/10.1177/1553350616687434.
Zheng CH, Lu J, Zheng HL, Li P, Xie JW, Bin WJ, et al. Comparison of 3D laparoscopic gastrectomy with a 2D procedure for gastric cancer: a phase 3 randomized controlled trial. Surg (United States). 2018;163:300–4. https://doi.org/10.1016/j.surg.2017.09.053.
Lui MW, Cheung VYT. Three-dimensional versus two-dimensional laparoscopy for ovarian cystectomy: a prospective randomised study. Hong Kong Med J. 2018;24:245–51. https://doi.org/10.12809/hkmj176846.
Yoon J, Il KS, Kim MH, Kim MJ, Oh HK, Kim DW, et al. Comparison of short-term outcomes between 3D and 2D imaging laparoscopic colectomy with D3 lymphadenectomy for colon cancer. J Laparoendosc Adv Surg Tech. 2019;29:340–5. https://doi.org/10.1089/lap.2018.0317.
Wang Y, Chen W, Xia S, Wang T, Wang S, Zhang F, et al. Three-dimensional versus two-dimensional laparoscopic-assisted transanal pull-through for hirschsprung’s disease in children: preliminary results of a prospective cohort study in a tertiary hospital. J Laparoendosc Adv Surg Tech. 2019;29:557–63. https://doi.org/10.1089/lap.2018.0537.
Yang C, Mo L, Ma Y, Peng G, Ren Y, Wang W, et al. A comparative analysis of lung cancer patients treated with lobectomy via three-dimensional video-assisted thoracoscopic surgery versus two-dimensional resection. J Thorac Dis. 2015;7:1798–805. https://doi.org/10.3978/j.issn.2072-1439.2015.10.59.
Yang CL, Wang W, Mo LL, Zhang L, Peng GL, Yu ZW, et al. Short-term outcome of three-dimensional versus two-dimensional video-assisted thoracic surgery for benign pulmonary diseases. Ann Thorac Surg. 2016;101:1297–302. https://doi.org/10.1016/j.athoracsur.2015.10.042.
Jiao P, Wu QJ, Sun YG, Ma C, Tian WX, Yu HB, et al. Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer. 2017;8:3–7. https://doi.org/10.1111/1759-7714.12387.
Dong S, Yang XN, Zhong WZ, Nie Q, Liao RQ, Lin JT, et al. Comparison of three-dimensional and two-dimensional visualization in video-assisted thoracoscopic lobectomy. Thorac Cancer. 2016;7:530–4. https://doi.org/10.1111/1759-7714.12361.
Padin EM, Santos RS, Fernández SG, Jimenez AB, Fernández SE, Dacosta EC, et al. Impact of Three-Dimensional Laparoscopy in a Bariatric Surgery Program: Influence in the Learning Curve. Obes Surg. 2017;27:2552–6. https://doi.org/10.1007/s11695-017-2687-5.
Bagan P, De Dominicis F, Hernigou J, Dakhil B, Zaimi R, Pricopi C, et al. Complete thoracoscopic lobectomy for cancer: comparative study of three-dimensional high-definition with two-dimensional high-definition video systems. Interact Cardiovasc Thorac Surg. 2015;20:820–4. https://doi.org/10.1093/icvts/ivv031.
Komatsuda A, Matsumoto K, Miyajima A, Kaneko G, Mizuno R, Kikuchi E, et al. Technical improvement using a three-dimensional video system for laparoscopic partial nephrectomy. Asian Pac J Cancer Prev. 2016;17:2475–8. https://doi.org/10.7314/APJCP.2016.17.5.2475.
Abou-Haidar H, Al-Qaoud T, Jednak R, Brzezinski A, El-Sherbiny M, Capolicchio JP. Laparoscopic pyeloplasty: initial experience with 3D vision laparoscopy and articulating shears. J Pediatr Urol. 2016;12:426.e1–5. https://doi.org/10.1016/j.jpurol.2016.08.027.
Patankar SB, Padasalagi RG. Three-dimensional versus two-dimensional laparoscopy in urology: A randomized study. Indian J Urol. 2017;33:207–14. https://doi.org/10.4103/iju.IJU.
Bove P, Iacovelli V, Celestino F, De Carlo F, Vespasiani G, Agrò EF. 3D vs 2D laparoscopic radical prostatectomy in organ-confined prostate cancer: Comparison of operative data and pentafecta rates: A single cohort study. BMC Urol. 2015;15:4–11. https://doi.org/10.1186/s12894-015-0006-9.
Ruan Y, hai WX, Wang K, yang ZY, jie XS, D liang X. Clinical evaluation and technical features of three-dimensional laparoscopic partial nephrectomy with selective segmental artery clamping. World J Urol. 2016;34:679–85. https://doi.org/10.1007/s00345-015-1658-5.
Code Availability
Not applicable.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the design of this study, acquisition, and analysis of data. All the authors participated in revising it critically for intellectual content and for final version to be published.
Corresponding author
Ethics declarations
Ethics Approvals and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Publication
The authors consent this study publication.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Vilaça, J., de Azevedo, J.M., Louro, H.C. et al. Clinical Use of Third-Generation 3D Imaging Systems in Endoscopic Surgery—a Systematic Review. SN Compr. Clin. Med. 3, 879–896 (2021). https://doi.org/10.1007/s42399-021-00774-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00774-x