Abstract
In this review, we summarize advances in knowledge derived from the application of magnetic resonance imaging (MRI)-based techniques to patients with multiple sclerosis (MS) published in the Journal of Neurology over the past year. We highlight the pivotal role played by conventional MRI techniques for a correct and early diagnosis of this condition and the exclusion of alternative disorders. Advanced MR methods have contributed to demonstrating how damage to selected brain structures is related to disease clinical manifestations, thus contributing to overcome the well-known “clinical-radiological” paradox of MS, and ameliorating the understanding of the mechanisms underlying the accumulation of irreversible disability. Finally, we discuss the use of MRI to assess treatment efficacy and optimize therapeutic approaches in this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to its exquisite sensitivity in detecting multiple sclerosis (MS) abnormalities, magnetic resonance imaging (MRI) has become an established tool to diagnose this disease and to monitor its evolution. MRI has been formally included in the diagnostic workup of patients at presentation with clinically isolated syndromes (CIS) suggestive of MS, and ad hoc criteria have been proposed and are updated on a regular basis [40]. On the contrary, in patients with established MS, the ability of MR measures to explain patients’ clinical status and progression of disability is still suboptimal [15]. This has prompted the development and extensive application of modern MR-based technologies to estimate overall MS burden in patients at different stages of the disease.
In this review, we report the most recent findings from papers published in the Journal of Neurology over the past year for a correct and early diagnosis of MS, a better understanding of the pathophysiological processes underlying disability accumulation as well as monitoring and optimization of therapeutic approaches in this condition.
Diagnosis, differential diagnosis and early prognosis
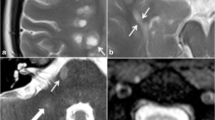
The diagnosis of MS is based on the demonstration of disease dissemination in space and time as well as the exclusion of alternative neurological conditions. As discussed in a review article published in the May issue, the differential diagnosis of MS includes a virtually endless list of potential mimickers, encompassing infectious, inflammatory, rheumatologic, metabolic, nutritional, and degenerative entities [10]. MRI is one of the most helpful tools for excluding alternative disorders that may mimic MS in routine clinical practice. Indeed, focal MS lesions have some typical features in terms of appearance, distribution, extension, and shape that may support the diagnosis or direct the physician toward alternative etiologies. Brain MS lesions are frequently located asymmetrically in the periventricular and juxtacortical white matter (WM), the corpus callosum (CC), and infratentorial areas (with the pons and cerebellum more frequently affected than the medulla and midbrain), and they are sometimes characterized by oval or elliptical shapes [34]. A series of MRI “red flags,” derived from evidence-based findings and educated guesses, have also been identified in the setting of clinically suspected MS, which should alert the clinicians to prompt the performance of “non-routine” tests and to reconsider the differential diagnosis more extensively [7, 32]. Despite this, in a few cases a correct diagnosis may be challenging. For instance, in the September issue, Kleinfeld et al. [28] described five middle-aged patients with progressive spastic quadriparesis and dementia who were initially diagnosed as suffering from MS. Brain MRI showed diffuse T2 hyperintensities in the subcortical and periventricular WM, without gadolinium (Gd) enhancement, and diffuse cerebral and CC atrophy. All these cases had a normal cerebrospinal fluid (CSF) examination. Pathological assessment (based on biopsy or autopsy) and genetic analysis led to a diagnosis of adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia. Although the diagnosis of this condition is based on genetic analysis, some of the MRI features found in these patients were not typical for MS, including the presence at MRI of extensive symmetric confluent WM changes and the absence of Gd enhancement.
Considering the frequent involvement of the spinal cord in MS, MRI features of MS cord lesions have also been identified [5]. MS lesions in the cord are more frequently observed in the cervical than in other sections, are usually peripheral, limited to two vertebral segments in length or less, occupy less than half the cross-sectional area of the cord, and are not seen as T1-hypointensities. Acute plaques can cause a swelling of the cord and enhance after Gd administration. In the July issue, Patrucco and coworkers showed that the quantification of spinal cord lesions contributes to identifying CIS patients with a higher risk of developing definite MS independently of the presence of oligoclonal bands (OB) and an abnormal brain MRI, with a threefold increase if there is only one lesion and almost sixfold increase if there is more than one lesion in this population [38].
In some conditions, the features of cord lesions can help in the differential diagnosis from MS. For instance, in neuromyelitis optica, myelitis is usually accompanied in the acute phase by a T2-weighted spinal cord lesion extending over three or more spinal segments, which may be hypointense on T1-weighted MRI and associated with varying degrees of Gd-enhancement [7, 32]. There are, however, selected cases, in which MRI is not helpful. Nociti et al. [33] described ten patients with partial or complete transverse myelitis, preceded 2–3 weeks before by an infectious event. At disease onset, spinal cord MRI showed lesions extending for less than two segments and at follow-up detected a progressive spinal cord atrophy without formation of any new lesion. All these patients experienced an increased visual evoked potential (VEP) latency and two or more clinical episodes of myelitis, evolving later in a progressive clinical course. Although these patients did not fulfill the criteria for a diagnosis of MS [40], since it was not possible to make an alternative diagnosis, the diagnosis of an atypical form of MS was made.
Optic neuritis (ON) is frequently the first clinical manifestation in CIS patients suspected of having MS. The risk of developing MS in these patients is increased if multiple WM lesions are detected [1]. VEPs are abnormal in 80–90 % of ON patients at onset, but this finding is not specific [25]. On the contrary, an abnormal CSF examination (IgG OBs and an elevated IgG index) in these patients has a high specificity for the presence of a demyelinating condition. In the December issue, Horwitz et al. [26] showed that ON patients with normal MRI and VEP had a 96 % probability of a normal CSF examination, suggesting a limited role of this analysis in the evaluation of this subgroup of patients.
Understanding MS clinical manifestations and heterogeneity
In patients with definite MS, the strength of the association of conventional MRI findings with the subsequent clinical manifestations of the disease remains modest, at best. This is likely due to the relative lack of specificity of conventional MRI in the evaluation of the heterogeneous pathological substrates of the disease, its inability to provide accurate estimates of damage outside focal lesions, and the fact that it cannot be used to identify the mechanisms through which the central nervous system recovers after tissue injury has occurred. Structural, metabolic, and functional MR techniques have provided new markers, more closely linked to the pathological features of the disease, which may in part overcome the aforementioned limitations of conventional MRI. The use of these techniques is also likely to contribute in characterizing the phenotypical manifestations of MS, such as the primary progressive (PP) MS [45]. As recently reviewed in the April issue [45], the application of quantitative and functional imaging techniques has led to hypothesize that the rate of accumulation of disability in PPMS might not only be a function of tissue loss, but also of progressive failure of the adaptive capacity of the cortex.
As discussed by a recent review published in this journal, these techniques might also contribute to shed light on the potential role of energy failure as an important cause of disability in MS [35], thus providing new outcome measures for trials of novel treatments. As pointed out in the October issue, further gains for the understanding of MS pathophysiology are likely to derive from the use of resting state fMRI [11], which provides task-free and unbiased findings on the role of brain reorganization in this condition, as well as from improvements in methods of analysis, which have allowed assessing the distribution of damage in the normal-appearing (NA) WM and gray matter (GM) at a regional level. However, the future of MRI is likely to reside in the use of ultra-high field scanners. Several studies performed at 7.0 T showed the ability of MRI to depict the morphological characteristics of MS lesions at a resolution that resembles that of pathological assessment. These studies also allowed a better definition of the relationship between demyelinating lesions and the deep venous system to be achieved and showed in vivo that MS plaques are centered around the microvasculature. In addition, ultra-high field MRI has the potential to improve quantitative, metabolic, and fMRI studies of MS [15].
Clinical disability
The poor pathological specificity of conventional MRI measures, as well as the intrinsic limitations of the clinical scale that is most commonly applied to measure disability in MS, the Expanded Disability Status Scale (EDSS), which is strongly weighted toward impairment of ambulation for values higher than 4.0 [29], is thought to explain the so-called clinical/MRI paradox of MS.
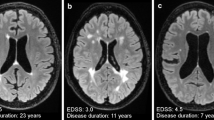
In the October issue, Hackmack et al. [18] applied multivariate analysis algorithms to investigate to what extent it was possible to predict individual disease profiles from conventional MRI measures of 40 MS patients. They demonstrated that local tissue intensity patterns extracted from conventional MRI of MS patients encode clinically relevant information about symptom severity (disease duration and motor and cognitive dysfunction) and overall clinical disability. Importantly, these patterns were more informative than the total lesion load.
The use of objective and quantitative measures of disability may be a valuable strategy to overcome the MS paradox. In this respect, grip force variability (GFV) has been proposed as a measure of motor disability in these patients [42]. In a preliminary study of 27 MS patients published in the August issue [42], increased GFV was correlated with reduced fractional anisotropy of WM in the vicinity of the somatosensory and visual cortices. GFV also correlated, but to a lesser degree, with the EDSS.
In the August issue, a multiparametric MRI study of 117 MS patients, which compared conventional MRI measures of lesions and atrophy with diffusion tensor (DT) MRI and magnetization transfer (MT) MRI metrics derived from three tracts of interest (CC, corticospinal tract and optic radiation) for the prediction of patients' disability, showed that DT MRI indices in the optic radiations were more strongly correlated with EDSS and with low-contrast visual acuity than conventional MRI measures [19]. These findings suggest that integration of more disability-specific measures into clinical trials, as opposed to global markers of pathology, may be a rewarding exercise [19].
Cognitive impairment, fatigue, and quality of life
Cognitive impairment affects a large proportion of MS patients, with a prevalence rate ranging from 40 to 70 % [8, 41]. Several studies have consistently demonstrated that GM atrophy, which starts early and in distinct cortical regions in MS, is related to cognitive impairment [2, 6]. Furthermore, assessment of GM structures critical for specific cognitive functions could provide additional pieces of information. In line with this, hippocampal atrophy has been associated with deficits in memory encoding and retrieval [48]. In the January issue, the relationship between deep GM atrophy and slowing of information-processing speed (IPS), quantified using the Paced Auditory Serial Addition Test (PASAT) and the Symbol Digit Modalities Test (SDMT), was explored [4]. After controlling for the effect of neocortical volume, poor performance at SDMT was associated with atrophy of the putamen and thalamus, whereas PASAT performance correlated with atrophy of the putamen only.
Fatigue is another frequent complaint of MS patients, leading to a severely impaired quality of life. The pathophysiology of MS-related fatigue is not fully understood. However, functional MRI studies [14, 46] have demonstrated an abnormal recruitment of fronto-thalamic circuits in MS patients with this symptom, thus supporting its central origin. Consistent with this, voxel-based morphometry (VBM) [43, 47] and DT MRI [37] studies have shown atrophy [43, 47] and reduced fractional anisotropy values [37] in regions of the frontal lobes in fatigued MS patients. A recent investigation published in this journal showed that progression of CC atrophy over a mean period of 5 years after the diagnosis of MS is associated with the occurrence and severity of fatigue, independently of the degree of disability [53].
An important aspect that remains to be established is whether MRI-detected brain pathology may have an impact on quality of life (QoL). In the February issue, by analyzing the clinical and MRI data of secondary progressive (SP) MS patients included in a double-blinded placebo-controlled trial using lamotrigine (a sodium channel blocker), Hayton et al. [22] found that clinical and MRI measures have a limited association with QoL. Timed walk (TW) was the only clinical measure independently correlated with the physical component of the MS impact scale (MSIS), an index of the impact of MS on patients’ QoL. The best model that explained the psychological component (MSIS-psych) of the MSIS included the performance at PASAT and T1/T2 lesion volume (LV) ratio. Over a 2-year period, TW and MT MRI measures of damage to the NAWM and GM correlated with changes in the MSIS.
MRI to monitor MS treatment
In patients with MS, disease activity measured using dual-echo and post-contrast T1-weighted scans is higher than what would be detected by clinical assessment of relapses. This is why these MRI measures are used for monitoring response to treatment. In the context of clinical trials, MRI is used as a primary outcome measure in phase II studies, where serial scans are acquired to measure disease activity (new/enlarged T2 lesions, Gd-enhancing lesions) [3]. In phase III trials, given the uncertainty of the relationship between conventional MRI and clinical evolution, imaging measures (absolute or percentage increases in total T2 lesion load) are used as secondary outcomes and typically performed on yearly scans [12]. Despite this, two studies have shown that conventional MRI measures of disease activity are valid surrogate markers of clinical activity [17, 49]. In addition, recent meta-analyses [49, 50] of randomized, placebo-controlled clinical trials of relapsing-remitting (RR) MS found a strong correlation between the effect of treatment on relapses and on MRI activity; this relationship was weaker when considering EDSS worsening.
It is clear that the assessment of response to treatment with MRI requires a rigorous and validated approach, with standardized imaging protocols and consistent evaluation procedures.
At present, the monitoring of an individual MS patient’s response to treatment using MRI measures remains a challenge. First line treatments in MS include interferons (IFN) and glatiramer acetate (GA). In the past few years, several new classes of drugs have been approved and are currently available. Among them, natalizumab is an α4-integrin antagonist approved for RRMS patients that has shown a marked effect in reducing the annualized relapse rate and the risk of sustained disability progression [39]. In patients treated with natalizumab, the concept “disease activity free” has been defined as no clinical (relapses and disability progression) or radiological (Gd-enhancing lesions and new/enlarging T2-hyperintense lesions) activity [21]. A study published in the June issue [31] assessed the radiological and clinical efficacy of natalizumab treatment over a 2-year period in 180 RRMS patients and compared it with results previously obtained in the AFFIRM trial [21]. In this study, the proportion of patients remaining free from clinical activity was lower than in the AFFIRM study (37.8 vs. 64.3 %), whereas the proportion of patients remaining free from radiological activity was higher than in the AFFIRM study (68.9 vs. 57.7 %). Overall, the proportion of patients remaining free from disease activity during the 2 years of treatment was comparable to the AFFIRM study (33.3 vs. 36.7 %). These results suggest that natalizumab is effective in a real-life setting and have important practical implications, considering the risk of progressive multifocal leukoencephalopathy (PML) associated with such a treatment [54]. Indeed, as discussed in a recent review [24], there is an urgent need to develop surrogate markers that may help to define the risk of PML in individual patients. Since the risk of PML increases after 2 years of treatment [30], a risk management plan has been established by the European Medicines Agency and the US Food and Drug Administration. An early identification of MRI patterns suggestive of this condition in natalizumab-treated patients is also critical. As pointed out in a recent paper that included some of the MS patients who developed post-natalizumab PML, the MRI features observed in these patients are different from those that have been described in patients developing PML following HIV infection. In particular, the presence of contrast enhancement and the subcortical location of lesions may be useful for making an early diagnosis of PML in natalizumab-treated MS patients [55].
At present, reports on recurrence of disease activity after natalizumab discontinuation are controversial [51, 52]. In a study published in this journal, Havla et al. [20] described disease activity in 13 MS patients who stopped natalizumab therapy and either remained without disease-modifying therapy (DMT) or switched to GA. In both groups, patients presented with relapses and marked MRI activity within 12 months after cessation of natalizumab. Three patients without DMT and one patient on GA experienced severe relapses with sustained EDSS worsening, suggesting a possible rebound of disease activity after natalizumab has been withdrawn.
Modern MR techniques still need to be validated and evaluated in the context of treatment monitoring. MT MRI has been incorporated into clinical trials to provide additional outcome measures in limited subgroups of patients. Lamotrigine has been tested as a possible neuroprotective agent. In a 2-year, randomized, double-blinded, placebo-controlled trial of lamotrigine in SPMS, no effect was detected on central cerebral volume, which was the primary end-point of the study [27]. In the March issue, MT MRI changes in brain NAWM, GM, and focal T2 were assessed in the same cohort of patients [23], and, disappointingly, a significant reduction of MT MRI values was detected in GM and lesions in treated patients.
Another measure that has been proposed as a tool to monitor neuroprotection is based on the assessment of the evolution of active lesions into permanent black holes (PBH), which correspond to areas of severe and irreversible tissue damage. Studies with GA, IFN, and natalizumab have shown that these treatments can modify, to different extents, the percentage of lesions evolving into PBH [9, 13, 16]. On the contrary, BHT-3009, a DNA plasmid-encoding myelin basic protein (MBP) that might induce antigen-specific tolerance, was found to have no effect on the evolution of new inflammatory lesions into PBH in RRMS patients, as published in the July issue [36].
Conclusions
The extensive application of conventional and advanced MR methods to study MS patients has contributed greatly to improving our ability to diagnose and monitor the disease as well as to our understanding of its pathophysiology. From the data available, it is clear that combining different MR modalities, which are sensitive to different aspects of MS pathology, is a promising way to improve further our understanding of the mechanisms accounting for the accumulation of irreversible disability in this disease. Such an approach should include not only the assessment of brain damage, but also that of the spinal cord. Finally, the precision and accuracy of quantitative MRI scans in detecting longitudinal, MS-related changes also need to be defined, and ad hoc, large-scale, prospective studies are warranted. This is a central issue for a future application of quantitative MRI as a way to monitor MS evolution in clinical trials, as acknowledged in a recent position paper published in this journal [44].
References
The Optic Neuritis Study Group (2008) Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 65:727–732
Amato MP, Portaccio E, Goretti B, Zipoli V, Battaglini M, Bartolozzi ML, Stromillo ML, Guidi L, Siracusa G, Sorbi S, Federico A, De Stefano N (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64:1157–1161
Barkhof F, Filippi M, Miller DH, Tofts P, Kappos L, Thompson AJ (1997) Strategies for optimizing MRI techniques aimed at monitoring disease activity in multiple sclerosis treatment trials. J Neurol 244:76–84
Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG, Weinstock-Guttman B, Benedict RH (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259:139–146
Bot JC, Barkhof F (2009) Spinal-cord MRI in multiple sclerosis: conventional and nonconventional MR techniques. Neuroimaging Clin N Am 19:81–99
Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M (2009) Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 66:1144–1150
Charil A, Yousry TA, Rovaris M, Barkhof F, De Stefano N, Fazekas F, Miller DH, Montalban X, Simon JH, Polman C, Filippi M (2006) MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol 5:841–852
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Dalton CM, Miszkiel KA, Barker GJ, MacManus DG, Pepple TI, Panzara M, Yang M, Hulme A, O’Connor P, Miller DH (2004) Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol 251:407–413
Eckstein C, Saidha S, Levy M (2012) A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol 259:801–816
Filippi M, Agosta F, Spinelli EG, Rocca MA (2012) Imaging resting state brain function in multiple sclerosis. J Neurol. doi:10.1007/s00415-012-6695-z
Filippi M, Horsfield MA, Ader HJ, Barkhof F, Bruzzi P, Evans A, Frank JA, Grossman RI, McFarland HF, Molyneux P, Paty DW, Simon J, Tofts PS, Wolinsky JS, Miller DH (1998) Guidelines for using quantitative measures of brain magnetic resonance imaging abnormalities in monitoring the treatment of multiple sclerosis. Ann Neurol 43:499–506
Filippi M, Rocca MA, Camesasca F, Cook S, O’Connor P, Arnason BG, Kappos L, Goodin D, Jeffery D, Hartung HP, Comi G, Wolinsky JS, Bogumil T, Pohl C, Beckmann K, Sandbrink R, Croze E, Brown C, Desimone TM, Arnold DL, Cutter G, Knappertz V (2011) Interferon beta-1b and glatiramer acetate effects on permanent black hole evolution. Neurology 76:1222–1228
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15:559–567
Filippi M, Rocca MA, De Stefano N, Enzinger C, Fisher E, Horsfield MA, Inglese M, Pelletier D, Comi G (2011) Magnetic resonance techniques in multiple sclerosis: the present and the future. Arch Neurol 68:1514–1520
Filippi M, Rovaris M, Rocca MA, Sormani MP, Wolinsky JS, Comi G (2001) Glatiramer acetate reduces the proportion of new MS lesions evolving into “black holes”. Neurology 57:731–733
Goodin DS (2006) Magnetic resonance imaging as a surrogate outcome measure of disability in multiple sclerosis: have we been overly harsh in our assessment? Ann Neurol 59:597–605
Hackmack K, Weygandt M, Wuerfel J, Pfueller CF, Bellmann-Strobl J, Paul F, Haynes JD (2012) Can we overcome the ‘clinico-radiological paradox’ in multiple sclerosis? J Neurol 259:2151–2160
Harrison DM, Shiee N, Bazin PL, Newsome SD, Ratchford JN, Pham D, Calabresi PA, Reich DS (2012) Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol. doi:10.1007/s00415-012-6638-8
Havla J, Gerdes LA, Meinl I, Krumbholz M, Faber H, Weber F, Pellkofer HL, Hohlfeld R, Kumpfel T (2011) De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, O’Connor PW, Giovannoni G, Phillips JT, Lublin FD, Pace A, Kim R, Hyde R (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 8:254–260
Hayton T, Furby J, Smith KJ, Altmann DR, Brenner R, Chataway J, Hunter K, Tozer DJ, Miller DH, Kapoor R (2012) Clinical and imaging correlates of the multiple sclerosis impact scale in secondary progressive multiple sclerosis. J Neurol 259:237–245
Hayton T, Furby J, Smith KJ, Altmann DR, Brenner R, Chataway J, Hunter K, Tozer DJ, Miller DH, Kapoor R (2012) Longitudinal changes in magnetisation transfer ratio in secondary progressive multiple sclerosis: data from a randomised placebo controlled trial of lamotrigine. J Neurol 259:505–514
Hellwig K, Gold R (2011) Progressive multifocal leukoencephalopathy and natalizumab. J Neurol 258:1920–1928
Hickman SJ, Dalton CM, Miller DH, Plant GT (2002) Management of acute optic neuritis. Lancet 360:1953–1962
Horwitz H, Degn M, Modvig S, Larsson HB, Wanscher B, Frederiksen JL (2012) CSF abnormalities can be predicted by VEP and MRI pathology in the examination of optic neuritis. J Neurol 259:2616–2620
Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, Chataway J, Hughes RA, Miller DH (2010) Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 9:681–688
Kleinfeld K, Mobley B, Hedera P, Wegner A, Sriram S, Pawate S (2012) Adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia: report of five cases and a new mutation. J Neurol. doi:10.1007/s00415-012-6680-6
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, Martin C (2009) Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med 361:1081–1087
Melin A, Outteryck O, Collongues N, Zephir H, Fleury MC, Blanc F, Lacour A, Ongagna JC, Berteloot AS, Vermersch P, de Seze J (2012) Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 259:1215–1221
Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, Galetta SL, Hutchinson M, Johnson RT, Kappos L, Kira J, Lublin FD, McFarland HF, Montalban X, Panitch H, Richert JR, Reingold SC, Polman CH (2008) Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 14:1157–1174
Nociti V, Batocchi AP, Luigetti M, Conte A, Lorusso VS, Roiati S, Tartaglione T, Del Grande A, Sabatelli M (2011) Progressive ascending myelopathy: atypical forms of multiple sclerosis or what else? J Neurol 258:1965–1970
Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, Moseley IF, Johnson G, Tofts PS, Halliday AM et al (1987) The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quant study. Brain 110(Pt 6):1579–1616
Paling D, Golay X, Wheeler-Kingshott C, Kapoor R, Miller D (2011) Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol 258:2113–2127
Papadopoulou A, von Felten S, Traud S, Rahman A, Quan J, King R, Garren H, Steinman L, Cutter G, Kappos L, Radue EW (2012) Evolution of MS lesions to black holes under DNA vaccine treatment. J Neurol 259:1375–1382
Pardini M, Bonzano L, Mancardi GL, Roccatagliata L (2010) Frontal networks play a role in fatigue perception in multiple sclerosis. Behav Neurosci 124:329–336
Patrucco L, Rojas JI, Cristiano E (2012) Assessing the value of spinal cord lesions in predicting development of multiple sclerosis in patients with clinically isolated syndromes. J Neurol 259:1317–1320
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 69:292–302
Rao SM, Leo GJ, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41:685–691
Reilmann R, Holtbernd F, Bachmann R, Mohammadi S, Ringelstein EB, Deppe M (2012) Grasping multiple sclerosis: do quantitative motor assessments provide a link between structure and function? J Neurol. doi:10.1007/s00415-012-6639-7
Riccitelli G, Rocca MA, Forn C, Colombo B, Comi G, Filippi M (2011) Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. AJNR Am J Neuroradiol 32:874–879
Rieckmann P, Boyko A, Centonze D, Coles A, Elovaara I, Havrdova E, Hommes O, Lelorier J, Morrow SA, Oreja-Guevara C, Rijke N, Schippling S (2012) Future MS care: a consensus statement of the MS in the 21st Century Steering Group. J Neurol. doi:10.1007/s00415-012-6656-6
Rocca MA, Absinta M, Filippi M (2012) The role of advanced magnetic resonance imaging techniques in primary progressive MS. J Neurol 259:611–621
Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G, Filippi M (2007) fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum Brain Mapp 28:373–382
Sepulcre J, Masdeu JC, Goni J, Arrondo G, Velez de Mendizabal N, Bejarano B, Villoslada P (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 15:337–344
Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P (2009) Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 65:268–275
Sormani MP, Bonzano L, Roccatagliata L, Mancardi GL, Uccelli A, Bruzzi P (2010) Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-anal approach. Neurol 75:302–309
Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK (2006) Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 59:743–747
Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH (2008) Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 70:1150–1151
Yaldizli O, Glassl S, Sturm D, Papadopoulou A, Gass A, Tettenborn B, Putzki N (2011) Fatigue and progression of corpus callosum atrophy in multiple sclerosis. J Neurol 258:2199–2205
Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue EW, Jager HR, Clifford DB (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354:924–933
Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, Filippi M (2012) MRI pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. doi:10.1002/ana.23676
Conflicts of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocca, M.A., Messina, R. & Filippi, M. Multiple sclerosis imaging: recent advances. J Neurol 260, 929–935 (2013). https://doi.org/10.1007/s00415-012-6788-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6788-8