Abstract
Information-processing speed (IPS) slowing is a primary cognitive deficit in multiple sclerosis (MS). Basal ganglia, thalamus and neocortex are thought to have a key role for efficient information-processing, yet the specific relative contribution of these structures for MS-related IPS impairment is poorly understood. To determine if basal ganglia and thalamus atrophy independently contribute to visual and auditory IPS impairment in MS, after controlling for the influence of neocortical volume, we enrolled 86 consecutive MS patients and 25 normal controls undergoing 3T brain MRI and neuropsychological testing. Using Sienax and FIRST software, neocortical and deep gray matter (DGM) volumes were calculated. Neuropsychological testing contributed measures of auditory and visual IPS using the Paced Auditory Serial Addition Test (PASAT) and the Symbol Digit Modalities Test (SDMT), respectively. MS patients exhibited significantly slower IPS relative to controls and showed reduction in neocortex, caudate, putamen, globus pallidus, thalamus and nucleus accumbens volume. SDMT and PASAT were significantly correlated with all DGM regions. These effects were mitigated by controlling for the effects of neocortical volume, but all DGM volumes remained significantly correlated with SDMT, putamen (r = 0.409, p < 0.001) and thalamus (r = 0.362, p < 0.001) having the strongest effects, whereas for PASAT, the correlation was significant for putamen (r = 0.313, p < 0.01) but not for thalamus. We confirm the significant role of thalamus atrophy in MS-related IPS slowing and find that putamen atrophy is also a significant contributor to this disorder. These DGM structures have independent, significant roles, after controlling for the influence of neocortex atrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment is now widely recognized as a common and early manifestation of multiple sclerosis (MS). Information-processing speed (IPS) is one of the core cognitive domains involved [1, 2], underlying other cognitive deficits [3, 4], and contributing to disruption of daily living instrumental activities and work capacity [5]. The slowness in processing speed is independent of stimulus modality, as documented in studies examining performance on both visual and auditory processing speed tests [6].
In MS, it is increasingly recognized that degeneration of cerebral gray matter (GM) is strongly associated with psychometric evidence of slowed IPS. Demyelinating white matter (WM) disease, and disruption of the WM pathways mediating the transmission of information across distributed brain networks, is also contributory [7, 8]. However, in several studies comparing multiple MRI measures, GM volume indices have accounted for most variance in MS-associated cognitive decline [9]. The volume or thickness of the neocortex is correlated with mental processing efficiency in MS [9, 10], and the basal ganglia and thalamus are in a unique position to modulate rapid processing of information [11].
MS studies examining deep GM (DGM) structures and cognition [12, 13] reveal robust correlation between thalamus and performance. However, these studies have not assessed the influence of all candidate DGM structures. Moreover, in studies assessing the influence of multiple GM compartments via linear regression, most often only a single predictor is retained, suggesting that in general, GM predicts impairment and the independent significance of specific regions is either not important or poorly understood.
In the present study, using the same MRI volumetric analysis for all DGM structures, we tested the hypothesis that basal ganglia and thalamic atrophy in MS contributes independently to slowed IPS, after controlling for the effects of neocortical volume.
Methods
Participants
We enrolled 86 consecutive patients with clinically definite MS [14], paid for research in our clinic, and 25 healthy volunteers that served as normal controls (NC), matched on demographic characteristics (Table 1). All participants provided written informed consent to participate in the study, which was approved by the local institutional review board.
Exclusion criteria for patients were current or past major medical, neurologic, or psychiatric disorder (other than MS related), previous or current substance abuse, relapse or steroid pulse treatment within 8 weeks preceding evaluation. Depressive disorders emerging after the onset of MS were permitted, but patients meeting criteria for current major depressive episode were excluded. NC were free of developmental delay and medical history which could impact on cognitive ability. The disease course was relapsing–remitting (RR) in 59 patients (68.6%) and secondary progressive (SP) in 27 patients (31.4%). The mean disease duration was 10.8 ± 7.6 years and the median Expanded Disability Status Scale (EDSS) score was 3.5 (range 0–6.5).
MRI acquisition and analysis
All subjects were examined on a 3T GE Signa Excite HD 12.0 Twin Speed 8-channel scanner (General Electric, Milwaukee, WI), with a maximum slew rate of 150 T/m/s and maximum gradient amplitude in each orthogonal plane of 50 mT/m (zoom mode). A multi-channel head and neck (HDNV) coil manufactured by GE was used to acquire the following sequences: 2D multi-planar dual fast spin-echo (FSE) proton density (PD) and T2-weighted image (WI); Fluid-Attenuated Inversion Recovery (FLAIR); 3D high resolution (HIRES) T1-WI using a fast spoiled gradient echo (FSPGR) with magnetization-prepared inversion recovery (IR) pulse and spin echo (SE) T1-WI. One average was used for all pulse sequences. All sequences were acquired with a 256 × 192 matrix (freq × phase), field-of-view (FOV) of 25.6 cm × 19.2 cm (256 × 256 matrix with Phase FOV = 0.75), for an in-plane resolution of 1 mm × 1 mm. For all 2D scans (PD/T2, FLAIR and SE T1), 48 slices were collected, thickness of 3 mm, and no gap between slices. For the 3D HIRES IR-FSPGR, 128 locations were acquired, 1.5 mm thick. Other relevant parameters were as follows: for dual FSE PD/T2, echo and repetition times (TE and TR) E1/TE2/TR = 9/98/5,300 ms, flip angle (FA) = 90, echo train length (ETL) = 14; for FLAIR, TE/TI/TR = 120/2,100/8,500 ms (TI-inversion time), FA = 90, ETL = 24; for SE T1-WI, TE/TR = 16/600 ms, FA = 90 and for 3D HIRES T1-WI, TE/TI/TR = 2.8/900/5.9 ms, FA = 10.
Scans were prescribed in an axial-oblique orientation, parallel to the sub-callosal line. The T2- and T1-lesion volumes (LVs) were measured using a semi-automated edge detection contouring/thresholding technique previously described [15]. Global and tissue-specific atrophy measures were acquired via brain extraction and tissue segmentation techniques separating GM and white matter. The SIENAX cross-sectional software tool was applied (version 2.6), with corrections for T1-hypointensity misclassification using an in-house developed in-painting program. Normalized volumes of the whole brain (NBV), GM volume (NGMV), neocortical volume (NCV) and WM volume (NWMV) were assessed as previously described [16]. We also calculated central atrophy measures, including normalized lateral ventricle volume (NLVV) and third ventricle width (TVW) [9].
We used FMRIB’s FIRST software to segment high resolution 3D T1-weighted images (http://www.fmrib.ox.ac.uk/fsl/first/index.html) [17]. Briefly, FIRST is a model-based segmentation/registration program that uses shape/appearance models constructed from manually segmented images. The manual labels are parameterized as surface meshes and modeled as a point distribution model. Deformable surfaces are used to automatically parameterize the volumetric labels in terms of meshes; the deformable surfaces are constrained to preserve vertex correspondence across the training data. Normalized intensities along the surface normals are sampled and modeled. The shape and appearance model is based on multivariate Gaussian assumptions. Shape is then expressed as a mean with modes of variation (principal components). Based on the learned models, FIRST searches through linear combinations of shape modes of variation for the most probable shape instance given the observed intensities in the input image. Volumetric data for DGM structures (caudate, putamen, globus pallidus, thalamus, nucleus accumbens) were obtained (Fig. 1). We also calculated T1- and T2-LVs present within each DGM region by overlaying the FIRST maps with their respective lesion masks. We next investigated the relationship in healthy controls between the total DGM volume, as estimated by FIRST, and head size, as estimated by SIENAX. A Pearson r of −0.817 (p < 0.0001) demonstrated that absolute DGM volumes vary with head size independently of any disease process. Thus, normalized DGM volumes were obtained by multiplying the estimated volumes from FIRST by the volumetric scaling factor from SIENAX.
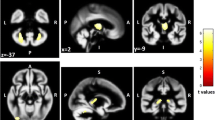
Representative images of 3T brain MRI, T1-weighted images, with deep gray matter structures segmented using FMRIB’s FIRST software, in a patient with relapsing-remitting multiple sclerosis (44-year old female with 12 years of disease duration and an Expanded Disability Status Scale of 5.5) (a) and in an age- and sex-matched healthy control subject (b). Note the apparent volume reduction of basal ganglia and thalamus in the multiple sclerosis patient (a) comparatively to the healthy control subject (b). Caudate (light blue), globus pallidus (dark blue), putamen (pink) and thalamus (green)
Neuropsychological testing
Neuropsychological testing (NP) was performed under the supervision of a board certified neuropsychologist blinded to MR imaging findings. Tests were chosen according to the recommendations of a consensus panel [18] recently validated in a large prospective MS sample [19]. Auditory and visual IPS was assessed using the gold standard Rao adaptations [20] of the Paced Auditory Serial Addition Test (PASAT) [21] and the Symbol Digit Modalities Test (SDMT) [22], respectively. The PASAT (3-s presentation rates) required participants to monitor audiotaped digits while adding each consecutive digit to the preceding one during a continuous stream of 60 stimuli. On the SDMT, participants were presented with a series of nine symbols, each paired with a single digit in a key at the top of an 8-1/2 × 11-in. sheet and were asked to respond orally with the digit associated with each symbol as rapidly as possible for 90 s. The number of correct responses was tabulated for each test. Both tests have well established reliability and validity in the MS population [23]. Auditory/verbal episodic memory was evaluated using learning and delayed recall indexes derived from the California Verbal Learning Test–Second Edition (CVLT2) [24]. Visuospatial memory was assessed using analogous indexes from the Brief Visuospatial Memory Test-Revised (BVMT-R) [25]. Tests emphasizing spatial perception, language, and executive function were also administered, including the Judgment of Line Orientation Test [26], Controlled Oral Word Association Test (COWAT) [26] and the Delis–Kaplan Executive Function System (DKEFS) Sorting Test [27].
The Beck Depression Inventory Fast Screen (BDIFS) [28] and the Fatigue Severity Scale (FSS) [29], both validated in MS samples, were used to determine the influence of depression and fatigue, respectively.
Statistical analysis
We compared the MS and control groups across MR imaging findings and NP testing results using one-way analysis of variance (ANOVA) with significance set at p < 0.05. Pearson product moment correlation were examined between DGM volumes and neuropsychological tests with significance set at p < 0.05. As there was a modest trend toward a significant difference between patients and controls on age, we incorporated age as a covariate in all analyses. As the right and left DGM structures were strongly correlated (r values ranging from 0.602 for the nucleus accumbens to 0.967 for the thalamus), they were combined by calculating mean values in order to reduce the number predictor variables to the smallest possible set.
Only DGM volumes that were significantly correlated with PASAT and SDMT were carried forward into subsequent regression analyses. Forward stepwise linear regression models (entrance criterion p < 0.05 and exit criterion p = 0.10) were generated in order to determine the strongest DGM predictors of SDMT and PASAT performance. In each case, age was entered as covariate and retained in block 1, and the significant DGM structures were entered in block 2 using the forward stepwise technique. Finally, each model was then repeated with NCV included in block 1.
Analyses were conducted using SPSS for Windows version 17.0 (SPSS Inc, Chicago, IL). Multiple regression and ANOVA models were evaluated using standard procedures to ensure that final models met the underlying assumptions required by these statistical techniques.
Results
As expected, MS patients performed significantly worse than NCs on both auditory and visual IPS tests, i.e. PASAT and SDMT, respectively. The same was verified on the other NP tests (Table 2).
The mean BDIFS for patients was 2.9 ± 3.4 and the mean FSS was 4.9 + 1.6, representing mild to moderate degrees of depression and subjectively reported fatigue, while for NC BDFIS was 1.7 ± 1.9 and FSS was 2.8 ± 1.1. There were no significant correlations between the cognitive measures and either BDIFS or FSS, and consequently these measures were not analyzed further.
MRI data are presented in Table 3. There was a significant reduction of NCV in MS patients, as well of all DGM structures assessed: caudate, putamen, globus pallidus, thalamus and nucleus accumbens. The volumes of the DGM were negatively correlated with the total T2 lesion volume, ranging from r = −0.319 (globus pallidus, p < 0.01) to r = −0.657 (putamen, p < 0.001), and with the total T1 lesion volume (r = −0.305 for globus pallidus p = 0.004, to r = −0.476 for putamen, p < 0.001), but not with the T2 and T1 lesion volume present within each DGM structure. Furthermore, the disease duration was also correlated with the DGM volumes, effects ranging from r = −0.269 (nucleus accumbens, p < 0.05) to r = −0.494 (thalamus, p < 0.001). When comparing RR and SP subgroups of MS patients, the DGM volumes were not significantly different (data not shown). NCV was significantly correlated with all DGM volumes, ranging from r = −0.267 (globus pallidus, p < 0.05) to r = 0.556 (thalamus, p < 0.001), the difference between the coefficients being significant (p < 0.01).
Correlations between the regional brain MRI and cognitive tests in MS patients are presented in Table 4. All of the significant correlations were in the expected direction with higher tissue volumes being associated with better cognitive ability. When controlling for age, SDMT performance showed a significant correlation with NCV (r = 0.542, p < 0.001) and all DGM volumes, the largest effect for thalamus (r = 0.543, p < 0.001). For PASAT, correlations were similarly significant: NCV (r = 0.431, p < 0.001), largest DGM coefficient for putamen (r = 0.435, p < 0.001). After controlling for NCV, as expected, the strength of these correlations was mitigated due to covariation between NCV and DGM volume.
The regression models predicting SDMT and PASAT first controlled for the effects of age, and then both age and NCV (Table 5). In the first tier, thalamus and putamen volumes were both retained as SDMT predictors (R 2 = 0.35, p < 0.001), while for PASAT only putamen was retained (R 2 = 0.19, p < 0.001). In the second tier, only putamen was retained both for SDMT (R 2 = 0.43, p < 0.0001) and PASAT (R 2 = 0.27, p < 0.001).
Discussion
Although it is well known that IPS decrease is a primary cognitive deficit in MS, the underlying neural mechanisms remain undetermined. Basal ganglia and thalamus are thought to have a key role for efficient information-processing; nevertheless the specific contribution of these structures for MS-related IPS impairment is unknown. In this context, the purpose of this study was to explore specifically the IPS slowing in MS and its relation with basal ganglia and thalamus atrophy.
The results obtained confirm a significant reduction of basal ganglia and thalamus volumes in MS patients relative to normal controls, as reported previously [12, 13]. More importantly, we demonstrate for the first time that the atrophy of these DGM structures provides an additional and independent contribution, relative to neocortex, for predicting MS-related slowed IPS.
DGM atrophy was most prominent for thalamus where the effect size was 1.1 and the relative decrease of volume was 12.4%. Similar degrees of volume reduction were observed by Cifelli et al. [30] and Houtchens et al. [12], respectively 17 and 16.8%. In the latter work, thalamus volume was strongly correlated with SDMT and PASAT. However, these earlier studies did not control for the influence of NCV and other DGM structures were not assessed. We found that in explaining reduced IPS in MS, the role of the thalamus appears to be closely tied to the cortex, as controlling for neocortex volume removes thalamus from regression models, revealing a stronger independent contribution of putamen. This impression is further corroborated by the stronger correlation between NCV and thalamus, compared to for example the putamen. In this study, we found that DGM volumes were not significantly different when comparing RR and SP subgroups of MS patients. This null result is probably due, in part, to type 2 error as there were few SP patients enrolled in the study. Future studies adequately powered should be performed in order to provide further information according to MS clinical phenotypes.
On the other hand, it is also noteworthy that WM volumes were not significantly decreased as compared with those of NC, particularly considering that the patients had on average a long-standing disease course. The disruption of the WM pathways mediating the transmission of information across distributed brain networks is a well recognized mechanism contributing to the slowed IPS in MS [7, 8]; however, accordingly to the results of this study the GM pathology seems to be the main contributory factor.
Since the original conceptualization by Alexander et al. [31] of parallel cortico-basal ganglia-thalamocortical loops connecting basal ganglia and thalamus with frontal cortex, the role of these structures in cognition has been emphasized. In line with this, basal ganglia and thalamus are recognized to be in a unique position to modulate information processing efficiency, and may have a key role for an efficient and rapid processing during the execution of complex attention and executive function tasks [11]. Therefore, it seems reasonable that atrophy of these DGM structures, as shown here, significantly contributes to slowed or inefficient processing of information in MS.
When considering visual and auditory IPS separately, although some of the cortico-subcortical circuits are probably shared, there are some unique findings. For visual processing, thalamus and putamen account independently for the main variance in our SDMT results. After controlling for neocortical volume, the effect of thalamus seems to be moderated, perhaps because of its important reciprocal connections with widespread areas of the neocortex. In addition to the role of the anterior nucleus of the thalamus, our findings might also be related, in part, to the recognized importance of pulvinar nuclei in visual cognitive processing. Pulvinar represents a substantial portion of thalamic volume. It has been recently postulated that a second visual pathway mediates visual information, from retina to the superficial layers of the superior colliculus and from there to the pulvinar nucleus which projects to multiple cortical visual areas [32]. In addition, pulvinar nuclei may actively participate in the processing of visual information in conjunction with cortical areas, not being merely a passive relay station for sensory signals en route to the cerebral cortex [33].
On the other hand, this is the first study to report correlation between putamen and processing speed in MS. Our finding may be explained by the participation of this structure in the frontal eye field circuitry. This circuitry passes through the striatum, primarily the putamen, and on to the substantia nigra pars reticulata, to thalamic nuclei and then returning to the frontal eye fields, being responsible for voluntary fixational control and saccades. This circuitry would necessarily be recruited in the performance of planning tasks with a component of visual searching [34]. Moreover, certain developmental disorders such as attention deficit/hyperactivity disorder, also exhibit deficits in this circuitry [35]. For auditory information processing speed as measured by PASAT, putamen was the most robust predictor of performance in this study. Similarly, in Parkinson’s disease, it has been reported in several studies that auditory processing is affected due to structural alterations in striatum [36, 37]. Moreover, in a fMRI study by Saft et al. [38] it was reported that the occurrence of altered auditory stimulus processing in Huntington’s disease (HD) that was related to an abnormal pattern of activation in basal ganglia–thalamic circuits compared to controls. This finding is supported by a recent work of Opitz et al. in healthy subjects, showing that basal ganglia–thalamic circuits are involved in attentive processing of auditory stimulus, with putamen considered a central structure [39].
There are some limitations of our study to be considered. First of all, we did not assess other MRI variables that may have accounted for variance in information processing speed impairment, such as measures of diffuse occult disease like MR spectroscopy, magnetization transfer, and diffusion tensor imaging of normal appearing brain tissue. Moreover, recent work has shown evidence of demyelinating lesions in the grey matter in patients with MS and we did not account for the influence of these lesions in our analysis [40]. Another limitation is that while PASAT and SDMT are gold standard neuropsychological measures, they are not pure measures of information-processing speed, as to some extent they involve working memory [4].
These concerns apart, our study furthers the knowledge about the underlying mechanisms of MS-related cognitive impairment. We conclude that basal ganglia and thalamus atrophy independently contribute to visual and auditory information processing speed defects in MS, in addition to the well established contribution of the cerebral cortex.
References
Denney DR et al (2004) Cognitive impairment in relapsing and primary progressive multiple sclerosis: mostly a matter of speed. J Int Neuropsychol Soc 10(7):948–956
Rao SM, St Aubin-Faubert P, Leo GJ (1989) Information processing speed in patients with multiple sclerosis. J Clin Exp Neuropsychol 11(4):471–477
DeLuca J et al (2004) Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol 26(4):550–562
Forn C et al (2008) Information-processing speed is the primary deficit underlying the poor performance of multiple sclerosis patients in the Paced Auditory Serial Addition Test (PASAT). J Clin Exp Neuropsychol 30(7):789–796
Barker-Collo SL (2006) Quality of life in multiple sclerosis: does information-processing speed have an independent effect? Arch Clin Neuropsychol 21(2):167–174
Archibald CJ, Fisk JD (2000) Information processing efficiency in patients with multiple sclerosis. J Clin Exp Neuropsychol 22(5):686–701
Sanfilipo MP et al (2006) Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 66(5):685–692
Dineen RA et al (2009) Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132(Pt 1):239–249
Benedict RHB et al (2006) Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 63(9):1301–1306
Amato MP et al (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64(8):1157–1161
Leyden J, Kleinig T (2008) The role of the basal ganglia in data processing. Med Hypotheses 71(1):61–64
Houtchens MK et al (2007) Thalamic atrophy and cognition in multiple sclerosis. Neurol 69(12):1213–1223
Benedict RHB et al (2009) Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry 80(2):201–206
Polman CH et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58(6):840–846
Zivadinov R et al (2001) Effects of IV methylprednisolone on brain atrophy in relapsing-remitting MS. Neurol 57(7):1239–1247
Zivadinov R et al (2007) Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum Mol Genet 16(22):2659–2668
Patenaude B et al (2008) Improved surface models for FIRST. In: human brain mapping conference
Benedict RHB et al (2002) Minimal neuropsychological assessment of ms patients: a consensus approach. Clin Neuropsychol 16:381–397
Benedict RHB et al (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 12:549–558
Rao SM (1991) A manual for the brief, repeatable battery of neuropsychological tests in multiple sclerosis. National Multiple Sclerosis Society, New York
Gronwall DM (1977) Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 44(2):367–373
Smith A (1982) Symbol digit modalities test: manual. Western Psychological Services, Los Angeles
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151
Delis DC et al (2000) California verbal learning test manual: second edition, adult version. Psychological Corporation, San Antonio
Benedict RHB (1997) Brief visuospatial memory test-revised: professional manual. Psychological Assessment Resources, Odessa
Benton AL et al (1994) Contributions to neuropsychological assessment. Oxford University Press, New York
Delis DC, Kaplan E, Krammer JH (2001) Delis–Kaplan executive function system. Psychological Corporation, San Antonio
Beck AT, Steer RA, Brown GK (2000) BDI-fast screen for medical patients: manual. Psychological Corporation, San Antonio
Krupp LB et al (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46(10):1121–1123
Cifelli A et al (2002) Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 52(5):650–653
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Berman RA, Wurtz RH (2008) Exploring the pulvinar path to visual cortex. Prog Brain Res 171:467–473
Casanova C et al (2001) Higher-order motion processing in the pulvinar. Prog Brain Res 134:71–82
Koziol L, Budding DE (eds) (2010) Subcortical structures and cognition: implications for neuropsychological assessment. Springer, Berlin
Feifel M et al (2004) Inhibitory deficits in ocular motor behavior in adults with attention-defficit/hyperactivity disorder. Biol Psychiatry 56:333–339
Grossman M et al (2002) Information processing speed and sentence comprehension in Parkinson’s disease. Neuropsychology 16(2):174–181
Vieregge P et al (1994) Auditory selective attention is impaired in Parkinson’s disease–event-related evidence from EEG potentials. Brain Res Cogn Brain Res 2(2):117–129
Saft C et al (2008) fMRI reveals altered auditory processing in manifest and premanifest Huntington’s disease. Neuropsychology 46(5):1279–1289
Opitz B, Schroger E, von Cramon DY (2005) Sensory and cognitive mechanisms for preattentive change detection in auditory cortex. Eur J Neurosci 21(2):531–535
Pirko I et al (2007) Gray matter involvement in multiple sclerosis. Neurology 68(9):634–642
Acknowledgments
Supported by National MS Society grant RG4060A3/1.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batista, S., Zivadinov, R., Hoogs, M. et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259, 139–146 (2012). https://doi.org/10.1007/s00415-011-6147-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6147-1