Abstract
Fatigue is one of the most disabling symptoms in multiple sclerosis (MS) patients. There is no or only weak correlation between conventional magnetic resonance imaging (MRI) parameters and level of fatigue. The aim of this study was to investigate the relationship between progression of corpus callosum (CC) atrophy and fatigue in MS patients. This was a cohort study in 70 patients with relapsing form of MS (RRMS) and serial MRIs over a mean follow-up of 4.8 years [67% female, mean age 42 ± 11 years, mean disease duration 9.7 ± 7.6 years, mean Expanded Disability Status Scale (EDSS) 2.8 ± 1.6]. Fatigue was assessed by the Fatigue Severity Scale (FSS). CC size was measured with the CC index (CCI). In total, 40% of the patients suffered from fatigue (mean FSS score 5.3 ±1.1) and 60% patients had no fatigue (mean FSS score of 2.1 ± 1). Patients with fatigue had higher EDSS scores (p = 0.01) and CC atrophy was more pronounced in patients with fatigue (−21.8 vs. −12.1%, p = 0.005). FSS correlated with CCI change over time (r = −0.33; p = 0.009) and EDSS (p = 0.008; r = 0.361). The association between annualized CCI change and FSS was independent from EDSS, disease duration, gender and age in a multivariate linear regression analysis (p < 0.001). Progression of CC atrophy may play a role in the evolution of MS-related fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis associated fatigue is defined as a “subjective lack of physical or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities” [7]. Fatigue is one of the most common symptoms encountered in MS, leading to a severe level of disability and impaired quality of life [13, 15, 20, 21]. Fatigue occurs in all stages of the disease and usually does not correlate with demographic or disease characteristics such as age, gender, disability or disease severity [2, 3, 18, 22, 23, 28, 40]. The pathophysiology of MS-related fatigue is not fully understood and seems to be multifactorial [7]. Functional imaging studies [12, 34, 35] have demonstrated an abnormal recruitment of cortical and subcortical networks in MS patients with fatigue supporting the theory of a central origin. It has been suggested that diffuse wide-spread axonal loss in MS patients might play a role in the pathogenesis of fatigue. However, structural magnetic resonance imaging (MRI) studies have provided conflicting results concerning the relation between fatigue severity and brain pathology [4]. Since the corpus callosum (CC) is the largest and functionally most important interhemispheric connection bundle, commonly affected in MS, we hypothesized an association between CC atrophy and fatigue.
Patients and methods
This is an exploratory observational MS cohort study with the analysis of CC size on brain MRI as measured at two timepoints, at diagnosis and at time of study. The study was approved by the local ethic committee of the Cantonal Hospital St. Gallen, Switzerland.
Patients
We assessed MRI and fatigue in 97 consecutive patients attending our outpatient clinic and fulfilling the following inclusion criteria: age between 18 and 60 years, diagnosis of a relapsing form of MS according to McDonald criteria (revised version 2005 [31]) and an actual cranial MRI scan (within 3 months before visit). Exclusion criteria are given in Table 1.
We excluded four patients due to co-medication supposed to have an effect on fatigue, including one patient with zopiclone, one patient with mirtazapine and two patients with amitryptiline. Moreover, we excluded one patient with pure spinal manifestation of disease, and 22 patients because of depressive symptoms. Thus, this analysis included data of 70 MS patients. Disease duration was defined as time since first manifestation of MS. Relapses were defined as the development of new or recurrent neurological symptoms not associated with fever or infection lasting at least 24 h confirmed by a Swiss or German board-certified neurologist. EDSS and fatigue were assessed with a maximum time distance of 1 month.
Fatigue assessment
Fatigue was assessed in all patients using the Fatigue Severity Scale (FSS; see appendix). FSS is a 9-item measure. The patients had to rate the degree of agreement of a given statement on a 7-point ordinal scale ranging from 1 (strongly disagree) to 7 (strongly agree). Scores were calculated by deriving an arithmetic mean. FSS scores of ≥4 where suggested to be indicative of “fatigue” whereas FSS <4 characterizes “no fatigue” [21]. Depressive symptoms were assessed by Beck’s Depression Inventory [5].
MRI analysis
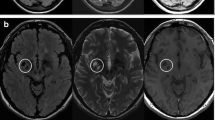
All MRI scans were performed in the radiological institute of the Cantonal Hospital St. Gallen (1.5 Tesla Sigma Magnetom Scanner, Siemens AG, Germany) including an axial pre- and post-Gadolinium T1 weighted, sagittal T1 weighted, sagittal fluid attenuated inversion recovery sequence (FLAIR), axial T2 weighted and axial FLAIR sequences. The first MRI represented the MRI at diagnosis. The last MRI was performed within 3 months before fatigue assessment. All MRI scans were analysed by the same examiner (OY). For CC measurement we used the CC index (CCI) on Picture Archiving Communicating System and if on-screen MRIs are not available on hardcopies. CCI is an established linear measurement technique, published elsewhere (Fig. 1) [11, 41].
Calculation of corpus callosum index (CCI). CCI is calculated as (aa′ + bb′ + cc′)/ab′. Lower CCI values indicate a lower CC volume [11]
Higher CCI values indicate a higher CC volume. OY was blinded to the clinical data of the patients. Forty-seven MRIs (33.6%) were evaluated by a second rater (SG), on-screen blinded to the results of OY and clinical data. The concordance rate was 0.91. In 38 patients (54.3%) actual MRI were both available on-screen and hardcopies. In these patients, hardcopy MRIs were additionally evaluated by SG who blinded to the results from on-screen measurement with a concordance rate of 0.88. T2 and T1 lesion load and contrast enhancement were determined by Swiss or German board-certified radiologists. The T2 lesion load was dichotomized as <9 T2 lesions or ≥9 T2 lesions. T1 lesions and contrast enhancing lesions were categorized as present or absent.
Statistical analysis
Demographic data are presented as mean, standard deviation and 95% confidence interval (CI). Curtosis was calculated for continued variables. We used the Mann–Whitney U test for independent samples for comparison of continued variables and applied the non-parametric rank correlation analysis Spearman-Rho. The significance level was corrected for multiple testings by Bonferroni (adjusted p value: 0.05/5 = 0.01). We used a multivariate linear regression analysis (backward directed regression model) to analyse the interdependence of potential confounding variables. FSS was set as continuous outcome parameter. Clinically meaningful confounders included disease duration, gender, EDSS, age, CCI and annualized CCI change since diagnosis of MS. We used SPSS (MAC version 18; SPSS, Chicago, IL) for all statistical analysis.
Results
Mean FSS in all patients was 3.37 ± 1.88. Forty percent of the patients (n = 28) had fatigue according to the definition (mean FSS score of 5.27 ± 1.1). Sixty percent of the patients (n = 42) had no fatigue (mean FSS score of 2.1 ± 1.0). Patients’ demographic and clinical characteristics in total and group-wise are given in Table 2.
Group-wise univariate comparisons of baseline characteristics
Most of the patients' characteristics were similar in both groups including gender, age, disease duration, annualized relapse and steroid treatment rate, treatment with disease modifying drugs and conventional MRI characteristics. However, patients with fatigue had higher EDSS scores (p = 0.01; Table 2).
CC atrophy in patients with and without fatigue
In all patients, mean CCI at diagnosis was 0.36 and decreased to 0.31 in the last MRI (annual CCI decrease −0.8%). CCI values on first and last MRI with refer to time since first manifestation of MS are given in Fig. 2.
CCI at diagnosis and CCI on last MRI were not significantly different in both groups but CCI change over mean of 4.8 years was more pronounced in patients with fatigue: −21.8% compared to those without −12.1% (p = 0.005; Fig. 3a). This difference remains significant after correction for the time between first and last MRIs: annual CCI decrease was −1.1% in patients with fatigue compared to 0.6% in patients without (p = 0.02). Results of CCI assessment are given in Table 3.
a Box-and-whisker diagram of corpus callosum index (CCI) change from baseline (1.0) in fatigued and non-fatigued MS patients. Fatigue is associated with a more pronounced CCI decrease within the last 5 years (88 vs. 79%, mean values; Mann–Whitney U test; p = 0.005). b Scatter plot of annualized CCI change and fatigue severity
Correlation analysis between CCI and fatigue
CCI change over time correlated with FSS at last MRI (ρ = −0.333; p = 0.009; Fig. 3b), EDSS (ρ = −0.367; p = 0.007) and disease duration (ρ = −0.378; p = 0.003). Moreover, EDSS correlated with FSS at study time-point (ρ = 0.361; p = 0.008) and with disease duration (ρ = 0.489; p < 0.001). FSS did not correlate with disease duration (ρ = 0.121; p = 0.34).
Linear regression model
Multivariate linear regression analysis using FSS as the dependent continuous variable and disease duration, gender, EDSS, age, last CCI and annualized CCI (aCCI) change from first to last MRI as co-variates revealed both EDSS and annualized CCI change as independent factors associated with FSS (p value of the regression model = 0.006; adjusted Nagelkerkes’ R square = 0.23; Table 4).
Discussion
The main finding of our study is that progression of CC atrophy over a mean of 5 years after diagnosis is associated with the occurrence and severity of MS-related fatigue.
In MS patients, CC atrophy is a common finding [17, 38] and correlates with whole brain atrophy [38]. Among others, we recently showed that CC atrophy correlates with progression of disability in MS [29, 36, 41]. In recent decades, several studies could demonstrate cognitive dysfunction in patients with CC abnormalities not only due to MS [11, 26, 32]. In our study, CC atrophied by a mean of approximately 0.8% per year which was in the range of studies using other methods, such as brain parenchymal fraction method (0.5–1.8% annual brain volume loss [1, 6, 16, 19]). Like in our study, Martola et al. [25] did not find any association between midsagittal CC area and gender, age and disease duration investigating 37 MS patients. That fatigue severity is independent from disease duration is well-known. There are studies reporting a fatigue prevalence of more than 50% in patients with early forms of MS [37]. The association between progression of CC atrophy and fatigue severity in our study was independent from disability. The common scientific viewpoint is that EDSS does not correlate with fatigue despite of some studies which found a weak association [14, 30]. However, the weak correlation between EDSS and fatigue in our study is probably due to patient selection. In the last decade several studies were conducted investigating the association between fatigue and brain pathology. Most of these studies are designed cross-sectional and found, similar to our results, no correlation between fatigue severity and conventional MRI parameters such as the extent and location of T2 lesions [2, 8, 9, 27, 37, 39, 40], the occurrence of contrast enhancing lesions [23] or brain atrophy [2, 40]. Only one study could demonstrate higher T2 lesion burden in 15 non-disabled MS patients with fatigue compared to 15 patients without [10] with a correlation coefficient of 0.5 (p < 0.005). Recently, a study found in 24 patients with RRMS and 14 healthy controls reduced grey matter volume in the frontal lobe [33].
Results from our regression model suggest that progression of corpus callosum atrophy contributes independently to fatigue severity in MS patients. Similar findings were found in another study investigating the association between progression of brain atrophy and fatigue severity: Marrie et al. [24] investigated 134 MS patients and found that an increase of fatigue correlated with changes of brain volume over 8 years while short term changes in brain volume and fatigue were not correlated. In contrast to our study, fatigue was measured by the Sickness Impact Profile Sleep and Rest Scale. They also found that the association between progression of brain atrophy and fatigue was independent from conventional MRI parameters such as contrast enhancement and T2 lesion load.
Strengths and limitations
This is an observational and explorative clinical study with retrospective assessment of CC size. However, the rater were blinded to clinical characteristics. The measurement technique is valid and reliable [11, 41]. It has been shown to correlate with brain parenchymal fraction [11]. The concordance rate between two independent raters and between measurement on screen and hardcopies was excellent and similar to results published recently [41]. An advantage of this study is the inclusion of a large community-based patient population, close timing of MRI and fatigue assessment and careful selection criteria to insure the measurement of MS-related fatigue. We excluded patients with progressive forms of MS to avoid a heterogeneous study population with a small number of patients in one group. We used a validated depression scale to exclude patients with depressive symptoms [5] and used FSS because of its high reliability, validity and internal consistency. FSS is the most widely used fatigue scale in cross-sectional and longitudinal MS studies [22]; initial studies of the FSS to assess test–retest reliability of the FSS in MS provided a correlation coefficient of 0.84. The internal consistency of the FSS using Cronbach in MS was 0.81 [21]. However, FSS remains a subjective parameter. Another disadvantage of this study is the relatively imprecise assessment of disease burden on MRI. Further studies should include exact volumetric measurements of MS lesions.
Conclusions
Progression of CC atrophy over time seems to be an independent risk factor of MS related fatigue. However, our multivariate regression model could explain only about one-fourth of the variance of fatigue severity. MS-related fatigue remains largely unpredictable suggesting other important confounder not yet identified. There is high need of more objective assessment tools for fatigue. Our results should be reproduced by further studies using innovative quantitative MR-based techniques such as voxel-based morphometry or diffusion tensor imaging.
References
Anderson VM, Fox NC, Miller DH (2006) Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging 23:605–618
Bakshi R, Miletich RS, Henschel K, Shaikh ZA, Janardhan V, Wasay M, Stengel LM, Ekes R, Kinkel PR (1999) Fatigue in multiple sclerosis: cross-sectional correlation with brain MRI findings in 71 patients. Neurology 53:1151–1153
Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, Janardhan V, Dubey N, Kinkel PR (2000) Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler 6:181–185
Barkhof FJ, Elton M, Lindeboom J, Tas MW, Schmidt WF, Hommes OR, Polman CH, Kok A, Valk J (1998) Functional correlates of callosal atrophy in relapsing-remitting multiple sclerosis patients. A preliminary MRI study. J Neurol 245:153–158
Beck AT, Brown GK, Steer RA (1997) Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther 35:1039–1046
Chard DT, Griffin CM, Rashid W, Davies GR, Altmann DR, Kapoor R, Barker GJ, Thompson AJ, Miller DH (2004) Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult Scler 10:387–391
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet 363:978–988
Codella M, Rocca MA, Colombo B, Martinelli-Boneschi F, Comi G, Filippi M (2002) Cerebral grey matter pathology and fatigue in patients with multiple sclerosis: a preliminary study. J Neurol Sci 194:71–74
Codella M, Rocca MA, Colombo B, Rossi P, Comi G, Filippi M (2002) A preliminary study of magnetization transfer and diffusion tensor MRI of multiple sclerosis patients with fatigue. J Neurol 249:535–537
Colombo B, Martinelli BF, Rossi P, Rovaris M, Maderna L, Filippi M, Comi G (2000) MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol 247:506–509
Figueira FF, Santos VS, Figueira GM, Silva AC (2007) Corpus callosum index: a practical method for long-term follow-up in multiple sclerosis. Arq Neuropsiquiatr 65:931–935
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15:559–567
Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ (1994) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21:9–14
Ford H, Trigwell P, Johnson M (1998) The nature of fatigue in multiple sclerosis. J Psychosom Res 45:33–38
Freal JE, Kraft GH, Coryell JK (1984) Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil 65:135–138
Ge Y, Grossman RI, Udupa JK, Babb JS, Nyul LG, Kolson DL (2001) Brain atrophy in relapsing-remitting multiple sclerosis: fractional volumetric analysis of gray matter and white matter. Radiology 220:606–610
Gean-Marton AD, Vezina LG, Marton KI, Stimac GK, Peyster RG, Taveras JM, Davis KR (1991) Abnormal corpus callosum: a sensitive and specific indicator of multiple sclerosis. Radiology 180:215–221
Giovannoni G, Thompson AJ, Miller DH, Thompson EJ (2001) Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology 57:676–681
Hardmeier M, Wagenpfeil S, Freitag P, Fisher E, Rudick RA, Kooijmans-Coutinho M, Clanet M, Radue EW, Kappos L (2003) Atrophy is detectable within a 3-month period in untreated patients with active relapsing remitting multiple sclerosis. Arch Neurol 60:1736–1739
Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC (1988) Fatigue in multiple sclerosis. Arch Neurol 45:435–437
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Krupp LB, Pollina DA (1996) Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol 9:456–460
Mainero C, Faroni J, Gasperini C, Filippi M, Giugni E, Ciccarelli O, Rovaris M, Bastianello S, Comi G, Pozzilli C (1999) Fatigue and magnetic resonance imaging activity in multiple sclerosis. J Neurol 246:454–458
Marrie RA, Fisher E, Miller DM, Lee JC, Rudick RA (2005) Association of fatigue and brain atrophy in multiple sclerosis. J Neurol Sci 228:161–166
Martola J, Stawiarz L, Fredrikson S, Hillert J, Bergstrom J, Flodmark O, Kristoffersen WM (2007) Progression of non-age-related callosal brain atrophy in multiple sclerosis: a 9-year longitudinal MRI study representing four decades of disease development. J Neurol Neurosurg Psychiatry 78:375–380
Mesaros S, Rocca MA, Riccitelli G, Pagani E, Rovaris M, Caputo D, Ghezzi A, Capra R, Bertolotto A, Comi G, Filippi M (2009) Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp 30:2656–2666
Niepel G, Tench C, Morgan PS, Evangelou N, Auer DP, Constantinescu CS (2006) Deep gray matter and fatigue in MS: a T1 relaxation time study. J Neurol 253:896–902
Nocentini U, Rossini PM, Carlesimo GA, Graceffa A, Grasso MG, Lupoi D, Oliveri M, Orlacchio A, Pozzilli C, Rizzato B, Caltagirone C (2001) Patterns of cognitive impairment in secondary progressive stable phase of multiple sclerosis: correlations with MRI findings. Eur Neurol 45:11–18
Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, Lyon-Caen O, Cherif AA (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol 58:105–111
Pittion-Vouyovitch S, Debouverie M, Guillemin F, Vandenberghe N, Anxionnat R, Vespignani H (2006) Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci 243:39–45
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L (1989) Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology 39:161–166
Riccitelli G, Rocca M, Forn C, Colombo B, Comi G, Filippi M (2010) Definition of regional distribution of grey matter loss in multiple sclerosis patients with fatigue: a voxel-based morphometry study. J Neurol 257:S1–S246
Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G, Filippi M (2007) fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum Brain Mapp 28:373–382
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, Plohmann A, Dellas S, Maguire RP, Missimer J, Radu EW, Steck A, Leenders KL (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48:1566–1571
Schreiber K, Sorensen PS, Koch-Henriksen N, Wagner A, Blinkenberg M, Svarer C, Petersen HC (2001) Correlations of brain MRI parameters to disability in multiple sclerosis. Acta Neurol Scand 104:24–30
Simioni S, Ruffieux C, Bruggimann L, Annoni JM, Schluep M (2007) Cognition, mood and fatigue in patients in the early stage of multiple sclerosis. Swiss Med Wkly 137:496–501
Simon JH, Holtas SL, Schiffer RB, Rudick RA, Herndon RM, Kido DK, Utz R (1986) Corpus callosum and subcallosal-periventricular lesions in multiple sclerosis: detection with MR. Radiology 160:363–367
Tartaglia MC, Narayanan S, Francis SJ, Santos AC, De SN, Lapierre Y, Arnold DL (2004) The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol 61:201–207
Van Der Werf SP, Jongen PJ, Nijeholt GJ, Barkhof F, Hommes OR, Bleijenberg G (1998) Fatigue in multiple sclerosis: interrelations between fatigue complaints, cerebral MRI abnormalities and neurological disability. J Neurol Sci 160:164–170
Yaldizli O, Atefy R, Gass A, Sturm D, Glassl S, Tettenborn B, Putzki N (2010) Corpus callosum index and long-term disability in multiple sclerosis patients. J Neurol 257:1256–1264
Conflict of interest
OY reports receiving travel grants, advisory, consulting and lecture fees from Bayer Schering, Biogen Idec, Novartis and Merck Serono which are exclusively used for research at the University of Basel, department of neurology. NP reports that he is now an employee of Biogen Idec. He had previously received honoraria, travel grants, research grants and personal compensation from Bayer Healthcare, Biogen Idec, GSK, TEVA, Sanofi Aventis, Merck Serono and Novartis. BT has received travel grants and honoraria from Merck Serono, Biogen Idec and Bayer Healthcare. AG and AP had nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2011_6091_MOESM1_ESM.doc
Fatigue severity scale. Each patient is asked to respond to the following nine statements by choosing a number between one and seven, where one indicates strongly disagree and seven indicates strongly agree. FSS mean score >4 indicates severe fatigue [21]
Rights and permissions
About this article
Cite this article
Yaldizli, Ö., Glassl, S., Sturm, D. et al. Fatigue and progression of corpus callosum atrophy in multiple sclerosis. J Neurol 258, 2199–2205 (2011). https://doi.org/10.1007/s00415-011-6091-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6091-0