Abstract
“Disease activity free” in relapsing-remitting multiple sclerosis (RRMS) is a new concept introduced by the results of the AFFIRM study. Our objective was to analyze the clinical and radiological efficacy of natalizumab treatment in actual clinical practice and compare it with the post hoc analysis of the AFFIRM study. All patients with RRMS who began treatment with natalizumab at our two French MS centres between April 2007 and May 2008 were included and followed-up for at least 2 years. No measurable disease activity (“disease activity free”) was defined as no activity on clinical measures (no relapses and no sustained disability progression) and radiological measures (no gadolinium-enhancing lesions and no new T2-hyperintense lesions on cerebral MRI). A total of 193 patients were included. Natalizumab was discontinued in 25.9% of cases before the completion of 2 years of treatment. In our cohort, we observed patients with more severe disease than in the AFFIRM study. The proportion of patients remaining free of clinical activity during 2 years of treatment was lower than in the AFFIRM study (37.8% vs. 64.3%). The proportion of patients remaining free of radiological activity during 2 years of treatment was higher than in the AFFIRM study (68.9% vs. 57.7%), while the proportion of patients remaining free of disease activity during 2 years of treatment was comparable to the AFFIRM study (33.3% vs. 36.7%). Natalizumab seems to be as effective in a real-life setting as in pivotal and post hoc studies. The confirmation of such benefits is important because of the progressive multifocal leukoencephalopathy risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natalizumab is the first treatment of a new class of selective adhesion molecule inhibitors used in relapsing-remitting multiple sclerosis (RRMS). The results of the AFFIRM study demonstrated that this α4-integrin antagonist reduced the annualized relapse rate (ARR) over 2 years by 68% and the risk of sustained disability progression by 42–54% in RRMS [1]. A post hoc analysis of the AFFIRM study introduced a new concept of “disease activity free” in MS [2]. “Disease activity free” can be defined as absence of measurable disease activity: no activity on clinical (no relapses and no sustained disability progression) and radiological measures (no gadolinium-enhancing lesions and no new or enlarging T2-hyperintense lesions on cranial MRI).
Natalizumab has been available in France since April 2007. Thus, in our two French MS centres (University Hospital of Lille and University Hospital of Strasbourg), at the time of the study (June 2010), a substantial proportion of our patients have already reached 2 years of treatment. We identified those patients who initiated natalizumab treatment over 2 years ago and analyzed the clinical and radiological efficacy of natalizumab treatment in actual clinical practice and compared it with the post hoc analysis of the AFFIRM study.
Methods
All patients with RRMS treated with natalizumab at our two MS centres in France between April 2007 and May 2008 were included and were prospectively followed-up for at least 2 years. Natalizumab was applied intravenously once every 4 weeks. All patients were clinically followed up (complete clinical and neurological evaluation for relapses; disability scored on the Expanded Disability Status Scale [EDSS] every 6 months). All patients receiving natalizumab underwent cMRI just before initiation of treatment and after completion of 1 and 2 years of treatment.
We used the same criteria as in the post hoc analysis of the AFFIRM study to determine “clinical disease activity”: occurrence of new relapses and/or sustained disability progression [2]. Progression was defined as an increase of at least 1.0 point (or at least 1.5 points from a baseline score of 0.0) on the EDSS. Concerning radiological disease activity, all cerebral MRI (cMRI) performed included at least axial T2-FLAIR and axial T1-weighted gadolinium-enhanced (T1-Gd) sequences. For geographical and practical reasons, these examinations were not all performed in the same MRI centre and were not all analysed with T2 lesion load measurement software. However, all were performed on a 1.5 T MRI. All cMRI were analyzed by experienced neurologists in each MS centre. We defined “radiological disease activity” as the appearance of any new T2 hyperintense lesions and/or the presence of any gadolinium-enhancing lesions. Unlike the post hoc analysis of the AFFIRM study [2], we were unable to measure enlarging T2 hyperintense lesions, which is a relatively rare phenomenon with poorly reproducible calculation methods. Patients without clinical and radiological activity were considered as free of combined activity and designated as disease-free patients.
We defined “patient with highly active disease” as a patient presenting at least two relapses in the year before natalizumab initiation and at least one Gd-enhanced lesion on cMRI within the 3 months preceding natalizumab initiation. All other patients were considered “non-highly active”.
We analyzed disease activity during the first year, during the second year and after 2 successive years of treatment. Subsequent analysis (reduction/increase of ARR and EDSS) included comparisons between different subgroups: EDSS ≤3/EDSS >3 and highly active disease/non-highly active disease.
In our study, patients who were lost to follow-up due to pregnancy planning or because they moved away were not considered as treatment failure and were excluded from analysis. Any other causes of treatment discontinuation were considered as treatment failure with clinical and radiological disease activity in the 2 years of treatment if disability progression or discontinuation occurred in the first year, and only in the second year if discontinuation occurred in the second year of treatment.
Statistical analyses were performed with SPSS 13.0 software. We used Student’s t test to compare mean values, the Wilcoxon non-parametric test to compare median values and the Chi-squared test to compare data distribution. The level of statistical significance (p) was set at 0.05.
Results
Cohort characteristics (Table 1)
A total of 193 patients began natalizumab therapy between April 2007 and May 2008. After excluding 13 patients (6 for pregnancy planning and 7 who moved away), as described in Methods, our final French cohort comprised 180 patients. The demographic data of the final cohort were similar to those of patients in the AFFIRM study but clinical status at baseline was more severe (French cohort/AFFIRM): higher median disease duration (7 years [range 0–34]/5.0 years [range 0–34]), higher EDSS score (mean ± SD, 3.8 ± 1.5/2.3 ± 1.2) and higher ARR (Mean ± SD, 2.2 ± 1.20/1.53 ± 0.91) [1]. In our cohort, the proportion of patients having received more than 6 months of immunomodulatory treatment prior to natalizumab initiation (86%) was totally different from the AFFIRM study (0%) [1]. Mean ± SD and median number of immunomodulatory/immunosuppressive treatments previous to natalizumab initiation were 1.45 ± 1.03 and 1 (range 0–6), respectively. The proportion of patients with gadolinium enhancement was similar but with smaller mean number of gadolinium-enhanced lesions in the French cohort.
Fifty patients (25.9%) stopped treatment within less than 2 years, for various reasons: conversion to the secondary progressive form of MS (SPMS; n = 15; 7.7%), immunoallergic side-effects (n = 10; 5.2%), lost to follow-up due to moving away (n = 7; 3.6%), pregnancy planning (n = 6; 3.1%), relapse(s) with antibodies against natalizumab (n = 3; 1.6%), noncompliance (n = 3; 1.6%), serious adverse event (SAE; n = 4; 2.0%), or psychiatric disorder (n = 2; 1.0%). Most of the immunoallergic side-effects occurred at the second perfusion (70%) and all of them in the first year of treatment. Detection of anti-natalizumab antibodies (NAb; n = 3) occurred in all cases in the first year of treatment. Serum samples were collected after 6 months of treatment, as recommended [3]. Only patients with relapses, disease progression or repeated adverse event were tested for NAb. Fifteen patients converted to SPMS. Discontinuation of natalizumab occurred at a mean 15.9 ± 9.3 months but almost all the patients who discontinued (86.7%) were suspected of SPMS, with disability progression as defined above and a reduction of walking distance in the first year of treatment. Four patients discontinued treatment due to an SAE: 1 patient due to breast cancer (patient with a family history of breast cancer who had regular mammographic examinations before and during natalizumab therapy, 20 months of treatment); 1 patient due to bacterial pleuropneumonia (13 months of treatment); 1 patient due to autoimmune haemolytic anaemia, leading us to discontinue natalizumab and initiate rituximab (9 months of treatment); and 1 patient due to exacerbation of extended atopic dermatitis with hypereosinophilia (up to 4,500/mm3, 11 months of treatment). No case of progressive multifocal leukoencephalopathy (PML) was observed in our cohort. After natalizumab discontinuation, patients received either interferon beta (n = 8), glatiramer acetate (n = 4) or immunosuppressive agents (n = 6); 11 received non-combined immunosuppressive and immunomodulatory agents; 5 did not receive any treatment and 3 were included in a phase III therapeutic trial. After natalizumab discontinuation, the most frequently used immunosuppressive therapies were cyclophosphamide and mycophenolate mofetil for patients who converted to SPMS and mitoxantrone for patients with highly active disease.
Comparison between patients with 2 years of natalizumab therapy and patients who discontinued natalizumab within 2 years (Table 2)
Proportion of male/female, disease duration, ARR pre-natalizumab and baseline cMRI characteristics were similar in these 2 populations, although age, EDSS pre-natalizumab, EDSS at 2 years and ARR at 2 years were significantly higher in the population with natalizumab discontinuation. The majority (86.5%) of patients with discontinuation of natalizumab had a baseline EDSS >3.0. As most of the patients who discontinued natalizumab had no control cMRI after treatment discontinuation, we had insufficient data to compare radiological evolution.
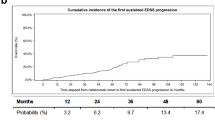
Analysis of disease activity in the final cohort and comparison with the post hoc analysis of the AFFIRM study (Fig. 1a–c)
Eighty-seven patients (48.3%) were free of clinical activity during the first year, 100 patients (55.5%) during the second year and 68 patients (37.8%) over the 2 successive years of treatment (Fig. 1a). Statistical analysis showed that there were significantly fewer patients free of clinical activity in our cohort than in the post hoc analysis of the AFFIRM study [2].
One hundred and thirty-two patients (73.3%) were free of radiological activity during the first year, 135 patients (75%) during second year and 124 patients (68.9%) over the 2 successive years (Fig. 1b). Statistical analysis showed that significantly more patients were free of radiological activity in our cohort than in the AFFIRM cohort during the first year and over the 2 successive years, whereas significantly fewer were free of radiological activity during the second year [2].
Eighty patients (44.4%) were free of combined activity during the first year, 95 patients (52.8%) during the second year and 60 patients (33.3%) over the 2 successive years (Fig. 1c). Statistical analysis did not show any significant difference in the proportion of disease activity free patients between our cohort and the post hoc analysis of the AFFIRM study, except during the second year, where we observed a higher proportion of disease activity free patients in AFFIRM cohort [2].
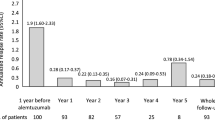
Analysis of disease activity in subgroups with highly and non-highly active disease and comparison with the post hoc analysis of the AFFIRM study (Fig. 2a, b)
Sixty-three patients in the final cohort were considered as presenting highly active disease. Treatment was interrupted in 12 patients (19%; 5 allergies, 3 SPMS, 1 Nab, 3 others). Thirty-one patients (49.2%) were free of combined activity during the first year, 37 patients (58.7%) during the second year and 24 patients (38.1%) over 2 successive years (Fig. 2a). One hundred and seventeen patients were considered as presenting non-highly active disease. Treatment was discontinued in 25 patients (21.4%; SPMS [n = 12], allergies [n = 4], NAb [n = 2], SAE [n = 4], others [n = 3]). Forty nine patients (41.9%) were free of combined activity during the first year, 58 patients (49.6%) during the second year and 36 patients (30.8%) during 2 successive years (Fig. 2b). Among patients with highly active disease, we observed a higher proportion of patients free of combined activity than in the AFFIRM cohort (27.4%) but this result did not reach statistical significance [2]. Among patients with non-highly active disease, we observed a lower rate of patients free of combined activity than in the AFFIRM post hoc study (39.6%); however this result did not reach statistical significance [2].
Analysis of subgroups in final cohort and cohort who continued natalizumab for at least 2 years (Table 3)
As expected, the reduction in ARR and EDSS was greater in the cohort that continued natalizumab than in the final cohort. The ARR of the two cohorts and of all subgroups of these cohorts was significantly reduced after 2 years of treatment (75–84.6%). The highest reductions of ARR were observed in patients with highly active disease, within the final cohort (79.4%) and in the cohort who continued natalizumab (84.6%). Only patients who continued natalizumab and had a baseline EDSS >3.0 had a significant reduction of EDSS (9%). For the two cohorts and for all other subgroups, EDSS remained stable at 2 years.
Discussion
Overall, the proportion of patients free of clinical and radiological disease in our cohort seems to be almost comparable to the post hoc analysis of the AFFIRM study [2]. As in all post-marketing studies, one could discuss that the lack of blinding might make the treatment effect appear greater than it really is. We observed a better radiological response and a worse clinical response than that reported in the AFFIRM cohort.
As in all observational post-marketing studies [4–9], our population of patients treated with natalizumab had a higher disease duration, baseline EDSS, ARR and more often previously been treated by immunomodulatory drugs than in AFFIRM study [1]. Nevertheless, our cohort had a shorter disease duration, higher baseline EDSS and similar ARR compared to other post-marketing studies. This is mainly due to differences between the marketing authorization criteria in European countries and inclusion criteria for the AFFIRM study [1].
The proportion of patients who discontinued treatment was higher in our cohort than in other observational studies, but in the other studies not all patients had reached 2 full years of treatment, thus inducing an underestimation of discontinuation [4–9]. The reasons for discontinuing natalizumab were numerous in our study, as in other studies, but the classification and distribution could be relatively different, thus making any comparison difficult. Patients who discontinued treatment that we considered as non-responders were significantly older and had a slightly longer disease duration. This is in complete agreement with recently published data showing that patients <40 years of age were better responders than patients ≥40 years of age [10]. In fact, the probability of suffering from the SP form of MS is higher after 40 years of age than before.
The efficacy of natalizumab on ARR and disability progression is confirmed by our post-marketing study. A significant reduction of EDSS was only observed in the subgroup of patients who continued treatment and had a baseline EDSS >3.0, because the majority of patients who discontinued treatment (non-responders) had a baseline EDSS >3.0 and were excluded from this analysis. However it is important to consider that the EDSS remained stable in a very large proportion of patients. After 2 years of treatment, we observed a significant reduction of ARR (75%) similar to that reported in the AFFIRM study (85% by comparison of ARR year 0 and ARR year 2 of the natalizumab group) [1]. Nevertheless, we also noted that the proportion of patients free of clinical activity was smaller than in the AFFIRM cohort, regardless of the period analyzed (year 0–1, year 1–2 or year 0–2) [2]. This difference is likely explained by the higher clinical disease activity in our cohort at baseline. In contrast to the setting in which the AFFIRM study was conducted [1], the prescription of natalizumab in France is authorized for the active form of RRMS without any limit on the EDSS score, and for a large majority of patients it has been proposed as a second line treatment. Among the patients who discontinued treatment, we considered those with immunoallergic effects, SAE, noncompliance, and psychiatric disorders as non-responders. Thus, we considered the worst scenario and may well have slightly underestimated the number of patients free of clinical disease. We also noted that patients in our cohort were more likely to be free of radiological activity than those in the AFFIRM post hoc study [2]. We assume that this was because radiological activity at baseline was lower than in the AFFIRM study [1]. At baseline, the proportion of patients with Gd-enhanced lesions was almost similar in our cohort and the AFFIRM study [1], but significantly fewer Gd-enhanced lesion(s) were found. We may have underestimated radiological activity by not measuring enlarging T2 lesions. In contrast to clinical trials, the cMRI parameters were not standardized between the MRI centres in our study, and this could have reduced the detection of some new T2 lesions and Gd-enhanced lesions. However if we consider the proportion of patients free of combined activity in our cohort, we observed a result similar to the AFFIRM post hoc study [2]. As in the AFFIRM post hoc study [2], the ability of natalizumab to induce more disease activity free patients (clinical or radiological activity, or both) seems to be greater during the second year of treatment. No explanation for this has yet been provided [2]. In line with the AFFIRM post hoc study [2], the patients with the greatest reduction in ARR in our study were those with highly active disease; however in contrast to that study, the ability of natalizumab to induce more disease activity free patients was also greater in our highly active disease patients than in our non-highly active disease patients. These results confirm that the greatest benefit is obtained in patients with highly active disease [10].
In our post-marketing study, natalizumab seems to have been as effective as in pivotal and post hoc studies. This study may help clinicians to decide to treat a patient with natalizumab, taking into account the PML risk.
References
Polman CH, O’Connor PW, Havrdova E et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Havrdova E, Galetta S, Hutchinson M et al (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the natalizumab safety and efficacy in relapsing-remitting multiple sclerosis (AFFIRM) study. Lancet Neurol 8:254–260
Calabresi PA, Giovannoni G, Confavreux C et al (2007) The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology 69:1391–1403
Prosperini L, Borriello G, Fubelli F et al (2010) Natalizumab treatment in multiple sclerosis: the experience of S. Andrea MS Centre in Rome. Neurol Sci 31(Suppl 3):S303–S307
Fernandez O, Papais Alvarenga O, Guerrero M et al (2011) The efficacy of natalizumab in patients with multiple sclerosis according to level of disability: results of an observational study. Mult Scler 17:192–197
Mancardi GL, Tedeschi G, Amato MP et al (2011) Three years of experience: the Italian registry and safety data update. Neurol Sci 31(Suppl 3):S295–S297
Piehl F, Holmen C, Hillert J et al (2011) Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci 31(Suppl 3):S289–S293
Sangalli F, Moiola L, Bucello S et al (2011) Efficacy and tolerability of natalizumab in relapsing-remitting multiple sclerosis patients: a post marketing observational study. Neurol Sci 31(Suppl 3):S299–S302
Holmen C, Piehl F, Hillert J et al (2011) A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler 17:708–719
Hutchinson M, Kappos L, Calabresi PA et al (2009) The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 256:405–415
Conflict of interest
Dr Melin has received funding for travel from Biogen Idec and Merck Serono and research support from Biogen Idec. Dr Outteryck has received funding for travel from Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd, and Sanofi-Aventis and speaker honoraria from Bayer Schering Pharma, Biogen Idec, and Sanofi- Aventis. Dr Collongues has received funding for travel from Novartis and Merck Serono. Dr Zéphir has received funding for travel from Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd, and Sanofi-Aventis and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Teva Pharmaceutical Industries Ltd, and Sanofi-Aventis. Dr Lacour has received funding for travel from Teva Pharmaceutical Industries Ltd, Biogen Idec, Genzyme and speaker honoraria from Genzyme and Sanofi-Aventis. Dr Berteloot has received funding for travel from Teva Pharmaceutical Industries Ltd, Bayer-Schering Pharma, Biogen Idec, and Novartis. Prof. Vermersch serves on scientific advisory boards for Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd, and Sanofi-Aventis; has received funding for travel and speaker honoraria from Biogen Idec, Bayer Schering Pharma, Novartis, Teva Pharmaceutical Industries Ltd, Sanofi-Aventis and Merck Serono; and receives research support from Biogen Idec, Merck Serono, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, and Bayer Schering Pharma. Prof. de Sèze serves on scientific advisory boards for Biogen Idec, Bayer Schering Pharma, Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd, and Sanofi-Aventis; has received funding for travel and speaker honoraria from Biogen Idec, Bayer Schering Pharma, Novartis, Teva Pharmaceutical Industries Ltd, Sanofi-Aventis, and Merck Serono; and receives research support from Biogen Idec, Merck Serono, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, and Bayer Schering Pharma.
The remaining authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Melin and O. Outteryck contributed equally to this work.
Rights and permissions
About this article
Cite this article
Melin, A., Outteryck, O., Collongues, N. et al. Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 259, 1215–1221 (2012). https://doi.org/10.1007/s00415-011-6339-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6339-8