Abstract
With the advent of subtype-selective ligands and dopamine receptor knockout mice, the last two decades have seen an explosion in research on the role of dopamine receptor subtypes in reward and relapse to drug-seeking behavior. This chapter represents a relatively comprehensive review of this literature, beginning with the ability of D1-like and D2-like receptor agonists to support self-administration behavior and produce conditioned preference, and followed by the modulation of natural reward, brain stimulation reward, and conditioned reward with subtype-selective ligands and dopamine receptor knockout mice. Subsequent sections describe the modulation of drug and alcohol self-administration by dopamine receptor subtypes, and role of dopamine receptor subtypes in relapse to drug and alcohol seeking in animal models. Finally, down-regulation in dopamine receptors following chronic drug self-administration is discussed in reference to differential changes in dopamine receptor-mediated behavior, suggesting that better integration between biological and behavioral data is needed in future studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Dopamine involvement in natural and drug reward is well established, but the role of specific dopamine receptor subtypes in reward processes remains a topic of intensive investigation. The most common approach to investigate dopamine receptor function in reward employs pharmacological ligands with relatively good selectivity for D1-like and D2-like receptor classes, although their potential actions at other neurotransmitter receptors (e.g., serotonergic or noradrenergic) are often neglected. Unfortunately, there have been far fewer studies targeting specific D1-like (D1 and D5) or D2-like (D2, D3 and D4) receptor subtypes due to a lack of selective ligands, and promising candidates that exhibit good selectivity under artificial conditions in vitro are notorious for their lack of selectivity in vivo with further scrutiny. The ability to differentiate between related dopamine receptor subtypes is even more difficult when they play similar roles in behavior, but enhanced when related subtypes mediate different behavioral effects. Another potential caveat is that opposing behavioral effects are often mediated by pre- and post-synaptic receptors in the mesolimbic dopamine system, leading to biphasic dose–effect curves for behavioral responses. Given these considerations, recent advances in antagonist selectivity profiles, especially in differentiating the effects of D2 and D3 receptor blockade, have yielded compelling evidence for distinct receptor subtype actions in reward and reward-seeking behavior.
Genetic deletion generally is more definitive for assessing dopamine receptor involvement in natural and drug reward, although developmental compensation must be considered especially when negative results are found. Another important consideration is that behavioral responses in mice can differ substantially from rats and primates, a caveat highlighted by uniformly inhibitory effects of D2-like agonists on psychomotor behavior in mice over a wide dose range [1]. Dopamine receptor knockout mice can be used to demonstrate definitive selectivity of novel and putative subtype-selective ligands in vivo, most notably when their behavioral effects are completely attenuated with the deletion of a specific dopamine receptor subtype. However, the interpretation of behavioral changes that result from brain-wide receptor deletion can be complicated by competing effects of dopamine receptors in different brain regions on neural circuits regulating natural and drug reward. The recent advent of inducible and localized genetic deletion strategies in mice is a powerful approach that circumvents problems relating to developmental compensation, and provides anatomically discrete loss of specific dopamine receptor subtypes, but this approach has yet to be applied to studies on natural and drug reward. Conversely, transgenic approaches to investigate gain of dopamine receptor function have not been widely used due to the need to limit expression to cells that naturally express the dopamine receptor subtypes. Recent advances in D1 and D2 promoter-driven transgenics will be pivotal to modulate dopamine receptor function in the appropriate cell types in future studies.
Using anatomically discrete microinfusion of dopaminergic ligands, studies have found that dopamine receptors play different roles in mediating or modulating reward processes in different brain regions. For example, dopamine receptors in ventral striatal regions mediate primary rewarding effects. Thus, transient dopamine receptor activation in these regions is sufficient to support self-administration behavior when receptor activation is a consequence of the behavior. In contrast, dopamine receptors in neocortical or amygdala regions can modulate reward evaluation, choice, or the formation of conditioned environmental (Pavlovian) associations that acquire their own rewarding properties secondary to the role of dopamine receptors in primary reward. These regional effects, in turn, are determined by dopamine receptor localization on specific neuronal subpopulations such as interneurons or projection neurons, and differential coupling to G protein signaling pathways, further illustrating the complexities of delineating the mechanism of dopamine receptor subtypes in reward.
In addition to reward, dopamine receptors play a prominent role in eliciting appetitive or approach behavior, a phenomenon distinguishable from reward itself by the fact that behavioral responses follow rather than precede dopamine receptor stimulation. The seminal work of Schultz and colleagues [2, 3] found that environmental predictors of reward availability activate midbrain dopamine neurons, and other studies have shown that dopamine release in forebrain regions is sufficient to elicit appetitive behavior directed at obtaining reward. This behavior often is referred to as reward-seeking behavior, and is widely studied in animal models of drug and alcohol addiction to simulate craving and relapse in humans. Thus, dopamine receptors mediate two crucial aspects of addictive behavior: (1) they mediate primary reward when stimulated consequential to self-administration behavior and (2) they elicit reward-seeking behavior during periods of forced abstinence.
This chapter reviews the role of dopamine receptor subtypes in reward and reward-seeking behavior. Initial sections review earlier work establishing that dopamine receptor agonists serve as primary rewards in self-administration studies. Other studies that use dopamine receptor agonists to induce a conditioned place preference are described; a more commonly used but indirect measure of drug reward. Subsequent sections discuss the role of dopamine receptor subtypes in natural reward, brain stimulation reward, conditioned reward, and self-administration of abused drugs and alcohol. In each section, intracranial studies that elucidate regional sites of dopamine receptor action and genetic deletion of specific receptor subtypes are reviewed where available with an emphasis on differential roles for D1-like and D2-like receptors. In later sections, the role of dopamine receptor subtypes in relapse to drug- and alcohol-seeking behavior is discussed. In a final section, alterations in dopamine receptors produced by chronic drug self-administration are highlighted together with discrepant changes in dopamine receptor-mediated behavior, suggesting the need for better integrative models of biological and behavioral change in drug addiction. Clearly, dopamine receptors play a role in several other aspects of reward-related behavior that are beyond the scope of this chapter.
17.2 Dopamine Receptor Subtypes that Mediate Primary Reward
The fact that selective and directly acting dopamine receptor agonists will support self-administration behavior indicates that dopamine receptors mediate a primary rewarding stimulus. The earliest study supporting this notion found that drug-naïve female rats will learn to perform a novel lever-press response to receive intravenous injections of the direct dopamine receptor agonist apomorphine, and will maintain stable dose-dependent responding for several weeks after acquisition [4]. Apomorphine self-administration is abolished by pretreatment with the dopamine receptor antagonist pimozide. A subsequent study found that depletion of endogenous brain catecholamines with alpha-methylparatyrosine treatment has no effect on apomorphine self-administration, but attenuates self-administration of the indirect agonist amphetamine [5]. These early studies clearly established a role for dopamine receptor stimulation in mediating primary reward and are often overlooked in more modern theoretical formulations of dopamine receptor function in reward-related behavior. Thus, in addition to serving as a primary reward, various other functions ascribed to dopamine include signaling reward prediction error [6], increasing incentive salience or wanting [7], enhancing the impact of conditioned environmental stimuli on instrumental responding [8], and reducing psychological effort requirements to obtain rewards [9]. These and other attributes suggest a complex multi-functional role for dopamine in reward processing and execution of instrumental behavior, but they do not belie the fact that dopamine receptor stimulation is in itself sufficient to mediate primary rewarding events.
(a) Self-administration of the D1-like agonist SKF 82958 in drug-naive rats. Data points show the mean daily number of self-injections (± S.E.M.) of groups self-administering SKF 82958 at a dose of 10 μg/kg/injection or saline over 15 consecutive test sessions (*SKF 82958 differs from saline, P < 0 05). High response rates in the initial test sessions are due to lever-press training for food pellets prior to self-administration testing. (b) In choice tests, rats prefer to self-administer a moderate dose of cocaine (800 μg/kg/injection) to either the D1-like agonist SKF 82958 (10 μg/kg/injection) or the D2-like agonist (+)-PHNO (1 μg/kg/injection), but exhibit a similar preference for cocaine and a mixture of the D1- and D2-like agonists. Rats prefer the mixture of the D1- and D2-like agonists to a lower dose of cocaine (267 μg/kg/injection). *Significantly different from 50%, P < 0.05. (c) Intranucleus accumbens (NAc) shell self-administration of D1- and D2-like agonists. Groups of rats self-administered 100 nl infusions containing 0.5 mM of the D1-like agonist SKF 38393, the D2-like agonist quinpirole, or a mixture during the first three sessions. The SKF and quinpirole groups were switched to the mixture for sessions 4 and 5, while the group initially trained on the mixture self-administered vehicle in session 4. During sessions 1–3, rats receiving SKF + quinpirole obtained more infusions than did rats receiving either drug alone (P < 0.001). The SKF and quinpirole groups exhibited higher levels of self-administration in session 5, when SKF + quinpirole was given in place of SKF or quinpirole alone, than in session 3 (P = 0.01). The replacement of SKF + quinpirole with vehicle in session 4 diminished self-administration (P = 0.02). (d) The D1-like agonist SKF 82958 induces dose-dependent conditioned place preferences with minimal pairings (2 drug, 2 saline). Data are expressed as mean ± S.E.M. difference in preference for the drug- minus saline-paired side. Symbols indicate post-test scores differ from saline/saline pairing (SAL; * P < 0.05, ** P < 0.01), or from pretest scores (†† P < 0.01). Reproduced with permission from (a) Self and Stein [11], (b) Manzardo et al. [21], (c) Ikemoto et al. [25], and (d) Graham et al. [43]
17.2.1 Self-Administration of D1-Like and D2-Like Receptor Agonists
In addition to apomorphine, animals will self-administer intravenous injections of agonists selective for either D1-like or D2-like receptors. Early studies with the prototypical but partial D1-like receptor agonist found that intravenous infusions of SKF 38393 fail to support self-administration behavior [10]. Subsequent studies found that higher efficacy D1-like agonists such as SKF 82958 and SKF 81297 will support self-administration in rats, mice, and monkeys, and even in drug-naïve animals (Fig. 17.1a) [11–16]. Similarly, several studies found that D2-like
receptor agonists are self-administered intravenously by rats, mice and monkeys (reviewed by [17]), including highly D2-like selective agonists such as quinpirole, 7-OH-DPAT, and (+)-PHNO [10, 18–22]. In contrast to the self-administration of D1-like agonists in drug-naïve animals, D2-like agonists are self-administered only when substituted for drugs such as cocaine in animals with extensive prior self-administration training. Whether drug-naïve animals will learn to self-administer D2-like agonists has not been systematically studied, and there are no reports of experimentally or drug-naive animals acquiring D2-like agonist self-administration, but studies have found that quinpirole and 7-OH-DPAT generally fail to support self-administration in monkeys without prior cocaine self-administration training [23, 24]. These findings indicate that D1-like receptor stimulation is sufficient to mediate primary rewarding effects, but it is not clear whether selective D2-like receptor stimulation is capable of mediating primary reward. One possible explanation is that D2-like agonists maintain self-administration behavior once acquired by enhancing the conditioned rewarding properties of lever-press behavior associated with primary rewards during previous training (see Section 17.3.3). Furthermore, D2-like receptors apparently can augment the magnitude of reward mediated by D1-like receptor stimulation, since rats prefer to self-administer co-injections of D2-like with D1-like receptor agonists over cocaine, but cocaine is preferred over injections of D1-like receptor agonists alone (Fig. 17.1b) [21]. Moreover, genetic deletion of D1 receptors abolishes the reinforcing effects of a D2-like agonist in mice [16], further indicating that D2-like receptors fail to independently mediate primary reward.
Intracranial self-administration studies indicate that the ventral striatum contains D1-like and D2-like receptors that are important for mediating primary rewarding effects. Rats will learn to self-administer a cocktail containing both D1-like and D2-like receptor agonists into the nucleus accumbens shell (Fig. 17.1c), but not the core subregion [25]. While neither agonist alone supports self-administration behavior, only the partial D1-like receptor agonist SKF 38393 has been tested, and so it is possible that high efficacy and selective D1-like receptor agonists would support self-administration behavior in the nucleus accumbens shell. Interestingly, infusion of cocaine into more ventral olfactory tubercle regions more effectively supports self-administration behavior than infusion in the nucleus accumbens shell, an effect blocked by co-infusion with either D1-like or D2-like antagonists [26]. Dopamine receptors in the medial prefrontal cortex also support self-administration behavior, since rats will self-administer dopamine (or cocaine) into this region, and the effects are blocked by co-infusion with a D2-like but not a D1-like antagonist [27–29]. Whether animals would self-administer selective D1-like or D2-like agonists directly into the medial prefrontal cortex or other brain regions is unknown. Other studies found that cocaine is self-administered directly into the dopamine cell body region of the posterior ventral tegmental area in mice and rats, which may be mediated by D1-like receptor modulation of serotonergic excitation of dopamine neurons [30, 31].
Some studies suggest that the hedonic impact of rewards may be dissociable from dopamine receptor stimulation and its role in goal-directed behavior. Thus, for example, depletion of central dopamine has no effect on hedonic responses to natural reward in animals [7]. In humans, indirect dopamine agonists such as cocaine and amphetamine induce euphoria, but the dopamine receptor agonist apomorphine produces nausea and dysphoria, an effect that may involve peripheral dopamine receptor activation [32]. The D2-like receptor agonist bromocriptine produces little subjective effects on its own [33], and neuroleptic drugs that block D2-like receptors fail to consistently attenuate subjective reports of euphoria with amphetamines or cocaine in humans [34, 35]. In contrast, however, acute blockade of D1-like receptors with ecopipam (SCH 39166) attenuates cocaine-induced euphoria in human cocaine addicts [36], although chronic blockade of D1-like receptors actually enhances cocaine euphoria potentially due to compensatory changes [37]. These findings suggest that the primary rewarding effects of direct dopamine receptor stimulation may be dissociable from hedonic responses in animals and that D1-like, but not D2-like, receptors may play a role in drug-induced euphoria in humans.
17.2.2 Conditioned Place Preference with D1-Like and D2-Like Receptor Agonists
Conditioned place preference has been widely used as an indirect measure of the rewarding properties of dopamine receptor agonists. In conditioned place preference, animals will prefer an environment associated with primary rewarding stimuli over those paired with neutral stimuli. The environmental context acquires conditioned rewarding properties that reflect the primary rewarding properties of the dopamine receptor agonists. Several decades of research has shown differential abilities of D1-like and D2-like receptor agonists to produce conditioned place preference [17, 38]. Early studies found that systemic administration of high doses of the partial D1-like agonist SKF 38393 actually produces a place aversion [39, 40], reflecting dysphoric effects, and this effect is blocked by a D1-like but not a D2-like antagonist [39, 41]. However, subsequent studies found that the higher efficacy D1-like agonists SKF 82958 (Fig. 17.1d), SKF 81297, and the non-benzazepine agonist ABT-431 will produce a conditioned place preference [42, 43]. Interestingly, the long-acting full D1-like agonist A-77636 produces a place aversion potentially due to profound receptor internalization [43, 44]. In this sense, place aversions with A-77636 and the partial D1-like agonist SKF 38393 resemble strong place aversions that are found with D1-like receptor blockade [45], suggesting that a loss of D1-like receptor tone produces dysphoria in animals. Together, these studies generally agree with self-administration studies indicating that selective D1-like receptor stimulation is sufficient to mediate primary rewarding effects.
Systemic treatment with D2-like agonists such as quinpirole, 7-OH-DPAT, or bromocriptine can produce a conditioned place preference, a place aversion, or have no effect depending on rat strain, dose, and other experimental conditions. Systemic administration of quinpirole at doses thought to activate post-synaptic receptors produces a weak conditioned place preference in Wistar [39, 41], Lister-hooded [46], and Long–Evans rats [40], while it is ineffective in Sprague Dawley rats unless they have received repeated cocaine injections prior to conditioning [43]. Similarly, 7-OH-DPAT has been shown to produce a conditioned place preference in Wistar [47] and Sprague Dawley rats [48, 49] in some studies, but no effect in Swiss Webster mice or Wistar rats in other studies [50, 51]. Place conditioning with low doses that are thought to selectively activate D2-like autoreceptors actually produce conditioned place aversions [52, 53]. The less selective D2-like agonist bromocriptine has been shown to produce place preference in both rats and mice [54, 55]. Similarly, certain full efficacy and putative D3-selective ligands produce either a place preference [56] or an aversion [52], while a novel D4 agonist is without effect [57]. Together these discrepancies highlight the impact of strain differences, intralaboratory variability in place conditioning procedures, or the potential differential influences of D2 and D3 receptors on reward substrates.
When infused into the nucleus accumbens, both D1-like and D2-like agonists produce conditioned place preferences [40], whereas negative findings have been found in the hippocampus and amygdala. However, dopamine receptors in the hippocampus and amygdala can modulate place conditioning with other drugs of abuse [58, 59], suggesting that dopamine receptors in these regions can alter associative learning between rewarding stimuli and conditioned environments. Results with place conditioning differ from self-administration studies by suggesting that either D1-like or D2-like receptors in the nucleus accumbens can produce rewarding effects in drug-naïve animals.
17.3 Modulation of Natural and Endogenous Reward by Dopamine Receptor Subtypes
Given the fact that natural rewards, including highly palatable foods, water, and sexual interaction, all increase dopamine levels in terminal regions, it is not surprising that dopamine receptors play a role in natural reward. Moreover, the rewarding effect of electrical brain stimulation, or brain stimulation reward, also is mediated by dopamine receptors to a major extent. While both D1-like and D2-like receptors are implicated in natural reward, D1-like receptors in particular play an important role in reward-related learning during initial establishment of stimulus–response associations, whereas D2-like receptors play a prominent role in augmenting the motivational salience of conditioned rewards after learning has occurred. These differences suggest that D1- and D2-like receptors are involved in different phases of acquisition and expression of rewarded behavior.
17.3.1 Modulation of Food, Water, and Sexual Reward by Dopamine Receptor Subtypes
Pretreatment with either dopamine agonists or antagonists can reduce instrumental responding for food rewards (e.g. [60]), but these effects usually reflect performance or other rate-altering effects unrelated to reward impact. In sham-fed rats, where sucrose is ingested without instrumental requirements but prevented from remaining in the stomach, both D1- and D2-like antagonists reduce preference for rewarding sucrose [61–63]. Pretreatment with either D1- and D2-like agonists also reduces food intake, but only D1-like agonists reduce sucrose-sham feeding, consistent with a role for D1-like receptor stimulation in satiety mechanisms for highly palatable reward [64]. In contrast, instrumental responding for high sucrose content food pellets is increased by the D2-like antagonist raclopride, consistent with an antagonist-like effect on reward processes surmountable by increased self-administration behavior [65].
Water intake in thirsty mice is not affected by D1-like antagonists [66], while it is reduced by both D1-like and D2-like antagonists in rats [67]. However, the conditioned place preference to water availability in deprived rats is blocked by either the D1-like antagonist SCH 23390 or the D2-like antagonist raclopride at doses that have no effect on water intake during conditioning [67]. These findings suggest that both D1- and D2-like receptors could play a role in primary water reward, although blockade of Pavlovian learning processes independent of alterations in primary reward strength also could account for these results.
In addition, dopamine receptors are implicated in the rewarding effects of sexual interaction. Dopamine D2-like agonists promote while antagonists reduce motivational indices of copulatory behavior in male rats [68]. In contrast, the partial D1-like agonist SKF 38393 does not influence copulation [69]. When male rats perform an instrumental response to gain access to a receptive female, non-selective blockade of D1- and D2-like receptors impairs this response [70], and the pursuit of receptive females is reduced by systemic pretreatment with either D1- or D2-like antagonists [71]. In female rats, high doses of the D2-like agonist quinpirole elicit sexual behavior (lordosis) in non-receptive rats, while lower (presumably autoreceptor doses) are effective in receptive rats [72], suggesting that receptivity disrupts the sensitivity to post-synaptic D2-like stimulation. D1-like ligands are ineffective at modulating this behavior. Many sexual responses in female rats may reflect actions in brain regions unrelated to brain reward and motivational systems. However, a recent study found that the preference for male pheromones in female mice is not blocked by D1-like or D2-like antagonists, but is blocked by the D1-like agonist SKF 38393 [73], potentially reflecting a masking of the pheromone reward stimulus by tonic D1-like receptor stimulation.
D1-receptor knockout mice are capable of acquiring instrumental responding for sucrose or sweetened food reward, but acquire self-administration more slowly despite no difference in sucrose intake when freely available [16, 74]. Thus, these decrements may reflect performance rather than motivational deficits. In female D5 knockout mice, the ability of apomorphine to facilitate sexual receptivity is blocked, whereas male D5 knockout mice show an impaired conditioned place preference to environments paired with intromission, but not ejaculation [75]. D2 receptor knockout mice show impaired acquisition of instrumental response for sweetened milk most likely due to Parkinson-like effects [76], but another study found no effect on water self-administration [77]. D3 receptor knockout mice show no changes in responding for food or water rewards [78]. The inability to regulate the degree of dopamine receptor inactivation, or to localize effects to specific brain regions, probably accounts for behavioral impairment and makes it difficult to assign a role for specific dopamine receptor subtypes in natural reward in these knockout studies.
Dopamine levels in the nucleus accumbens are elevated by unfettered sucrose consumption, but reverse microdialysis of either D1- or D2-like antagonists into the nucleus accumbens fails to alter this behavior [79]. In contrast, microinfusions of both D1- or D2-like antagonists into the nucleus accumbens core, but not shell, reduce instrumental responding for normal chow pellets under more demanding progressive ratio schedules, which could reflect performance rather than motivational effects [80]. Putative D3 and D4 antagonists are ineffective. When regular chow is available under free-feeding conditions in food-deprived rats, neither D1- nor D2-like receptor blockade in the nucleus accumbens impairs food intake, but the number of feeding bouts is reduced [81], consistent with a role for nucleus accumbens dopamine receptors in incentive motivational responses, but not the regulation of food intake. Kelley and colleagues found that a low dose of a D1-like antagonist that does not impair instrumental performance will attenuate acquisition of responding rewarded by sucrose pellets when infused into the nucleus accumbens, but only when co-infused with a similar low dose of an NMDA glutamate receptor antagonist [82]. These results suggest that coincident activation of D1-like and NMDA receptor signals are needed for reward-related learning. Post-trial infusions of the D1-like and NMDA antagonists fail to alter acquisition of sucrose self-administration, but post-trial infusion of a protein kinase A inhibitor (PKA) does impair acquisition, suggesting that prior D1-like receptor activation of cAMP is important for consolidating these learned stimulus–response associations [83]. A similar relationship for coincident D1-like and NMDA receptor activation and dependence on protein kinase A is found in the medial prefrontal cortex [84]. Blockade of intra-amygdala D1-like receptors also impairs acquisition of instrumental responses for sucrose reward without affecting performance, suggesting that amygdala D1-like activity is important in reward-related learning [85].
17.3.2 Modulation of Brain Stimulation Reward by Dopamine Receptor Subtypes
Electrical stimulation of several brain regions will support self-stimulation behavior, including the ventral tegmental area where dopaminergic cell bodies are located, and the lateral hypothalamus where descending fibers of the medial forebrain bundle activate dopamine neurons through direct and indirect pathways [86]. Forebrain regions including the medial prefrontal cortex also support self-stimulation behavior potentially through activation of dopamine neurons in the ventral tegmental area [87]. The magnitude of brain stimulation reward can be determined in a response rate-independent manner by measuring stimulus frequency (or intensity) thresholds, or by parallel shifts in stimulus–response curves, valuable procedures given that dopaminergic ligands can produce substantial effects on performance. Systemic administration of either D1- or D2-like antagonists attenuate the rewarding impact of electrical brain stimulation (e.g.[88, 89]), whereas D3 antagonists are ineffective [48, 90]. The rate–frequency curve is shifted rightward in D1 receptor knockout mice [91], and D2 knockout mice require 50% more stimulus intensity to support self-stimulation [92]. Together these studies suggest that both D1 and D2 receptor subtypes play a necessary role in the rewarding effects of electrical brain stimulation.
In contrast to antagonists, systemic administration of D1- and D2-like receptor agonists produces differing effects on brain stimulation reward. Thus, the prototypical but partial D1-agonist SKF 38393 is without effect [89] or inhibits brain stimulation reward [93], although the facilitation of ventral tegmental self-stimulation by SKF 38393 has been reported [94]. The full efficacy D1-agonist SKF 81297 elevates brain stimulation reward thresholds in rats [95], while the less selective D1-agonist SKF 82958 reduces thresholds in mice [96]. Pretreatment with the non-benzazepine D1-agonist A-77636 also lowers thresholds in rats [97]. Given the differential ability of these compounds to support self-administration behavior and produce a conditioned place preference (SKF 81297 and SKF 82958), or fail self-administration tests and produce a conditioned place aversion (SKF 38393 and A-77636), the relationship between facilitation of brain stimulation reward and inherent rewarding properties of these compounds is unclear.
In contrast, pretreatment with the D2-like agonist quinpirole lowers the threshold of brain stimulation reward [89, 98], while lower presumably autoreceptor doses increase thresholds [98]. Although the D1-like agonist SKF 38393 is without effect, co-administration with quinpirole augments the threshold lowering effects of quinpirole alone, while the D1-like antagonist SCH 23390 attenuates this facilitation, consistent with synergistic and enabling interactions between D1- and D2-like receptors [99]. Pretreatment with the D2-like agonist 7-OH-DPAT has inconsistent or biphasic effects [48, 95, 98, 100], with low doses attenuating and high doses facilitating brain stimulation reward, consistent with biphasic activation of pre-and post-synaptic D2-like receptors with increasing dose [101]. Similarly, 7-OH-DPAT produces biphasic effects using a progressive ratio schedule of brain stimulation reward that measures the amount of effort animals will exert to obtain brain stimulation; low doses reduce and high doses increase the amount of effort exerted [102]. The less selective D2-like agonist bromocriptine also lowers self-stimulation thresholds [103]. These results clearly indicate that stimulation of post-synaptic D2-like receptors enhances the rewarding effects of electrical brain stimulation.
Intracranial infusion studies have identified the nucleus accumbens as a major site for modulation of brain stimulation reward by D1- and D2-like receptors. Nucleus accumbens infusions of either the D1-like antagonist SCH 23390 or the D2-like antagonist raclopride attenuate brain stimulation reward, while the D3 antagonist (+)-UH232 and the D4 antagonist clozapine are ineffective [104, 105]. In contrast, agonist infusion studies have found that intra-accumbens infusions of SKF 38393 (D1-like) enhance but infusions of quinpirole (D2-like) attenuate brain stimulation reward, whereas medial prefrontal cortex infusions are ineffective [106]. Similarly, infusions of the D1-like agonist A-77636 into more caudal nucleus accumbens regions lower stimulation thresholds, while quinpirole infusions elevate thresholds [97]. Another study found that nucleus accumbens infusions of the D2-like agonist 7-OH-DPAT have no effect on brain stimulation reward thresholds unless co-infused with AMPA glutamate receptor antagonists to remove glutamatergic tone [107]. While the effect of D2-like agonists differs from systemic administration, it is possible that local effects mediated by D2-like autoreceptors on dopamine terminals in the nucleus accumbens reduce dopamine release in response to brain stimulation reward. Given the reliance of D2-like receptor-mediated reward on D1-like receptor tone in the nucleus accumbens, local inhibition of dopamine release and D1-like tone could explain a lack of facilitation with direct activation of post-synaptic D2-like receptors in the nucleus accumbens.
In this regard, infusions of either D1- or D2-like agonists into the dopamine cell body region of the ventral tegmental area attenuate self-stimulation of the ventral tegmental area, but not self-stimulation of the lateral hypothalamus [106]. Local D1-like receptor stimulation in the ventral tegmental area induces GABA release that inhibits dopamine neurons [108], while D2-like autoreceptors directly inhibit dopamine neuron firing. Infusions of D1- or D2-like agonists into the frontal cortex or caudate-putamen are ineffective at modulating the rewarding effects of ventral tegmental area stimulation [97]. However, infusions of the D2-like antagonists spiperone and pimozide into the medial prefrontal cortex will decrease self-stimulation of the same site [109]. Together these studies implicate both D1 and D2 receptors, especially in the nucleus accumbens, in mediating the rewarding effects of electrical stimulation of midbrain dopamine neurons, while a role for D3 receptors awaits further testing in D3 knockout mice or the availability of more selective ligands. D4 and D5 receptors are not highly expressed in the nucleus accumbens [110–112], but these receptors in other brain regions may play a role in modulating brain stimulation reward.
17.3.3 Modulation of Conditioned Reward by Dopamine Receptor Subtypes
The repeated temporal pairing of hedonically neutral and temporally discrete environmental stimuli (e.g., tones and lights) with response-independent delivery of primary rewards (natural or drug) leads to the formation of conditioned reward. Through Pavlovian conditioning, these stimuli acquire rewarding properties of their own, and animals subsequently will learn to perform a novel instrumental response when rewarded by presentation of the conditioned stimulus. The phenomenon has major implications for control over behavior exerted by conditioned cues in drug addiction, since these cues can trigger craving and relapse to drug use. Studies by Robbins and Taylor showed that psychostimulants greatly enhance conditioned reward by elevating dopamine levels in the nucleus accumbens and potentially other regions [113–115].
The facilitation of conditioned reward by amphetamine is blocked by systemic treatment with either D1- or D2-like antagonists [116]. When given alone, low doses of D2-like antagonists can potentiate conditioned rewards, probably due to enhanced dopamine release with autoreceptor blockade, while higher post-synaptic doses of D1-like and D2-like antagonists attenuate responding [117]. Systemic administration of D1-like agonists also attenuate responding for conditioned reward, potentially due to masking of temporally discrete reward-related signals with presentation of the conditioned reward [118]. In contrast, responding for conditioned rewards is markedly enhanced by systemic pretreatment with D2-like agonists [119]. Interestingly, the opposite effects of D1- and D2-like agonists on conditioned reward are remarkably similar to their effects on drug-seeking behavior as discussed in Section 17.5.1. The facilitation of conditioned reward by the D2-like agonist bromocriptine is blocked by pretreatment with the D1-like antagonist SCH 23390 [120], indicating the necessary role for D1-like receptor tone on the expression of D2-like receptor-mediated effects.
However, when directly infused into the nucleus accumbens, either the D1-like agonist SKF 38393 or the D2-like agonist quinpirole facilitates conditioned reward [121, 122], suggesting that the attenuation of conditioned reward by D1-like receptor stimulation involves multiple or other brain regions. In any event, a necessary role for both D1- and D2-like receptors in the nucleus accumbens is indicated by the fact that antagonists for either receptor will block conditioned reward when infused in this brain region [121]. Interestingly, infusions of the D2-like agonist 7-OH-DPAT into the amygdala during the conditioning phase prevent the subsequent expression of conditioned reward in a drug-free state [123], potentially due to D2-like autoreceptor inhibition of reward-related dopamine release in the amygdala. Similarly, blockade of either D1- or D2-like receptors in the amygdala during conditioning prevents the formation of conditioned reward for cocaine-associated cues [124]. These latter results suggest that stimulation of dopamine receptors in the amygdala is important for establishing enduring learned associations between primary and conditioned rewards, whereas the former results suggest that the expression of conditioned reward requires stimulation of dopamine receptors in the nucleus accumbens.
17.4 Modulation of Drug Self-Administration by Dopamine Receptor Subtypes
Many studies have utilized dopaminergic ligands to study the role of dopamine receptors in the rewarding effects of abused drugs. Given this vast literature, this section will focus on drug self-administration studies, arguably the most pertinent animal model of human drug abuse available. The schedule requirements for drug delivery in self-administration studies are an important consideration when modeling distinct symptoms of addictive behavior. In studies where each drug injection requires a low fixed number of instrumental responses (fixed ratio), an animal’s preferred level of drug intake is not encumbered by performance demands or by prolonged intervals of drug unavailability. Under these circumstances, animals titrate their preferred level of drug intake in a highly stable and dose-sensitive manner. For example, animals will compensate for a lowering in the injection dose by increasing the rate of self-administration, and decrease self-administration rates when the injection dose is increased. This relationship is reflected by an inverted U-shaped self-administration dose–response curve, spanning subthreshold doses that are too low to support self-administration, moderate but short acting suprathreshold doses that are self-administered at increased rates, and higher doses that are self-administered at reduced rates due to prolonged effects of the drug injections. Systemic pretreatment with dopaminergic agonists such as apomorphine reduces the rate of amphetamine self-administration by prolonging the interval between successive self-injections [18]. Since this reduction resembles the effect of increasing the injection dose of amphetamine, it is thought that generalized dopamine receptor stimulation potentiates the satiating or other rate limiting effects of self-administered amphetamine in an additive manner. Conversely, blockade of post-synaptic dopamine receptors with systemic neuroleptic treatment increases the rate of cocaine intake by shortening the time interval between successive self-injections [125], similar to the effect of lowering the cocaine injection dose. Thus, dopamine receptor blockade is thought to antagonize the impact of the cocaine injections in a manner surmounted by volitional increases in cocaine intake, and a similar effect is produced when animals transit to more addicted biological states [126]. An advantage of this approach is that the performance degrading effects of dopamine receptor blockade are negated by increases in self-administration behavior.
While the stability of fixed ratio drug self-administration has its advantage, self-administration rates on these schedules are not directly related to the magnitude of reward. For assessing reward magnitude, self-administration studies often employ progressive ratio schedules, where the degree of effort (e.g., lever pressing) an animal will exert to obtain drug is used as an index of reward, rather than the actual amount of drug consumed. In progressive ratio testing, the response demands for each successive drug injection increase progressively during active self-administration, and the highest ratio of lever presses/injection achieved before animals quit responding (break point) measures a drug’s rewarding efficacy. Thus, while dopamine receptor blockade can increase drug self-administration on fixed ratio schedules (Fig. 17.2a), the same treatment can decrease self-administration on progressive ratio schedules (Fig. 17.2b). The use of both schedules of drug reward is a powerful combination for clarifying the contribution of specific dopamine receptor subtypes in the regulation of drug intake (fixed ratio) and the motivation for drugs when obtaining reward is more demanding (progressive ratio).
(a) Intranucleus accumbens shell infusions of the D1-like antagonist SCH 23390 (3.0 μg/side) or the D2-like antagonist eticlopride (10.0 μg/side) increase stabilized cocaine self-administration (0.5 mg/kg/injection) on a fixed ratio 1:timeout 15 s schedule of reward. Representative self-administration records for individual animals during baseline self-administration one day prior to tests with intranucleus accumbens antagonist treatment. Upward hatchmarks denote times of self-injection. (b) Similar intranucleus accumbens shell infusions of SCH 23390 (1.25 μg/side) or eticlopride (10.0 μg/side) reduce cocaine self-administration on a progressive ratio self-administration schedule. Individual cumulative response records show that a saline-treated control achieves a ratio of almost 200 responses per injection (vertical distance between dotted lines on left) before quitting self-administration behavior, whereas animals treated with SCH 23390 or eticlopride achieve less than 40 responses per injection. Adapted with permission from (a) Bachtell et al. [157] and (b) Bari and Pierce [80]
17.4.1 Modulation of Psychostimulant Self-Administration by Dopamine Receptor Subtypes
The rewarding effects of psychostimulants such as cocaine and amphetamine involve their ability to function as indirect dopamine receptor agonists. Given that both D1- and D2-like receptors play a role in mediating or modulating reward processes, it is not surprising that systemic pretreatment with either D1- or D2-like selective antagonists leads to compensatory increases in cocaine intake on fixed ratio self-administration schedules in rats [127–130], and monkeys [131]. These findings suggest that both D1- and D2-like receptors contribute to negative feedback regulation of cocaine intake. Conversely, both D1- and D2-like receptor antagonists decrease the amount of effort animals will exert to obtain cocaine or amphetamine when self-administered on a progressive ratio schedule [130, 132–134]. Since these treatments increase self-administration rates on fixed ratio schedules, decreased self-administration on progressive ratio schedule is likely due to reduced motivational rather than performance effects. In contrast, systemic pretreatment with putative D3 and D4 antagonists fails to increase cocaine intake on fixed ratio schedules [135–138], but the D3 antagonists NGB 2904 and SB-277011A attenuate cocaine reward on progressive ratio schedules [137, 138]. This differential sensitivity to D3 antagonists with fixed and progressive ratio cocaine self-administration distinguishes these compounds from generalized D2-like receptor antagonists. Thus, D3 receptors may play a necessary role along with D1- and D2 receptors in cocaine reward, while D1- and D2 (but not D3) receptors may be preferentially involved in negative feedback regulation of cocaine intake.
Genetic deletion of D1 receptors dramatically decreases the number of mice that will acquire cocaine self-administration [16]. In contrast, D2 receptor knockout mice acquire cocaine self-administration, and show increased cocaine intake on fixed ratio schedules similar to the effect of D2-like receptor antagonists [135]. However, the ability of the D2-like receptor antagonist eticlopride to further elevate cocaine intake is absent in D2 receptor knockout mice, indicating the effect primarily reflects blockade of D2 and not D3 or D4 receptors as indicated by antagonist studies in rats. These results support the notion that D1 receptors play a major role in the primary rewarding properties of cocaine, while D2 receptors contribute to cocaine’s rate-reducing effects.
In contrast to antagonist pretreatment, pretreatment with full efficacy D1- or D2-like receptor agonists reduces cocaine self-administration on unrestricted fixed ratio schedules, but the qualitative aspects of this reduction differ. Thus, pretreatment with D1-like agonists suppress the initiation of cocaine self-administration, and produce downward shifts in the inverted U-shaped dose–response function in rats and monkeys [139–142]. In contrast, pretreatment with D2-like agonists prolongs the time interval between successive cocaine injections (post-injection pause), similar to the effect of increasing the unit cocaine dose/injection, and produces a leftward shift in the dose–response curve [20, 140, 141, 143–145]. As suggested above, these results are consistent with the idea that tonic D1-like receptor stimulation suppresses cocaine self-administration by supplanting and occluding the primary rewarding effects of cocaine, whereas tonic stimulation of D2-like receptors potentiates the rewarding effects of self-administered cocaine. Systemic pretreatment with the D2-like agonist quinpirole has no effect on amphetamine self-administration on a progressive ratio schedule [134]. However, when D1- or D2-like agonists are combined with cocaine injections in monkeys self-administering on a progressive ratio schedule, a situation where agonists are delivered in a discrete response-contingent manner, both D1-like and D2-like agonists produce leftward shifts in the dose–response curve compared to cocaine alone, indicating that either receptor contributes to cocaine reward [146].
While studies with D3-selective antagonists have been informative, studies with putative D3-selective agonists have been complicated by the fact that the behavioral profile often resembles that of general D2-like agonists on fixed ratio cocaine self-administration, i.e., a prolonging of the interval between successive cocaine injections consistent with an additive interaction with cocaine [136, 147]. Previous studies suggested that the potency for this effect in vivo correlates with activity at D3 but not D2 receptor activation in vitro using a bioassay in cells expressing D2 or D3 receptors [148, 149]. However, other evidence suggests that D3 receptors actually would oppose cocaine effects by opposing D1 receptor function when co-localized on similar striatal neurons [150–152]. Furthermore, the ability to study the functional effects of D3 receptors using partial D3-preferring agonists such as BP 897 is complicated by their agonist/antagonist profiles at D2 and D3 receptors [153].
Intracranial infusion studies suggest that D1- and D2-like receptors in multiple brain regions contribute to the modulation of psychostimulant self-administration by dopamine receptor ligands. Infusions of either D1- or D2-like antagonists into the nucleus accumbens mimic the rate-increasing effects of systemic antagonist administration on fixed ratio cocaine and amphetamine self-administration [154–157], an effect illustrated in Fig. 17.2a. However, local stimulation of nucleus accumbens D1- or D2-like receptors fails to recapitulate the reduction in cocaine intake produced by systemic agonist administration [157], although infusion of dopamine itself does reduce cocaine intake on fixed ratio self-administration schedules [158]. These results may suggest that D1- and D2-like receptors in the nucleus accumbens are saturated with dopamine during cocaine self-administration, or that co-activation of both receptor classes and possibly in multiple brain regions is needed to produce an additive interaction with cocaine to reduce self-administration rates. However, viral-mediated over-expression of D2 receptors in the nucleus accumbens effectively reduces the rate of fixed ratio cocaine self-administration [159], suggesting that the amount of D2 receptors in the nucleus accumbens plays an important role in regulating preferred levels of cocaine intake.
Increases in fixed ratio cocaine self-administration produced by local blockade of nucleus accumbens D1- or D2-like receptors are paralleled by decreases in the motivation for cocaine (but not food) on progressive ratio schedules (Fig. 17.2b) [80, 160]. The motivational effects are selective for cocaine when infused in the shell subregion, but responding for both rewards is blocked when antagonists are infused in the core subregion potentially reflecting performance deficits [80]. Nucleus accumbens infusions of the D3 antagonist U99194A or the D4 antagonist L-750,667 have no effect on cocaine reward in progressive ratio testing.
Fixed ratio cocaine self-administration is increased by infusing the D1-like antagonist SCH 23390 into several other dopamine terminal regions, including the medial prefrontal cortex [161], insular cortex [162], bed nucleus of the stria terminalis [163], and amygdala [156, 160, 162, 164]. Similar infusions of SCH 23390 in medial prefrontal cortex reduce cocaine reward on progressive ratio schedules [161], but not when infused in the amygdala [160]. These results illustrate that regulation of cocaine intake and the motivation for cocaine are distinct behavioral phenomena mediated by separate neural substrates. Interestingly, infusions of SCH 23390 in the dopamine cell body region of the ventral tegmental area also increase cocaine intake on fixed ratio schedules, and decrease the motivation for cocaine on progressive ratio schedules [165], presumably acting on D1-like receptors located on the axons of GABAergic, serotonergic, or glutamatergic input to dopamine neurons. Local blockade of D2-like receptors in the arcuate nucleus of the hypothalamus increases fixed ratio cocaine intake potentially due to attenuation of cocaine-induced β-endorphin release in the nucleus accumbens [166]. Together, these findings suggest that dopamine receptors in multiple brain regions regulate cocaine reward through vastly different mechanisms.
17.4.2 Modulation of Opiate and Nicotine Self-Administration by Dopamine Receptor Subtypes
Like most drugs of abuse, opiate drugs including heroin and morphine, along with nicotine, stimulate mesolimbic dopamine release that plays a major role in their rewarding properties, although dopamine-independent mechanisms also exist for opiate reward [167, 168]. Opiate and nicotine self-administration on fixed ratio schedules can show compensatory increases in drug intake when the primary receptors (opioid and nicotinic cholinergic) are blocked with antagonists in a surmountable manner. However, the positive effects of dopamine receptor blockade on opiate and nicotine self-administration, where present, generally show reductions rather than increases in drug self-administration on fixed ratio schedules. This difference in the response may reflect an insurmountable blockade produced by blocking dopamine receptors downstream from the primary opioid and nicotinic receptors in reward pathways. Reductions in fixed ratio drug self-administration with dopamine receptor blockade also are found when the rewarding effects are relatively weak, and exhibit shallow inverted U-shaped dose–response curves as found with nicotine compared cocaine or heroin self-administration. Thus, it is important to control for possible impairments in response performance by dopamine antagonists in these studies. Given these caveats, there are far fewer reports of the modulation of opiate or nicotine self-administration with subtype-selective dopamine receptor ligands than with psychostimulant self-administration, which also could suggest that many negative effects have not been reported.
The D1-like receptor antagonist SCH 23390 blocks the acquisition of heroin self-administration in rats when given as a systemic pretreatment prior to daily acquisition trials, but this treatment also reduces motor behavior and so performance deficits are a consideration [169]. However, similar pretreatments with SCH 23390 infused directly into the nucleus accumbens fail to impair acquisition of heroin self-administration despite decreases in motor behavior, suggesting that D1-like receptors in the nucleus accumbens do not mediate opiate reward in self-administration paradigms (but see [170]). Interestingly, D1 receptor knockout mice readily self-administer the opioid receptor agonist remifentanil similar to wild-type controls, despite impairments in cocaine self-administration [16]. Discrepancies between pharmacological blockade and D1 receptor knockout could suggest a role for D5 receptors, or involve species differences or compensatory changes with a total loss of D1 receptors. Regarding the latter, chronic blockade of dopamine receptors causes a compensatory sensitization in dopamine-independent opiate reward in rats self-administering heroin [171].
In contrast, D2 receptor knockout eliminates intravenous morphine self-administration on either fixed or progressive ratio schedules, without affecting acquisition of water self-administration [77]. Since dopamine-dependent opiate reward is mediated by disinhibition of dopamine neurons in the ventral tegmental area, the loss of both pre- and post-synaptic D2 receptors complicates the interpretation of these findings. A loss of autoreceptor inhibition of dopamine neurons could occlude disinhibition by opiates. Pre- rather than post-synaptic sites of action are supported by the finding that systemic administration of the D2-like receptor antagonist sulpiride leads to extinction of intracranial morphine self-administration directly into the ventral tegmental area, but sulpiride fails to alter morphine self-administration directly into the nucleus accumbens [172]. The acquisition of morphine self-administration infused directly into the lateral septum is prevented by systemic administration of either D1- or D2-like receptor antagonists using a spatial discrimination Y-maze task less sensitive to performance issues [173].
The ability of D1-like receptor stimulation to augment heroin reward is indicated by the fact that co-injections of D1-like agonists with heroin cause an additive leftward shift in the self-administration dose–response curve on a progressive ratio schedule in monkeys, similar to their effect on cocaine self-administration [146]. However, in contrast to cocaine self-administration, heroin co-injected with D2-like agonists causes a rightward shift of the dose–response curves, again, potentially due to D2-like autoreceptor stimulation counteracting heroin’s ability to disinhibit midbrain dopamine neurons. Together, these findings suggest that while dopamine-dependent opiate reward may involve post-synaptic D1-like receptors similar to psychostimulant reward, dopamine-independent opiate reward may play a major role especially when dopamine systems are chronically compromised.
Intravenous nicotine is self-administered at modest rates by animals, but inhibition of monoamine oxidase, as produced by compounds found in tobacco, dramatically enhances nicotine self-administration in rats [174]. The enhanced nicotine self-administration is blocked by systemic pretreatment with D1-like receptor antagonist SCH 23390. When nicotine is self-administered directly into the ventral tegmental area, self-administration is reduced by systemic administration of SCH 23390 [175], or by co-infusion with the D2-like receptor agonist quinpirole [176], the latter reflecting autoreceptors counteracting the ability of nicotine to excite dopamine neurons. A high dose of the D3 antagonist SB-277011A reduces nicotine but not food self-administration on a more demanding progressive ratio schedule, even though this dose also impairs motor activity [177]. Another study found that SB-277011A has no effect on stable fixed ratio nicotine self-administration at doses that block nicotine-primed relapse to nicotine seeking in the absence of reward [178]. Oral nicotine self-administration is increased by clozapine but not haloperidol, potentially reflecting surmountable blockade of D4 receptors [179]. Together, these studies suggest that both D1-like and D3 receptors play a role in the rewarding efficacy of nicotine, although the role of D2, D4, and D5 receptors remains undetermined.
17.4.3 Modulation of Alcohol Self-Administration by Dopamine Receptor Subtypes
Studies on alcohol self-administration also support a role for dopamine receptors in modulating alcohol intake and reward. Most studies measure volitional but freely available alcohol consumption in a choice over water or sucrose, but some investigators employ instrumental responses rewarded by alcohol presentation. Previous studies have found that either D1- or D2-like receptor blockade with SCH 23390 or raclopride can reduce alcohol consumption, but with the caveat that similar doses also reduce water intake [180]. Another study found that SCH 23390 and spiperone (D2-like) reduce water but not alcohol consumption [181]. Other studies using alcohol-preferring rats found that D2-like blockade with spiperone slightly increases alcohol intake, while the D1-like receptor antagonist SCH 23390 reduces consumption [182]. The D1-like receptor antagonist SCH 31966, devoid of serotonergic receptor activity, also reduces intake in alcohol-preferring rats, and at doses that reduce sucrose but not water intake [183]. In contrast, the D2-like antagonist remoxipride fails to affect alcohol intake when instrumental responses are required, even at doses that reduce appetitive responding in non-rewarded sessions [184]. Thus, studies in rats suggest that D1- rather than D2-like receptors may be necessary for alcohol reward.
In mice, pretreatment with either the D1-like agonist SKF 38393 or the D2-like agonist bromocriptine reduces alcohol consumption, but the effect of bromocriptine is reduced after mice are sensitized to alcohol exposure [185]. In alcohol-preferring rats, SKF 38393 reduces alcohol intake similar to the D1-like antagonist SCH 23390, potentially reflecting the partial agonist properties of SKF 38393 or satiating effects with D1-like stimulation [182]. Pretreatment with the D2-like agonist quinpirole also reduces alcohol intake via dopamine autoreceptor effects as discussed below. However, pretreatment with the D2-like agonist 7-OH-DPAT at very low presumably autoreceptor doses increases alcohol drinking [180]. The putative D3 antagonist U99194A fails to alter preference for alcohol in mice [186], while a high dose of the D3 antagonist SB-277011A reduces alcohol intake in rats [187, 188]. It is interesting that both D1 and D2 receptor knockout mice actually show an aversion to alcohol [189, 190]; while loss of pre-synaptic D2 autoreceptors could occlude the rewarding effects of alcohol that otherwise disinhibit dopamine neurons, post-synaptic D2 receptor responses also could be involved in D2 receptor knockout mice. In any event, findings in knockout mice strongly implicate dopamine in the rewarding effect of alcohol.
When alcohol is self-administered directly into the ventral tegmental area, co-infusion with quinpirole reduces self-administration to control levels, reflecting the ability of direct autoreceptor stimulation to oppose alcohol-mediated disinhibition of dopamine neurons [191]. However, blockade of D1-like receptors in dopamine terminal region of the nucleus accumbens with SCH 2390 also decreases alcohol intake without altering the overall rate of instrumental responding [192]. Nucleus accumbens infusions of the D2-like receptor antagonist raclopride reduce both intake and instrumental response rates [192, 193], potentially due to motor impairment. In the bed nucleus of the stria terminalis, D1- but not D2-like receptor blockade reduces instrumental responding for alcohol while producing lesser reductions in responding for sucrose [194].
Conversely, in a free choice procedure, intranucleus accumbens infusions of SCH 23390 have little effect on alcohol preference in alcohol-preferring rats, while the D2-like antagonist sulpiride dose dependently increases consumption of alcohol without altering intake of sucrose or saccharine [195]. Microinfusion of sulpiride into the ventral pallidum also increases alcohol intake under free choice conditions, while SCH 23390 has little effect, although both antagonist treatments elevate extracellular dopamine levels [196]. These findings suggest that alcohol intake is differentially sensitive to blockade of dopamine receptors under free choice or instrumental response procedures. D1-like receptors in the nucleus accumbens and bed nucleus of the stria terminalis may play a necessary role in regulating alcohol reward when instrumental responses are required. In contrast, D2-like receptors in the nucleus accumbens and ventral pallidum may mediate inhibitory feedback regulation of alcohol intake under free choice conditions.
Stimulation of nucleus accumbens D2-like receptors with quinpirole infusions increases instrumental responding for alcohol at low doses, opposite to the effect of receptor blockade, but decreases responding at high doses [193, 197]. Infusions of the D1-like agonist SKF 38393 have no effect. The increase in self-administration with low dose quinpirole is prevented by co-infusion of either SKF 38393 or SCH 23390 [197], suggesting that D1-like receptor tone is important for the expression of post-synaptic D2-like-mediated increases in alcohol self-administration. In contrast to instrumental responding for alcohol, viral vector-mediated increases in nucleus accumbens D2 receptors decrease both alcohol preference and intake in preferring and non-preferring rats [198], indicating that the D2 receptor itself is sufficient to regulate alcohol reward. Together these findings suggest that while stimulation of D2-like receptors in the nucleus accumbens is sufficient to facilitate instrumental responding for alcohol, increases in the amount of D2 receptors reduce alcohol intake under free choice conditions consistent with negative feedback regulation of intake discussed above.
17.5 Dopamine Receptor Subtypes in Relapse to Drug-Seeking Behavior
In addition to playing a critical role in drug reward, the mesolimbic dopamine system is a major neural substrate for drug-seeking behavior. The mesolimbic dopamine system is activated by exposure to drug-related environmental cues, stress, and other pharmacological stimuli that trigger relapse to drug seeking during withdrawal [199–204]. Dopamine release in forebrain regions such as the nucleus accumbens is sufficient to trigger relapse to drug seeking. Moreover, in some but not all cases, such dopamine release is necessary for environmental or pharmacological stimuli to trigger drug-seeking behavior.
Relapse to drug seeking is reflected by approach behavior aimed at performing responses that delivered drug injections on prior occasions during self-administration. Like self-administration on progressive ratio schedules, most studies measure the level of effort animals will exert to obtain drug when reward is withheld as an index of drug-seeking behavior, and this behavior is thought to reflect wanting or craving that would precipitate relapse to drug use in humans [7, 205]. An important distinction from self-administration behavior is that responding is measured in the non-rewarded or drug-free state either without or before reward delivery.
The most commonly used method to model the propensity for relapse to drug seeking is the extinction/reinstatement paradigm. The paradigm has face validity because environmental and pharmacological stimuli that reinstate drug seeking in animals also trigger drug craving in humans [206–208]. In the extinction phase of this procedure, drug-seeking behavior is elicited by environmental and contextual cues associated with drug use in the self-administration test chambers, and ultimately diminishes with repeated training in the absence of drug reward. Following extinction of drug-seeking behavior, the ability of specific experimenter-delivered stimuli to elicit or “reinstate” drug-paired lever responding is measured. The reinstatement of drug-seeking behavior can be induced by priming injections of drugs, presentation of discrete or environmental cues associated with drug injections, and by brief exposure to moderate intermittent footshock stress. There are numerous extinction/reinstatement studies in rats and monkeys. However, there are very few reports in mice, since commonly used strains do not exhibit effective reinstatement of drug seeking with non-contingent priming injections of drugs [209, 210], although alcohol-primed reinstatement of alcohol seeking has been reported in mice [188]. More recently, the reinstatement paradigm has been adapted to the Pavlovian conditioned place preference model of drug reward, but it is difficult to conceive that conditioned place preference reflects drug-seeking behavior without volitional drug self-administration on prior occasions. Furthermore, the role of dopamine receptor subtypes in the reinstatement of volitional drug seeking markedly differs from their role in the reinstatement of a drug-induced conditioned place preference [43, 139].
Another model of drug-seeking behavior involves the use of second-order schedules of drug self-administration, where initial responding is rewarded by discrete cues predictive of ultimate drug availability. The second-order schedule illustrates the powerful control over drug seeking exerted by these cues. However, since this cue-rewarded drug seeking declines without ultimate delivery of the drug reward, it has been used less extensively to model long-term drug withdrawal. Reinstatement paradigms, on the other hand, suffer from the fact that drug seeking is triggered after extinction of the behavior, a situation vastly different from human drug abuse where craving and relapse occur without extinction experience.
17.5.1 Modulation of Cocaine Seeking by Dopamine Receptor Subtypes: Systemic Administration
Most work on the role of dopamine receptors in drug-seeking behavior has been conducted in animals self-administering cocaine. As discussed above, cocaine self-administration is reduced by systemic pretreatment with either D1- or D2-like receptor agonists, suggesting that both receptors provide inhibitory feedback regulation of cocaine intake during self-administration. Cocaine seeking in the absence of reward also is strongly regulated by both D1- and D2-like dopamine receptor classes, except that they mediate opposite effects on this relapse behavior (Fig. 17.3a). Thus, selective stimulation of post-synaptic D2-like receptors is a powerful trigger of cocaine-seeking behavior during or after responses extinguish [19, 139, 211–216]. Conversely, selective D1-like receptor stimulation is virtually without effect, even when locomotor activation is similar to D2-like receptor stimulation [139, 211, 214, 215]. In addition, pretreatment with full efficacy D1-like receptor agonists will block the ability of a single cocaine priming injection, or the presentation of cocaine-associated cues, to reinstate cocaine seeking in the absence of increased stereotypy [139, 142, 216–218]. Conversely, pretreatment with D2-like agonists facilitates cocaine-primed reinstatement [139]. A similar D1-/D2-like dichotomy regulates cocaine seeking in monkeys [219, 220] and also has been shown in some studies to suppress and stimulate craving responses in humans, respectively [221, 222]. The D3-preferring agonist PD 128,907 fails to mimic the reinstating effects of D2-like agonists in monkeys [219]. Together, these studies suggest that D2 receptors play a major role in eliciting relapse to cocaine seeking when environmental stimuli such as cocaine-related cues or stress activate the mesolimbic dopamine system [223], while high D1 receptor tone may provide inhibitory regulation over cocaine seeking by satiating primary reward processes.
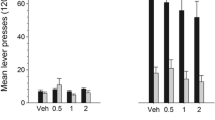
(a) Left, systemic subcutaneous (sc) pretreatment with the highly selective D1-like dopamine agonist SKF 81297 attenuates the ability of intravenous cocaine (2.0 mg/kg) priming to reinstate cocaine-seeking behavior (non-rewarded responding on the drug-paired lever) in rats. The priming injections of cocaine were given 30 min after pretreatment with SKF 81297 following extinction of cocaine-seeking behavior in the reinstatement paradigm. Right, intraperitoneal (ip) priming injections with the D2-like agonist 7-OH-DPAT trigger cocaine-seeking behavior (* P < 0.05 compared to vehicle pretreatment). (b) When infused directly into the nucleus accumbens, both D1- and D2-like agonists reinstate cocaine-seeking behavior in rats, with greater effects in the medial core than medial shell subregions of nucleus accumbens (* P < 0.05 compared to inactive lever responses or vehicle-infused controls # P < 0.05). Reinstatement of non-rewarded lever-press responding at the drug-paired lever is thought to model relapse behavior. Adapted with permission from (a) Self et al. [139] and (b) Bachtell et al. [157]
While systemic administration of D2-like but not D1-like receptor agonists is sufficient to trigger cocaine-seeking behavior, both receptor types are necessary for the expression of cocaine seeking. Thus, systemic pretreatment with either D1- or D2-like receptor antagonists attenuates cocaine-seeking behavior elicited by priming injections of cocaine [217, 219, 224], discrete cues associated with prior cocaine injections [217, 225], cues predictive of cocaine availability [213, 226], or exposure to a cocaine-associated environmental context [227]. In monkeys, cocaine-primed reinstatement is not reduced by the D3-preferring antagonists UH 232 and AJ-76 [219]. In rats, however, the D3 antagonist SB-277011-A reduces cocaine seeking during initial extinction conditions [228], and reduces responding maintained by cocaine-associated but not sucrose-associated cues with a second-order self-administration schedule [229]. Similarly, the D3 antagonists SB-277011A and/or NGB 2904 dose dependently attenuate cue-primed [230], cocaine-primed [138, 231], and footshock stress-primed [232] reinstatement of cocaine seeking following extinction, without altering reinstatement of sucrose-seeking behavior [232]. Since these compounds also reduce cocaine self-administration on progressive ratio self-administration schedules, but fail to increase cocaine intake on fixed ratio schedules
(unlike general D2-like antagonists), D3 receptors may play a distinct role in the motivation for cocaine independent of regulation of cocaine intake as discussed in Section 17.4.1.
17.5.2 Modulation of Cocaine Seeking by Dopamine Receptor Subtypes: IntraCranial Administration
Although systemic D1-like agonist administration attenuates cocaine-seeking behavior, infusion of SKF 81297 directly into the nucleus accumbens actually triggers cocaine seeking in reinstatement paradigms [157, 233]. This effect involves D1-like modulation of calcium/calmodulin-mediated kinase II and activation of L-type calcium channels [234]. Nucleus accumbens infusions of D2-like agonists also reinstate cocaine seeking [157, 233], an effect that may involve D2-like receptor-mediated inhibition of cyclic AMP-protein kinase A signaling [235]. In both cases, D1- and D2-like agonists induce more robust cocaine seeking when infused into the medial core rather than the shell subregion as shown in Fig. 17.3b, while lateral core infusions are ineffective [157, 233]. Such enhanced sensitivity of dopamine receptor responses in the medial nucleus accumbens core may be important for cocaine seeking elicited by cocaine-associated cues, since unanticipated cues elevate dopamine levels in the core rather than shell [203, 236].
As shown in Fig. 17.4a, the reinstating effects of D2-like agonists in the nucleus accumbens probably involve the D2 subtype, since the D3 agonist PD 128,907 and the D4 agonist PD 168,077 fail to mimic reinstatement of cocaine seeking elicited by the D2-like agonist quinpirole [233]. Synergistic interactions between D1- and D2-like receptors are demonstrated by the ability of subthreshold doses of SKF 81297 and quinpirole to elicit far greater cocaine-seeking behavior when co-infused in the nucleus accumbens than when infused alone (Fig. 17.4b) [237]. Similarly, cooperativity between D1- and D2-like receptors and a role for endogenous dopamine receptor tone is indicated by the ability of the D1-like antagonists to block cocaine seeking induced by D2-like agonists, while D2-like antagonists block cocaine seeking induced by D1-like agonists, when co-infused in the nucleus accumbens (Fig. 17.4c) [157, 237].
(a) Intranucleus accumbens shell infusions of the D2-like agonist quinpirole, but not the D3 agonist PD 128,907 or the D4 agonist PD 168,077, elicit cocaine-seeking behavior in a reinstatement paradigm in rats. Total number of responses (mean ± S.E.M.) on the drug-paired lever differs from saline (* P < 0.05). (b) Co-infusion of subthreshold doses of SKF 81297 and quinpirole into the nucleus accumbens shell reinstates cocaine seeking. Total number of drug-paired lever responses differs from either SKF 81297 or quinpirole alone (* P < 0.05). (c) Cross blockade of reinstatement induced by intra-accumbens D1- and D2-like agonists by D1- and D2-like antagonists. Co-infusion of SCH 23390 or eticlopride in the nucleus accumbens blocks SKF 81297- and 7-OH-DPAT-induced reinstatement of cocaine seeking. # P < 0.05 compared to vehicle/agonist co-infused controls. Adapted with permission from (a) Schmidt et al. [233], (b) Schmidt and Pierce [237], and (c) Bachtell et al. [157]
In contrast to direct activation of dopamine receptors, systemic priming injections of cocaine preferentially elevate dopamine levels in the shell subregion of the nucleus accumbens [238, 239]. Blockade of either D1- or D2-like receptors in the nucleus accumbens shell attenuates cocaine seeking elicited by systemic cocaine priming injections [157, 240, 241]. Nucleus accumbens infusions of the D3 antagonist U99194A and the D4 antagonist L-750667 are ineffective [241]. However, nucleus accumbens infusion of the D3 antagonist SB-277011A is effective at blocking reinstatement of cocaine seeking induced by footshock stress [232], but SB-277011A fails to block cue-maintained cocaine seeking on a second-order self-administration schedule [242]. Since response-contingent cue presentation in second-order schedules does not increase dopamine levels in the nucleus accumbens [236], it is not clear whether local D3 blockade would attenuate reinstatement of cocaine
seeking induced by unanticipated cues that do elevate nucleus accumbens dopamine. In any event, it appears that D1- and D2-like receptor stimulations in the nucleus accumbens is both sufficient and in most cases necessary for induction of cocaine-seeking behavior. Given the ability of systemic D3 antagonist administration to block cocaine- and cue-mediated cocaine seeking, these findings suggest that D3 receptors in other brain regions may be involved, while D3 receptors in the nucleus accumbens could play a role in stress-induced relapse. Conversely, the failure to block cue- and cocaine-primed cocaine seeking with nucleus accumbens infusions of D3 antagonists indirectly implicates D2 receptors in these behaviors, since D4 receptors are only marginally expressed in the nucleus accumbens [110, 111]. Clearly, further investigation with multiple and selective D3 ligands is needed.
Reinstatement of cocaine seeking by response-contingent cues is blocked by local infusion of the D1-like receptor antagonist SCH 23390 in the amygdala, while the D2-like antagonist raclopride is ineffective [243]. However, amygdala infusions of the D3 antagonist SB-277011A reduce cue-maintained responding using a second-order self-administration schedule [242]. These results suggest that D1-like and D3 receptors in the amygdala may be critical for the expression of conditioned reward mediated by cocaine-associated cues, similar to their role in the formation of these associations during conditioning as discussed in Section 17.3.3. Cocaine-primed reinstatement of cocaine seeking also is blocked by amygdala infusions of the D1-like antagonist SCH 23390 [162].
Modulation of neocortical dopamine receptors also regulates cocaine-seeking behavior. Local blockade of D1- or D2-like receptors in the medial prefrontal cortex with SCH 23390 and eticlopride, respectively, attenuates cocaine-primed relapse to cocaine seeking without affecting food self-administration [244]. Another study found no effect when specifically targeting the prelimbic subregion with SCH 23390 and raclopride [245]. However, this study found that blockade of D1-like, but not D2-like, receptors in the medial prefrontal cortex attenuates relapse to cocaine seeking induced by footshock stress. Cocaine- and cue-primed reinstatement of cocaine seeking also is attenuated by D1-like blockade in the insular cortex [162]. Interestingly, stimulation of D1-like receptors in the agranular insular area of prefrontal cortex with SKF 81297 actually reduces cocaine seeking maintained by cues on a second-order schedule of cocaine self-administration [246]. In this sense, the agranular insular subregion of cortex represents the only brain site known to date where a D1-like agonist attenuates cocaine seeking in a manner consistent with systemic administration. However, focal D1-like receptor stimulation in regions such as the nucleus accumbens may have different effects on limbic circuits than when the same accumbens D1-like receptors are stimulated concomitant with brain-wide D1-like receptor activation. For example, D1-like receptor stimulation of prefrontal cortical afferents to nucleus accumbens alters the physiological response to coincident D1-like receptor stimulation in nucleus accumbens neurons, producing excitatory rather than inhibitory effects [247]. If so, the behavioral responses of such isolated and localized receptor activation could be epiphenomenal to more natural situations.
17.5.3 Modulation of Heroin, Nicotine, and Alcohol Seeking by Dopamine Receptor Subtypes
Far fewer studies have been conducted on dopamine receptor involvement in relapse behavior with other drugs of abuse. As found with cocaine-trained animals, reinstatement of heroin-seeking behavior is induced by systemic administration of the D2-like receptor agonist quinpirole, but only as long as animals remain sensitized to D2-like receptor stimulation in withdrawal [19, 248]. Similarly, the D1-like agonist SKF 82958 is ineffective and tends to reduce heroin seeking [211]. Systemic pretreatment with the D1-like antagonist SCH 23390 or the D2-like agonist raclopride blocks reinstatement induced by non-contingent heroin priming injections, but fails to block relapse elicited by footshock stress [249]. However, generalized blockade of both D1- and D2-like receptors with flupenthixol effectively blocks this behavior [249], suggesting a role for dopamine in stress-induced relapse. Systemic administration of SCH 23390, but not raclopride or the D3 antagonist NGB 2904, attenuates reinstatement of heroin seeking induced by food deprivation [250], indicting a specific role for D1-like receptors in the motivational effects of hunger on heroin-seeking behavior. However, stimulation of D2-like receptors with 7-OH-DPAT effectively reinstates food-seeking behavior in animals self-administering food pellets, although D2-like antagonists fail to block food seeking elicited by non-contingent priming with food pellets [251]. These results suggest that D2-like receptor activation may underlie a fundamental mechanism for reward-seeking behavior in the absence of primary reward, whereas both D1- and D2-like receptors are necessary for expression of heroin seeking induced by heroin priming and footshock stress.
Blockade of D1-like receptors in the nucleus accumbens shell reduces reinstatement of heroin seeking induced by environmental contextual cues, whereas heroin seeking elicited by discrete cues paired with prior heroin injections is reduced by blockade of D1-like receptors in the nucleus accumbens core [252], consistent with the ability of discrete cocaine-associated cues to induce dopamine release in the core but not in the shell [236]. Cues that predict food availability will reinstate food-seeking behavior, and this effect is blocked by local D1-like receptor blockade in the nucleus accumbens [253].
Systemic administration of a novel D3 antagonist or SB-277011A attenuates nicotine-primed reinstatement of nicotine seeking in rats, without affecting cue-primed reinstatement or fixed ratio nicotine self-administration [178, 254]. Since D3 antagonists also reduce nicotine self-administration on a progressive ratio schedule (see Section 17.4.2), D3 receptors may be critical for the incentive motivational effects of nicotine. Pretreatment with SB-277011A also blocks alcohol- and cue-primed reinstatement of alcohol seeking in rats and mice [188, 255], and the withdrawal-induced binge in alcohol consumption known as the alcohol deprivation effect [255]. More generalized D2-like receptor blockade with remoxipride reduces alcohol-seeking behavior under initial extinction conditions at doses that have no effect on instrumental responding for alcohol [184]. The effects of systemic remoxipride on alcohol seeking are recapitulated by infusions of raclopride directly into the nucleus accumbens at doses that have no effect on alcohol consumption [256, 257]. D1- and D2-like antagonists also attenuate alcohol seeking induced by cues that predict alcohol availability in rats [258], and the D1-like antagonist SCH 23390 blocks renewal of alcohol seeking elicited by an environmental context associated with alcohol self-administration [259]. These findings suggest that both D1- and D2-like receptors, potentially in the nucleus accumbens, are important for relapse to nicotine- and alcohol-seeking behavior, similar to cocaine relapse. A role for D3 receptors in the motivation for nicotine and alcohol during withdrawal also is strongly indicated.
17.6 Future Directions
With the advent of subtype-selective ligands and knockout mice, a vast body of work on dopamine receptor function in natural and drug reward and in relapse to drug-seeking behavior has accumulated in the past two decades. An equally expansive literature has shown that chronic drug and alcohol use can change the amount of D1- and D2-like receptors in specific brain regions in animals and more recently, in humans using neuroimaging procedures. A major challenge for future work will involve integrating observed changes in the amount of dopamine receptors in specific brain regions with changes in dopamine receptor regulation of reward and relapse. The breadth of our current knowledge will facilitate this integration, but it is important for future research to track changes in the biochemical, physiological, and behavioral responses mediated by D1- and D2-like receptors as animals transit from initial drug self-administration to more addicted biological states. It is also important to identify premorbid alterations in D1- and D2-like receptors that predispose individual vulnerability to drug and alcohol addiction.
For example, decreases in the amount of D2-like receptors in the nucleus accumbens of rats are predictive of impulsive behavior and early escalation of cocaine self-administration [260], whereas increases in D2-like receptors that accompany social dominance in monkeys are associated with resistance to initial propensity for cocaine self-administration [261]. After self-administration is initiated, prolonged daily access to drugs leads to profound escalation in drug intake, along with a greater propensity for relapse to drug seeking [126, 262, 263]. Animals that escalate cocaine self-administration also show enhanced sensitivity to the rate-altering effects of generalized dopamine receptor blockade on a fixed ratio self-administration schedule[264] suggesting a loss of D1- and/or D2-like dopamine receptor function with the transition to addiction. These results are consistent with enduring reductions in cortical and striatal D2-like receptors that are found in human cocaine, methamphetamine, heroin, and alcohol addicts [265–268].
Differential development of D1- and D2-like receptor sensitization in low and high intake rats during withdrawal from chronic cocaine self-administration. (a) Low intake animals develop D1-like receptor sensitization from early to late withdrawal, whereas high intake animals develop greater D2-like receptor sensitization. Data in top panels show changes in cumulative locomotor responses for dose–response tests (0.1–1.0 mg/kg) with the D1-like agonist SKF 81297 and the D2-like agonist quinpirole conducted before self-administration (pretest) and at early (2–3 days) and late (28–29 days) withdrawal (WD) times. (b) Locomotor dose–response data for low and high intake animals challenged with the D1-like and D2-like agonists at late withdrawal. Asterisks indicate that high differ from low intake animals * p < 0.05, ** p < 0.01. (c) Average daily cocaine intake during the last 6 days of acquisition training is negatively correlated with the change (Δ) in D1 responsiveness from early to late withdrawal (r = –0.507, p = 0.001), but positively correlated with the change in D2 responsiveness (r = 0.686, p < 0.001) among individual animals based on cumulative locomotor responses in dose–response tests. Reproduced with permission from Edwards et al. [216]
These decreases in D2-like receptors are intriguing given that D2-like receptor stimulation triggers relapse to drug-seeking behavior as discussed above. Furthermore, animal data clearly show that chronic cocaine or heroin self-administration [211, 216, 248], and opiate withdrawal [269], increases sensitivity to D2-like receptor-mediated behaviors. Similarly, rats with higher preferred levels of cocaine intake are more sensitive to D2-like agonist-induced relapse to cocaine seeking [216], consistent with the development of greater sensitization in D2-like receptor responses in more addicted biological states. Conversely, rats with lower preferred levels of cocaine intake are more sensitive to the ability of a D1-like agonist to suppress cocaine-primed relapse to cocaine-seeking behavior [216], potentially reflecting a compensatory neuroadaptation that prevents low intake animals from developing a more addicted phenotype. These opposing differences in D1- and D2-like receptor regulation of cocaine seeking are paralleled by changes in the unconditioned locomotor response to D1- and D2-like receptor challenge after 4 weeks withdrawal, where both D1- and D2-like receptors mediate directionally similar behavioral effects (Fig. 17.5a–b). Furthermore, higher levels of cocaine intake are positively correlated with sensitization in D2-like receptor responsiveness after 4 weeks withdrawal from self-administration, and negatively correlated with changes in D1-like receptor responsiveness (Fig. 17.5c).
Thus, a challenge for future work will be to relate changes in the amount of D1- and D2-like receptors in human drug abusers or in animal studies to seemingly discrepant changes in behavioral responses mediated by dopamine receptors in animal models of drug addiction. One possible explanation for these discrepancies could involve the finding that chronic drug administration increases the amount of high affinity (G protein-coupled) D2-like receptors in striatal regions, despite decreases in the total amount of D2-like receptors [270–273]. Thus, drug-induced neuroadaptations that reduce the total amount of D2-like receptors often are associated with increases in the amount of functional D2-like receptors. It would be interesting to determine whether the amount of functionally coupled D2-like receptors parallels or diverges from sensitization in D2-like receptor-mediated relapse in the same study. In this regard, decreases in the amount of total D2-like receptor binding in striatal subregions in human cocaine abusers fail to correlate with cocaine-induced euphoria or cocaine-primed self-administration [274], which could reflect an inability to measure functionally coupled D2-like receptors in vivo.
Given that D1- and D2-like receptors play such integral roles in reward and relapse behavior, alterations in these receptors due to genetic or environmental factors will remain a major focus for addiction research. It is increasingly apparent that changes in the amount of D1- and D2-like receptors in drug and alcohol addiction belie a straightforward interpretation that integrates these findings with their functional roles in reward and relapse phenomena. More work is needed to compare changes in the amount of receptors with changes in D1-like and D2-like receptor function using biochemical and physiological measures. It is imperative that findings from multiple approaches ultimately converge with changes in behavioral responses mediated by D1- and D2-like receptors before an unambiguous understanding of alterations in dopamine receptor function in drug and alcohol addiction can be appreciated.
References
Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse 1997;26:81–92.
Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 1996;379:449–51.
Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 1998;80:1–27.
Baxter BL, Gluckman MI, Stein L, Scerni RA. Self-injection of apomorphine in the rat: positive reinforcement by a dopamine receptor stimulant. Pharmacol Biochem Behav 1974;2:387–91.
Baxter BL, Gluckman MI, Scerni RA. Apomorphine self-injection is not affected by alpha-methylparatyrosine treatment: support for dopaminergic reward. Pharmacol Biochem Behav 1976;4:611–2.
Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci 2007;30:259–88.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 1998;28:309–69.
Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev 2001;36:129–38.
Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 2007;191:461–82.
Woolverton WL, Goldberg LI, Jinos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 1984;230:678–83.
Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 1992;582:349–52.
Weed MR, Vanover KE, Woolverton WL. Reinforcing effect of the D1 dopamine agonist SKF 81297 in rhesus monkeys. Psychopharmacology 1993;113:51–2.
Weed MR, Woolverton ML. The reinforcing effects of D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 1995;275:1367–74.
Grech DM, Spealman RD, Bergman J. Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology 1996;125:97–104.
Self DW, Belluzzi JD, Kossuth S, Stein L. Self-administration of the D1 agonist SKF 82958 is mediated by D1, and not D2, receptors. Psychopharmacology 1996;123:303–6.
Caine SB, Thomsen M, Gabriel KI, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci 2007;27:13140–50.
Self DW, Stein L. Receptor subtypes in opioid and stimulant reward. Pharmacol Toxicol 1992;70:87–94.
Yokel RA, Wise RA. Amphetamine-type reinforcement by dopaminergic agonists in the rat. Psychopharmacology 1978;58:289–96.
Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology 1990;100:355–60.
Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 1993;260:1814–6.
Manzardo AM, Del Rio JA, Stein L, Belluzzi JD. Rats choose cocaine over dopamine agonists in a two-lever self-administration preference test. Pharmacol Biochem Behav 2001;70:257–65.
Woolverton WL, Ranaldi R. Comparison of the reinforcing efficacy of two dopamine D2-like receptor agonists in rhesus monkeys using a progressive-ratio schedule of reinforcement. Pharmacol Biochem Behav 2002;72:803–9.
Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology 1996;125:13–22.
Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend 1999;54:97–110.
Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 1997;17:8580–7.
Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci 2003;23:9305–11.
Goeders NE, Smith JE. Reinforcing properties of cocaine in the medical prefrontal cortex: primary action on presynaptic dopaminergic terminals. Pharmacol Biochem Behav 1986;25:191–9.
Goeders NE, Dworkin SI, Smith JE. Neuropharmacological assessment of cocaine self-administration into the medial prefrontal cortex. Pharmacol Biochem Behav 1986;24:1429–40.
Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science 1983;221:773–5.
David V, Segu L, Buhot MC, Ichaye M, Cazala P. Rewarding effects elicited by cocaine microinjections into the ventral tegmental area of C57BL/6 mice: involvement of dopamine D1 and serotonin1B receptors. Psychopharmacology 2004;174:367–75.
Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther 2005;313:134–45.
Corsini GU, Del Zompo M, Gessa GL, Mangoni A. Therapeutic efficacy of apomorphine combined with an extracerebral inhibitor of dopamine receptors in Parkinson’s disease. Lancet 1979;1(8123):954–6.
Kumor K, Sherer M, Jaffe J. Effects of bromocriptine pretreatment on subjective and physiological responses to i.v. cocaine. Pharmacol Biochem Behav 1989;33:829–37.
Brauer LH, Goudie AJ, de Wit H. Dopamine ligands and the stimulus effects of amphetamine: animal models versus human laboratory data. Psychopharmacology 1997; 130:2–13.
Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend 2002;68:23–33.
Romach MK, Glue P, Kampman K, et al. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166). Arch Gen Psychiat 1999;56:1101–6.
Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology 2001;155:330–7.
Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 1998;56:613–72.
Hoffman DC, Beninger RJ. Selective D1 and D2 dopamine agonists produce opposing effects in place conditioning but not in conditioned taste aversion learning. Pharmacol Biochem Behav 1988;31:1–8.
White NM, Packard MG, Hiroi N. Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology 1991;103:271–6.
Hoffman DC, Beninger RJ. The effects of selective dopamine D1 or D2 receptor antagonists on the establishment of agonist-induced place conditioning in rats. Pharmacol Biochem Behav 1989;33:273–9.
Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol 1998;343:111–8.
Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology 2007;191:719–30.
Blanchet PJ, Grondin R, Bedard PJ, Shiosaki K, Britton DJ. Dopamine D1 receptor desensitization profile in MPTP-lesioned primates. Eur J Pharmacol 1996;309:13–20.
Shippenberg TS, Herz A. Place preference reveals the involvement of D-1 dopamine receptors in the motivational properties of μ- and κ-opioid agonists. Brain Res 1987;436:169–72.
Papp M, Muscat R, Willner P. Subsensitivity to rewarding and locomotor stimulant effects of a dopamine agonist following chronic mild stress. Psychopharmacology 1993;110:152–8.
Mallet PE, Beninger RJ. 7-OH-DPAT produces place conditioning in rats. Eur J Pharmacol 1994;261:R5–6.
Kling-Petersen T, Ljung E, Wollter L, Svensson K. Effects of dopamine D3 preferring compounds on conditioned place preference and intracranial self-stimulation in the rat. J Neural Trans 1995;101:27–39.
Biondo AM, Clements RL, Hayes DJ, Eshpeter B, Greenshaw AJ. NMDA or AMPA/kainate receptor blockade prevents acquisition of conditioned place preference induced by D(2/3) dopamine receptor stimulation in rats. Psychopharmacology 2005;179:189–97.
Rodriguez De Fonseca F, Rubio P, Martin-Calderon JL, Caine SB, Koob GF, Navarro M. The dopamine receptor agonist 7-OH-DPAT modulates the acquisition and expression of morphine-induced place preference. Eur J Pharmacol 1995;274:47–55.
Frances H, Smirnova M, Leriche L, Sokoloff P. Dopamine D3 receptor ligands modulate the acquisition of morphine-conditioned place preference. Psychopharmacology 2004;175:127–33.
Gyertyan I, Gal K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport 2003;14:93–8.
Khroyan TV, Baker DA, Neisewander JL. Dose-dependent effects of the D3-preferring agonist 7-OH-DPAT on motor behaviors and place conditioning. Psychopharmacology 1995;122:351–7.
Hoffman DC, Dickson PR, Beninger RJ. The dopamine D2 receptor agonists, quinpirole and bromocriptine produce conditioned place preferences. Prog Neuropsychopharmacol Biol Psychiatry 1988;12:315–22.
Kuz’min AV, Zvartau EE. The reinforcing properties of psychostimulating preparations on models of conditioned-reflex place preference in mice. Zh Vyssh Nerv Deiat Im I P Pavlova 1995;45:802–10.
Khroyan TV, Fuchs RA, Baker DA, Neisewander JL. Effects of D3-preferring agonists 7-OH-PIPAT and PD-128,907 on motor behaviors and place conditioning. Behav Pharmacol 1997;8:65–74.
Woolley ML, Waters KA, Reavill C, et al. Selective dopamine D4 receptor agonist (A-412997) improves cognitive performance and stimulates motor activity without influencing reward-related behaviour in rat. Behav Pharmacol 2008;19:765–76.
Rezayof A, Zarrindast MR, Sahraei H, Haeri-Rohani A. Involvement of dopamine receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. J Psychopharmacol 2003;17:415–23.
Zarrindast MR, Rezayof A, Sahraei H, Haeri-Rohani A, Rassouli Y. Involvement of dopamine D1 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Brain Res 2003;965:212–21.
Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 2004;47(Suppl 1):256–73.
Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacol Biochem Behav 1990;37:317–23.
Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacol Biochem Behav 1995;50:121–5.
Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav 2000;65:635–47.
Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obes Res 1995;3(Suppl 4):515S–23S.
Phillips G, Willner P, Muscat R. Suppression or facilitation of operant behaviour by raclopride dependent on concentration of sucrose reward. Psychopharmacology 1991;105:239–46.
Price KL, Middaugh LD. The dopamine D1 antagonist reduces ethanol reward for C57BL/6 mice. Alcohol Clin Exp Res 2004;28:1666–75.
Agmo A, Federman I, Navarro V, Padua M, Velazquez G. Reward and reinforcement produced by drinking water: role of opioids and dopamine receptor subtypes. Pharmacol Biochem Behav 1993;46:183–94.
Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev 1995;19: 19–38.
Beck J, Bialy M, Kostowski W. Effects of D(1) receptor agonist SKF 38393 on male rat sexual behavior and postcopulatory departure in the goal compartment-runway paradigm. Physiol Behav 2002;76:91–7.
Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 1990;14:217–32.
Pfaus JG, Philipps AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat: I. Effects of systemic administration of dopamine antagonists. Behav Neurosci 1991;105:727–43.
Grierson JP, James MD, Pearson JR, Wilson CA. The effect of selective D1 and D2 dopaminergic agents on sexual receptivity in the female rat. Neuropharmacology 1988;27:181–9.
Agustin-Pavon C, Martinez-Ricos J, Martinez-Garcia F, Lanuza E. Effects of dopaminergic drugs on innate pheromone-mediated reward in female mice: a new case of dopamine-independent "liking." Behav Neurosci 2007;121:920–32.
El-Ghundi M, O‘Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci 2003;17:851–62.
Kudwa AE, Dominguez-Salazar E, Cabrera DM, Sibley DR, Rissman EF. Dopamine D5 receptor modulates male and female sexual behavior in mice. Psychopharmacology 2005;180:206–14.
Fowler SC, Zarcone TJ, Vorontsova E, Chen R. Motor and associative deficits in D2 dopamine receptor knockout mice. Int J Dev Neurosci 2002;20:309–21.
Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci 2002;22:RC224.
Boyce-Rustay JM, Risinger FO. Dopamine D3 receptor knockout mice and the motivational effects of ethanol. Pharmacol Biochem Behav 2003;75:373–9.
Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res 2001;904:76–84.
Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 2005;135:959–68.
Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 2002;137:165–77.
Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 2000;20:7737–42.
Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol Learn Mem 2002;77:44–62.
Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci 2002;22:1063–71.
Andrzejewski ME, Spencer RC, Kelley AE. Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience 2005;135:335–45.
Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol 2005;493:115–21.
You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci 1998;18:6492–500.
Gallistel CR, Davis AJ. Affinity for the dopamine D2 receptor predicts neuroleptic potency in blocking the reinforcing effect of MFB stimulation. Pharmacol Biochem Behav 1983;19:867–72.
Nakajima S, O‘Regan NB. The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacol Biochem Behav 1991;39:465–8.
Carr KD, Yamamoto N, Omura M, Cabeza de Vaca S, Krahne L. Effects of the D(3) dopamine receptor antagonist, U99194A, on brain stimulation and d-amphetamine reward, motor activity, and c-fos expression in ad libitum fed and food-restricted rats. Psychopharmacology 2002;163:76–84.
Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA 2005;102:2117–22.
Elmer GI, Pieper JO, Levy J, et al. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology 2005;182:33–44.
Hunt GE, Atrens DM, Jackson DM. Reward summation and the effects of dopamine D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacol Biochem Behav 1994;48:853–62.
Singh J, Desiraju T, Raju TR. Dose-response functions of apomorphine, SKF 38393, LY 171555, haloperidol and clonidine on the self-stimulation evoked from lateral hypothalamus and ventral tegmentum. Indian J Physiol Pharmacol 1996;40:15–22.
Baldo BA, Jain K, Veraldi L, Koob GF, Markou A. A dopamine D1 agonist elevates self-stimulation thresholds: comparison to other dopamine-selective drugs. Pharmacol Biochem Behav 1999;62:659–72.
Gilliss B, Malanga CJ, Pieper JO, Carlezon WA, Jr. Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology 2002;163:238–48.
Ranaldi R, Beninger RJ. The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Res 1994;651:283–92.
Depoortere R, Perrault G, Sanger DJ. Behavioural effects in the rat of the putative dopamine D3 receptor agonist 7-OH-DPAT: comparison with quinpirole and apomorphine. Psychopharmacology 1996;124:231–40.
Nakajima S, Liu X, Lau CL. Synergistic interaction of D1 and D2 dopamine receptors in the modulation of the reinforcing effect of brain stimulation. Behav Neurosci 1993;107:161–5.
Panagis G, Spyraki C. Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology 1996;123:280–8.
Gilbert DB, Millar J, Cooper SJ. The putative dopamine D3 agonist, 7-OH-DPAT, reduces dopamine release in the nucleus accumbens and electrical self-stimulation to the ventral tegmentum. Brain Res 1995;681:1–7.
Depoortere R, Perrault G, Sanger DJ. Intracranial self-stimulation under a progressive-ratio schedule in rats: effects of strength of stimulation, d-amphetamine, 7-OH-DPAT and haloperidol. Psychopharmacology 1999;142:221–9.
Knapp CM, Kornetsky C. Bromocriptine, a D2 receptor agonist, lowers the threshold for rewarding brain stimulation. Pharmacol Biochem Behav 1994;49:901–4.
Kurumiya S, Nakajima S. Dopamine D1 receptors in the nucleus accumbens: involvement in the reinforcing effect of tegmental stimulation. Brain Res 1988;448:1–6.
Nakajima S, Patterson RL. The involvement of dopamine D2 receptors, but not D3 or D4 receptors, in the rewarding effect of brain stimulation in the rat. Brain Res 1997;760:74–9.
Singh J, Desiraju T, Raju TR. Dopamine receptor sub-types involvement in nucleus accumbens and ventral tegmentum but not in medial prefrontal cortex: on self-stimulation of lateral hypothalamus and ventral mesencephalon. Behav Brain Res 1997;86:171–9.
Choi KH, Clements RL, Greenshaw AJ. Simultaneous AMPA/kainate receptor blockade and dopamine D(2/3) receptor stimulation in the nucleus accumbens decreases brain stimulation reward in rats. Behav Brain Res 2005;158:79–88.
Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature 1993;366:344–7.
Ferrer JM, Sanguinetti AM, Vives F, Mora F. Effects of agonists and antagonists of D1 and D2 dopamine receptors on self-stimulation of the medial prefrontal cortex in the rat. Pharmacol Biochem Behav 1983;19:211–7.
Van Tol HH, Bunzow JR, Guan HC, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 1991;350(6319):610–4.
Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci 1994;15:264–70.
Tiberi M, Jarvie KR, Silvia C, et al. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA 1991;88:7491–5.
Robbins TW. The potentiation of conditioned reinforcement by psychomotor stimulant drugs: A test of Hill’s hypthesis. Psychopharmacolgy 1975;45:103–14.
Taylor JR, Robbins TW. Enhanced behavioral control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology 1984;84:405–12.
Taylor JR, Robbins TW. 6-hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology 1986;90:390–7.
Ranaldi R, Beninger RJ. Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology 1993;113:110–8.
Smith JK, Neill JC, Costall B. Bidirectional effects of dopamine D2 receptor antagonists on responding for a conditioned reinforcer. Pharmacol Biochem Behav 1997;57:843–9.
Beninger RJ, Rolfe NG. Dopamine D1-like receptor agonists impair responding for conditioned reward in rats. Behav Pharmacol 1995;6:785–93.
Beninger RJ, Ranaldi R. The effect of amphetamine, apomorphine, SKF 38393, quinpirole and bromocriptine on responding for conditioned reward in rats. Behav Pharmacol 1992;3:155–63.
Ranaldi R, Beninger RJ. Bromocriptine enhancement of responding for conditioned reward depends on intact D1 receptor function. Psychopharmacology 1995;118:437–43.
Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology 1993;110:355–64.
Phillips GD, Robbins TW, Everitt BJ. Mesoaccumbens dopamine-opiate interactions in the control over behaviour by a conditioned reinforcer. Psychopharmacology 1994;114:345–59.
Hitchcott PK, Phillips GD. Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. II. Instrumental conditioning. Psychopharmacology 1998;140:310–8.
Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience 2006;137:699–706.
Roberts DCS, Vickers G. Atypical neuroleptics increase self-administration of cocaine: an evaluation of a behavioral screen for antipsychotic activity. Psychopharmacology 1984;82:135–9.
Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science 1998;282:298–300.
Koob GF, Le HT, Creese I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett 1987;79:315–20.
Britton DR, Mackenzie RG, Kebabian JW, Williams JEG, Kerkmen D. Evidence for involvement of both D1 and D2 receptors in maintaining cocaine self-administration. Pharmacol Biochem Behav 1991;39:911–5.
Corrigall WA, Coen KM. Cocaine self-administration is increased by both D1 and D2 dopamine receptor antagonists. Pharmacol Biochem Behav 1991;39:799–802.
Hubner CB, Moreton JB. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology 1991;105:151–6.
Glowa JR, Wojnicki FH. Effects of drugs on food- and cocaine-maintained responding, III: Dopaminergic antagonists. Psychopharmacology 1996;128:351–8.
Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav 1993;45:539–48.
Fletcher PJ. A comparison of the effects of risperidone, raclopride, and ritanserin on intravenous self-administration of d-amphetamine. Pharmacol Biochem Behav 1998;60:55–60.
Izzo E, Orsini C, Koob GF, Pulvirenti L. A dopamine partial agonist and antagonist block amphetamine self-administration in a progressive ratio schedule. Pharmacol Biochem Behav 2001;68:701–8.
Caine SB, Negus SS, Mello NK, et al. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 2002;22:2977–88.
Gal K, Gyertyan I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull 2003;61:595–601.
Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr., Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 2005;21:3427–38.
Xi ZX, Newman AH, Gilbert JG, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 2006;31:1393–405.
Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science 1996;271:1586–9.
Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther 1999;291:353–60.
Caine SB, Negus SS, Mello NK. Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology 2000;148:41–51.
Self DW, Karanian DA, Spencer JJ. Effects of the novel D1 agonist ABT-431on cocaine self-administration and reinstatement. Annl NY Acad Sci 2000;909:133–44.
Hubner CB, Koob GF. Bromocriptine produces decreases in cocaine self-administration in the rat. Neuropsychopharmacology 1990;3:101–8.
Pulvirenti L, Koob GF. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol Sci 1994;15:374–9.
Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol 1995;6:333–47.
Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 2007;321:1135–43.
Parsons LH, Caine SB, Sokoloff P, Schwartz J-C, Koob GF, Weiss F. Neurochemical evidence that post-synaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem 1996;67:1078–89.
Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport 1997;8:2373–7.
Le Foll B, Schwartz JC, Sokoloff P. Dopamine D3 receptor agents as potential new medications for drug addiction. Eur Psychiatry 2000;15:140–6.
Xu M, Koeltzow TE, Santiago GT, et al. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 1997;19:837–48.
Karasinska JM, George SR, Cheng R, O‘Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci 2005;22(7):1741–50.
Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D(1) and D(3) receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J Neurochem 2007;103:840–8.
Pilla M, Perachon S, Sautel F, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 1999;400:371–5.
Maldonado R, Robledo P, Chover AJ, Caine SB, Koob JF. D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav 1993;45:239–42.
Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology 1994;114:477–85.
Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res 1995;692:47–56.
Bachtell RK, Whisler K, Karanian DA, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology 2005;183:41–53.
Hurd YL, Ponten M. Cocaine self-administration behavior can be reduced or potentiated by the addition of specific dopamine concentrations in the nucleus accumbens and amygdala using in vivo microdialysis. Behav Brain Res 2000;116:177–86.
Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse 2008;62:481–6.
McGregor A, Roberts DCS. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res 1993;624:245–52.
McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behav Brain Res 1995; 67:75–80.
Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology 2006;31:363–74.
Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res 1998;784:105–15.
Hurd YL, McGregor A, Ponten M. In vivo amygdala dopamine levels modulate cocaine self-administration behaviour in the rat: D1 dopamine receptor involvement. Eur J Neurosci 1997;9:2541–8.
Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 2001;21:5841–6.
Doron R, Fridman L, Yadid G. Dopamine-2 receptors in the arcuate nucleus modulate cocaine-seeking behavior. Neuroreport 2006;17:1633–6.
Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 1982;78:204–9.
Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 1984;84:167–73.
Gerrits MA, Ramsey NF, Wolterink G, van Ree JM. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology 1994;114:486–94.
Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 1993;265:53–9.
Stinus L, Nadaud D, Deminiere JM, Jauregui J, Hand TT, Le Moal M. Chronic flupentixol treatment potentiates the reinforcing properties of systemic heroin administration. Biol Psychiatry 1989;26:363–71.
David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology 2002;160:307–17.
Le Merrer J, Gavello-Baudy S, Galey D, Cazala P. Morphine self-administration into the lateral septum depends on dopaminergic mechanisms: Evidence from pharmacology and Fos neuroimaging. Behav Brain Res 2007;180:203–17.
Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology 2007;52:1415–25.
David V, Besson M, Changeux JP, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 2006;50:1030–40.
Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci 2006;26:723–30.
Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol 2007;559:173–9.
Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA. Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 2003;28:1272–80.
Kameda G, Dadmarz M, Vogel WH. Influence of various drugs on the voluntary intake of nicotine by rats. Neuropsychobiology 2000;41:205–9.
Silvestre JS, O‘Neill MF, Fernandez AG, Palacios JM. Effects of a range of dopamine receptor agonists and antagonists on ethanol intake in the rat. Eur J Pharmacol 1996;318:257–65.
Linseman MA. Effects of dopaminergic agents on alcohol consumption by rats in a limited access paradigm. Psychopharmacology 1990;100:195–200.
Dyr W, McBride WJ, Lumeng L, Li TK, Murphy JM. Effects of D1 and D2 dopamine receptor agents on ethanol consumption in the high-alcohol-drinking (HAD) line of rats. Alcohol 1993;10:207–12.
Panocka I, Ciccocioppo R, Mosca M, Polidori C, Massi M. Effects of the dopamine D1 receptor antagonist SCH 39166 on the ingestive behaviour of alcohol-preferring rats. Psychopharmacology 1995;120:227–35.
Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol 2002;28:39–46.
Ng GY, George SR. Dopamine receptor agonist reduces ethanol self-administration in the ethanol-preferring C57BL/6 J inbred mouse. Eur J Pharmacol 1994;269:365–74.
Boyce JM, Risinger FO. Dopamine D3 receptor antagonist effects on the motivational effects of ethanol. Alcohol 2002;28:47–55.
Thanos PK, Katana JM, Ashby CR, Jr., et al. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav 2005;81:190–7.
Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR, Jr. Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol 2007;12:35–50.
El-Ghundi M, George SR, Drago J, et al. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol 1998;353:149–58.
Phillips TJ, Brown KJ, Burkhart-Kasch S, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci 1998;1:610–5.
Rodd ZA, Melendez RI, Bell RL, et al. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci 2004;24:1050–7.
Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res 1997;21:1083–91.
Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav 2003;79:581–90.
Eiler WJ, 2nd, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse 2003;48:45–56.
Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl 1991;1:417–20.
Melendez RI, Rodd ZA, McBride WJ, Murphy JM. Dopamine receptor regulation of ethanol intake and extracellular dopamine levels in the ventral pallidum of alcohol preferring (P) rats. Drug Alcohol Depend 2005;77:293–301.
Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res 1996;20:1631–8.
Thanos PK, Taintor NB, Rivera SN, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res 2004;28:720–8.
Self DW, Nestler EJ. Relapse to drug seeking: neural and molecular mechanisms. Drug Alcohol Depend 1998;51:49–60.
Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav 1999;64:327–36.
Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiat Neurosci 2000;25:125–36.
Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 2002;54:1–42.
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature 2003;422:614–8.
Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J Neurosci 2004;24:2825–31.
Wise RA. The brain and reward. In: Liebman JM, Cooper SJ, eds. The Neuropharmacological Basis of Reward. New York: Oxford University Press; 1989:377–424.
Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology 1989;97:59–64.
Robbins SJ, Ehrman RN, Childress AR, O‘Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav 1997;22:157–67.
Sinha R, Catapano D, O‘Malley S. Stress induced craving and stress response in cocaine dependent individuals. Psychopharmacology 1999;142:343–51.
Fuchs RA, See RE, Middaugh LD. Conditioned stimulus-induced reinstatement of extinguished cocaine seeking in C57BL/6 mice: a mouse model of drug relapse. Brain Res 2003;973:99–106.
Yan Y, Nitta A, Mizoguchi H, Yamada K, Nabeshima T. Relapse of methamphetamine-seeking behavior in C57BL/6 J mice demonstrated by a reinstatement procedure involving intravenous self-administration. Behav Brain Res 2006;168:137–43.
De Vries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren LJMJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal if IV drug self-administration. Psychopharmacology 1999;143:254–60.
Fuchs RA, Tran-Nguyen LT, Weber SM, Khroyan TV, Neisewander JL. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacol Biochem Behav 2002;72:623–32.
Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology 2003;28:1150–9.
Dias C, Lachize S, Boilet V, Huitelec E, Cador M. Differential effects of dopaminergic agents on locomotor sensitisation and on the reinstatement of cocaine-seeking and food-seeking behaviour. Psychopharmacology 2004;175:414–27.
Koeltzow TE, Vezina P. Locomotor activity and cocaine-seeking behavior during acquisition and reinstatement of operant self-administration behavior in rats. Behav Brain Res 2005;160:250–9.
Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology 2007;32:354–66.
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 2002;159:284–93.
Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology 2003;168:109–17.
Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther 2000;294:680–7.
Khroyan TV, Platt DM, Rowlett JK, Spealman RD. Attenuation of relapse to cocaine seeking by dopamine D1 receptor agonists and antagonists in non-human primates. Psychopharmacology 2003;168:124–31.
Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology 1998;137:15–24.
Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology 1999;143:102–10.
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature 2003;422:614–8.
Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacology 2003;168:118–23.
Weissenborn R, Deroche V, Koob G, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology 1996;126:311–22.
Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 2001;98:1976–81.
Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 2002;27:1006–15.
Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend 2006;81:63–70.
Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology 2003;28:329–38.
Gilbert JG, Newman AH, Gardner EL, et al. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse 2005;57:17–28.
Vorel SR, Ashby CR, Jr., Paul M, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 2002;22:9595–603.
Xi ZX, Gilbert J, Campos AC, et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology 2004;176:57–65.
Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core,of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci 2006;23:219–28.
Anderson SM, Famous KR, Sadri-Vakili G, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 2008;11:344–53.
Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci 1998;18:1848–59.
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 2000;20:7489–95.
Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience 2006;142:451–61.
Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci USA 1995;92:12304–8.
Hedou G, Feldon J, Heidbreder CA. Effects of cocaine on dopamine in subregions of the rat prefrontal cortex and their efferents to subterritories of the nucleus accumbens. Eur J Pharmacol 1999;372:143–55.
Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology 2003;168:132–8.
Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology 2006;31:1452–61.
Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci 2008;122(1):129–39.
See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology 2001;154:301–10.
Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology 2005;177:315–23.
Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 2003;168:66–74.
Di Pietro NC, Mashhoon Y, Heaney C, Yager LM, Kantak KM. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology 2008;200:81–91.
O‘Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci 2003;17:429–35.
De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology 2002;26:18–26.
Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology 1996;125:385–91.
Tobin S, Newman AH, Quinn T, Shalev U. A role for dopamine D1-like receptors in acute food deprivation-induced reinstatement of heroin seeking in rats. Int J Neuropsychopharmacol 2008:1–10.
Duarte C, Biala G, Le Bihan C, Hamon M, Thiebot MH. Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacology 2003;166:19–32.
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 2007;27:12655–63.
Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci 2004;20:249–63.
Micheli F, Bonanomi G, Blaney FE, et al. 1,2,4-triazol-3-yl-thiopropyl-tetrahydroben-zazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem 2007;50:5076–89.
Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, et al. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB J 2006;20:2223–33.
Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res 2001;25:1431–40.
Samson HH, Chappell AM. Effects of raclopride in the core of the nucleus accumbens on ethanol seeking and consumption: the use of extinction trials to measure seeking. Alcohol Clin Exp Res 2004;28:544–9.
Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther 2002;300:882–9.
Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 2007;146:525–36.
Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007;315:1267–70.
Morgan D, Grant KA, Gage HD, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 2002;5:169–74.
Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 2000;22:413–21.
Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology 2007;32:616–24.
Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology 2004;172:450–54.
Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993;14:169–77.
Volkow ND, Wang GJ, Fowler JS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 1996;20:1594–8.
Wang GJ, Volkow ND, Fowler JS, et al. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 1997;16:174–82.
Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiat 2001;158:2015–21.
Druhan JP, Walters CL, Aston-Jones G. Behavioral activation induced by D(2)-like receptor stimulation during opiate withdrawal. J Pharmacol Exp Ther 2000;294:531–8.
Seeman P, Tallerico T, Ko F, Tenn C, Kapur S. Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse 2002;46:235–9.
Seeman P, Weinshenker D, Quirion R, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 2005;102:3513–8.
Seeman P, McCormick PN, Kapur S. Increased dopamine D2(High) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse 2007;61:263–7.
Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacol 2008;18:551–6.
Martinez D, Broft A, Foltin RW, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 2004;29:1190–202.
Acknowledgements
This work is supported by United States Public Health Service Grants DA 010460 and DA 018743 and by the Wesley Gilliland Professorship in Biomedical Research (UTSW).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Self, D.W. (2010). Dopamine Receptor Subtypes in Reward and Relapse. In: Neve, K. (eds) The Dopamine Receptors. The Receptors. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60327-333-6_17
Download citation
DOI: https://doi.org/10.1007/978-1-60327-333-6_17
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60327-332-9
Online ISBN: 978-1-60327-333-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)