Abstract
Rationale
The dopamine (DA) D3 receptor is preferentially expressed in the mesolimbic system. We have previously shown that selective D3 receptor blockade by the novel D3 antagonist SB-277011A inhibits cocaine’s reinforcing action and cocaine-induced reinstatement of cocaine-seeking behavior.
Objective
In the present study, we investigated whether SB-277011A similarly inhibits stress-induced reinstatement of cocaine-seeking behavior.
Methods
Rats were allowed to self-administer cocaine (0.5 mg/kg per infusion, 3 h per session) for 10–14 days, followed by a once-daily extinction session for 7–14 days during which saline was substituted for cocaine. Extinction criteria were fewer than ten lever-presses per 3-h session for at least 3 consecutive days. After cocaine-seeking behavior was extinguished, each animal was tested twice for footshock-stress-induced reinstatement, once with vehicle (25% hydroxypropyl-β-cyclodextrin) and once with one of three doses of SB-277011A in counterbalanced fashion.
Results
During the last 3 days of cocaine self-administration (SA), active lever-presses were approximately 100 per session under fixed-ratio 2 reinforcement (∼25 mg/kg cocaine per session). After extinction, intermittent footshock (10 min, 0.5 mA, 0.5 s on with a mean inter-shock interval of 40 s) robustly reinstated the cocaine-seeking behavior (8.4±3.6 active lever-presses in last extinction session to 35.3±5.2 in animals after footshock stress). Intraperitoneal (IP) injections of SB-277011A (3, 6, and 12 mg/kg) dose-dependently blocked stress-induced reinstatement of cocaine-seeking. Reinstatement was also blocked by microinjections of SB-277011A (1.5 μg/0.5 μl per side) bilaterally into the nucleus accumbens, but not into the dorsal striatum.
Conclusions
The mesolimic DA D3 receptor plays an important role in mediating stress-induced reinstatement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug addiction is characterized by high rates of relapse to drug use (Wallace 1992; Schuckit 1994; O’Brien 1997). Despite advances in understanding the neurobiology of addiction (Wise 1996; Nestler 2001) and relapse (Stewart 2000; Shalev et al. 2002), no broadly effective anti-relapse pharmacotherapies exist.
Relapse to drug taking behavior in abstinent addicts can be triggered by addictive drugs, stress, or drug-associated environmental cues (Carter and Tiffany 1999; Sinha et al. 1999). These three classes of triggers also reinstate extinguished drug-seeking in animals (Shalev et al. 2002). The involvement of mesolimbic dopamine (DA) in cocaine addiction and relapse (Wise 1996; Stewart 2000; Nestler 2001; Shalev et al. 2002; Volkow et al. 2002) has implicated DA receptors as targets for the development of anti-cocaine medication (Rothman and Glowa 1995; Platt et al. 2002). Among the five DA receptor subtypes identified (Greengard 2001), the D3 receptor has attracted special attention because it is highly localized in the nucleus accumbens (NAcc) and adjacent mesolimbic loci (Bouthenet et al. 1991; Landwehrmeyer et al. 1993; Diaz et al. 1995). In addition, the D3 receptor has the highest binding affinity for DA of all DA receptors (30-fold over D1 or D2) (see Sokoloff et al. 1992; Levant 1997 for review). These data suggest that the D3 receptor is highly activated by endogenous DA and may be crucial to DA’s neurobiological functions. In support of this hypothesis, blockade of the D3 receptor with SB-277011A, a novel D3-selective antagonist (Reavill et al. 2000), dose-dependently attenuates cocaine-enhanced brain stimulation reward, cocaine-induced conditioned place preference, and cocaine-induced reinstatement of cocaine-seeking in rats (Vorel et al. 2002), as well as drug-associated cue-controlled cocaine-seeking under second-order reinforcement (Di Ciano et al. 2003). However, it remains unclear if SB-277011A can similarly block stress-induced reinstatement of drug-seeking.
Acute stress rapidly increases DA release in the NAcc and prefrontal cortex (PFC) (Sorg and Kalivas 1993; Kalivas and Duffy 1995; Shaham and Stewart 1996), possibly by activating an excitatory glutamate projection from PFC to Ventral Tegmental Area (VTA) DA neurons (Moghaddam 2002). Stress may also activate mesolimbic DA by releasing corticotrophin-releasing factor (CRF) in midbrain and amygdala (Lavicky and Dunn 1993; Shaham et al. 1997). Moreover, such stress-induced elevation of NAcc DA correlates temporally with reinstatement of heroin-seeking (Shaham and Stewart 1995), which can be blocked by the mixed DA antagonist flupenthixol, but not by the D1 antagonist SCH-23390 or the D2 antagonist raclopride (Shaham and Stewart 1996). These data support the involvement of DA in stress-induced reinstatement, while the receptor mechanism remains unidentified. In the present study, we sought to determine whether the D3 antagonist SB-277011A can block stress-induced reinstatement of cocaine-seeking in rats.
Materials and methods
Subjects
Male Long-Evans rats (250–300 g; Charles River, Raleigh, N.C., USA) were housed individually in a climate-controlled animal colony on a reverse light–dark cycle (lights on at 7:00 p.m., lights off at 7:00 a.m.) with access to food and water ad libitum. The experimental procedures followed the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse.
Surgery
Intravenous catheterization
Intravenous microrenathane catheters (Braintree Scientific, Inc., Braintree, Mass., USA) were surgically placed in the right jugular veins of rats under sodium pentobarbital anesthesia (65 mg/kg, IP). The right jugular vein was exposed and the catheter was inserted into the vein and sutured to it. The catheter then was passed subcutaneously to the skull top, where it exited into a connector (a modified 22 gauge cannula; Plastics One, Roanoke, Va., USA) mounted to the skull with jeweler’s screws and dental acrylic. A cannula cap was placed over the opening of the connector during the recovery period and at all other times when rats were not in an SA session. To prevent clogging, catheters were flushed daily with sterile saline and every 3rd day with 0.1 ml of sterile saline–heparin solution (15 IU/ml heparin; ICN Biochemicals, Cleveland, Ohio, USA).
Intracranial cannulation
In some animals, guide cannulae (20 gauge; Plastics One) were stereotaxically implanted into each hemisphere, aimed at either the NAcc (n=7) or dorsal striatum (n=6). Coordinates for the NAcc were: AP +1.6 mm, ML ±1.7 mm, DV −4.7 mm relative to bregma (Paxinos and Watson 1998) using a 6° off-vertical angle of approach. Coordinates for the dorsal striatum were: AP +2.0 mm, ML ±2.8 mm, DV −3.0 mm relative to bregma (Paxinos and Watson 1998). The sterotaxic coordinates were calculated for an internal injector protruding 2.0 mm beyond the tip of guide cannulae. Animals were allowed for at least 7 days to recover from surgery.
Apparatus
Experiments were conducted in operant response test chambers (32×25×33 cm), each equipped with a house light, ventilator fan, drug infusion pump (3.33 rpm motor, 10 ml syringe), and liquid swivel with counterbalanced arm. Each test chamber had two levers located 6.5 cm above the floor—one “active” and one “inactive.” Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no other consequence. A cue light and a speaker were located 12 cm above the active lever. At the start of each 3-h test session, the house-light was turned on. When animals made a lever-pressing response that resulted in a drug infusion, the cue-light was illuminated and a cue-sound (tone) was turned on for the duration of the infusion. All equipments were obtained from Med Associates (Georgia, Vt., USA). Scheduling of experimental events and data collection was accomplished using Med Associates software.
Procedure
The general protocol of cocaine SA, extinction, and tests for stress-induced reinstatement is illustrated in Fig. 1.
Self-administration
After recovery from surgery, rats were placed into the test chambers and allowed to lever-press on a fixed-ratio 1 (FR1) reinforcement schedule for intravenous cocaine (0.5 mg/kg per infusion) delivered in 0.08 ml over 4.6 s. Each session lasted for 3 h. This schedule was used for 3–5 days until regular SA behavior was established. Rats were then changed to a FR2 schedule for the same cocaine dose until the following criteria of stable cocaine-maintained responding were met: a minimum of 20 presses on the active lever per test session, less than 10% variability in inter-response interval, less than 10% variability in number of infusions taken, and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. The maximum number of cocaine infusions was limited to 50 per session to avoid accidental overdose. Responses made on the active lever during infusions were recorded but did not lead to additional infusions. The dose of cocaine was chosen on the basis of previous findings that rats trained with 0.5 mg/kg per infusion displayed rapid and reliable acquisition of SA behavior. Total SA training lasted for 10–14 days. At the end of each daily 3-h session, rats were returned to the colony room.
Extinction and tests for reinstatement
During extinction, the active lever-pressing response was extinguished by replacing cocaine with saline, without the cue-light and tone that were associated with cocaine during the SA phase. Daily 3-h extinction continued until the rats lever-pressed fewer than 10 times per 3-h session for at least 3 consecutive days. Testing for reinstatement began immediately after successful extinction. Each rat was given two reinstatement tests in random order, once with one dose of SB-277011A and once with vehicle [25% (2-hydroxypropyl)-β-cyclodextrin]. The interval between receiving vehicle and SB-277011A was 5–7 days (Fig. 1). SB-277011A (3, 6, and 12 mg/kg, IP) was given 60 min before reinstatement testing, as its peak drug level in rat brain is achieved approximately 60 min after systemic administration (Austin et al. 2001). In the intracranial microinjection experiments, reinstatement testing began after 30 min, based on pilot studies showing maximal SB-277011A effects 30 min after being locally administered into NAcc. Vehicle (0.5 μl/side) or SB-277011A (1.5 μg/0.5 μl per side) was manually injected over a 1-min period into the brain 2 mm below the guide cannula tip via an internal injection needle. After drug administration, rats were returned to the testing chambers immediately and, after a delay of 60 or 30 min (depending on whether SB-277011A was given systemically or intracranially, see above), were exposed to 10 min of intermittent footshock stress (0.5 mA; 0.5 s on; mean off period of 40 s) (Shaham et al. 2000b), delivered by a computer-controlled system. Following termination of footshock, reinstatement testing began with onset of the house light and introduction of the active and inactive levers. During each reinstatement test session, conditions were identical to those in extinction sessions. Each reinstatement test session lasted 3 h.
Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, Mo., USA) was dissolved in physiological saline. SB-277011A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydro-2-isoquinolinyl)ethyl]-cyclohexyl]-4-[2-3H]quinolinylcarboxamine) was provided by GlaxoSmithKline Pharmaceuticals (Verona, Italy, and Harlow, Essex, UK). As SB-277011A is not water soluble, we dissolved it in 25% (2-hydroxypropyl)-β-cyclodextrin (Sigma), which acts as a carrier into the brain and then slowly releases SB-277011A from inside the fullerene-shaped β-cyclodextrin molecules. The SB-277011A/β-cyclodextrin solution was prepared as follows: a 25% solution of (2-hydroxypropyl)-β-cyclodextrin was prepared by adding (2-hydroxypropyl)-β-cyclodextrin to distilled water, stirring for 1 h, sonicating, and filtering the clear solution through a 0.22 μm filter. SB-277011A was then added and stirred for 1 h. The resulting clear solution was again filtered through a 0.22 μm filter and stored at 4°C for up to 1 week. The 25% (2-hydroxypropyl)-β-cyclodextrin vehicle was used for the intracranial SB-277011A microinjections as well as for the systemic (IP) SB-277011A injections. The dose of SB-277011A for the intracranial microinjections was chosen on the basis of a ratio of approximately 10,000:1 for systemic/intracranial microinjection dosing that we have successfully used previously (e.g. Chen et al. 1993). The intracranial injected volume of 0.5 μl was similarly chosen on the basis of prior experience. We chose to locally administer SB-277011A into the NAcc or the dorsal striatum 30 min before the reinstatement testing based upon preliminary experiments demonstrating a maximal effect 30 min after intra-NAcc SB-277011A administration.
Histology
At the experiment’s end, animals with intracranial guide cannulae were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 10% formalin–saline solution. Brains were removed and fixed in a 10% formalin–saline-25% sucrose solution for at least 24 h before sectioning. Brains were sectioned by cryostat at 30 μm thickness, and stained with cresyl violet. Animals were discarded from the experiment if injector tips fell beyond the anatomic boundaries of the intended site. Brain slices through the NAcc and dorsal striatum were examined by visual microscopy to verify the location of the cannulae tips and to look for possible neurotoxicity induced either by the (2-hydroxypropyl)-β-cyclodextrin vehicle or by SB-277011A.
Statistical analyses
All behavioral data are presented as means±SEM, and were subjected to standard multivariate statistical analyses (Winer 1962). Three-way analysis of variance (ANOVA) (main effects: different doses of SB-277011A×stress×drug) with repeated measures on the last two factors was used to determine statistical significance of overall main effects on reinstatement and interactions between main effects (for the data in Fig. 2). Three-way ANOVA with repeated measures on the last two factors was similarly used to assess statistical significance of the NAcc and dorsal striatal SB-277011A microinjections on stress-induced reinstatement (main effects: anatomical locus×stress×drug) and to assess interactions between main effects. Two-way ANOVAs (main effects: stress×drug) with repeated measures on both main factors were used to determine the significance of the effects of local administrations of SB-277011A into the NAcc or dorsal striatum separately on stress-induced reinstatement (for the data in Fig. 3). The Tukey b-test was used to assess the statistical significance of post hoc individual group comparisons.
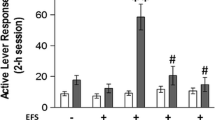
Effects of systemic administration of the potent and highly selective DA D3 receptor antagonist SB-277011A on footshock-induced reinstatement of cocaine-seeking behavior. A Mean responding on the active lever during the last session of cocaine self-administration (SA), during the last session of extinction prior to testing with either vehicle or SB-277011A, and during the test session for footshock-induced reinstatement in the presence of vehicle or SB-277011A. Statistical analyses revealed a significant stress-triggered reinstatement effect, and a significant dose-dependent protective effect against stress-triggered reinstatement by the highly selective DA D3 receptor antagonist SB-277011A. B Mean responding on the inactive lever. See Results for details of the statistical analyses and findings
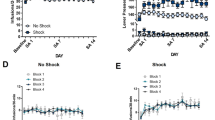
Effects of local microinjections of the potent and the highly selective DA D3 receptor antagonist SB-277011A into the nucleus accumbens (NAcc) (A) or dorsal striatum (B) on footshock-induced reinstatement of cocaine-seeking behavior. Statistical analyses revealed a significant stress-triggered reinstatement effect, and a significant protective effect against stress-triggered reinstatement by the highly selective DA D3 receptor antagonist SB-277011A when microinjected bilaterally (1.5 μg/0.5 ml per side) into the NAcc but not into the dorsal striatum. See Results for details of the statistical analyses and findings
Results
Effects of systemic administration of SB-277011A on stress-induced reinstatement
Acquisition of cocaine self-administration
Most rats rapidly acquired cocaine SA. Rats not showing stable cocaine SA were excluded from the study (n=4). Twenty-five rats showed stable cocaine SA and were divided into three groups based on SB-277011A dose to be given: 3 mg/kg (n=9), 6 mg/kg (n=7), 12 mg/kg (n=9). Figure 2 shows mean number of lever-presses on last day of cocaine SA, last day of extinction prior to test, and the reinstatement test day in the presence of vehicle or SB-277011A. During the last 3 days of cocaine SA prior to extinction, mean active lever-presses were 104.5±5.6 per 3 h across all 25 subjects (Fig. 2A). Responding on the inactive lever was 19.2±3.4 per 3 h. There was no difference in cocaine infusions, active lever-presses, or inactive lever-presses across the three different SB-277011A dose groups during the last 3 days of cocaine SA.
Extinction training
During extinction, responding on the active lever progressively decreased in all three groups of rats. There were no differences in extinction responding between the three groups (Fig. 2A) or between vehicle and SB-277011A treatment days within each group. Responding on the inactive lever during extinction was approximately 50% of that seen during the cocaine SA phase (Fig. 2B).
Stress-induced reinstatement
Testing for stress-induced reinstatement began 24 h following the last extinction session. Intermittent footshock for 10 min reliably reinstated extinguished cocaine-seeking behavior in most rats. Compared with the last session of extinction prior to reinstatement testing (8.4±3.6), active-lever responding during reinstatement was significantly increased overall in the three groups under vehicle conditions (35.3±5.2), and there were no differences in reinstatement responding between these three different groups (Fig. 2A). However, stress-induced reinstatement was dose-dependently attenuated by SB-277011A. Three-way ANOVA with repeated measures on the last two factors revealed a statistically significant main effect of stress [F(1,22)=21.65, P<0.0001], a statistically significant drug×dose interaction [F(2,22)=3.61, P<0.05], and most importantly a statistically significant drug×dose×stress interaction [F(2,22)=4.28, P<0.05]. Individual group comparisons using the Tukey b post hoc test revealed statistically significant stress-induced reinstatement of cocaine-seeking behavior (Fig. 2A, bar “b” versus bar “e”: q=8.09, df=22, P<0.001; Fig. 2A, bar “d” versus bar “e”: q=8.21, df=22, P<0.001). This stress-induced reinstatement of cocaine-seeking behavior was significantly attenuated dose-dependently by SB-277011A (Fig. 2A bar “c” versus bar “o”: q=5.88, df=22, P<0.05; Fig. 2A bar “e” versus bar “o”: q=9.24, df=22, P<0.001; Fig. 2A bar “h” versus bar “o”: q=6.81, df=22, P<0.01; Fig. 2A bar “j” versus bar “o”: q=5.69, df=22, P<0.05). This SB-277011A-induced attenuation of stress-triggered reinstatement to cocaine-seeking behavior became significantly protective against stress-triggered reinstatement at 12 mg/kg SB-277011A (Fig. 2A bar “m” versus bar “o”: q=6.25, df=22, P<0.05). Thus, at doses of 3 or 6 mg/kg, SB-277011A had no significant effect on stress-induced reinstatement. When the dose was increased to 12 mg/kg, stress-induced reinstatement was completely blocked (Fig. 2A). There were no significant effects of stress or SB-277011A on inactive lever responses (Fig. 2B).
Effects of intracranial administration of SB-277011A on stress-induced reinstatement
Thirteen rats that showed stable SA behavior were used in this experiment and were divided into two groups: intra-NAcc (n=7) and intra-striatum (n=6). When administered bilaterally into NAcc, SB-277011A (1.5 μg/0.5 μl per side) significantly blocked stress-induced reinstatement, compared with vehicle injection in the same rats (Fig. 3A). Three-way ANOVA with repeated measures on the last two factors revealed significant main effects of anatomical site (NAcc versus dorsal striatum) [F(1,11)=13.41, P<0.005] and stress [F(1,11)=24.08, P<0.0001]. Two-way ANOVAs with repeated measures on both factors revealed a significant main effect of stress [F(1,6)=17.52, P<0.01] and a significant stress×drug interaction [F(1,6)=6.04, P<0.05] in NAcc-injected rats. In the dorsal striatum-injected rats, a significant main effect of stress [F(1,5)=11.91, P<0.025] was seen, but—importantly—no stress×drug interaction. Individual group comparisons using the Tukey b post hoc statistic revealed significant stress-induced reinstatement in NAcc-injected rats (Fig. 3A bar “b” versus bar “c”: q=5.29, df=11, P<0.05) and dorsal striatum-injected rats (Fig. 3B bar “b” versus bar “c”: q=5.20, df=11, P<0.05). Individual group comparisons also revealed a significant protective effect of SB-277011A against stress-triggered reinstatement in NAcc-injected rats (Fig. 3A bar “c” versus bar “e”: q=5.69, df=11, P<0.05), but not in the dorsal striatum group (Fig. 3B bar “c” versus bar “e”: q=0.47, df=11, NS). Thus, when given locally into the NAcc, SB-277011A protected against stress-induced reinstatement, but when given into the dorsal striatum did not.
Histology
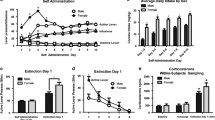
Figure 4A shows a representative track left by a microinjector tip in the NAcc. Microinjector tips aimed at the NAcc were found to be either within or on the medial borders of the NAcc shell or within the medial portion of the NAcc core (Fig. 4C). Microinjector tips aimed at the dorsal striatum (caudate-putamen) were found to be in the dorsal striatum, near the border of the corpus callosum (Fig. 4D). Figure 4B shows a high-power (×10) micrograph corresponding to the area in the box in Fig. 4A, revealing mechanical damage caused by the penetration of the guide cannula and the internal microinjector tip that protruded beyond the cannula tip, but no vehicle-induced or drug-induced tissue damage in the injection field 1–2 mm below the tip of the guide cannula. Detailed microscopic examination of injection areas in NAcc and dorsal striatum showed no detectable tissue damage within the microinjection fields of either brain structure, other than the mechanical damage caused by the insertion of the guide cannulae and microinjectors.
Histological verification of microinjector tip locations in the nucleus accumbens (NAcc) and the dorsal striatum. A Photomicrograph showing a representative microinjector tip location in the NAcc. B High-power (×10) photomicrograph of the area in the box in A illustrating mechanical damage caused by the penetration of a guide cannulae and microinjector needle tip, but without other patent cellular damage or neurotoxicity in the injection field 1–2 mm below the tip of the guide cannula. C and D Locations of microinjector tips in the NAcc and dorsal striatum, plotted on drawings of coronal sections of rat brain taken from the atlas of Paxinos and Watson (1998)
Discussion
The present data demonstrate that the selective D3 antagonist SB-277011A, when given systemically at 12 mg/kg or microinjected directly into the NAcc bilaterally at 1.5 μg/0.5 μl per side, attenuates stress-triggered reinstatement of cocaine-seeking behavior. Local administration of SB-277011A into the dorsal striatum had no effect on stress-triggered reinstatement. This is the first report implicating mesolimbic DA and D3 receptors in stress-triggered reinstatement of drug-seeking behavior. If these results can be extrapolated to humans, highly selective D3 receptor antagonists may be worthy of controlled investigation in humans as potential treatments for stress-induced relapse to drug abuse.
As noted in the Introduction, the unique distribution and high binding affinity of the D3 receptor suggest a role for D3 receptors in drug reinforcement and drug-seeking behavior. In support of this hypothesis, previous studies have shown that D3 receptor activation appears to enhance, while D3 blockade attenuates cocaine’s rewarding effects (Caine and Koob 1993, 1995; Parsons et al. 1996; Caine et al. 1997). For example, the D3-preferring agonists PD-128,907, pramipexole, or quinelorane dose-dependently decrease cocaine SA (Caine et al. 1997), similar to what is seen after increases in cocaine’s unit dose (Caine and Koob 1995). Also, the D3-preferring agonist 7-OH-DPAT increases the progressive-ratio “break-point” for cocaine SA (Caine and Koob 1995; Depoortere et al. 1999), and substitutes for cocaine in drug discrimination tests (Lamas et al. 1996; Nader and Mach 1996). Conversely, D3 receptor antagonism shifts cocaine’s SA dose-effect curve rightward (Roberts and Ranaldi 1995; Le Foll et al. 2000), suggesting attenuation of cocaine reward. However, because such putative “D3-preferring” compounds have poor D3/D2 selectivity (see Levant 1997 for review), the reported effects cannot be convincingly attributed to D3 receptor mediation. Although evidence from the use of BP-897, a putative D3 partial agonist, supports the involvement of D3 receptors in cocaine’s actions (Pilla et al. 1999; see Preti 2001 for review), the exact role of D3 receptors in drug reward as revealed by such studies remains elusive, as BP-897 may have more antagonist than partial agonist properties (Wood et al. 2000; Wicke and Garcia-Ladona 2001). In addition, BP-897 also binds to other receptors such as D2, D4, 5-HT1A, or α1 receptors (Pilla et al. 1999; Campiani et al. 2003).
In contrast to BP-897, the present novel D3-selective antagonist SB-277011A has high potency, full antagonist properties, and high selectivity (100-fold) for D3 over D2 receptors and 66 other receptors, enzymes, and ion channels (Reavill et al. 2000). The present results show that SB-277011A-induced blockade of mesolimbic D3 receptors attenuates stress-induced reinstatement, extending our previous findings that SB-277011A attenuates cocaine-enhanced brain stimulation reward, cocaine-induced conditioned place preference, and cocaine-induced reinstatement of cocaine-seeking behavior (Vorel et al. 2002). In our view, the presently observed inhibition of stress-induced reinstatement by SB-277011A is unlikely to have resulted from possible antinociceptive effects of SB-277011A, as we saw no evidence that SB-277011A affected footshock-induced species-typical rodent escape or avoidance behaviors such as foot-flicking, foot-withdrawal, or jumping (also see Shaham and Stewart 1996).
When microinjected directly into the NAcc, but not into dorsal striatum, SB-277011A blocked stress-induced reinstatement. These findings strongly suggest involvement of NAcc D3 receptors in stress-triggered reinstatement of cocaine-seeking. Provocatively, the NAcc contains some of the highest D3 receptor densities in the brain (Bouthenet et al. 1991; Landwehrmeyer et al. 1993; Diaz et al. 1995), whereas dorsal striatum has very low D3 receptor density (Bouthenet et al. 1991; Landwehrmeyer et al. 1993). An important caveat, however, is that the NAcc is near the bed nucleus of the stria terminalis (BNST), a region well implicated in stress-triggered reinstatement (Shaham et al. 2000a; Stewart 2000; Shalev et al. 2002). Although it seems unlikely that the microvolume (0.5 μl) used in the present study was sufficient to diffuse from NAcc to BNST, the latency between microinjections and testing in the present experiments was sufficient for such diffusion. Thus, additional work is needed to localize the site of action for SB-277011A’s protective effects against stress-triggered reinstatement of drug-seeking behavior. Consistent with our present findings, recent studies show that SB-277011A attenuates cue-controlled cocaine-seeking behavior as assessed by second-order reinforcement (Di Ciano et al. 2003), nicotine-triggered nicotine-seeking behavior (Andreoli et al. 2003), and heroin-induced conditioned place preference (Ashby et al. 2003). All these findings are congruent with D3 receptors playing an important role in drug reward and drug-seeking.
Stress provokes craving and relapse to drug-seeking behavior in abstinent cocaine addicts (Sinha et al. 1999; Sinha 2001). Stress also reliably reinstates drug-seeking in animals after extinction of heroin-taking, cocaine-taking, nicotine-taking, or alcohol-taking behavior (Shaham et al. 2000a). Although noradrenergic and CRF neurotransmitter mechanisms appear to underlie stress-triggered reinstatement (Lavicky and Dunn 1993; Shaham et al. 1997; Shaham et al. 2000a; Leri et al. 2002 for review), a role for DA has not been ruled out. The present study demonstrates that selective D3 receptor antagonism blocks stress-induced reinstatement of cocaine-seeking behavior, supporting the involvement of DA and D3 receptors in stress-induced reinstatement. Congruent with this conclusion, Shaham and Stewart (1995) showed that elevations of NAcc DA produced by footshock stress correlate temporally with reinstatement of heroin-seeking behavior. Such reinstatement can be blocked by the mixed DA antagonist flupenthixol, but not by the D1 antagonist SCH-23390 or the D2 antagonist raclopride (Shaham and Stewart 1996). Thus, the possibility remains that combined D1/D2 antagonism could block stress-induced reinstatement, or that other DA receptors (e.g. D3) could play a role. In addition, further studies are needed to ascertain whether SB-277011A antagonizes heroin-induced reinstatement of heroin-seeking behavior in manner similar to our previous finding that SB-277011A antagonizes cocaine-induced reinstatement of cocaine-seeking behavior (Vorel et al. 2002).
The present findings, taken together with other recent reports (Vorel et al. 2002; Andreoli et al. 2003; Ashby et al. 2003; Di Ciano et al. 2003), suggest that the D3 receptor may constitute a new target for anti-addiction medications. At the same time, caution along these lines is warranted. The seeming face validity of the reinstatement animal model has not been shown to have predictive validity for human relapse (Epstein and Preston 2003; Katz and Higgins 2003; however, for more sanguine views of the utility of the reinstatement model, see Shaham and Miczek 2003; Shaham et al. 2003).
Notably, SB-277011A shows none of the side effects commonly associated with D1 or D2 antagonism (Rothman and Glowa 1995; Platt et al. 2002). For example, SB-277011A (up to 90 mg/kg, PO) has no effect on spontaneous or stimulant-induced locomotion, does not alter memory, and is non-cataleptogenic (Reavill et al. 2000; Vorel et al. 2002). It also has no effects on free-feeding, food-reinforced operant behavior, or food-conditioned place preference (Vorel et al. 2002). It does not significantly alter responding for sucrose under second-order reinforcement (Di Ciano et al. 2003). SB-277011A appears to have no addictive liability, as it cannot by itself (1.5 mg/kg per injection) maintain intravenous SA by rats (Xi et al., unpublished observations).
In conclusion, the present data (taken together with other recent published findings using SB-277011A-induced D3 receptor blockade) suggest that highly selective D3 receptor antagonists merit further study as potential pharmacotherapeutic agents for the treatment of drug addiction, with the above-noted caveats regarding the lack of established predictive validity to humans of the reinstatement model’s seeming face validity in laboratory animals.
References
Andreoli M, Tessari M, Pilla M, Valerio E, Hagan JJ, Heidbreder CA (2003) Selective antagonism at dopamine D3 receptors prevents nicotine-triggered relapse to nicotine-seeking behavior. Neuropsychopharmacology 28:1272–1280
Ashby CR Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ (2003) Acute administration of the selective D3 receptor antagonist SB-277011-A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse 48:154–156
Austin NE, Baldwin SJ, Cutler L, Deeks N, Kelly PJ, Nash M, Shardlow CE, Stemp G, Thewlis K, Ayrton A, Jeffrey P (2001) Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica 31:677–686
Bouthenet M-L, Souil E, Martres M-P, Sokoloff P, Giros B, Schwartz J-C (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Caine SB, Koob GF (1993) Modulation of cocaine self-administration in the rat through D3 dopamine receptors. Science 260:1814–1816
Caine SB, Koob GF (1995) Pretreatment with the dopamine agonist 7-OH DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol 6:333–347
Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz J-C, Sokoloff P (1997) D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport 8:2373–2377
Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, Gemma S, Nacci V, Novellino E, Stark JA, Cagnotto A, Fumagalli E, Carnovali F, Cervo L, Mennini T (2003) Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J Med Chem 46:3822–3839
Carter BL, Tiffany ST (1999) Meta-analysis of cue-reactivity in addiction research. Addiction 94:327–340
Chen J, Marmur R, Pulles A, Paredes W, Gardner EL (1993) Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana’s psychoactive ingredient. Brain Res 621:65–70
Depoortere R, Perrault G, Sanger DJ (1999) Intracranial self-stimulation under a progressive-ratio schedule in rats: effects of strength of stimulation, d-amphetamine, 7-OH-DPAT and haloperidol. Psychopharmacology 142:221–229
Diaz J, Lévesque D, Lammers CH, Griffon N, Martres M-P, Schwartz J-C, Sokoloff P (1995) Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65:731–745
Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ (2003) Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology 28:329–338
Epstein DH, Preston KL (2003) The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology 168:31–41
Greengard P (2001) The neurobiology of dopamine signaling. Biosci Rep 21:247–269
Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Lamas X, Negus SS, Nader MA, Mello NK (1996) Effects of the putative dopamine D3 receptor agonist 7-OH-DPAT in rhesus monkeys trained to discriminate cocaine from saline. Psychopharmacology 124:306–314
Landwehrmeyer B, Mengod G, Palacios JM (1993) Differential visualization of dopamine D2 and D3 receptor sites in rat brain. A comparative study using in situ hybridization histochemistry and ligand binding autoradiography. Eur J Neurosci 5:145–153
Lavicky J, Dunn AJ (1993) Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem 60:602–612
Leri F, Flores J, Rodaros D, Stewart J (2002) Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci 22:5713–5718
Levant B (1997) The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev 49:231–252
Le Foll B, Schwartz J-C, Sokoloff P (2000) Dopamine D3 receptor agents as potential new medications for drug addiction. Eur Psychiatry 15:140–146
Moghaddam B (2002) Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 51:775–787
Nader MA, Mach RH (1996) Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology 125:13–22
National Academy of Sciences (1996) Guide for the care and use of laboratory animals. National Academy, Washington, D.C.
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128
O’Brien CP (1997) A range of research-based pharmacotherapies for addiction. Science 278:66–70
Parsons LH, Caine SB, Sokoloff P, Schwartz J-C, Koob GF, Weiss F (1996) Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem 67:1078–1089
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic, San Diego
Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P (1999) Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400:371–375
Platt DM, Rowlett JK, Spealman RD (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology 163:265–282
Preti A (2001) BP-897 bioproject. Curr Opin Invest Drugs 1:110–115
Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ (2000) Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294:1154–1165
Roberts DCS, Ranaldi R (1995) Effect of dopaminergic drugs on cocaine reinforcement. Clin Neuropharmacol 18:S84–S95
Rothman RB, Glowa JR (1995) A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909. Mol Neurobiol 11:1–19
Schuckit MA (1994) The treatment of stimulant dependence. Addiction 89:1559–1563
Shaham Y, Miczek KA (2003) Reinstatement—toward a model of relapse. Psychopharmacology 168:1–2
Shaham Y, Stewart J (1995) Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology 119:334–341
Shaham Y, Stewart J (1996) Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology 125:385–391
Shaham Y, Funk D, Erb S, Brown TJ, Walker C-D, Stewart J (1997) Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614
Shaham Y, Erb S, Stewart J (2000a) Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33
Shaham Y, Highfield D, Delfs J, Leung S, Stewart J (2000b) Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci 12:292–302
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359
Sinha R, Catapano D, O’Malley S (1999) Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology 142:343–351
Sokoloff P, Martres M-P, Giros B, Bouthenet M-L, Schwartz J-C (1992) The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol 43:659–666
Sorg BA, Kalivas PW (1993) Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience 53:695–703
Stewart J (2000) Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci 25:125–136
Volkow ND, Fowler JS, Wang G-J (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13:355–366
Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22:9595–9603
Wallace BC (1992) Treating crack cocaine dependence: the critical role of relapse prevention. J Psychoact Drugs 24:213–222
Wicke K, Garcia-Ladona J (2001) The dopamine D3 receptor partial agonist, BP 897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. Eur J Pharmacol 424:85–90
Winer BJ (1962) Statistical principles in experimental design. McGraw-Hill, New York
Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251
Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ (2000) Evidence for antagonist activity of the dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur J Pharmacol 407:47–51
Acknowledgments
The authors thank Yavin Shaham for advice on technical aspects of the footshock procedure and for insightful criticisms and suggestions regarding the manuscript, and Richard Taylor for assistance with the data analyses. This work was supported by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, US. Department of Health and Human Services, and by GlaxoSmithKline Pharmaceuticals. Equipment support was provided by the Aaron Diamond Foundation of New York, the Julia Sullivan Medical Research Fund, and the Old Stones Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xi, ZX., Gilbert, J., Campos, A.C. et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology 176, 57–65 (2004). https://doi.org/10.1007/s00213-004-1858-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1858-y