Abstract
Rationale
The role of ventral tegmental area (VTA) in mediating the rewarding effects of cocaine has not been extensively studied.
Objectives
We used the intracranial self-administration (ICSA) procedure to assess the involvement of the VTA in the rewarding effects of cocaine, and the effect of dopamine (DA) D1- and serotonin (5-HT)1B-receptor antagonists on ICSA of cocaine.
Methods
Adult male C57BL/6 mice were stereotaxically implanted, unilaterally, with a guide cannula either 1.5 or 2.3 mm above the VTA. After 1 week, mice were trained to discriminate between the two arms of a Y-maze over seven daily sessions, one arm being reinforced by intracranial cocaine microinjections. Starting from session 8, the D1 and 5-HT1B-receptor antagonists were injected IP pre-test each day over five consecutive sessions.
Results
Mice injected into the VTA rapidly exhibited a preference for the cocaine-reinforced arm, whatever the dose of cocaine available (30 pmol or 150 pmol per injection), reaching optimum ICSA performance within 5 days. In contrast, mice injected 0.8 mm above the VTA did not discriminate between the arms of the maze and performed at random, except for one subject. Once the ICSA response was acquired, systemic pre-injections of either the D1 (SCH23390; 25 μg/kg IP) or 5-HT1B (GR127935; 0.5 mg/kg IP) antagonist disrupted this behavior. Replacement of each antagonist by vehicle led to the reinstatement of intra-VTA cocaine self-administration.
Conclusions
The results of the present study suggest that VTA neurons play a critical role in mediating the rewarding effects of acute cocaine and that both D1 and 5-HT1B receptors modulate these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The positive reinforcing effects of cocaine are currently considered to be mediated via its blocking action on neuronal membrane transporters for dopamine (DAT), at mesolimbic dopaminergic (DA) synapses within the nucleus accumbens (NAc); as was initially proposed by Kuhar and colleagues (Ritz et al. 1987). Behavioral and pharmacological data in animals now support a role for the involvement of DA in the reinforcing effect of cocaine. Disruption of intravenous cocaine self-administration by intra-NAc injection of DA receptor antagonists or lesions of the NAc (Zito et al. 1985; Robledo et al. 1992; Maldonado et al. 1993), as well as a combined behavioral and intra-NAc electrophysiological study of cocaine reinforcement (Uzwiak et al. 1997; Carelli and Ijames 2000) also support the concept of an involvement of mesolimbic DA in the reinforcing effects of cocaine. Accordingly, intra-NAc cocaine self-administration has been reported in both rats and mice (Carlezon and Wise 1996; David et al. 2001). However, cocaine reinforcement can be modified independently of the DA system (Hubner and Koob 1990; Robledo and Koob 1993). Recent studies using genetically engineered mice lacking, under-expressing or over-expressing the DAT gene have generated conflicting data on this issue. Cocaine-induced hyperlocomotion is absent in DAT knockout mice (Giros et al. 1996; Sora et al. 1998, 2001). DAT knockout mice display normal place preference and cocaine intravenous self-administration (Rocha et al. 1998a; Sora et al. 1998). The emerging view from these genetic studies is that mechanisms of cocaine-induced changes in locomotion, but not of cocaine-induced reward, are consistent with the DAT hypothesis (Uhl et al. 2002).
There is evidence that extra-striatal brain regions may be involved in the rewarding effects of acute cocaine, in particular other targets of the DA system such as the medial prefrontal cortex (Goeders and Smith 1983; McGregor et al. 1996), or the origin of the system itself, i.e. the ventral tegmental area (VTA) (Roberts and Koob 1982). However, with the exception of an early study that reported negative results (Goeders and Smith 1983), the putative rewarding effects of cocaine injections within the VTA have not been much studied. Intra-VTA injection of the GABAB antagonist, baclofen, or the D1 receptor antagonist, SCH23390, disrupted intravenous cocaine self-administration, as measured with either a fixed or progressive ratio paradigm (Brebner et al. 2000; Ranaldi and Wise 2001). Cocaine exhibits higher affinity for the 5-HT than the DA transporter, and the VTA is densely innervated by 5-HT fibers (Van Bockstaele et al. 1994). Combined DA and 5-HT transporter knockout in mice eliminates cocaine-induced place preference (Sora et al. 2001). Therefore, the action of cocaine within the VTA may also involve an inhibition of 5-HT re-uptake. Among the various 5-HT receptor types found in the VTA, the 5-HT1B receptor has been reported to be involved in the locomotor stimulant effects of cocaine, sensitization (Przegalinski et al. 2001), discriminative stimulus effects (Callahan and Cunningham 1995; Filip et al. 2003) and the reinforcing effect of cocaine (Parsons et al. 1998). Activation of 5-HT1B receptors is thought to decrease local GABA release, thereby weakening GABAergic control over DA neuronal activity, an effect that may be potentiated by the effect cocaine on 5-HT re-uptake (Johnson et al. 1992; Cameron and Williams 1994). A viral-mediated gene transfection method was used to increase the expression of 5-HT1B receptors in NAc efferent neurons, presumably projecting to the VTA. This increased expression was shown to result in sensitization of cocaine-induced place preference (Neumaier et al. 2002). Mice lacking the gene for this receptor subtype failed to display any preference for stimuli paired with cocaine (Belzung et al. 2000). However, they exhibited an increased locomotor response to this drug and appeared to be more motivated to self-administer it, thus suggesting an increased vulnerability to cocaine (Rocha et al. 1997, 1998a). Given that the behavior of these mice was not similar to that of normal mice treated with 5-HT1B receptor antagonists, compensatory mechanisms increasing sensitivity to cocaine have been suspected to develop in 5-HT1B knockout mice (Parsons et al. 1998; Rocha et al. 1998b; Castanon et al. 2000). In the present report, we have used the intracranial self-administration paradigm (David et al. 2002) to assess the role of VTA neurons in cocaine reinforcement. To test whether the D1 or 5-HT1B receptors might be involved in the reinforcing effects of cocaine microinjections into the VTA, we also studied the effects of selective antagonists of both receptor types on intra-VTA cocaine self-administration.
Materials and methods
Ethical statement
All surgical and experimental procedures were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Animals and surgery
The experiments used male C57BL/6JiCo mice (Iffa-Credo, Lyon, France). At 12 weeks of age, they were housed individually with ad libitum access to food and water in a temperature-controlled room (23°C) with a light/dark cycle (12 h/12 h, light on at 0800 hours) and sawdust bedding changed weekly. The animals were aged 3–4 months (27–30 g) at the beginning of the experiments. The subjects were deeply anaesthetized with tribromoethanol (Avertin®, 300 mg/kg, IP). Moreover, lidocaine HCl (Xylocaine®, 5%) was applied locally both before opening the scalp and trepanation. The animals were implanted in a counterbalanced, left and right order, and unilaterally, since it has previously been demonstrated that the magnitude of the motivational effects of unilaterally applied opioids is equivalent to that observed when bilateral injections are used (Phillips and Le Piane 1980; Bozarth 1987). The tip of the cannula guide (outer diameter 0.460 mm; inner diameter 0.255 mm) was positioned either 1.5 mm (VTA groups) or 2.3 mm (dorsal control or D-VTA group) above the VTA. The stereotaxic coordinates used were: 0.40 mm anterior to the interaural line; ±0.30 mm lateral to the sagittal line; 2.5 or 3.30 mm vertically below the surface of the skull. The incisor bar was level with the interaural line. Mice were allowed to recover from surgery for at least 1 week.
Materials and experimental protocol
Intracranial self-injection procedure
Self-administration behavior was studied in a gray Plexiglas Y-maze, the two arms of which were separated by an angle of 90°. The stem and the arms were 31 cm long and 12 cm high. The starting box (14×8 cm) was separated from the stem by a sliding door. A photoelectric cell was situated 6 cm from the end of each arm. On each day of the experimental period, a stainless-steel injection cannula (outer diameter 0.229 mm, inner diameter 0.127 mm) was inserted into the VTA and held in a fixed position by means of a small connector. The injection cannula was connected by flexible polyethylene tubing to the microinjection system, which housed a 5-µl Hamilton syringe. The tip of the injection cannula projected beyond the guide cannula by 1.5 mm. By interrupting the photocell beam in one of the two target arms, mice could trigger an injection of one of two doses (30 or 150 pmol) of cocaine HCl (Coopération Pharmaceutique Française) dissolved in polyionic Ringer-Aguettant solution (pH 6.1). The other arm was neutral (no injection). Intracranial injections were carried out using an automatic computer-controlled apparatus, which provided, via a micro-vernier system, a precise and highly reproducible descent of the microsyringe piston. Each self-injection (50 nl) lasted 4 s. Normal drug flow was verified visually both before and after each ICSA session for each animal. The movements of the animals in the Y-maze were detected using an optical system. This information was transmitted to a computer, which rotated in turn the injector in the same direction as each animal’s movement. This process avoided the twisting of the flexible tubing. The number of self-administrations per daily session was noted and automatic equipment, triggered by opening the door to the stem, recorded the latency to enter the reinforced arm (injection latency) or the neutral arm for each subject.
Behavioral protocol
Four groups were constituted as follows: VTA cocaine 30 pmol (n=5); VTA cocaine 150 pmol (n=13) and D-VTA cocaine 150 pmol (n=6). In the VTA cocaine 150 pmol group, two out of 13 mice displayed unstable performances and were thus removed from the experiments. The D-VTA cocaine group refers to animals having received the cannula guide 0.8 mm above the VTA cocaine group. A fourth group of animals (n=6) served as controls, having only vehicle (Ringer) available.
The protocol consisted of three phases:
-
1.
Acquisition phase. This phase lasted for seven daily sessions. Each session comprised the following steps. To begin a trial, a mouse was placed in the start box and, after 1 min, the door to the stem was opened. In each group, half of the animals were assigned to the right arm to trigger the injection of cocaine, whereas the remainder were assigned to the left arm. Each daily session was composed of ten trials each separated by a 1-min inter-trial interval. Therefore, a maximum of ten injections could be obtained by each subject per daily session. During the first four trials of the first session only, if an animal made an error in choosing the neutral arm, it was immediately allowed to access to the arm enabling an injection of cocaine. From the fifth trial onward and during the following sessions, if an animal entered into the neutral arm, it was replaced into the start box for the following trial.
-
2.
DA and 5-HT antagonist challenge (extinction test). This phase lasted 5 days. Half (n=5) of all subjects that exhibited robust cocaine self-administration with the 150 pmol dose were injected with the D1 receptor antagonist (+)-SCH 23390 HCl [(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,-5-tetrahydro-1H-3-benzazepine; 25 μg/kg IP, dissolved in isotonic NaCl 0.9% to the required final concentration] 10 min prior to the self-administration session. The other half (n=6) was injected with the selective 5-HT1B/1D receptor antagonist/partial agonist GR127935 (n-[4-methoxy-3-(4-methyl-1-piperizin-1-yl)phenyl]-2’-methyl-4’(5-methyl-1,2,4-oxadiazol-3-yl)[1,1’-biphenyl]-4-carboxamide); 0.5 mg/kg IP, dissolved in sterile water) 20 min prior to the self-administration session. Injections were administered in a volume of 0.1 ml/10 g body weight.
-
3.
Replacement of antagonist treatment with vehicle (relapse test). Following the last session using SCH23390 or GR127935 pretreatments, both antagonists were then replaced by pre-trial injections of vehicle alone for three consecutive daily sessions.
Histology
At the end of the experimental period, animals were killed with an overdose of Avertin. The head was removed, with the guide cannula attached, and placed into 10% formol for a 72-h period. The guide cannula was then withdrawn, the brain dissected and placed in a solution of formol containing 30% sucrose for an additional week. Brains were then frozen and cut in a microtome to provide 60 µm frontal sections, which were stained using 0.1% thionine to identify the injection site.
Statistical analyses
To analyze the acquisition of cocaine self-administration, a repeated measures ANOVA of the number of cocaine self-injections and self-injection latency were conducted with Drug (cocaine versus Ringer) as a between-subjects factor and Session (training day) as a within-subjects repeated measure. Since the 150 pmol dose group (n=13) was subsequently divided into separate groups, the number of subjects is over 2-fold higher than in the 30 pmol dose group (n=5). For this reason, we compared each group separately to control (vehicle) mice. The same parameters were analysed following pharmacological challenges of intra-VTA cocaine self-administration using each antagonist (D1 or 5-HT1B) as a between-subjects factor and Session as a within-subjects repeated measure. One-way ANOVA with the repeated factor session was used to examine the evolution of the number of self-injections for each group. Significant main effects were further analysed using Fisher’s PLSD tests. Paired Student t-tests were used to compare either the number of self-administrations or time to trigger the injection between two sessions in the same group (ex: session E5 versus R1). A minimum significance level of P<0.05 was required for all statistical analyses.
Results
Acquisition of cocaine ICSA
Spatial discrimination
No discrimination between the two arms of the Y-maze was observed in subjects having access only to vehicle (Ringer) for intra-VTA self-injections. Choice behavior did not alter significantly over the seven acquisition sessions [F(6,30)=1.89, ns], demonstrating the absence of any non-specific (chemical or mechanical) stimulating effects of the microinjection system and confirming previous observations obtained using a different inbred strain (David et al. 1998, 2002). In marked contrast, mice rapidly exhibited a preference for the cocaine-associated arm of the Y-maze. Although the acquisition phase lasted a total of 7 days, optimum choice performance was reached within five sessions. These observations are supported by a two-way ANOVA, revealing main effects of cocaine on choice performance, whatever the dose used [dose of 30 pmol: main effect of drug F(1,9)=50.42, P<0.0001; session: F(6,54)=4.93, P<0.001; drug×session interaction: F(6,54)=10.65, P<0.0001; dose of 150 pmol: main effect of drug: F(1,15)=53.00, P<0.0001; main effect of session: F(6,90)=1.91, ns; drug×session interaction: F(6,90)=4.62, P<0.001 (Fig. 1A, left panel]. Although non-significantly, the level of discrimination performance tended to be higher with the low cocaine dose (30 pmol), than with the high cocaine dose (150 pmol). When the injection cannula was raised 0.8 mm dorsally (D-VTA group), cocaine self-administration was no longer observed. Only one mouse exhibited a slight preference for the cocaine-reinforced arm. Therefore, it is likely that neurons within the VTA or a 0.8 mm radius sphere around the injection cannula tip, are responsible for this behavior as attested by a main effect of injection site on spatial discrimination [F(1,15)=53.91, P<0.0001; main effect of session: F(6,90)=1.79, ns; site×session interaction: F(6,90)=2.77, P<0.02].
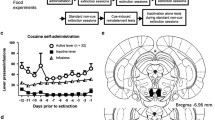
A Mean number (±SEM) of self-administrations of cocaine (30 or 150 pmol) into the VTA or 0.8 mm dorsally to the VTA (D-VTA group). An additional control group had only vehicle (Ringer solution) available for intra-VTA injections. B Mean value of the latency (seconds±SEM) to trigger the injection of cocaine (30 pmol or 150 pmol) or Ringer solution into the VTA or D-VTA groups. *P<0.05; **P<0.01; ***P<0.001: comparison with controls (Ringer). ¥ Comparison of SCH23390 and GR127935 groups. †Significantly different from the last acquisition (A7) or extinction (E5) session
Self-injection latencies
Analyses of the time taken to trigger cocaine self-injections revealed that there was a significant decrease of this parameter over the seven acquisition sessions in both cocaine groups, while the same parameter increased progressively in the vehicle-injected group yielding a robust drug×session interaction [two-way ANOVA, main effect of drug (30 pmol versus Ringer): F(1,9)=7.89, P<0.05; main effect of session: F(6,54)=0.76, ns; drug×session interaction F(6,54)=2.12, P=0.06; main effect of drug (150 pmol versus Ringer): F(1,15)=10.34, P<0.01; main effect of session: F(6,90)=1.47, ns; interaction drug×session F(6,90)=2.91, P<0.01 (Fig. 1B, left panel)]. There was no dose effect for cocaine on this parameter. In contrast, when infused 0.8 mm dorsally to the VTA, cocaine produced an inverse effect on the time taken to trigger injections as compared to intra-VTA administration, i.e. strongly increasing instead of decreasing it [main effect of site: F(1,15)=20.76, P<0.001; main effect of session: F(6,90)=3.85, P<0.002; site×session interaction: F(6,90)=3.73, P<0.01]. It should be noted that among the D-VTA group, the mice exhibiting discrimination performance above chance level did not complete trials any faster than the remaining subjects of that group.
Effects of SCH23390 and GR127935
Spatial discrimination
Before pre-treatment with the antagonists, the two groups used in this experiment, injected with the higher dose of cocaine (150 pmol), exhibited identical self-administration performance during the acquisition phase [F(1,9)=0.076, ns]. Pre-treatment with either SCH 23390 (25 μg/kg, IP, n=5) or GR127935 (0.5 mg/kg IP, n=6), respectively, 10 min and 20 min before each session, altered cocaine ICSA in a similar manner. In both groups, a significant reduction of the number of entries into the reinforced arm was observed over sessions 8–12 (Fig. 1A, middle panel). In support of these observations, a two-way ANOVA comparing the effect of systemic injections of SCH23390 or GR127935 systemic injections revealed no differences for the effect of either antagonist on this parameter [F(1,9)=0.08, ns; main effect of sessions: F(4,36)=16.95, P<0.0001; with no group×sessions interaction: F(4,36)=0.05, ns]. Post-hoc analysis revealed that significant decreases in ICSA performance occurred starting from the third session under treatment in GR127935-injected mice [comparison with the performance recorded during the last acquisition session using paired Student t-test in the GR127935 group: t(5)=5.39, P<0.01; and from the second pre-treatment session in the SCH 23390 group t(4)=3.31, P<0.02]. In both groups, the performance level rapidly fell to chance level and at a rate similar to subjects undergoing extinction, i.e. when an addictive drug is replaced by vehicle (Cazala et al. 1987). These data demonstrate that both SCH23390 and GR127935 disrupted cocaine ICSA, and that the time-course of their effects on this behavior were quite similar.
Self-injection latencies
Concomitantly with the decrease in preference for the cocaine-reinforced arm, both antagonist treatments significantly increased the time to trigger intra-VTA cocaine self-injections over the 5 consecutive days of treatment (Fig. 1B, middle panel). However, in contrast to their identical effects on discrimination performance, SCH23390 and GR127935 produced contrasting effects on the latency to trigger injections of cocaine into the VTA. While GR127935-preinjected mice exhibited a progressive but significant increase of this parameter over the five consecutive sessions, SCH23390-preinjected mice were much slower than GR127935 mice over days 2–5. Nevertheless all mice were able to complete each of the sessions. These observations were supported by statistical analysis (two-way ANOVA), revealing a main effect of the type of antagonist [F(1,9)=22.84, P<0.001; main effect of session: F(4,36)=4.82, P<0.01; but no drug×session interaction: F(4,36)=1.25, ns].
Replacement of antagonist pretreatment by vehicle
The replacement of either antagonist by injection of their respective vehicle led to immediate reinstatement of intra-VTA cocaine self-administration, as measured by the preference for the cocaine-associated arm as well as decrease in the time to trigger cocaine self-injections (Fig. 1A,B right panel, respectively). Effects on choice accuracy were significant starting from the first vehicle pretreatment session in GR127935 and SCH23390 groups [comparison of mean number of self-administrations during E5 and R1 for GR127935 group: t(5)=2.98 P<0.05; and SCH23390 group: t(4)=3.77, P<0.02]. Latency was also reduced from the first session of antagonist replacement by vehicle, although this was significant only for the SCH23390 group [comparison of mean time to trigger self-injection during E5 versus R1 in SCH23390 group: t(4)=4.26 P<0.02].
Histological control
Injection sites were precisely located by following the track of each injection cannula. Figure 2 shows the most ventral and dorsal placements in the VTA group, and the most ventral and dorsal placements in the D-VTA group (0.8 mm dorsal to the VTA). Concerning the VTA, most of the injection sites were located in the posterior part of the structure according to the atlas of Franklin and Paxinos (1997). In the D-VTA group, one subject appeared to be implanted closer to the VTA than remaining animals of the same group. It is interesting to note that this mouse exhibited a level of discrimination performance comparable to VTA mice (Fig. 2D).
Photomicrographs of thionin-stained frontal brain sections (60 μm) through the guide-cannula tracks and the injection site into the VTA (left) and 0.8 mm dorsally to the VTA (D-VTA group, right). High and low placements are shown for both groups (VTA group: A and C; D-VTA group: B and D). Picture D shows the only subject of the D-VTA group that tended to exhibit a spatial discrimination toward the cocaine-reinforced arm
Discussion
Cocaine microinjections into the VTA served as a powerful reinforcer at both doses used in the present study, leading to the acquisition of robust self-administration within only a few days of training. Since raising the injection cannula 0.8 mm dorsally eliminated cocaine ICSA, it is probable that neurons within the VTA are the primary target of the drug. Therefore, the VTA may be a critical component of the neural substrate underlying cocaine reinforcement. However, an early study did not observe any cocaine self-administration into the VTA of the rat (Goeders and Smith 1983). Among the differences with the present report that could account for this discrepancy, anatomical localization is likely to be a key factor. In the study of Goeders and Smith (1983), injection sites were located in the anterior part of the VTA, while in the present report cocaine was administered into the posterior VTA. Previous ICSA studies have revealed regional heterogeneity of the VTA region, functional differences being especially marked within the anteroposterior axis (Ikemoto et al. 1998; Zangen et al. 2002). The hypothesis that VTA could be involved in cocaine reinforcement is not inconsistent with the observation that intra-VTA cocaine injections do not substitute for systemic cocaine (De La Garza et al. 1998). Drug discrimination studies have provided valuable information on the subjective effects of drugs. However, subjective effects may be related to aversive as well as to rewarding effects. Indeed, the lack of effect of intra-VTA cocaine injections is consistent with the observation that intra-VTA cocaine self-administration did not produced any of the anxiogenic side effects observed with intravenous or intra-accumbens injections of cocaine (Ettenberg 1991; David et al. 2001). Consistently, intra-accumbens infusions of cocaine have been demonstrated to substitute fully for systemic cocaine (Callahan et al. 1997). It should be noted also that cocaine shares with other local anesthetics such as procaine, the properties to induce anesthesia by blocking Na+ channels. However, injection of procaine in reward-relevant brain regions, at concentrations equipotent to a cocaine concentration effective in blocking Na+, did not induce any rewarding effects (Ikemoto 2003). Therefore, it is unlikely that anesthetic effects are a key mechanism underlying cocaine-induced reward.
Until recently, cocaine reinforcement was thought to depend on an inhibition of mesolimbic DA re-uptake within the nucleus accumbens (NAc) (cf. Introduction). In the present study, blockade of cocaine self-administration by systemic pre-injection of the D1 receptor antagonist SCH23390 confirmed an involvement of the DA system. Previous studies of the effects of SCH23390 on intravenous cocaine self-administration have reported either a decrease or an increase, depending on the reinforcement schedule (Glowa and Wojnicki 1996). Increases in cocaine-maintained responding using a low schedule (FR1–5) are often interpreted as an antagonism of cocaine reinforcement. Since replacement of an intracranially self-administered drug by its vehicle typically led to progressive extinction of the preference for the drug-associated arm of the maze (Cazala et al. 1987; David et al. 2002), we interpret the decrease in discrimination performance following antagonist treatment as a decrease in cocaine reward. Our observations are therefore consistent with a compensatory increase in the number of lever-presses for intravenous cocaine following SCH23390 treatment. Since antagonist treatment was started only when discrimination performance was optimum, it is difficult to observe any significant further increase in our paradigm, even initially. In rate-dependent paradigms, DA antagonists will increase self-administration when using a small ratio/high dose schedule, and decrease it with a large ratio and a low dose (Glowa and Wojnicki 1996). Alternatively, they could increase cocaine-maintained responding by blocking the rate-decreasing effects of cocaine. In the present study, concurrent measures of self-injection latencies controlled for any motor impairment. Our results are also consistent with the observation that intra-VTA injection of SCH23390 disrupted intravenous cocaine self-administration in rats (Ranaldi and Wise 2001). However, since SCH23390 is administered systemically, concurrent blockade of post-synaptic receptors in limbic or cortical areas targeted by tegmental DA neurons may account for the disruptive effect of SCH23390 on cocaine self-administration observed in the present study.
The selective 5-HT1B receptor antagonist GR127935 also disrupted intra-VTA cocaine self-administration, eventually leading to an extinction profile very similar to that observed following systemic injection of SCH23390. Extinction of ICSA following blockade of D1 or 5-HT1B receptors clearly results from a decrease in cocaine rewarding effects, and not from lesion due to the repeated insertion of injection cannulae or any deleterious effect on motor capacity, since the ICSA response reappeared when SCH23390 or GR127935 were replaced by vehicle. In contrast to what was previously observed with the D2/D3 antagonist sulpiride on ICSA of several drugs of abuse, extinction profiles produced by the injection of both antagonists were close to what would be expected from classical extinction, i.e. when the abused drug is replaced by its vehicle (Cazala et al. 1987; David et al. 1998, 2002). However, the effect of GR127935 on self-injection latency was significantly less as compared to SCH23390. The increase in the time taken to trigger the injection by SCH23390 cannot be explained by motor impairment, since this occurred progressively over the five sessions and not from the first day of antagonist treatment, and can therefore rather be associated mainly to a loss of motivation (David et al. 2001).
Increasing evidence implicates 5-HT1B receptors in the reinforcing effects of cocaine. 5-HT1B receptors are also involved in striatal c-fos expression elicited by cocaine (Lucas et al. 1997). Interestingly, stimulation of both DA and 5-HT receptors is required to fully mimic the effects of cocaine on striatal gene expression (Bhat and Baraban 1993). Activation of 5-HT1B receptors also potentiates the reinforcing effects of DA uptake inhibitors and enhances intravenous cocaine self-administration (Parsons et al. 1996, 1998). Although GR127935 displays also partial agonist properties (Pauwels 1997), the effects observed in the present study are more likely interpreted as being a result of its antagonist action on 5-HT1B receptors. GR127935 did not disrupt intravenous cocaine self-administration or intracranial self-stimulation when used alone, but blocked the facilitating effect of the agonist CP93129 (Parsons et al. 1998), or the elevating effect of the 5-HT1A/1B agonist RU24969 on self-stimulation threshold (Harrison et al. 1999). Fluoxetine (a 5-HT reuptake blocker), a 5-HT1B agonist but not a 5-HT1A agonist, enhanced the discriminative effects of cocaine (Callahan and Cunningham 1997). Using a two-lever, water-reinforced FR20 drug discrimination procedure, neither intra-VTA 5-HT1A receptors agonists nor antagonists substituted for cocaine (De la Garza et al. 1998). 5-HT1B is an axon terminal autoreceptor, which has less potency than 5-HT1A in controlling 5-HT release. A 5-HT1B antagonist would have a stimulating effect on 5-HT release and thus on cocaine reward, since cocaine increases 5-HT release. In the present study, we observed the opposite, i.e. a 5-HT1B antagonist decreased cocaine reward. Therefore, the effects of GR127935 might not be explained by an action on autoreceptors.
Cocaine increases 5-HT levels via its blockade of 5-HT re-uptake, which then stimulates 5-HT1B receptors located on GABAergic afferents causing a decrease of tegmental GABA release and GABAB synaptic potentials, thereby disinhibiting DA neurons within the VTA (Johnson et al. 1992; Cameron and Williams 1994; Yan and Yan 2001). Interestingly, cocaine-induced place conditioning was recently reported to depend on VTA glutamate transmission (Harris and Aston-Jones 2003). This latter study has also revealed that intra-VTA injections of a NMDA antagonist alone induce place conditioning, thus providing support for our previous observations of intra-VTA self-administration of NMDA antagonists (David et al. 1998). It is therefore conceivable that the action 5-HT1B receptors may involve presynaptic regulation of glutamate release. However, indirect VTA glutamate effects are thought to involve GABAA receptors, whereas 5-HT1B effects on VTA are due to their presynaptic inhibitory actions on GABA neurons, resulting in a decreased GABAB but not a GABAA synaptic potential on DA neurons (Johnson et al. 1992). If 5-HT1B receptors were located on VTA glutamatergic afferents, a 5-HT1B antagonist could block 5-HT tonic driving of those neurons onto VTA-GABA interneurons. This speculative mechanism clearly deserves further experimental attention. As for SCH23390, it cannot be excluded that 5-HT regulation of other pathways, such as the glutamatergic hippocampo-accumbens or GABAergic accumbo-pallidall projections, was affected by GR127935 (Bruinvels et al. 1994; Boulenguez et al. 1996). In the nucleus accumbens itself, 5-HT1B modulates glutamate release by acting at presynaptic sites located on glutamatergic terminals, an effect potentiated by cocaine or a selective serotonin reuptake inhibitor (Muramatsu et al. 1998). In the cingulate cortex, 5-HT1B activation reduced the excitatory postsynaptic potential of pyramidal neurons, an effect enhanced 10-fold by cocaine (Tanaka and North 1993). Nevertheless, recent evidence supports a role of VTA 5-HT in cocaine reward. Intra-VTA injections of the 5-HT1B antagonist GR55562 dose-dependently decreased, whereas the agonist CP93129 increased, the discriminative effects of systemic cocaine (Filip et al. 2003). Finally, transfection of an active 5-HT1B gene in the accumbens GABA efferent neurons was recently reported to increase expression of 5-HT1B receptors within the VTA, thereby sensitizing rats to cocaine place conditioning (Neumaier et al. 2002).
In summary, the results of the present study suggest that (i) cocaine acts within the VTA to elicit reinforcement, (ii) both DA and 5-HT systems, via D1 and 5-HT1B receptors, respectively, are involved in cocaine’s rewarding effects. We speculate that cocaine-induced inhibition of 5-HT re-uptake, leading to a reduction of GABA release through action on 5-HT1B receptors within the VTA, may contribute to its reinforcing effects. The hypothesis that the addictive properties of cocaine may also depend on its action within the VTA does not exclude the previously held view that cocaine-induced inhibition of mesolimbic DA re-uptake within the NAc participates in reinforcement. Rather, we suggest that cocaine could act synergistically within both the VTA and its terminal fields to elicit reward.
References
Belzung C, Scearce-Levie K, Barreau S, Hen R (2000) Absence of cocaine-induced place conditioning in serotonin 1B receptor knock-out mice. Pharmacol Biochem Behav 66:221–225
Bhat RV, Baraban JM (1993) Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther 267:496–505
Boulenguez P, Rawlins JN, Chauveau J, Joseph MH, Mitchell SN, Gray JA (1996) Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacology 35:1521–1529
Bozarth MA (1987) Neuroanatomical boundaries of the reward relevant opiate receptors field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res 414:77–84
Brebner K, Phelan R, Roberts DC (2000) Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology 148:314–321
Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM (1994) Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33:367–386
Callahan PM, Cunningham KA (1995) Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther 274:1414–1424
Callahan PM, De La Garza R 2nd, Cunningham KA (1997) Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 57:601–607
Cameron DL, Williams JT (1994) Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci 14:6763–6767
Carelli RM, Ijames SG (2000) Nucleus accumbens cell-firing during maintenance, extinction and reinstatement of cocaine self-administration behavior in rats. Brain Res 866:44–54
Carlezon WA, Wise RA (1996) Rewarding action of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci 16:3112–3122
Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R (2000) Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knock-outs and antagonists. Pharmacol Biochem Behav 67:559–566
Cazala P, Darracq C, Saint Marc M (1987) Self-administration of morphine into the lateral hypothalamus in the mouse. Brain Res 416:283–288
David V, Durkin TP, Cazala P (1998) Rewarding effects elicited by the microinjection of either AMPA or NMDA glutamatergic antagonists into the ventral tegmental area revealed by an intracranial self-administration paradigm in mice. Eur J Neurosci 10:1394–1402
David V, Gold LH, Koob GF, Cazala P (2001) Anxiogenic-like effects limit rewarding effects of cocaine in BALB/cByJ mice. Neuropsychopharmacology 24:300–318
David V, Durkin TP, Cazala P (2002) Differential effect of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology 160:307–317
De La Garza R 2nd, Callahan PM, Cunningham KA (1998) The discriminative stimulus properties of cocaine: effects of microinfusion of cocaine, a 5-HT1A agonist or antagonist, into the ventral tegmental area. Psychopharmacology 137:1–6
Ettenberg A, Geist TD (1991) Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology 103:455–461
Filip M, Papla I, Nowak E, Czepiel K, Przegalinski E. (2003) Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on cocaine discrimination in rats. Eur J Pharmacol 459:239–245
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. San Diego, Academic Press
Giros B, Jaber M, Jones SR Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996:379:606–612
Glowa JR, Wojnicki FH (1996) Effects of drugs on food- and cocaine-maintained responding, III: Dopaminergic antagonists. Psychopharmacology 128:351–358
Goeders NE, Smith JE (1983) Cortical dopaminergic involvement in cocaine reinforcement. Science 221:773–775
Harris GC, Aston-Jones G (2003) Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology 28:73–76
Harrison AA, Parsons LH, Koob GF, Markou A (1999) RU24969, a 5-HT1A/1B agonist, elevates brain stimulation reward thresholds: an effect reversed by GR127935, a 5-HT1B/1D antagonist. Psychopharmacology 141:242–250
Hubner CB, Koob GF (1990) The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res 508:20–29
Ikemoto S (2003) Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci 23:9305–9311
Ikemoto S, Murphy JM, McBride MC (1998) Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol Biochem Behav 61:87–92
Johnson SW, Mercuri NB, North RA (1992) 5-Hydroxytryptamine 1B receptors block the GABA B synaptic potential in rat dopamine neurons. J Neurosci 12:2000–2006
Lucas JJ, Segu L, Hen R (1997) 5-Hydroxytryptamine 1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine 1B knockout mice and the 5-hydroxytryptamine 1B/1D antagonist GR 127935. Mol Pharmacol 51:755–763
Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF (1993) D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav 45:239–242
McGregor A, Baker G, Roberts DC (1996) Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav 53:5–9
Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J (1998) Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci 10:2371–2379
Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA Jr (2002) Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci 22:10856–10863
Parsons LH, Weiss F, Koob GF (1996) Serotonin 1B receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacology 128:150–160
Parsons LH, Weiss F, Koob GF (1998) Serotonin 1B receptor stimulation enhances cocaine reinforcement. J Neurosci 18:10078–10089
Pauwels PJ (1997) 5-HT1B/1D receptor antagonists. Gen Pharmacol 29:293–303
Phillips AG, Le Piane FG (1980) Reinforcing effects of morphine micro-injection into the ventral tegmental area. Pharmacol Biochem Behav 12:965–968
Przegalinski E, Filip M, Papla I, Siwanowicz J (2001) Effect of serotonin (5-HT)1B receptor ligands on cocaine sensitization in rats. Behav Pharmacol 12:109–116
Ranaldi R, Wise RA (2001) Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 21:5841–5846
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223
Roberts DC, Koob GF (1982) Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav 17:901–904
Robledo P, Koob GF (1993) Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res 55:159–166
Robledo P, Maldonado-Lopez R, Koob GF (1992) Role of dopamine receptors in the nucleus accumbens in the rewarding properties of cocaine. Ann N Y Acad Sci 654:509–512
Rocha A, Ator R, Emmett-Oglesby MW, Hen R (1997) Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav 57:407–412
Rocha, BA Fumagalli F, Gainetdinov RR, Jones SR, Atos R, Giros B et al. (1998a) Cocaine self-administration in dopamine- transporter knockout mice. Nat Neurosci 1:132–137
Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R (1998b) Increased vulnerability to cocaine in mice lacking the serotonin 1B receptor. Nature 393:175–178
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R et al. (1998) Cocaine reward models: conditioned place preference can be established in dopamine and serotonin-transporter knockout mice. Proc Natl Acad Sci USA 95:7699–7704
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB et al. (2001) Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine preference. Proc Natl Acad Sci USA 98:5300–5305
Tanaka E, North RA (1993) Cocaine enhancement of the action of 5-hydroxytryptamine in rat cingulate cortex in vitro. Neurosci Lett 163:50–52
Uhl GR, Hall FS, Sora I (2002) Cocaine, reward, movement and monoamine transporters. Mol Psychiatry 7:21–26
Uzwiak AJ, Guyette FX, West MO, Peoples LL (1997) Neurons in accumbens subterritories of the rat: phasic firing time-locked within seconds of intravenous cocaine self-infusion. Brain Res 767:363–369
Van Bockstaele EJ, Cestari DM, Pickel VM (1994) Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neurons. Brain Res 647:307–322
Yan QS, Yan SE (2001) Serotonin-1B receptor-mediated inhibition of 3H-GABA release from rat ventral tegmental area slices. J. Neurochem 79:914–922
Zangen A, Ikemoto S, Zadina JE, Wise RA (2002) Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci 22:7225–7233
Zito KA, Vickers G, Roberts DC (1985) Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav. 23:1029–1036
Acknowledgements
We would like to thank Mrs. L. Decorte and D. Panzeri for their excellent technical assistance and Dr. T.P. Durkin for correction of the English text and useful discussions. This investigation was supported by the CNRS (UMR 5106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
David, V., Segu, L., Buhot, MC. et al. Rewarding effects elicited by cocaine microinjections into the ventral tegmental area of C57BL/6 mice: involvement of dopamine D1 and serotonin1B receptors. Psychopharmacology 174, 367–375 (2004). https://doi.org/10.1007/s00213-003-1767-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1767-5