Abstract
Rationale
Animal research has demonstrated a role of dopamine D1 and D3 receptors in cocaine reward and seeking.
Purpose and methods
Here, we investigated the potential interaction of these two dopamine receptors in cue-induced reinstatement of cocaine seeking, cocaine conditioned place preference (CPP), and cocaine self-administration in rats.
Results
The co-administration of a D3 receptor antagonist, NGB 2904 and a D1 partial agonist, SKF 77434, of doses which when administered individually produced no significant effects, prior to reinstatement or CPP tests significantly reduced lever pressing and time spent in the cocaine-paired environment, suggesting synergistic effects of the combined compounds on cocaine seeking. When given to rats self-administering cocaine under a progressive ratio schedule of reinforcement doses of NGB 2904 which were ineffective alone significantly enhanced the break point-reducing effects of SKF 77434.
Conclusions
Our results indicate that the combined treatment with a D1 receptor partial agonist and D3 receptor antagonist produces robust decreases in cocaine seeking and reward. This suggests an interaction between dopamine D1 and D3 receptors in cocaine-related behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A hallmark of cocaine addiction is that addicts experience intense craving making them susceptible to relapse, even after prolonged abstinence. Drug and drug cues can induce craving and precipitate relapse (Ehrman et al. 1992; Jaffe et al. 1989) by activating the mesolimbic dopamine (DA) system (Childress et al. 1999). This system’s biggest dopaminergic projections reach the nucleus accumbens (NAcc) (Fallon and Moore 1978), a region populated by medium spiny neurons with a dense expression of DA receptors (Weiner et al. 1991). In the NAcc, D1-like receptors are localized to GABAergic substance P-dynorphinergic medium spiny neurons (Le Moine et al. 1991) and have the ability to activate the second messenger enzyme, adenylyl cyclase, by coupling to G-protein Gs. D2-like receptors are localized to GABAergic enkephalinergic neurons (Le Moine and Bloch 1991) and can inhibit adenylyl cyclase by coupling to G-protein G i. In the striatum, D3 receptors are more concentrated in the shell of the NAcc and Islands of Calleja (Bouthenet et al. 1991; Diaz et al. 1994), where they are primarily found on GABAergic substance P-dynorphinergic neurons.
Interestingly, D1 receptors (D1Rs) and D3 receptors (D3Rs) are often co-localized on the same medium spiny neurons (Le Moine and Bloch 1996; Surmeier et al. 1996). These two receptors can interact at the physical level by forming heteromers that bring the two receptors together (Marcellino et al. 2008) and at the functional level by promoting changes in their ability to bind ligands and modulate intracellular signaling cascades. D3R stimulation potentiates the affinity of D1R agonists to their receptors and D1R-mediated cAMP when expressed on the same neuron (Fiorentini et al. 2008; Marcellino et al. 2008). D1R repeated stimulation can up-regulate both D1Rs and D3Rs, suggesting an interaction between the two receptors (Bordet et al. 1997; Levavi-Sivan et al. 1998). Of particular interest here is whether this interaction is evident in cocaine reward and cue-driven behavior.
A host of pharmacological studies has demonstrated the involvement of D1Rs and D3Rs individually in cocaine-related behaviors. D1R and D3R antagonists can reduce cocaine reward (Awasaki et al. 1997; Galaj et al. 2014a; McGregor and Roberts 1993; Xi et al. 2006) and reinstatement of cocaine seeking (Alleweireldt et al. 2002; Anderson et al. 2003; Capriles et al. 2003; Cervo et al. 2007; Galaj et al. 2014a; Vorel et al. 2002; Xi et al. 2004). In addition, D1Rs or D3Rs play a role in cocaine conditioned place preference (CPP) (Cervo et al. 2005; Hachimine et al. 2014; Nazarian et al. 2004; Sanchez et al. 2003).
Although a vast number of studies have demonstrated independent roles of D1Rs and D3Rs in cocaine-related behaviors, only a few have analyzed D1R-D3R interactions at a behavioral level. Genetically modified mice lacking both D1Rs and D3Rs showed a lack of cocaine CPP and enhanced spontaneous locomotor activity (Karasinska et al. 2005). In another study, the D3R agonist, PD 128907, potentiated D1-receptor mediated locomotor activity, an effect that was counteracted by D3R antagonism (Marcellino et al. 2008). Thus, co-localization of D1Rs and D3Rs appears to be important in regulating striatal behavioral functions.
Although animal research with D1R antagonists and full agonists has been promising, clinical trials with these agents have had limited success (Haney et al. 1999; Nann-Vernotica et al. 2001). This has led to some attention paid to D1R partial agonists as potential pharmacotherapeutic candidates. D1R partial agonists function as D1R antagonists under conditions of high dopamine levels such as in the presence of cocaine or cocaine cues and they can reduce cocaine-related behaviors. D1R partial agonists alter cocaine self-administration (Caine et al. 1999; Katz and Witkin 1992; Mutschler and Bergman 2002; Platt et al. 2001; Spealman et al. 1997). Also, compared to traditional D1R and D2R antagonists (Lublin et al. 1993; Peacock et al. 1999) D1R partial agonists produce milder extrapyramidal side effects (Platt et al. 2000) and, unlike D1R full agonists that are readily self-administered (Self et al. 1996; Weed et al. 1993), D1R partial agonists exhibit lower abuse potential [(Grech et al. 1996; Weed and Woolverton 1995) but see (Self and Stein 1992)]. Thus, because of the interest in D1R partial agonism, it would be interesting to investigate whether the effects of D1R partial agonism is modulated by action at D3Rs.
Given the D1R and D3R co-localization and heteromers in the NAcc and the independent involvements of these receptors in cocaine-related behavior, we were interested in understanding the D1R-D3R interaction in cocaine reward and seeking. In the present study, we investigated whether the combination of the DA D1R partial agonist, SKF 77434, and the D3R antagonist, NGB 2904, would interact on cocaine self-administration under a progressive ratio (PR) schedule of reinforcement, cue-induced reinstatement of cocaine seeking and cocaine CPP. SKF 77434 is a D1R selective ligand with nanomolar K i values of 10.5 and 1000 at D1R and D2R, respectively, and no affinity at other dopamine receptors (Neumeyer et al. 2003). NGB 2904 is a D3R selective compound, with >100-fold selectivity versus other dopamine receptors: K i (nM) values of >10,000 (D1R), 217 (D2R), 1.4 (D3R), >5000 (D4R), and >10,000 (D5R) (Yuan et al. 1998).
Methods
Subjects
Animal housing and care conditions were consistent with those specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011). The protocols used in the present experiments were approved by the Queens College Institutional Animal Care and Use Committee.
Subjects consisted of facility-bred, male Long Evans rats; breeders were obtained from Charles River Laboratories (Kingston, NY, USA). All animals were housed individually and maintained on a reversed 12:12 h light/dark cycle (lights turned off at 1 PM). Rats weighed between 350 and 450 g and had free access to food and water.
Surgery
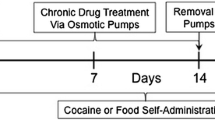
Rats were surgically implanted with an indwelling I.V. catheter under sodium pentobarbital (65 mg/kg, IP) anesthesia, as previously described (Galaj 2014a) and allowed to recover for 3 days before beginning self-administration training.
Apparatus
Self-administration chambers
Operant conditioning chambers were equipped with two retractable levers, a white light above each lever and a drug line, as previously described (Galaj et al. 2014a).
Conditioned place preference chambers
Conditioned place preference (CPP) chambers consisted of two compartments with distinct walls and floors, separated by a removable partition, and equipped with photo-emitters and detectors tracking the position of the rats. [For details see (Galaj et al. 2014b)].
Drugs
Cocaine (a gift from NIDA) was dissolved in 0.9 % physiological saline to achieve a dose of 0.75 mg/kg/injection for the self-administration experiments and a dose of 10 mg/kg for the CPP experiment. The selective D3R antagonist, NGB 2904 (Tocris, MO, USA), was dissolved in 10 % cyclodextrin to achieve the doses of 0.25, 1, 2 and 4 mg/kg, and the selective D1R partial agonist, SKF 77434 (Tocris, MO, USA), was dissolved in saline to achieve the doses of 0.5, 1, 2 and 4 mg/kg.
Procedures
Experiment 1: cue-induced reinstatement of cocaine seeking
Rats were trained to self-administer cocaine under a fixed ratio 1 (FR1) schedule of reinforcement during daily 3-h sessions. Responding on the active lever activated the injection pump for 4.5 s and turned on the light above the active lever for 20 s. During the 20-s interval, additional active lever presses did not activate the pump. Responding on the inactive lever was counted but had no consequences. Thirteen sessions of stable self-administration were required before beginning the extinction phase during which responding on either lever produced no consequences; cocaine was not delivered, and cocaine-related cues (light/pump activation) were not presented. This phase continued until extinction criteria were met; extinction criteria were defined as 9 or fewer lever presses per hour and less than 21 total lever presses in a 3-h session on the active or inactive lever for 3 consecutive sessions. Rats were randomly allocated to experimental conditions and treatments.
The cue-induced reinstatement test (120 min long) occurred 1 day following the last extinction session. Rats were injected with one of the doses of either SKF 77434 (15 min prior to the test session); [vehicle (n = 9), 0.5 (n = 8), 1 (n = 8) or 2 (n = 8) mg/kg], NGB 2904 (30 min prior to the test session); [vehicle, 0.25, 1 or 2 mg/kg (n = 8 in each)] or a combination of NGB 2904 and SKF 77434 [vehicle/vehicle (n = 9), 0.25/0.5 (n = 8), 1/0.5 (n = 8) or 1/1 (n = 8) mg/kg]. Non-contingent cocaine cues (20-s light and 4.5-s syringe pump) were presented twice, 2 min apart, at the beginning of the reinstatement session. Active lever presses were reinforced with the cocaine cues (light/pump) but cocaine was not delivered. Responding on the inactive lever produced no consequences.
To test the possibility that effects of NGB 2904/SKF 77434 on cue-induced reinstatement were due to motoric deficits, we evaluated the effects of NGB 2904/SKF 77434 (1/1 mg/kg) on lever pressing reinforced by food pellets, a procedure that produces many times more lever pressing than the reinstatement procedure. Seven rats were trained to lever press for food under a PR schedule of reinforcement, as described in Galaj et al. 2014a. After stable responding they were treated with NGB 2904/SKF 77434 (1/1 mg/kg) and the number of lever presses during 120 min, the same period of time as in the reinstatement test, was recorded.
Experiment 2: cocaine conditioned place preference
In session 1—the pre-exposure session—rats were placed in the CPP chamber with free access to both compartments for 15 min and time spent in each compartment was measured. Half of the rats were conditioned with cocaine to their preferred compartment (the compartment in which a rat spent most of its time during the pre-exposure session) and the remaining half to the non-preferred compartment. Prior to sessions 2, 4, 6 and 8, rats were injected with cocaine (IP) and immediately placed in one of the two compartments. Prior to sessions 3, 5, 7 and 9, rats received saline injections (IP) and were placed in the other compartment. Conditioning sessions were 30 min long. The order of conditioning was counterbalanced among rats. During session 10, rats were tested for their CPP. Prior to the CPP test, rats were injected with a dose of either SKF 77434 (15 min prior to the test [vehicle, 0.5, 1 or 2 mg/kg (n = 8 in each)], NGB 2904 (30 min prior to the test [vehicle, 0.25, 1 or 2 mg/kg (n = 8 in each)], or the combination NGB 2904/SKF 77434 [vehicle/vehicle, 0.25/0.5 (n = 8), 1/1 (n = 8) or 2/2 (n = 7) mg/kg]. Assignment to dose groups and treatments was random. The dividing partition was removed and rats were placed in the CPP chamber having free access to both compartments for 15 min. Time spent in each compartment was recorded.
Experiment 3: cocaine self-administration under a PR schedule of reinforcement (cocaine reward)
Rats were trained to self-administer cocaine initially under a FR1 schedule of reinforcement during daily 3-h sessions (as described in experiment 1). After rats demonstrated a steady rate of self-administration for three consecutive sessions, a PR schedule of reinforcement was implemented during which successive cocaine infusions required progressively more responses (i.e., 1, 2, 4, 6, 9, 12, 15, 20, 25…) until a break point (BP) was reached. The BP was operationally defined as the total number of infusions earned prior to a 1-h period without an infusion. Once rats demonstrated a baseline BP (three consecutive BPs that did not differ by more than two ratio steps and without descending or ascending trends), they were tested with a dose of either SKF 77434 [vehicle, 1, 2 or 4 mg/kg (n = 9 in each)], NGB 2904 [vehicle, 1, 2 or 4 mg/kg (n = 7 in each)] or a combination of NGB 2904/SKF 77434 [vehicle/vehicle, 1/1, 2/2 or 4/4 mg/kg (n = 9)]. Each rat assigned to a compound group was tested with all doses of the compound in random order; each test occurring after baseline BP was re-established.
Data analysis
For each compound/combination, the average number of active and inactive lever presses during the first 120 min of the last three extinction sessions and the number of active and inactive lever presses during the 120-min reinstatement test were compared using a separate three-way analysis of variance (ANOVA) [phase (extinction/reinstatement) and lever as repeated measures factors and dose as a between-groups factor]. A significant three-way interaction was followed by dose x phase interaction comparisons at each level of lever and by tests of simple effects of dose at each level of phase. Tests of simple effects of lever at each level of phase were also used to determine reinstatement effects on active lever responding. In the experiment addressing potential motoric deficits, rats treated with the 1/1 mg/kg dose of NGB 2904/SKF 77434 prior to lever pressing for food were compared on lever presses with the vehicle-treated group in the reinstatement experiment using a between-groups t test.
In experiment 2, three separate two-way ANOVAs with dose as a between-groups factor and phase (pre-exposure vs preference test) as a repeated measures factor were used to analyze the time spent in the cocaine compartment during the pre-exposure and test sessions for each dose group in each compound condition (SKF 77434, NGB 2904, and NGB 2904/SKF 77434). A significant interaction was followed by tests of simple effects of phase at each level of dose.
In experiment 3, for each compound condition BPs reached during the test session were compared to the baseline BPs for all doses using a two-way ANOVA with dose as a between-groups factor and phase as a repeated measures factor. Significant interactions were followed by tests of simple effects of phase at each dose.
Results
Experiment 1: cue-induced reinstatement of cocaine seeking
Total number of cocaine infusions during the self-administration phase in rats grouped according to the eventual treatment and doses they would receive ranged from 555.78 (± 26.44) to 648.75 (± 44.23). A two-way ANOVA with eventual treatment and eventual treatment dose as between-groups factors revealed no significant interaction or treatment or dose main effects. During extinction, responding on the active lever was similar among all dose/compound groups with slightly higher pressing on the active than inactive levers in all dose/compound groups (Fig. 1a, b and c, left panels). In all dose/compound groups, responding increased in the reinstatement test compared to extinction sessions and the increases on the active lever were greater than on the inactive lever. Rats treated with 0.25, 1 or 2 mg/kg of NGB 2904 showed a slight, but not significant, reduction in responding on the active lever as compared to NGB 2904 vehicle group (Fig. 1a, right panel). Responding on the inactive lever during the reinstatement test was similar in all NGB 2904 dose groups. Statistical analysis with a three-way ANOVA revealed a significant phase x lever interaction, regardless of the dose group [F(1,28) = 39.72, p < .001]. Simple effects of lever at each level of phase revealed a significant lever effect only during the reinstatement test [F(1,28) = 88.7, p < .001].
Mean (± SEM) number of presses on the active and inactive levers averaged across the last three extinction sessions (left panels; in rats grouped by the compound (SKF 77434, NGB 2904 or a combination of the two and the dose that they would eventually be treated with during the cue-induced reinstatement test) and during the cue-induced reinstatement test (right panels) for all dose and combination groups. Animals treated with a NGB 2904, b SKF 77434 or c combined NGB 2904/SKF 77434 prior to the start of the reinstatement test. Asterisk represents active lever pressing significantly different from vehicle at p < .05
Similarly, during the reinstatement test rats treated with SKF 77434 showed a slight, but not significant, reduction in active lever presses as compared to the vehicle group (Fig. 1b, right panel). Responding on the inactive lever during the reinstatement test was similar in all SKF 77434 dose groups. A three-way ANOVA revealed a significant phase x lever interaction, regardless of the dose group [F(1,29) = 86.39, p < .001]. Simple effects of lever at each phase revealed a significant lever effect during the reinstatement test [F(1,29) = 180.6, p < .001] but not extinction.
Rats treated with the combination of NGB 2904 and SKF 77434 showed much greater, dose-related reductions in active lever pressing than those treated with either compound alone (Fig. 1c, right panel). A three-way ANOVA revealed a significant phase x lever x dose interaction [F(3,29) = 5.34, p < .01]. Phase x dose interaction comparisons at each level of lever revealed a significant phase x dose interaction for the active lever [F(3,29) = 186.89, p < .001] but not for the inactive lever. Tests of simple effect of dose on active lever at each phase revealed a significant dose effect during the reinstatement test [F(3,29) = 44.17, p < .001]. Dunnett’s tests confirmed that the combinations of 1/0.5 and 1/1 mg/kg of NGB 2904/SKF 77434 were significantly different from the NGB 2904/SKF 77434 vehicle group in active lever pressing (ps < .05).
To address the possibility of motoric incapacitation due to the combined treatment, we tested this treatment in rats responding for food under a PR schedule of reinforcement. Rats treated with the 1/1 mg/kg of NGB 2904/SKF 77434 emitted on average 184.57 (± 44.73) lever presses, demonstrating that they could make at least as many, and in fact many times more, lever presses than the vehicle-treated group (63.44 ± 10.54) in the reinstatement experiment during a similar 120-min period (data not shown). A between-groups t test showed a significant difference in lever pressing between the groups [t(14) = −2.96, p < .01].
Experiment 2: cocaine conditioned place preference
During the pre-exposure session, all groups that would later be treated with one of the doses of SKF 77434, NGB 2904 or the combination of the two spent similar amounts of time in the CPP compartment that would later be paired with cocaine. Rats treated with NGB 2904 prior to the test session spent more time in the cocaine-paired compartment during the test session than they did during the pre-exposure session, regardless of the dose they were treated with (Fig. 2, left panel). A similar pattern was observed in rats treated with SKF 77434 such that rats treated with one of the SKF 77434 doses prior to the test session spent more time in the cocaine-paired compartment than they did during the pre-exposure session (Fig. 2, middle panel). Two separate two-way ANOVAs revealed a significant phase effect for NGB 2904 [F(1,28) = 85.03, p < .001] and for SKF 77434 [F(1,28) = 63.58, p < .05], but no dose x phase interactions for either.
Mean (± SEM) time spent in the cocaine-paired compartment of the CPP apparatus during pre-exposure and preference tests for all dose and combination dose groups. Prior to the CPP test animals were treated with NGB 2904 (left panel), SKF 77434 (middle panel) or NGB 2904/SKF 77434 (right panel). Asterisk represents the absence of cocaine CPP
For the combined treatments groups, rats treated with 0/0 or 0.25/0.5 mg/kg of NGB 2904/SKF 77434 spent more time in the cocaine-paired compartment during the test than they did during the pre-exposure sessions but rats treated with 1/1 or 2/2 mg/kg of NGB 2904/SKF 77434 spent as much time in the cocaine side during the test as they did during pre-exposure (Fig. 2, right panel). A two-way ANOVA revealed a significant dose x phase interaction [F(3,29) = 4.02, p < .05] and tests of simple effects of phase at each level of dose revealed phase effects only for the vehicle/vehicle and 0.25/ 0.5 mg NGB 2904/SKF 77434 groups [F(1,29) = 18.92, p < .01 and F(1,29) = 6.81, p < .05, respectively].
Experiment 3: cocaine self-administration under a PR schedule (cocaine reward)
During baseline testing, all dose/compound groups reached similar BPs. Rats treated with NGB 2904 showed no change in the BP, regardless of the dose they were treated with (Fig. 3, left panel). Rats treated with vehicle or 1 mg/kg of SKF 77434 reached similar BPs during the test and baseline sessions whereas rats treated with 2 or 4 mg/kg of SKF 77434 showed a reduction in BP compared to baseline (Fig. 3, middle panel). Two separate two-way ANOVAs revealed no significant dose x phase interaction or main effects for NGB 2904 but a significant dose x phase interaction for SKF 77434 [F(3,24) 5.30, p < .05]. Tests of simple effects of phase at each SKF 77434 dose revealed a significant phase effect for the 2 and 4 mg/kg doses [F(1,24) = 5.45 and F(1,24) = 32.04, respectively, ps < .05]. Similarly, rats treated with NGB 2904/SKF 77434 vehicle reached similar BPs during the test session as they did during the baseline whereas rats treated with 1/1, 2/2 or 4/4 mg/kg of NGB 2904/SKF 77434 showed reductions in BPs on the test day. A two-way ANOVA revealed a significant NGB 2904/SKF 77434 dose x phase interaction [F(3,24) = 3.32, p < .05]. Tests of simple effect of phase at each NGB 2904/SKF 77434 dose revealed a significant phase effect for 1/1, 2/2 and 4/4 mg/kg doses [F(1,24) = 21.67, F(1,24) = 7.97, F(1,24) = 14.15, respectively, ps < .05].
Mean (± SEM) BPs reached during the baseline and test sessions for all dose and combination dose groups. Prior to the start of the test session animals were treated with NGB 2904 (left panel), SKF 77434 (middle panel) or NGB 2904/SKF 77434 (right panel). Asterisk represents a significant reduction in BPs in the test session compared to baseline (ps < .05)
Interestingly, rats treated with 1/1 mg/kg of NGB 2904/SKF 77434 emitted on average 578.22 (± 177.65) lever presses within the first 2 h of the PR test session, demonstrating that they could make at least as many, and in fact many times more, lever presses than the vehicle-treated group (63.44 ± 10.54) in the reinstatement experiment during a similar 120-min period (data not shown).
Discussion
In the present study, we evaluated the effects of individual and simultaneous D1R partial agonism and D3R antagonism on cocaine self-administration and cue-driven behaviors. While NGB 2904 or SKF 77434 alone produced no significant changes in cue-induced reinstatement the simultaneous administration of the compounds caused a dose-dependent reduction in active lever pressing. The combined agents did not significantly affect responding on the inactive lever. Furthermore, rats treated with the NGB 2904/SKF 77434 combination and responding for food or cocaine under a PR schedule of reinforcement were able to emit at least as many, and in fact emitted significantly more, lever presses than the vehicle-treated group (and also much more than in the 1/1 mg/kg NGB 2904/SKF 77434 combination group) in the reinstatement experiment during a similar 120-min period. This demonstrates that the animals under this treatment (NGB 2904/SKF 77434 combination) in the reinstatement experiment were motorically capable of emitting many more responses than they did, and actually more than their corresponding vehicle group. Thus, the effect of combined NGB 2904/SKF 77434 treatment on cue-induced reinstatement is best understood as a reduction in cocaine seeking. In experiment 2, NGB 2904 or SKF 77434 administered individually failed to significantly affect cocaine CPP. However, when the ineffective doses of the compounds were administered simultaneously they abolished cocaine CPP. Again, these results suggest that the combined D1R partial agonist and D3R antagonist treatment reduced the incentive motivational effects of cocaine cues.
Interestingly, in both the reinstatement and CPP experiments, the combination of the two compounds appeared to produce an interactive, synergistic effect. For instance, when the compounds were administered alone the 1 mg/kg doses failed to produce effects (for both reinstatement and CPP) and the next highest dose—the 2 mg/kg dose—also failed to produce effects. If the effect of combining these 1 mg/kg doses was additive, one might expect to observe effects similar to the next higher doses of each compound alone. Instead, the combination of these doses (the 1/1 mg/kg combination) greatly surpassed effects observed at the next higher dose levels and produced highly significant effects. This finding suggests that the effects of the combined agents are synergistic rather than additive. This is a unique finding given that studies relevant to cocaine-related behaviors have focused primarily on the roles of D1Rs or D3Rs individually.
In the self-administration study, the co-administration of NGB 2904 and SKF 77434 caused a significant reduction in PR responding for cocaine, something that was also observed with SKF 77434 treatment but not with NGB 2904 alone. All of the combined doses reduced BPs whereas in the case of SKF 77434 alone only the two highest doses produced significant effects. Interestingly, the lowest dose of SKF 77434 alone, which was ineffective, reduced BP by 25 % when it was combined with the lowest, and ineffective, dose of NGB 2904. This is noteworthy because it suggests that a minimal D3R antagonism can potentiate D1R partial agonism.
D3R antagonists have been shown to have limited effects on cocaine self-administration (Gal and Gyertyan 2003; Martelle et al. 2007; Xi et al. 2005) but efficiently reduce cocaine seeking (Cervo et al. 2007; Galaj et al. 2014a; Gilbert et al. 2005; Vorel et al. 2002). D3Rs are primarily involved in behaviors driven by cues (Cervo et al. 2005; Galaj et al. 2014a; Gilbert et al. 2005; Hachimine et al. 2014) whereas D1Rs play a role in cocaine reward and conditioned reward (cues) (Alleweireldt et al. 2002; Awasaki et al. 1997; Galaj et al. 2014b; Nazarian et al. 2004). Here, we report that simultaneous D1R partial agonism and D3R antagonism produces synergistic effects on reduction of cue-induced reinstatement of cocaine seeking and CPP.
It is not clear whether the individual compounds of the combined treatment begin their effects through separate mechanisms producing cascades of events that converge at a later point or whether they initiate their effects through a common starting point. Perhaps, such robust effects are related to the D1R-D3R heteromers that promote changes in the ability of ligands to bind to the specific receptors and modulate intracellular signaling cascades (Fiorentini et al. 2008; Marcellino et al. 2008). D3R stimulation also results in the potentiation of D1R-mediated locomotor activity, an effect that was counteracted by a D3R antagonist (Marcellino et al. 2008). Deletion of D3Rs increases sensitivity to the administration of D1R and D2R agonists in mice (Xu et al. 1997) whereas deletion of both D1Rs and D3Rs impairs CPP with low doses of cocaine and alters locomotor activity (Karasinska et al. 2005). It is not clear how these and other D1R- and D3R-mediated cascades, some of which appear complimentary and some oppositional, might interact, if at all, to produce the effects observed here. But clearly, it is now necessary to delineate the neurophysiological and neurochemical mechanisms whereby the two receptors do interact to produce the synergistic and potentiating effects on cocaine seeking and reward observed in the present study.
Although it is likely that cumulative receptor interactions contribute to the overall effects of the drug combination, pharmacokinetic interactions of the drugs may also play a role and cannot be ruled out at this time. For example, the in vivo metabolism of one drug may be impaired or modified in the presence of the other, leading to a greater or more prolonged effect of the drug. Further studies will be needed to clarify the extent to which such possible drug-drug interactions between NGB 2904 and SKF 77434 influence the observed effects.
In conclusion, in the present study, we demonstrated that the simultaneous administration of a D1R partial agonist and D3R antagonist reduces cue-induced reinstatement, cocaine CPP, and PR responding to a significantly greater degree than the individual agents alone. Our results support the notion of complex functional interactions between D1Rs and D3Rs which begs further investigation. Finally, these results may be useful in the development of novel treatments for cocaine addiction.
References
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL (2002) Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 159:284–293
Anderson SM, Bari AA, Pierce RC (2003) Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology 168:132–138
Awasaki Y, Nishida N, Sasaki S, Sato S (1997) Dopamine D(1) antagonist SCH23390 attenuates self-administration of both cocaine and fentanyl in rats. Environ Toxicol Pharmacol 3:115–122
Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC (1997) Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A 94:3363–3367
Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Caine SB, Negus SS, Mello NK, Bergman J (1999) Effects of dopamine D1-like and D2-like agonists in rats that self-administer cocaine. J Pharmacol Exp Ther 291:353–360
Capriles N, Rodaros D, Sorge RE, Stewart J (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168:66–74
Cervo L, Burbassi S, Colovic M, Caccia S (2005) Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav 82:727–734
Cervo L, Cocco A, Petrella C, Heidbreder CA (2007) Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychopharmacol 10:167–181
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18
Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P (1994) Phenotypical characterization of neurons expressing the dopamine D 3 receptor in the rat brain. Neuroscience 65:731–745
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107:523–529
Fallon JH, Moore RY (1978) Catecholamine innervation of the basal forebrain. IV Topography of the dopamine projection to the basal forebrain and neostriatum Journal of Comparative Neurology 180: 545–580.
Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C (2008) Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol 74:59–69
Gal K, Gyertyan I (2003) Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull 61:595–601
Galaj E, Ananthan S, Saliba M, Ranaldi R (2014a) The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology 231:501–510
Galaj E, Manuszak M, Arastehmanesh D, Ranaldi R (2014b) Microinjections of a dopamine D1 receptor antagonist into the ventral tegmental area block the expression of cocaine conditioned place preference in rats. Behav Brain Res 272:279–285
Gilbert JG, Newman AH, Gardner EL, Ashby CR Jr, Heidbreder CA, Pak AC, Peng X-Q, Xi Z-X (2005) Acute administration of SB-277011 A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse 57:17–28
Grech DM, Spealman RD, Bergman J (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology 125:97–104
Hachimine P, Seepersad N, Ananthan S, Ranaldi R (2014) The novel dopamine D3 receptor antagonist, SR 21502, reduces cocaine conditioned place preference in rats. Neurosci Lett 569:137–141
Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW (1999) Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology 143:102–110
Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989) Cocaine-induced cocaine craving. Psychopharmacology 97:59–64
Karasinska JM, George SR, Cheng R, O'Dowd BF (2005) Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci 22:1741–1750
Katz JL, Witkin JM (1992) Selective effects of the D 1 dopamine receptor agonist, SKF 38393, on behavior maintained by cocaine injection in squirrel monkeys. Psychopharmacology 109:241–244
Le Moine C, Bloch B (1991) Rat striatal and mesencephalic neurons contain the long isoform of the D2 dopamine receptor mRNA. Brain Res Mol Brain Res 10:283–289
Le Moine C, Bloch B (1996) Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience 73:131–143
Le Moine C, Normand E, Bloch B (1991) Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A 88:4205–4209
Levavi-Sivan B, Park BH, Fuchs S, Fishburn CS (1998) Human D3 dopamine receptor in the medulloblastoma TE671 cell line: cross-talk between D1 and D3 receptors. FEBS Lett 439:138–142
Lublin H, Gerlach J, Peacock L (1993) Chronic treatment with the D1 receptor antagonist, SCH 23390, and the D2 receptor antagonist, raclopride, in cebus monkeys withdrawn from previous haloperidol treatment. Extrapyramidal syndromes and dopaminergic supersensitivity Psychopharmacology (Berl) 112:389–397
Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R (2008) Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem 283:26016–26025
Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA (2007) Effects of two novel D3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 321:573–582
McGregor A, Roberts DCS (1993) Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self- administration under fixed and progressive ratio schedules of reinforcement. Brain Res 624:245–252
Mutschler NH, Bergman J (2002) Effects of chronic administration of the D1 receptor partial agonist SKF 77434 on cocaine self-administration in rhesus monkeys. Psychopharmacology 160:362–370
Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL (2001) Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology 155:338–347
Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V (2004) The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull 63:295–299
Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ (2003) Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol 474:137–140
Peacock L, Jensen G, Nicholson KL, Gerlach J (1999) Extrapyramidal side effects during chronic combined dopamine D1 and D2 antagonist treatment in Cebus apella monkeys. Eur Arch Psychiatry Clin Neurosci 249:221–226
Platt DM, Rowlett JK, Spealman RD (2000) Dissociation of cocaine-antagonist properties and motoric effects of the D1 receptor partial agonists SKF 83959 and SKF 77434. J Pharmacol Exp Ther 293:1017–1026
Platt DM, Rowlett JK, Spealman RD (2001) Modulation of cocaine and food self-administration by low- and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology 157:208–216
Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA (2003) Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience 119:497–505
Self DW, Stein L (1992) The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 582:349–352
Self DW, Belluzzi JD, Kossuth S, Stein L (1996) Self-administration of the d 1 agonist skf 82958 is mediated by d 1, not d 2, receptors. Psychopharmacology 123:303–306
Spealman RD, Bergman J, Rosenzweig-Lipson S (1997) Differential modulation of behavioral effects of cocaine by low- and high-efficacy D1 agonists. Psychopharmacology 133:283–292
Surmeier DJ, Song WJ, Yan Z (1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16:6579–6591
Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22:9595–9603
Weed MR, Woolverton WL (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374
Weed MR, Vanover KE, Woolverton WL (1993) Reinforcing effect of the D1 dopamine agonist SKF 81297 in rhesus monkeys. Psychopharmacology 113:51–52
Weiner DM, Levey AI, Sunahara RK, Niznik HB, O'Dowd BF, Seeman P, Brann MR (1991) D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A 88:1859–1863
Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR Jr, Hagan JJ, Heidbreder CA, Gardner EL (2004) Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology 176:57–65
Xi Z-X, Gilbert JG, Pak AC, Ashby CR Jr, Heidbreder CA, Gardner EL (2005) Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 21:3427–3438
Xi Z-X, Newman AH, Gilbert JG, Pak A-C, Peng X-Q, Ashby CR Jr, Gitajn L, Gardner EL (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 31:1393–13405
Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S (1997) Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19:837–848
Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, Thurkauf A (1998) NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett 8:2715–2718
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galaj, E., Harding, W. & Ranaldi, R. Dopamine D1 and D3 receptor interactions in cocaine reward and seeking in rats. Psychopharmacology 233, 3881–3890 (2016). https://doi.org/10.1007/s00213-016-4420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4420-9