Abstract
Rationale
D1-Like agonists are self-administered by drug-naive animals, whereas D2-like agonists reinstate cocaine-seeking behavior, but the rewarding and reinstating effects of D1- and D2-like agonists in pavlovian-based conditioned place preference are equivocal.
Objective
To compare the ability of D1 and D2 agonists to produce conditioned place preference with their modulation of expression and reinstatement of an established cocaine place preference.
Methods
Using an unbiased procedure, we measured the place preference induced by the D1 receptor agonist SKF 81297 and the D2/D3 receptor agonist quinpirole in drug-naive or cocaine-exposed rats. The rewarding effects of the D1 agonists SKF 82958, ABT-431, A-77636, and the D2/D3 receptor agonist 7-OH-DPAT were also tested. Additionally, we tested the ability of SKF 81297 and quinpirole to modulate expression and reinstatement of an established cocaine place preference.
Results
The D1 receptor agonists SKF 81297, SKF 82958, and ABT-431 produced dose-dependent conditioned place preferences, whereas A-77636 produced only place aversion, and the D2/D3 agonists quinpirole and 7-OH-DPAT were without effect in drug naive rats. In cocaine-treated rats, SKF-81297-induced place preference was reduced, whereas quinpirole-induced place preference was revealed. Pretreatment using either D1 or D2/D3 agonists blocked expression of an established cocaine place preference, but only the D1 agonist SKF 81297 and cocaine dose-dependently reinstated an extinguished cocaine place preference, whereas the D2/D3 agonist quinpirole induced place aversion but failed to alter cocaine-induced reinstatement.
Conclusions
D1, but not D2/D3, agonists mediate rewarding effects and reinstatement of cocaine place preference, but the reinstating effects differ markedly from self-administration paradigms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine plays a major role in the primary rewarding effects of cocaine, but is also implicated in the motivational effects of environmental stimuli associated with primary rewards through pavlovian conditioning (Schultz 1998; Phillips et al. 2003; Wise 2004). Thus, certain environmental stimuli, through repeated and specific association with drug exposure, acquire incentive motivational properties and elicit approach behavior or act as secondary rewards in the absence of drug. The conditioned place preference procedure exemplifies this process in two distinct phases. During the induction phase, the primary rewarding effects of drugs are associated with a specific environment, as indicated by the subsequent expression of the place preference in the absence of drug exposure. Numerous studies have found that dopamine plays a role in both the induction (drug effects) and expression (environmental effects) phases of cocaine-conditioned place preference (for review, see Tzschentke 1998).
Dopamine mediates its effects through two subfamilies of dopamine receptors, the D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors, which are distinguishable by their pharmacology, signal transduction, and anatomical localization (hereafter referred to as D1 and D2 receptors). Previous studies found that both D1 and D2 dopamine receptor agonists are capable of producing a conditioned place preference, although results have been equivocal across laboratories. The D1 receptor agonist SKF 82958 was shown to produce a place preference at a low dose below threshold for psychomotor activation, and the more selective D1 agonist SKF 81297 was without effect (Abrahams et al. 1998). In contrast, two studies found that the D2 receptor agonists bromocriptine and quinpirole (D2/D3) can produce a place preference, whereas several other studies found that quinpirole and 7-OH-DPAT (D2/D3) fail to produce a place preference and, in some cases, produce a place aversion (Hoffman et al. 1988; Khroyan et al. 1995; Kling-Petersen et al. 1995; Rodriguez de Fonseca et al. 1995). Reasons for these discrepancies are unclear, but they may involve different rat strains, conditioning protocols, or other methodological differences.
Like most conditioned behaviors, cocaine-conditioned place preference can be extinguished by repeated exposure to the environmental context in the absence of drug reward. Following extinction, a priming injection of cocaine will reinstate the cocaine place preference (Mueller and Stewart 2000; Itzhak and Martin 2002; Kreibich and Blendy 2004), similar to reinstatement of cocaine-seeking behavior in self-administration studies. Because systemic administration of D1 agonists inhibits, and D2 agonists induces, reinstatement of cocaine seeking in self-administration studies (Self et al. 1996a,b; De Vries et al. 1999; Alleweireldt et al. 2002; Dias et al. 2004), it would be interesting to determine whether a similar dichotomy exists for reinstatement of a cocaine-conditioned place preference. Using the conditioned place preference procedure, we compared the rewarding properties of several D1 and D2/D3 receptor agonists in either cocaine-treated or drug-naive rats. We also investigated the ability of the D1 and D2/D3 agonists to modulate expression of a previously established cocaine-conditioned place preference and to reinstate a cocaine place preference following extinction of the preference.
Materials and methods
Animals and housing conditions
Male Sprague–Dawley rats (Charles River Labs, Kingston, NY, USA) initially weighing 275–300 g were housed in pairs in a climate-controlled environment (21–25°C) on a 12-h light–dark cycle (lights on at 0700 h) in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003). Animals were allowed 5–7 days acclimation and were subsequently tested during their light cycle.
Place conditioning apparatus
Place conditioning was conducted in three-compartment test chambers measuring 68 × 21 cm and 21 cm high (Med Associates, Georgia, VT). One side of the apparatus was black with a stainless steel rod floor, whereas the other side was white with a stainless steel mesh floor. The central compartment was 12 cm long with neutral gray walls and a smooth polyvinyl chloride (PVC) floor. Each side was equipped with three infrared photobeam detectors to measure entry and horizontal locomotion.
Place conditioning procedure
Experiment 1
The rewarding effects of the prototypical D1 and D2 agonists SKF 81297 and quinpirole were compared in drug-naive and cocaine-treated animals. Animals were handled and injected with saline (i.p.) or cocaine (20 mg/kg) once daily for 5 days in their home cages. This cocaine treatment regimen induces approximately 100% locomotor sensitization when challenged with a lower dose (10 mg/kg) after treatment (R.K. Bachtell and D.W. Self, unpublished observations). Drug- and saline-paired sides were assigned in a counterbalanced order 2 days after the last treatment, and animals were placed in the center chamber and allowed to explore all compartments for 30 min to determine pretest place preference scores (time in drug minus saline-paired sides). Agonist-induced place conditioning was conducted on days 3–6 posttreatment in a 2/2 conditioning procedure using subcutaneous injections of saline alternating with a single dose of SKF 81297 (saline, 1.0, 3.0, and 10.0 mg/kg) or quinpirole (saline, 0.3, 1.0, 3.0, and 10.0 mg/kg) immediately before confinement in one compartment for 30 min. The rewarding effects of another benzazepine-based D1 agonist, SKF 82958 (saline, 0.1, 0.3, 1.0, and 3.0 mg/kg), the nonbenzazepine D1 agonists ABT-431 and A-77636 (saline, 0.3, 1.0, and 3.0 mg/kg), and the D2/D3 receptor agonist 7-OH-DPAT (saline, 1.0, 3.0, and 10.0 mg/kg) were also assessed in drug-naive (saline-habituated) animals as described above.
Expression of place preference was assessed 24 h following the last conditioning session when animals were allowed free access to all compartments for a 30-min posttest session. Saline-conditioned controls received saline pairings on either side, with one side assigned as “drug-paired” in a counterbalanced manner. Each study group was composed of animals received and tested on at least three separate occasions to control for potential batch variation or other unknown procedural and environmental influences. The unbiased nature of the conditioning procedure is based on (1) drug-paired sides (counterbalanced for white and black sides) being chosen without reference to individual pretest preference scores, (2) a lack of pre- or posttest bias for white or black sides in saline/saline-conditioned animals, and (3) persistent neutral preference scores in saline/saline-conditioned animals despite habituation to novel and potential anxiogenic aspects of the test chambers (Table 1).
Experiment 2
The effects of pretreatment with the D1 agonist SKF 81297 and the D2/D3 agonist quinpirole on the expression of an established cocaine place preference were examined in animals conditioned with cocaine (15 mg/kg, i.p.) and saline in an extended 4/4 conditioning regimen to strengthen learned associations using an optimal dose for inducing cocaine place preference in the apparatus (D.W.S., unpublished observations). Separate groups of animals received 30-min pretreatments with saline, SKF 81297 (3.0 mg/kg, s.c.), or quinpirole (1.0 mg/kg, s.c.) 1 day after conditioning based on the peak dose for inducing place preference in experiment 1 (SKF 81297) or for reinstating cocaine-seeking behavior in self-administering animals for quinpirole (Edwards et al. 2006). In addition to preference scores, total horizontal locomotor activity (photobeam counts per minute) and locomotor activity in the cocaine-paired side were recorded during the posttests.
Procedure for reinstatement of place conditioning
Experiments 3 and 4
The ability of the D1 and D2 agonists SKF 81297 and quinpirole to reinstate an extinguished cocaine place preference was compared with cocaine in animals that displayed a positive preference score (56/61 rats) when conditioned with cocaine as described in experiment 2. Following the initial postconditioning test session, cocaine place preferences were extinguished in daily 30-min sessions when animals were allowed free access to all compartments of the apparatus. Animals received saline injections (i.p.) immediately before each extinction session, and preference scores for the first 15 min of the test session were used as an extinction baseline because animals typically rested in only one side for the remaining 15–30 min of the session precluding extinction criteria. Extinction training continued for a minimum of ten sessions and until animals achieved stable extinction criteria, with preference scores averaging less than ±120 s for the drug-paired side for 3 consecutive days. Cocaine-induced reinstatement was tested by injecting extinguished rats with cocaine (2.5, 5.0, and 10.0 mg/kg, i.p.) immediately before placement in the conditioning apparatus. Both preference scores and locomotor activity (total and drug-paired side) were recorded during the 15-min extinction and reinstatement test sessions.
Reinstatement induced by the D1 agonist SKF 81297 (0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg) or D2/D3 agonist quinpirole (0.1, 0.3, 1.0, and 3.0 mg/kg) was tested by giving a subcutaneous injection of agonist 30 min before placement in the apparatus (to allow for slower onset of action). In experiment 4, the ability of the D2/D3 agonist quinpirole to modulate cocaine-induced reinstatement was measured by administering quinpirole (saline, 0.1, 0.3, 1.0, and 3.0 mg/kg, s.c.) 30 min before a priming injection of cocaine (10.0 mg/kg, i.p.). Each reinstatement test was preceded by at least two extinction baseline (saline) sessions where animals met extinction criteria; these baselines served as within-subjects controls for each reinstatement test. Animals were tested serially with two of the four drug treatment regimens (cocaine, SKF 81297, quinpirole, and quinpirole/cocaine) in counterbalanced order for a maximum of seven to nine reinstatement tests. Reinstatement effects were similar whether animals received priming in early or later in the sequence. Each dose within a drug treatment was administered sequentially in counterbalanced order in a within-subjects design. Not all animals received the lowest dose of SKF 81297 that was added to determine a dose threshold, and these data were analyzed as between-subjects data.
Data analysis
Pre- and posttest preference scores for experiments 1 and 2 were analyzed by two-factor analysis of variance (ANOVA) (test session × dose/drug) with repeated measures on test session. Significant interactions were followed by one-factor ANOVA on dose followed by post hoc comparison to saline/saline-conditioned animals by Dunnett’s tests. Posttest preference scores from saline- and cocaine-treated rats were compared by two-factor ANOVA (pretreatment × dose) for SKF 81297 (1.0–10.0 mg/kg) and quinpirole (0.3–10.0 mg/kg) conditioning. In experiments 3 and 4, preference scores and locomotor activity (total and drug-paired side) during reinstatement tests were compared with extinction baselines by two-factor ANOVA (test session × dose) with repeated measures on both factors. Within-subjects comparisons (post- vs pretest; reinstatement vs extinction) utilized paired t tests corrected for multiple comparisons. Locomotor activity data from experiment 2 were analyzed by one-factor ANOVA (drug) followed by post hoc comparison with saline pretreatment by Dunnett’s tests.
Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA). SKF 81297 [(+/−)-6-chloro-7, 8-dihydroxy-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-benzazepine hydrobromide], SKF 82958 (6-chloro-7, 8-dihydroxy-3-allyl-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-benzazepine hydrobromide), A-77636 [(−)-(1R, 3s)-3-adamantyl-1-(aminomethyl)-3, 4-dihydro-5, 6-dihydroxy-1H-2-benzapyran hydrochloride], 7-OH-DPAT [(−)-7-hydroxy-N, N-di-n-propyl-2-aminotetralin], and quinpirole [4aR-trans-4, 4a, 5, 6, 7, 8, 8a, 9-octahydro-5-propyl-1H-pyrazolo(3, 4-g) quinoline hydrochloride] were obtained from Sigma (St. Louis, MO, USA). ABT-431 [(−)trans 9, 10-acetoxy-2-propyl-4, 5, 5a, 6, 7, 11-b-hexahydro-3-thia-5-azacyclopent-1-ena(c)phenanthrene hydrochloride] was obtained through a generous gift from Abbott Laboratories (Abbott Park, IL, USA). All drugs were injected in a 1 ml/kg volume of sterile-filtered 0.9% saline.
Results
Experiment 1: D1 and D2/D3 agonist-induced place conditioning in saline- and cocaine-treated rats
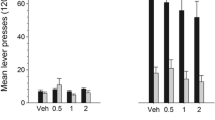
In drug-naive (saline-treated) animals, SKF 81297 produced a prominent conditioned place preference for the drug-paired side during the posttest compared with their pretest preference scores (Fig. 1a), resulting in a significant test session × dose interaction (F 3,39=4.727, P = 0.007). Subsequent analyses of posttest scores found that SKF 81297 (3.0 mg/kg) produced a place preference compared with either saline/saline-conditioned controls (F 3,39=3.473, P = 0.025) or to pretest scores corrected for multiple comparisons (T 9=−6.729, P<0.01). Similarly, Fig. 1c shows that animals treated with five daily cocaine injections (20 mg/kg) before conditioning also showed dose-dependent place conditioning with SKF 81297 (test session × dose interaction, F 5,61=2.840, P = 0.023), and a significant preference at the same 3.0-mg/kg dose compared with pretest scores (T 14=−3.237, P<0.05). However, SKF 81297 place preference scores were shifted downward in cocaine-treated compared with saline-treated rats (main effect of treatment on 1.0- to 10.0-mg/kg doses; F 1,22=8.436, P = 0.008), an effect reflected by a reduction in preference scores from 12±2.9 to 5.5±2.2 min at the peak dose of 3.0 mg/kg (T 22=2.904, P<0.05). In both saline- and cocaine-treated rats, the effects of SKF 81297 were biphasic because the highest dose (10 mg/kg) failed to produce a significant place preference.
The effects of D1 and D2/D3 receptor agonists on place conditioning in drug-naive and cocaine-treated rats. In drug naive (saline-treated) rats, the D1 agonist SKF 81297 induces dose-dependent conditioned place preference (a), whereas the D2/D3 agonist quinpirole is without effect (b). In cocaine-treated rats, SKF-81297-conditioned place preference is reduced at the 3.0-mg/kg dose (compare with a) (c), whereas quinpirole produces a place preference at the 3.0-mg/kg dose (compare with b) (d). Place preference data are expressed as the mean±SEM difference between time spent on drug- and saline-paired sides (n=8–23/group). Symbols indicate posttest scores for SKF 81297 differ from saline/saline pairing (SAL) by Dunnett’s test (**P<0.01) (a), or posttest scores in cocaine-treated animals differ from saline-treated animals by unpaired t tests corrected for multiple comparisons (*P<0.05) (c). Posttest scores differ from pretest scores by paired t tests corrected for multiple comparisons († P<0.05, ‡ P<0.01) (a, c, and d)
Conversely, drug-naive (saline-treated) animals failed to exhibit a place preference with the D2/D3 receptor agonist quinpirole across multiple test doses (Fig. 1b), whereas cocaine-treated animals did exhibit a small but significant place preference of 5.7±2.3 min at a single dose (3.0 mg/kg) of quinpirole (Fig. 1d) when compared with their pretest scores (T 16=−4.632, P<0.01). Therefore, repeated cocaine treatment attenuated place preference with the D1 agonist SKF 81297, while enabling a place preference with the D2/D3 agonist quinpirole.
Two of three other D1 agonists tested produced conditioned place preferences in drug-naive (saline-treated) rats (Fig. 2a–c). Thus, the D1 agonist SKF 82958 also induced dose-dependent and biphasic place preferences in post- vs pretest comparisons (test session × dose interaction, F 4,62 = 5.495, P = 0.001; main effects of dose, F 4,62 = 4.966, P = 0.002, and test session, F 1,62 = 22.002, P<0.001). Analysis of interactive effects found that animals conditioned with both 0.3 and 1.0 mg/kg SKF 82958 showed significant preference scores compared with saline-conditioned animals (F 4,62 = 7.625, P<.001), and compared with their pretest scores at the 1.0-mg/kg dose (T 13 = −6.884, P<0.01). In addition, the nonbenzazepine D1 agonist ABT-431 also produced dose-dependent increases in posttest preference scores, resulting in a test session × dose interaction (F 3,58 = 2.736, P = 0.05); the 3.0-mg/kg dose of ABT-431 increased preference scores compared with saline-conditioned animals (F 3,58 = 2.734, P = 0.05).
The D1 agonists SKF 82958 (a) and ABT-431 (b) induce dose-dependent conditioned place preferences, whereas A-77636 (c) induces a place aversion. In contrast, the D2/D3 agonist 7-OH-DPAT (d) is without effect. Data are expressed as mean±SEM preference scores (n=8–23/group). Symbols indicate posttest scores differ from saline/saline pairing (SAL) by Dunnett’s test (*P<0.05, **P<0.01) or from pretest scores by paired t test (‡ P<0.01)
Conversely, animals conditioned with the atypical D1 agonist A-77636 failed to show a place preference (Fig. 2c) and instead exhibited an aversion to the drug-paired side compared with their pretest scores (test session × dose interaction, F 3,40 = 3.992, P = 0.014; main effects of dose, F 3,40 = 3.042, P = 0.04; and test session, F 1,40 = 9.784, P = 0.035). A-77636 produced a significant place aversion at the 1.0-mg/kg dose compared with saline-conditioned animals (F 3,40 = 4.321, P = 0.01) and compared with their pretest preference scores (T 13 = 6.743, P<0.01). The place aversion to A-77636 was also biphasic, showing no significant effect at the highest dose tested. In contrast, animals conditioned with the D2/D3 receptor agonist 7-OH-DPAT failed to show evidence for place preference or aversion at all doses tested (Fig. 2d), similar to the lack of effect with the D2/D3 agonist quinpirole in drug-naive rats.
Experiment 2: effects of D1 and D2/D3 receptor agonists on the expression of cocaine-conditioned place preference
Given that dopaminergic mechanisms are involved in the expression of previously established cocaine place preference, we determined whether continuous D1 or D2 receptor activation would interfere with these expression mechanisms. Figure 3a shows that animals pretreated with saline 30 min before testing displayed a significant cocaine-conditioned place preference compared with their pretest preference scores (T 18 = −3.833, P<0.01), but expression of the preference was blocked by pretreatment with either SKF 81297 (3.0 mg/kg) or quinpirole (1.0 mg/kg), resulting in a significant test session × drug interaction (F 2,52 = 4.432, P = 0.017) and a significant reduction in posttest preferences scores compared with the saline pretreatment (F 2,52 = 5.623, P = 0.006). SKF 81297 blocked expression of place preference without altering total locomotion or locomotion in the drug-paired side (Fig. 3b), whereas quinpirole blocked expression concomitant with a reduction in both total locomotion (F 3,75 = 9.440, P < 0.001) and locomotor activity in the drug-paired side (F 3,75 = 3.982, P = 0.011). Expression of cocaine place preference was not associated with an increase in locomotion relative to saline/saline-conditioned animals.
Both the D1 agonist SKF 81297 (3.0 mg/kg) and the D2/D3 agonist quinpirole (1.0 mg/kg) block the expression of cocaine-conditioned place preference when administered 30 min before the postconditioning test session (a). Quinpirole, but not SKF 81297, reduces total locomotion and locomotion in the drug-paired side (DPS) while blocking expression of cocaine place preference (b). Symbols indicate posttest preference scores differ from saline pretreatment in cocaine-conditioned animals, or locomotion differs from saline/saline-conditioned controls by Dunnett’s test (*P<0.05, **P<0.01); pretest differs from posttest scores (‡ P<0.01) by paired t test (n=18–19/group)
Experiment 3: effects of D1 and D2/D3 receptor agonists on the reinstatement of cocaine-conditioned place preference
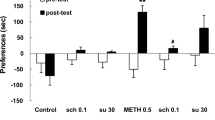
We determined whether the differential rewarding effects of the D1 agonist SKF 81297 and the D2/D3 agonist quinpirole would relate to their ability to reinstate a cocaine-conditioned place preference following extinction of the preference. As a positive control, we found that priming injections of cocaine dose-dependently reinstated cocaine place preferences when compared with saline-primed extinction baselines as shown in Fig. 4a (main effect of test session, F 1,9 = 95.954, P < 0.001). Significant place preferences were reinstated by both 5.0- (T 9 = −4.816, P < 0.01) and 10-mg/kg (T 9 = −5.190, P < 0.01) cocaine doses when corrected for multiple comparisons. Reinstatement by cocaine was associated with an increase in total locomotion at the highest test dose (T 9 = −5.200, P < 0.01), and a nonsignificant trend for increased locomotion in the drug-paired side. Similarly, Fig. 4b shows that the D1 agonist SKF 81297 also dose-dependently reinstated place preferences in cocaine-conditioned rats (main effect of test session, F 1,73 = 18.749, P < 0.001), resulting in significant reinstatement at doses of 0.1 (T 17 = −4.695, P < 0.05) and 1.0 mg/kg (T 15 = −3.893, P < 0.01). However, in contrast to cocaine, SKF-81297-induced reinstatement was associated with a decrease in total locomotion at doses of 1.0 (T 16 = 4.325, P < 0.01), and 3.0 mg/kg (T 17 = 2.904, P < 0.05). This reduction in locomotion also sharply contrasts with the failure of SKF 81297 to reduce locomotion when blocking expression of cocaine place preference (Fig. 3).
Priming injections of cocaine (a) and the D1 agonist SKF 81297 (b) dose-dependently reinstate an extinguished cocaine place preference. Conversely, the D2/D3 agonist quinpirole induces a place aversion (c). The right panels show that cocaine-induced reinstatement is associated with an increase in total locomotion compared with extinction sessions, whereas SKF-81297-induced reinstatement is associated with a decrease in total locomotion [drug-paired side (DPS)]. Preference scores in the first 15 min of the initial posttest session (PT) before extinction training are shown for reference. Symbols indicate reinstatement (Rstmt) differs from extinction baselines (Ext), or total locomotor activity during reinstatement differs from total locomotor activity during extinction († P<0.05, ‡ P<0.01) by paired t test (n=10–18/group)
Surprisingly, the D2/D3 agonist quinpirole induced dose-dependent place aversions to the cocaine-paired side of the conditioning apparatus (main effect of session, F 1,13 = 14.414, P = 0.002), resulting in significant place aversions at both 1.0-(T 13 = 4.177, P < 0.01) and 3.0-mg/kg (T 13 = 3.246, P < 0.05) doses. This effect is opposite to the reinstating effects of cocaine and SKF 81297 but also differs qualitatively from the ability of quinpirole to neutralize initial expression of place preference shown in Fig. 3. Thus, quinpirole induced an unconditioned aversion to the cocaine-paired side following extinction of the established cocaine preference. Quinpirole-induced place aversions were associated with substantially reduced total locomotor activity compared with extinction baselines at all doses tested (0.1 mg/kg, T 12 = 3.281, P < 0.05; 0.3 mg/kg, T 12 = 3.813, P < 0.01; 1.0 mg/kg, T 12 = 5.243, P < 0.01; and 3.0 mg/kg, T 12 = 5.776, P < 0.01), similar to reduced locomotion when neutralizing initial expression of place preference, but only a nonsignificant trend for reduced locomotion in drug-paired side was found at the highest dose tested.
Experiment 4: effects of the D2/D3 agonist quinpirole on cocaine-induced reinstatement of place preference
Given that the D2/D3 agonist quinpirole induced place aversions when given alone in reinstatement tests, we determined whether quinpirole would reverse the ability of cocaine to reinstate an extinguished cocaine place preference. Figure 5 shows that 30 min pretreatment with several doses of quinpirole failed to block cocaine-induced reinstatement (10.0 mg/kg) of cocaine place preference, resulting in a main effect of reinstatement compared with extinction baselines across all quinpirole doses (F 1,12 = 29.485, P<0.001). Animals pretreated with saline (T 12=−3.495, P<0.05), 0.3 mg/kg (T 12=−5.456, P<0.01), and 3.0 mg/kg (T 12=−4.339, P<0.01) quinpirole all showed significant cocaine-induced reinstatement compared with their extinction baselines. Thus, quinpirole-treated animals were capable of expressing a cocaine place preference despite the place aversions induced by quinpirole when given alone.
Pretreatment with quinpirole does not alter cocaine-induced (10 mg/kg, i.p.) reinstatement of cocaine place preference. The posttest preference score (PT) before extinction is shown for reference. Symbols indicate reinstatement (Rstmt) differs from extinction baselines (Ext) by paired t test († P<0.05, ‡ P<0.01; n=13)
We also determined whether quinpirole would induce an unconditioned place bias to black or white sides of the test chamber in drug-naive rats. Animals (n=16) were conditioned with saline on both sides (2/2), and half were pretreated with either saline or quinpirole (1.0 mg/kg) 30 min before a 15-min posttest, and then tested the next day with the alternate treatment. There was no evidence for place bias in quinpirole treated rats because mean preference, scores (black–white side) averaged 0.18±0.88 min for saline pretreatment and −1.47±1.57 min for quinpirole pretreatment (not shown).
Discussion
Differential rewarding effects of D1 and D2/D3 agonists measured in place conditioning
In drug-naive animals, the full efficacy benzazepine-based D1 receptor agonists SKF 81297 and SKF 82958 produced prominent rewarding effects with minimal conditioning (two pairings) in a place preference procedure. Similarly, ABT-431, a prodrug of the highly selective nonbenzazepine D1 agonist A-86929 (Shiosaki et al. 1996), also produced rewarding effects. The rewarding effects of SKF 82958 are consistent with an earlier report (Abrahams et al. 1998), although these effects occurred at doses an order-of-magnitude higher in our study. Others have found rewarding effects with intra-accumbens injections of the partial D1 agonist SKF 38393, but place aversions when this compound is injected systemically (Hoffman and Beninger 1988; White et al. 1991). Both SKF 81297 and SKF 81958 produced place preferences at doses at or above thresholds for locomotor activation (Self et al. 1996a; Edwards et al. 2006), suggesting that greater psychomotor activation may interfere with place preference at higher doses.
Conversely, the isochroman D1 agonist A-77636 produced only place aversions. A-77636 has an atypical D1 agonist profile due to a very long duration of action and tolerance with repeated administration (Kebabian et al. 1992; Blanchet et al. 1996; Lin et al. 1996). A-77636 dissociates very slowly from the D1 receptor, causing substantial receptor internalization and loss of D1 receptor responsiveness after even a single treatment (Blanchet et al. 1996). In this sense, the place aversion produced by A-77636 is similar to the place aversion produced by D1 receptor antagonists (Shippenberg et al. 1991), possibly reflecting receptor internalization with repeated treatment. Alternatively, the prolonged behavioral activation (>20 h) with initial treatment can produce an aversive stimulus.
In contrast, the lack of place conditioning with the D2 agonists quinpirole and 7-OH-DPAT in drug-naive animals agrees with several previous studies using doses thought to activate postsynaptic D2/D3 receptors (White et al. 1991; Rodriguez De Fonseca et al. 1995; Kivastik et al. 1996), whereas others have found either place preferences (Hoffman and Beninger 1988; Mallet and Beninger 1994; Kling-Petersen et al. 1995; Biondo et al. 2005) or place aversions at low doses thought to selectively activate D2 autoreceptors (Khroyan et al. 1995; Gyertyan and Gal 2003).
Repeated cocaine treatment differentially altered the rewarding effects of the D1 agonist SKF 81297 and the D2 agonist quinpirole when compared with drug-naive (saline-treated) animals. Thus, preference scores for SKF 81297 were downward-shifted by 54%, suggesting that repeated cocaine reduces the rewarding efficacy of the D1 agonist. Although this effect can involve the development of tolerance to D1-mediated reward, there was no rightward shift in the effective dose, but a narrower dose range is needed to rule out tolerance-like effects. The effects of repeated cocaine treatment can involve reduced striatal D1 receptor binding and D1-stimulated cyclic adenosine monophosphate (cAMP) formation that would reduce D1-mediated reward (Kleven et al. 1990; Tsukada et al. 1996; Graziella De Montis et al. 1998). However, others have found either no change (Mayfield et al. 1992) or increases in D1-mediated cAMP formation and electrophysiological responses (Henry and White 1991; Unterwald et al. 1996) using similar treatment and withdrawal protocols. Thus, the mechanism for a reduction in D1-mediated reward following repeated cocaine administration is unclear.
Conversely, repeated cocaine exposure enabled a significant place preference at a single dose of quinpirole, although this level of preference was less than the maximal preference levels found with D1 agonists in drug-naive animals, and was not found at a higher dose, possibly due to intense psychomotor activation as discussed above. Given that cocaine cross-sensitizes with D2 but not D1 receptor agonists (De Vries et al. 1999), it is possible that sensitization processes unmasked the rewarding potential of quinpirole. A previous study found that sensitizing regimens of quinpirole itself also increase sensitivity to quinpirole-induced place preference (Papp et al. 1993), and another study found that sensitizing regimens of amphetamine, but not morphine, cross-sensitize with quinpirole in locomotor tests (Vanderschuren et al. 1999). Thus, factors such as housing conditions or rat strain that influence the inherent level of sensitization in untreated animals can explain the discrepant findings with quinpirole in several previous studies. Similar to D1 receptors, studies on D2 receptor binding following repeated cocaine administration in rats are discrepant, finding either transient decreases (Kleven et al. 1990), no change (King et al. 1994), or increases in the nucleus accumbens concomitant with decreases in the dorsal striatum (Goeders and Kuhar 1987), despite substantial behavioral sensitization to D2 agonists. These discrepancies suggest that a reduction in D1 and sensitization in D2-receptor-mediated reward can involve alterations in network level responsiveness independent of dopamine receptor expression or changes in D1 and D2 receptors in other brain regions such as the amygdala that are critical to development of place conditioning (Brown and Fibiger 1993).
The ability of D1 agonists to produce rewarding effects is consistent with the ability of D1, but not D2, antagonists to block the induction of cocaine-conditioned place preference (Acquas and Di Chiara 1994; Cervo and Samanin 1995; Baker et al. 1996, 1998; Nazarian et al. 2004), and to their ability to function as primary rewards in drug-naive animals self-administering D1 agonists (Self and Stein 1992; Weed et al. 1993; Weed and Woolverton 1995; Grech et al. 1996; Self et al. 1996a,b). Together, these results suggest that D1 receptors effectively mediate primary rewarding effects. Similarly, the failure of D2/D3 agonists to produce place conditioning is consistent with their failure to support self-administration behavior in drug-naive animals (Nader and Mach 1996). However, cocaine-experienced animals readily self-administer D2/D3 agonists (Woolverton et al. 1984; Caine and Koob 1993; Nader and Mach 1996; Woolverton and Ranaldi 2002), consistent with our results in cocaine-exposed animals, and suggesting that cocaine exposure facilitates the rewarding properties of D2/D3 agonists. Given that D2/D3, but not D1, agonists reinstate cocaine-seeking behavior in self-administering animals (Self et al. 1996a,b; De Vries et al. 1999; Alleweireldt et al. 2002; Dias et al. 2004), it is possible that quinpirole evokes conditioned effects in cocaine-experienced animals that function as a secondary reward to produce place preference (second-order conditioning). In contrast, doses of D1 agonists that produce rewarding effects in place preference in our study are similar to doses that block reinstatement of cocaine-seeking behavior in self-administering animals (Self et al. 1996a,b, 2000; Alleweireldt et al. 2002), suggesting that the primary rewarding effects of D1 receptor stimulation satiate cocaine-seeking behavior.
Differential effects of D1 and D2/D3 agonists on expression and reinstatement of cocaine place preference
Simultaneous antagonism of both D1 and D2/D3 receptors is required to block expression of a cocaine-conditioned place preference (Lawley and Kantak 1990; Liao et al. 1998; Adams et al. 2001), indicating involvement of conditioned dopamine release in expression mechanisms. However, cocaine injections fail to alter the expression of cocaine-conditioned place preference in rats and mice (Nomikos and Spyraki 1988; Szumlinski et al. 2002), perhaps because blockade of dopamine uptake does not occlude detection of conditioned dopamine release in the drug-paired side. In contrast, we found that direct and selective D1 or D2/D3 receptor stimulation abolished expression of a cocaine-conditioned preference, consistent with a previous report with a D3 receptor preferring partial agonist (Duarte et al. 2003). In our study, D1 or D2/D3 agonists did not generally impair expression mechanisms because animals treated with either SKF 81297 or quinpirole displayed prominent preferences or aversions to the cocaine-paired side following extinction in reinstatement tests. Although it is difficult to reconcile the ability of SKF 81297 to block initial expression of place preference with its ability to reinstate place preference following extinction, the two phenomena may involve fundamentally distinct mechanisms. Thus, the primary rewarding properties of D1 receptor stimulation can mask the conditioned rewarding effects of the drug-paired side in initial expression tests, but after these effects extinguish, cocaine-like stimulus properties can induce reinstatement. In contrast, stimulation of D2/D3 autoreceptors can block expression by inhibiting conditioned dopamine release, consistent with a reduction in locomotor behavior.
The ability of cocaine to dose-dependently reinstate a cocaine place preference is consistent with previous reports (Mueller and Stewart 2000; Szumlinski et al. 2002), and is also consistent with cocaine’s ability to reinstate cocaine-seeking behavior in self-administering animals. However, the prominent ability of the D1 agonist SKF 81297 to reinstate place preference sharply contrasts with its failure to reinstate cocaine-seeking behavior and with its ability to attenuate cocaine- and cue-induced reinstatement in self-administering animals (Self et al. 1996a, b; Alleweireldt et al. 2002). SKF-81297-induced inhibition of reinstatement in self-administering animals is not due to interfering behaviors (Alleweireldt et al. 2003), and reinstatement of place preference is not specifically associated with psychomotor activation because cocaine- and SKF-81297-induced reinstatement was associated with increases and decreases in locomotor behavior, respectively. Thus, the role of D1 receptors in reinstatement of cocaine-seeking behavior using instrumental procedures fundamentally differs from their role in reinstatement of cocaine place preference.
Surprisingly, the D2/D3 agonist quinpirole induced strong aversion to the cocaine-paired side, which is also contrary to its ability to reinstate cocaine-seeking behavior in self-administering rats (Self et al. 1996a,b; De Vries et al. 1999; Edwards et al. 2006). Quinpirole-induced place aversion was revealed only after the competing influence of the cocaine place preference extinguished and was associated with a reduction in locomotion, suggesting that a similar mechanism can account for both blocking initial expression of place preference and induction of place aversion after extinction. D1 and D2/D3 agonists share similar cocaine-like discriminative stimulus properties in rats that cannot account for these opposite effects on reinstatement of cocaine place preference (Callahan et al. 1991; Witkin et al. 1991). Instead, agonist-induced reinstatement of cocaine place preference seems to be related to their ability to mediate primary reward.
In cocaine self-administration, dopamine signals are temporally linked in a response-contingent manner to instrumental behavior (Stuber et al. 2005). Thus, temporally diffuse D1 receptor activation would mask rather than mimic these discrete reward-related signals and prevent their ability to control instrumental behavior. Conversely, whereas D2/D3 agonists may lack inherent primary rewarding properties, D2/D3 receptor activation can augment control over instrumental behavior by conditioned stimuli in animals with prior cocaine self-administration experience. In this sense, D2/D3 agonists have been shown to facilitate instrumental responding for temporally discrete conditioned rewards, whereas D1 agonists block or mask conditioned reward (Beninger and Ranaldi 1992; Beninger and Rolfe 1995), similar to their opposing influence on reinstatement of cocaine seeking in self-administering animals. These behavioral differences parallel certain physiological differences of D1 and D2 receptor stimulation (Goto and Grace 2005), where lower-affinity D1 receptors mediate phasic responses to dopamine release and, thus, would be more susceptible to potential masking effects of D1 agonists. Conversely, higher-affinity D2 receptors are tonically activated by basal dopamine levels, and D2/D3 agonists would augment rather than mask this tonic regulation.
In conditioned place preference, there is no requirement for discrete timing of dopaminergic signals during induction of contextual conditioning, and so temporally diffuse D1 receptor activation during reinstatement would mimic rather than mask the cocaine stimulus experienced during induction of the place preference. In this sense, the failure of D2 receptor stimulation to reinstate cocaine place preference may be related to temporally indiscrete aspects the induction phase, cocaine-conditioned contextual stimuli, and the lack of behavioral control by response-contingent stimuli. Collectively, these results suggest that D1 and D2 receptors contribute motivationally distinct influences to cocaine reward, and that their role in reinstatement differs substantially in cocaine self-administration and conditioned place preference approaches to the study of drug addiction.
References
Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ (1998) Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol 343:111–118
Acquas E, Di Chiara (1994) D1 receptor blockade stereospecifically impairs the acquisition of drug-conditioned place preference and place aversion. Behav Pharmacol 5:555–569
Adams JU, Careri JM, Efferen TR, Rotrosen J (2001) Differential effects of dopamine antagonists on locomotor activity, conditioned activity and conditioned place preference induced by cocaine in rats. Behav Pharmacol 12:603–611
Alleweireldt AT, Weber SM, Kirschener KF, Bullock BL, Neisewander JL (2002) Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 159:284–293
Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL (2003) D1-Receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology (Berl) 168:109–117
Baker DA, Khroyan TV, O’Dell LE, Fuchs RA, Neisewander JL (1996) Differential effects of intra-accumbens sulpiride on cocaine-induced locomotion and conditioned place preference. J Pharmacol Exp Ther 279:392–401
Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL (1998) Effects of intra-accumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse 30:181–193
Beninger RJ, Ranaldi R (1992) The effect of amphetamine, apomorphine, SKF 38393, quinpirole and bromocriptine on responding for conditioned reward in rats. Behav Pharmacol 3:155–163
Beninger RJ, Rolfe NG (1995) Dopamine D1-like receptor agonists impair responding for conditioned reward in rats. Behav Pharmacol 6:785–793
Biondo AM, Clements RL, Hayes DJ, Eshpeter B, Greenshaw AJ (2005) NMDA or AMPA/kainate receptor blockade prevents acquisition of conditioned place preference induced by D(2/3) dopamine receptor stimulation in rats. Psychopharmacology (Berl) 179:189–197
Blanchet PJ, Grondin, R, Bedard PJ, Shiosaki, K, Britton DR (1996) Dopamine D1 receptor desensitization profile in MPTP-lesioned primates. Eur J Pharmacol 309:13–20
Brown EE, Fibiger HC (1993) Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 113:123–130
Caine SB, Koob GF (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816
Callahan PM, Appel JB, Cunningham KA (1991) Dopamine D1 and D2 mediation of the discriminative stimulus properties of d-amphetamine and cocaine. Psychopharmacology (Berl) 103:50–55
Cervo L, Samanin R (1995) Effects of dopaminergic and glutaminergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res 673:242–250
De Vries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren LJMJ (1999) Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal if IV drug self-administration. Psychopharmacology (Berl) 143:254–260
Dias C, Lachize S, Boilet V, Huitelec E, Cador M (2004) Differential effects of dopaminergic agents on locomotor sensitization and on the reinstatement of cocaine-seeking and food-seeking behavior. Psychopharmacology (Berl) 175:105–115
Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot M (2003) Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-morphine- and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology 28:1903–1915
Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW (2006) Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology doi:10.1038/sj.npp.1301062
Goeders NE, Kuhar MJ (1987) Chronic cocaine administration induces opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res 7:207–216
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8:805–812
Graziella De Montis M, Co C, Dworkin SI, Smith JE (1998) Modifications of dopamine D1 receptor complex in rats self-administering cocaine. Eur J Pharmacol 362:9–15
Grech DM, Spealman RD, Bergman J (1996) Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 125:97–104
Gyertyan I, Gal K (2003) Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. NeuroReport 14:93–98
Henry DJ, White FJ (1991) Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther 258:882–890
Hoffman DC, Beninger RJ (1988) Selective D1 and D2 dopamine agonists produce opposing effects in place conditioning but not in conditioned taste aversion learning. Pharmacol Biochem Behav 31:1–8
Itzhak Y, Martin JL (2002) Cocaine-induced conditioned place preference in mice: induction, extinction, and reinstatement by related psychostimulants. Neuropsychopharmacology 26:130–134
Kebabian JW, Britton DR, Deninno MP, Perner R, Smith L, Jenner P, Schoenleber R, Williams M (1992) A-77636: a potent and selective D1 receptor agonist with antiparkinsonian activity in marmosets. Eur J Pharmacol 229:203–209
Khroyan TV, Baker DA, Neisewander JL (1995) Dose-dependent effects of the D3-preferring agonist 7-OH-DPAT on motor behaviors and place conditioning. Psychopharmacology (Berl) 122:351–357
King GR, Ellinwood EH Jr, Silvia C, Joyner CM, Xue Z, Caron MG, Lee TH (1994) Withdrawal from continuous or intermittent cocaine administration: changes in D2 receptor function. J Pharmacol Exp Ther 269:743–749
Kivastik T, Vuorikallas K, Piepponen TP, Zharkovsky A, Ahtee L (1996) Morphine- and cocaine-induced conditioned place preference: effects of quinpirole and preclamol. Pharmacol Biochem Behav 54:371–375
Kleven MS, Pery BD, Woolverton WL, Seiden LS (1990) Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res 532:265–270
Kling-Petersen T, Ljung E, Wollter L, Svensson K (1995) Effects of dopamine D3 preferring compounds on conditional place preference and intracranial self-stimulation in the rat. J Neural Trans 101:27–39
Kreibich AS, Blendy JA (2004) cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci 24:6686–6692
Lawley SI, Kantak KM (1990) Postconditioning effects of magnesium on cocaine conditioned place preference in mice. Pharmacol Biochem Behav 36:531–538
Liao RM, Chang YH, Wang SH (1998) Influence of SCH23390 and spiperone on the expression of conditioned place preference induced by d-amphetamine or cocaine in the rat. Chin J Physiol 41:85–92
Lin CW, Bianchi BR, Miller TR, Stashko MA, Wang SS, Curzon P, Bednarz L, Asin KE, Britton DR (1996) Persistent activation of the dopamine D1 receptor contributes to prolonged receptor desensitization: studies with A-77636. J Pharmacol Exp Ther 276:1022–1029
Mallet PE, Beninger RJ (1994) 7-OH-DPAT produces place conditioning in rats. Eur J Pharmacol 261:R5–R6
Mayfield RD, Larson G, Zahniser NR (1992) Cocaine-induced behavioral sensitization in D1 dopamine receptor function in rat nucleus accumbens and striatum. Brain Res 573:331–335
Mueller D, Stewart J (2000) Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:39–47
Nader MA, Mach RH (1996) Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 125:13–22
Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V (2004) The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull 63:295–299
Nomikos GG, Spyraki C (1988) Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology (Berl) 94:119–125
Papp M, Willner P, Muscat R (1993) Behavioural sensitization to a dopamine agonist is associated with reversal of stress-induced anhedonia. Psychopharmacology (Berl) 110:159–164
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618
Rodriguez de Fonseca F, Rubio P, Martin-Calderon JL, Caine SB, Koob GF, Navarro M (1995) The dopamine receptor agonist 7-OH-DPAT modulates the acquisition and expression of morphine-induced place preference. Eur J Pharmacol 274:47–55
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Self DW, Barnhart WJ, Lehman DA, Nestler EJ (1996a) Opposite modulation of cocaine-seeking behavior by D1 and D2-like dopamine receptor agonists. Science 271:1586–1589
Self DW, Belluzzi JD, Kossuth S, Stein L (1996b) Self-administration of the D1 agonist SKF 82958 is mediated by D1, and not D2, receptors. Psychopharmacology (Berl) 123:303–306
Self DW, Karanian DA, Spencer JJ (2000) Effects of the novel D1 agonist ABT-431 on cocaine self-administration and reinstatement. Ann N Y Acad Sci 909:133–144
Self DW, Stein L (1992) The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res 582:349–352
Shiosaki, K, Jenner P, Asin KE, Britton DR, Lin CW, Michaelides M, Smith L, Bianchi B, Didomenico S, Hodges L, Hong Y, Mahan L, Mikusa J, Miller T, Nikkel A, Stashko M, Witte D, Williams M (1996) ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D1 receptor agonist: in vitro characterization and effects in animal models of Parkinson’s disease. J Pharmacol Exp Ther 276:150–160
Shippenberg TS, Bals-Kubik R, Herz A (1991) Neuroanatomical substrates mediating the aversive effects of D-1 dopamine receptor antagonists. Psychopharmacology (Berl) 103:209–214
Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863
Szumlinski KK, Price KL, Frys KA, Middaugh LD (2002) Unconditioned and conditioned factors contribute to the “reinstatement” of cocaine place conditioning following extinction in C57BL/6 mice. Behav Brain Res 136:151–160
Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, Kreek MJ (1996) Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: an in vivo study using positron emission tomography. J Neurosci 16:7670–7677
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Unterwald EM, Fillmore J, Kreek MJ (1996) Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol 318:31–35
Vanderschuren LJ, Schoffelmeer AN, Mulder AH, De Vries TJ (1999) Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology (Berl) 143:244–253
Weed MR, Vanover KE, Woolverton WL (1993) Reinforcing effect of the D1 dopamine agonist SKF 81297 in rhesus monkeys. Psychopharmacology (Berl) 113:51–52
Weed MR, Woolverton WL (1995) The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 275:1367–1374
White NM, Packard MG, Hiroi N (1991) Place conditioning with dopamine D1 and D2 agonists induced peripherally or into nucleus accumbens. Psychopharmacology (Berl) 103:271–276
Witkin JM, Nichols DE, Terry P, Katz J (1991) Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther 257:706–713
Wise RA (2004) Dopamine, learning, and motivation. Nat Rev Neurosci 5:483–494
Woolverton WL, Goldberg LI, Jinos JZ (1984) Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther 230:678–683
Woolverton WL, Ranaldi R (2002) Comparison of the reinforcing efficacy of two dopamine D2-like receptor agonists in rhesus monkeys using a progressive-ratio schedule of reinforcement. Pharmacol Biochem Behav 72:803–809
Acknowledgement
This work was supported by United States Public Health Services Grants DA 10460, DA 08227, DA 016857 (D.L.G.), and the Wesley Gilliland Professorship in Biomedical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, D.L., Hoppenot, R., Hendryx, A. et al. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology 191, 719–730 (2007). https://doi.org/10.1007/s00213-006-0473-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0473-5