Abstract
Molasses-based distilleries are amongst the most polluting industries worldwide generating huge volume of high strength wastewater. Discharge of this wastewater, enriched with toxic androgenic and carcinogenic pollutants including melanoidins, phenolics, endocrine disrupting chemicals, organic acids, heavy metals and other recalcitrant hazardous compounds, into the environment without adequate treatment posing a risk to human, animals, microorganisms, and plants. Thus, proper wastewater treatments are mandatory to remove contaminants before its discharge into the environment. Numerous physicochemical methods have been implemented for remediation or detoxification of distillery wastewater, which is viewed as a challenging job with respect to cost, technical complexity, and sludge generation in huge quantity with subsequent disposal problems. Therefore, there is an urgent need for safe management of hazardous distillery wastewater; the technologies must be economically viable, ecologically sound, and socially acceptable. This book chapter presents an overview of the generation of effluent, its chemical characteristics and environmental hazards. In addition, we have also discussed the existing treatment approaches and challenges for safe disposal of distillery wastewaer into the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
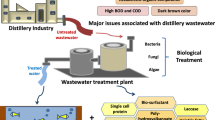
The worldwide demand for energy and the uncertainty of natural resources has led to the eco-friendly development of alternative liquid biofuels. Ethanol is one of the excellent candidates since it reduces dependence on fossil-fuel reserves. In developing countries like India, distilleries are one of the major agro-based polluting industries; in addition, they are a high consumer of fresh water and utilize the sugarcane molasses as the feedstock for ethanol making (Arimi et al. 2014; Kumar and Chandra 2018; Chandra and Kumar 2017a,b). However, there is serious environmental trouble with ethanol production from sugarcane molasses fermentation which is generally connected to the generation of dark brown-colored wastewater, known as a spent wash (SW) or raw wastewater/effluent (Kumar and Chandra 2018; Chandra and Kumar 2017a, b, c). It has been reported that SW produced from distilleries has a high organic load as compared to other raw material used for ethanol production (Kumar and Sharma 2019. There are three different organic wastes generated from the molasses based-distilleries which include yeast sludge, spent malt grain wash, and SW. A typical sugarcane molasses-based distillery generates 12–15 liters of SW for every liter of the ethanol produced. It stated that about 40.4 billion liters of SW are produced with a generation of 3.25 billion liters ethanol from 319 distilleries located in the tropical and subtropical region of India. SW is a dark brown-colored wastewater characterized by a specific obnoxious odor with high organic and inorganic load at acidic pH (Table 6.1). Wilkie et al. (2000) stated that COD is 4–5 times higher in sugarcane molasses-based SW as compared to sugarcane juice stillage. SW composition generally depends on the raw material used for sugar extraction as well as the distillation and fermentation processes adopted in distilleries for ethanol production. The dark brown-colored SW is one of the most obvious indicator of water and soil pollution. Apart from color, SW possesses a high concentration of reducing sugars, hemicelluloses, lignin, resins, dextrin, polysaccharides, organic acids, phenolic compounds, anthocyanins, tannins, fatty acids, sterols, and resins (Chandra and Kumar 2017a). High chemical oxygen demand (COD), biological oxygen demand (BOD), and persistent dark brown color of SW poses environmental, water, and soil pollution problems including a threat to plant and animal lives, and thus safe disposal of such kind of wastewater is challenging. In accordance with the environmental protection act and rules of the Ministry of Environment, Forests and Climate Change and Central Pollution Control Board (CPCB), Govt. of India, it is mandatory to treat hazardous SW before it is disposed into the environment. Indian government policies on pollution prevention have forced distilleries to look for an effective and sustainable technology for decreasing the SW characteristics. It is usually subjected to conventional aerobic and anaerobic secondary treatment approaches such as activated sludge, anaerobic digestion, and anaerobic lagoons processes, which easily remove organic matter (OM) and also reduce the BOD and COD of SW. However, these treatment methods do not decompose or decolorize melanoidins present in SW due to their recalcitrant nature and presence of other complex co-pollutants. Thus, adequate treatment is warranted before the wastewater is discharged into the environment. Hence, this book chapter is focused on the generation and characteristics of distillery wastewater (DW) pollutants, their environmental hazards as well as various existing physicochemical and biological treatment approaches used for DW. Further, the emerging treatment approaches used for DW have also been discussed.
6.2 Ethanol Manufacturing Process and Effluent Generation
Ethanol can be produced from various feedstock, including sugar-based materials (i.e., sugarcane juice and beet molasses), starch-based material (i.e., corn, barley, wheat, rice, and cassava), and cellulosic materials (i.e., crop residues and sugarcane bagasse). In India, distilleries used diluted sugarcane molasses (15–16%) as a chief feedstock material for ethanol production (7–8% v/v). In general, ethanol production consists of three steps: (i) feed preparation (fermentable sugar containing diluted molasses solution), (ii) fermentation (conversion of sugars to ethanol), and (iii) distillation (separation and purification of ethanol). For ethanol production, sugarcane molasses, and nitrogen and phosphate-containing food supplements are taken in a fermentation broth. Further, the fermentation process is carried out by yeast (Saccharomyces cerevisiae) culture, which converts the sugar into ethanol, and the yeast sludge settles down at the end of the process. The fermented mass contains 7–8% ethanol, which is separated in a distillation column as the top product, and brownish liquid as the bottom product known as SW (Fig. 6.1). In molasses-based distilleries, the fermentation process can be carried out by three modes; (i) batch, (ii) fed-batch, (iii) continuous mode. In a batch process, sugarcane molasses is diluted with water to reduce the sugar content from the existing 40–45% to 10–15%, and then yeast inoculum at about 10% concentration (v/v) is added with this diluted sugarcane molasses. Further, this diluted molasses is allowed to ferment for 30–40 hrs. After completion of fermentation, the yeast sludge is separate from the bottom of the fermenter (bioreactor) while the bioreactor wash is sent to the analyzer column for distillation with steam where a mixture of steam and ethanol vapors is collected at the top of the column and spent wash drained out from the bottom. The mixture of steam and ethanol is fed to the rectification column where rectified spirit (RS) is formed due to condensation of water and ethanol vapors. The condensed steam is discharged as spent lees (Fig. 6.1). In a fed-batch process, a combination of batch and a continuous mode, substrates (nutrients) are supplied (fed) to the bioreactor during cultivation and the product(s) remain in the bioreactor until the end of the run. Continuous mode is carried out by continually adding culture medium, substrates, and nutrients into a bioreactor containing microorganisms. During this process, the culture volume must be constant and the products formed after fermentation are continuously taken from the media. Absolute ethanol or fuel ethanol is generated by the dehydration of RS through molecular sieve technology where RS is passed through preheater to raise the temperature to 65 °C, then fed into the recovery column and heated through boiler by steam to produce alcohol vapors. Further, the alcohol vapors are superheated with steam and then passed through one of the pairs of molecular sieve beds in auto-mode. The water (H2O) molecules are absorbed by the molecular sieves beds and become saturated within 5–6 min. When the first molecular sieve bed is saturated with H2O, automatically ethanol vapors will pass through the second bed. The ethanol vapors having alcohol concentration of about 99.70% are condensed and stored in the collection tanks. The desorbed liquid contains 70–75% ethanol, which is partially used for creating a vacuum and remains recycled to the recovery column. During ethanol production, a huge amount of solid waste matter as a yeast sludge is formed in the distilleries, which cause pollution when it is disposed into the environment. Yeast sludge is rich in protein and contains a considerable amount of essential amino acids and drying sludge grains are marketed as livestock feed and make it the best source for the production of single cell protein. Generally, during ethanol production, distillery operations use water for various process and non-process applications. The process applications include preparation of sugarcane molasses for fermentation, yeast propagation, and steam requirements for distillation, while the non-process applications involve boiler water, wash water, cooling water used in making potable ethanol. The wastewaters discharged from the analyzer column, yeast sludge, spent less, water treatment plant, waste wash water, cooling water, boiler as blowdown, bottling plant, and other wastes as termed as SW. In distilleries, the major source of wastewater generation is the distillation step wherein a huge volume of dark brown-colored wastewater is generated. Average SW generation is highest in the batch process (11.1–15.0 liters per liter ethanol production), higher in the continuous process (8.5–11.0 liters per liter ethanol production), and lowest in the bio-still process (6–8 liters per liter ethanol production). Figure 6.1 illustrated the manufacturing process of ethanol from sugarcane molasses, along with the wastewater generation.
6.3 Distillery Wastewater: Nature and Chemical Characteristics
Over the last few decades, the occurrences of pollutants due to discharge of DW in the aquatic ecosystem and their toxic effects to human health as well as wildlife organisms have become major issues of increasing concern in India and other developing countries (Kumar and Sharma 2019). The majority of distilleries coexist with sugar mills and utilize sugarcane molasses as a starting material for ethanol production. In distilleries, a major fraction (~90%) of the fermented wash going to distillation column is discharged as SW (Chandra and Kumar 2017b). The characteristics of SW vary significantly according to the fermentation feedstock, and the fermentation/distillation processes adopted. SW is characterized with unpleasant odor; deep brown color; high level of BOD, COD, TDS, total solids (TS), total nitrogen (TN) sulfate and phosphate; and the presence of various heavy metals (HMs) ions such as iron (Fe3+), zinc (Zn2+), copper (Cu2+), nickel (Ni), manganese (Mn2+), and lead (Pb2+) and numerous endocrine-disrutping chemical (EDCs) (Tables 6.1 and 6.2).
The high organic load of SW is primarily composed of melanoidins (Maillard reaction products; MRPs), thermal degradation products (hexose alkaline degradation products; HADP), and sugar condensation reaction products (overheated sugars; caramels) (Hatano et al. 2013; Hatano and Yamatsu 2018; Kumar and Chandra 2018). The MRPs formed through the nonenzymatic browning reaction also known as Maillard reaction occurs between amino acids and reducing sugars and caramels at elevated temperatures that are responsible for deep brown color and odor in the SW (Kumar and Chandra 2018, 2020). Besides, the color of SW is also generally attributed to the existence of a wide variety of naturally polymeric colorants such as carotenoids, chlorophyll, resins, fatty acids, heme pigment, anthocyanins, tannins, riboflavin, betalains, quinone pigments, polyphenols, melanin, and metal sulfides (Borja et al. 1993; Arimi et al. 2014; Pant and Adholeya 2007). Among these colorants, melanoidins are major nitrogenous, high molecular weight (5–40 kDa), polymeric, acidic, negatively charged imparting organic compounds present at high concentration in SW. Melanoidins are toxic to microorganisms and recalcitrance to biological wastewater treatments; therefore, DW must be treated before disposal into the environment. It has been demonstrated that various HMs such as Mn2+, Co2+, Zn2+, Cr3+, Cu2+, Pb2+, and Fe3+ bind with melanoidins to make an organometallic complex and, consequently, enhance the toxicity of SW into the environment (Hatano et al. 2016; Migo et al. 1997; Chandra et al. 2018a, b, c, d). Polyphenols and melanoidins may also be the source of the formation of aromatic halogenated disinfection by-products (DBPs) during chlorine disinfection of DW. Liu and Zhang 2014 reported that aromatic halogenated DBPs showed higher developmental toxicity and growth inhibition than aliphatic halogenated DBPs. However, the colloidal nature of caramels makes the SW resistant to biological degradation and toxic to aquatic and terrestrial organisms. The obnoxious odor of DW mainly occurs due to the presence of indole, skatole, and other sulfur compounds. In addition, some toxic chemicals such as tricarballylic acid 3TMS; benzoic acid 3-methoxy-4-[(TMS)oxy], TMS ester; benzenepropanoic acid; α-[(TMS)oxy], TMS ester; 2-furancarboxylic acid, 5-[[(TMS)oxy] methyl], TMS ester; vanillylpropionic acid, bis(TMS); 2-hydroxysocaproic acid; and butanedioic acid bis(TMS) ester are also present in SW (Yadav and Chandra 2012). These organic compounds are well reported as potential EDCs by the US Environmental Protection Agency (USEPA) (2012). Hence, due to its toxic nature, SW must be treated properly before it is disposed into the environment (Tewari et al. 2007). In order to lower the high BOD and COD level, presently many distilleries are recycling this wastewater for getting fuel in the form of methane (Joshi et al. 1994). SW received after anaerobic digestion is called post-methanated distillery effluent (PMDE) or biomethanated distillery effluent (BMDE), which contains a higher level of BOD, COD, TDS, and phenols, with dark brown color, strong odor, and alkaline pH. Besides organic content, BMDE also contains a high level of nitrogen, potassium, sulfur, and phosphorus, which can lead to eutrophication of aquatic ecosystem. In addition, BMDE retains a high amount of various HMs (Table 6.1). This means that SW after anaerobic digestion retains high organic and inorganic load and it is not safe for discharge into the environment (Chandra et al. 2018a; Kaushik and Thankur 2009). Besides the effluent, sugarcane molasses-based distilleries produce huge amount of anaerobically digested SW sludge which has been reported for high concentration of phenolics, melanoidins, and complex OM along with metallic ions (i.e., Cd2+, Cu2+, Mn2+, Fe2+, Pb2+, Ni2+ and Zn2+) and nonmetallic ionic compounds (i.e., Na+, Cl−, SO2−4, PO3−4) (Table 6.1). Recently, distillery sludge is reported to contain high amount of plant-derived hexadecanoic acid; octadecanoic acid; n-pentadecanoic acid; stigmasterol; β-sitosterol trimethyl ether; heptacosane, lanosta-8, 24-dien-3-one, 1-phenyl-1-propanol, and 1-methylene-3-methyl butanol; dotriacontane; dodecanoic acid; 2-ethylthio-10-hydroxy-9-methoxy-1,4 anthraquinone; 5α-cholestane, 4-methylene; and campesterol TMS as potential EDCs reported by the USEPA (Chandra and Kumar 2017a, c; Chandra et al. 2018b). All these features combined with the huge volume of DW and sludge disposed of distilleries causes important environmental issues. Therefore, the elimination and biodegradation of organic and inorganic toxic compounds are necessary for the safe disposal of DW into the environment.
6.4 Environmental Pollution and Toxicity Profile of Distillery Wastewater
Distilleries generate a huge volume of wastewater during ethanol production, and most of the distilleries dispose their partially treated or untreated wastewater into water bodies causing environmental threats to organisms. Due to high pollution nature of DW, MoEF listed alcohol industries at the top among the “Red category” industries (Tewari et al. 2007). Regarding environmental pollution, the government of India made rules and regulations in 1976 and again revised them in 1983. The Bureau of Indian Standards (BIS) provides guidelines to state and central government authorities which would help to decide boundaries on effluent disposal and to the industry for selecting effective technology and the degree of treatment required for DW before their disposal. In an aquatic ecosystem, the DW reduced penetration of sunlight in lagoons, lakes, and rivers, which in turn decreases both dissolved oxygen and photosynthetic activity, thereby aquatic life suffers, resulting in deterioration of water quality and loss of productivity to such an extent that the water becomes unusable (Kumar and Gopal 2001; Chandralata et al. 2004; Ramakritinan et al. 2005; Kumar and Chandra 2006; Kumar and Sharma 2019). Disposal of DW on land is equally hazardous; it inhibits germination of seeds and depletes vegetation by decreasing the soil alkalinity, salinity, and manganese availability (Jadhav and Savant 1975; Chandraju and Basavaraju 2007; Bharagava and Chandra 2010; Narain et al. 2012; Srivastava and Jai 2010; Arora et al. 1992; Kannan and Upreti 2008). Chandra and Kumar (2017b) reported the toxic effects of SW at different concentrations on seedling growth of P. mungo L. and T. aestivum. In a another study, Chandra and Kumar (2017a) also reported the presence of androgenic-mutagenic compounds and potential autochthonous bacterial communities during in situ bioremediation of anaerobically digested distillery sludge and also tested the toxicity of in situ degraded sludge leachate by using Allium cepa L. root meristematic cell. They showed a reduction of toxicity in degraded samples of sludge and leachate, confirming the role of autochthonous bacterial communities in the bioremediation of distillery waste in situ. Nonjudicious use of PMDE adversely affected crop growth and decreased physicochemical properties (Jagdale and Sawant 1975; Joshi et al. 2000; Tripathi et al. 2011). However, the judicious application of PMDE improved crop productivity and alleviated environmental pollution problems (Devarajan et al. 1994; Davamani et al. 2006). The impact of distillery waste on the environment and their eco-friendly and advanced cleaner technologies used to combat the threat are illustares in Fig. 6.2.
6.5 Treatment Approaches for Distillery Wastewater
DW is a major threat to the environment, and it is therefore essential to adequately treat the DW prior to its safe disposal into the environment. This can be achieved by using biological, physical, and chemical approaches, either alone or in combination (Fig. 6.3).
6.5.1 Biological Treatment Approaches
Biological approaches have been recognized as eco-friendly and most effective methods for the treatment of highly polluted DW whereby organic substances are used as food by growing microorganisms such as bacteria, fungi, yeast, and cyanobacteria. The end result is a decrease in the number of organic pollutants and an increase in the number of microorganisms, carbon dioxide (CO2), water (H2O), and other by-products of microbial metabolism (Kumar et al. 2018; Kumar and Chandra 2020. The role of anaerobic and aerobic biological treatment approches in DW treatment are discussed in the following sections.
6.5.1.1 Anaerobic Treatment
Distillery SW retains high BOD/COD ratio (1.11–1.25), TDS, and high concentration of inorganic solids with low pH and high temperature. In order to decrease its high COD and BOD level, presently many distilleries are recycling this effluent for getting fuel in the form of methane (CH4) through anaerobic digestion (AD). AD is a multifaceted treatment process requiring the potential activity of different group of microorganisms interacting in a bioreactor. The breakdown of OM in an anaerobic reactor typically involves four major degradation phases: (i) hydrolysis, (ii) acetogenesis, (iii) acidogenesis, and (iv) methanogenesis. In the first three phases, organic pollutants are hydrolyzed and/or fermented into intermediate short-chain fatty acids which are further degraded to acetate and hydrogen and carbon dioxide. Further, in the fourth phase, acetate and H2/CO2 are converted into methane. Anaerobic treatment is a broadly accepted an effective exercise, and different high-rate anaerobic reactor designs have been tried for DW treatment. One of the methods that are used to treat DW is the application of upflow anaerobic sludge blanket (UASB) reactor. The UASB process is an attractive treatment because of low cost, high treatment efficiency, biogas generation, and ability to handle high organic loading rates (OLRs) and requires shorter hydraulic retention time (HRT) than other reactors (Keyser et al. 2003; Acharya et al. 2008). The UASB reactor has four major components, i.e., sludge bed, sludge blanket, gas-solid separator, and settlement compartment. A two-stage process with an anaerobic filter followed by a UASB reactor was investigated by Blonskaja et al. (2003). The acidogenic and methanogenic phases were clearly separated ensuring better conditions for the methanogens. COD reduction was 54% and 93% in the first and second stage, respectively. Table 6.3 provides a summary of different reactor configurations used for the anaerobic digestion of SW. The limitations of anaerobic treatment processes are the requirement of high dilution due to the presence of many antimicrobial compounds (Bharagava and Chandra 2010). AD can remove a substantial amount of organic load when applied in treating DW, but it is ineffective in color reduction and several recalcitrant pollutants. Therefore, further treatment is required to remove the remaining dark color and COD, BOD, etc. However, researchers have reported various alternatives for further treatment of BMDE through aerobic route and resource the recovery.
6.5.1.2 Aerobic Treatment
Aerobic processes are usually applied as post-aerobic treatment of BMDE, based on pollutant degradation by the utilization of specific microorganisms, either as pure strains or as a consortium. These processes generally depend on the oxidative activities of microorganisms, viz., fungi, yeast, bacteria, and cyanobacteria, used by the various researchers for the treatment of raw SW as well as BMDE in the presence of oxygen (Chandra and Kumar 2015a).
6.5.1.2.1 Fungal Treatment
In the last two decades, the fungi species belonging to basidiomycetes and ascomycetes class have been used in the decolorization of natural and synthetic melanoidins in connection with the color reduction of DW. The aim of fungal treatment is to reduce the COD and BOD of DW and at the same time to obtain some valuable products, such as fungal biomass for protein-rich animal feed, extracellular organic acids, or some specific fungal metabolites. Several fungi species such as Geotrichum candidum (Kim and Shoda 1999), Trametes sp. (Gonzalez et al. 2000), Coriolus hirsutus (Miyata et al. 2000), Flavadon flavus (Raghukumar and Rivonkar 2001), A. niveus (Angayarkani et al. 2003), Phanerochaete chrysosporium (Dhaiya et al. 2001; Thakkar et al. 2006), Pleurotus florida, Aspergillus flavus (Pant and Adholeya 2009a), Neurospora intermedia (Kaushik and Thakur 2013), Fusarium verticillioides (Pant and Adholeya 2009b) and yeast Citeromyces sp., Candida tropicalis (Tiwari et al. 2012), and Candida glabrate (Mahgoub et al. 2016) have been reported for the degradation and decolourisation of melanoidins containing DW. A list of yeast and fungi species used by various researchers for decolorization and degradation of DW is given in Table 6.4. The degradation and decolorization of melanoidins by fungus have occurred due to the prevalence of ligninolytic enzymes, i.e., manganese peroxidase (MnP), laccase (Lac), and lignin peroxidase (LiP), which metabolize melanoidins and other refractory organic compounds present in the DW as sole carbon and nitrogen sources (Miyata et al. 2000; Bonugli-Santos et al. 2012; Pant and Adholeya 2007, 2009a, b). The Lac and MnP have a broad range of substrate oxidizing enzymes able to cleave large varieties of several chemical bonds present in phenolic and nonphenolic recalcitrant compounds (Wong 2009). Miyata et al. (1998) demonstrated that synthetic melanoidin is decolorized by the sharing of manganese-dependent and -independent peroxidases of Trametes (Coriolus) hirsutus pellets, and the extracellular H2O2 along with the participation of Lac enzyme. MnP and Lac protein both act on pollutants in synergy resulting in the degradation and depolymerization of melanoidins (Miyata et al. 2000; González et al. 2008). González et al. 2008 and Miyata et al. (2000) have demonstrated that the presence of melanoidins and other similar compounds can induce the expression of MnP and Lac genes. González et al. (2008) report the induction of Lac by molasses wastewaters and molasses melanoidins in the Trametes sp. I-62. Tapia-Tussell et al. (2015) also reported the expression of Lac genes in the T. hirsutus strain Bm-2, in the presence of phenolic compounds, as well as its effectiveness in removing colorants from vinasse. In the presence of all phenolic compounds (i.e., guaiacol), increased levels of laccase-encoding mRNA were 40 times higher than those in the control. So far ligninolytic enzymes have been known to be produced by various fungi, but most of them have failed to bring about complete mineralization of melanoidins and other organic pollutants present in DW. However, the long growth cycle; long hydraulic retention time, requiring nitrogen limiting conditions; and low pH range (3.0–5.0) for complete decolorization of DW still limit the performance of the fungal decolorization system.
6.5.1.2.2 Bacterial Treatment
Bacterial cultures have a very high potential for biodegradation and decolorization of DW due to their higher environmental adaptability, faster growth rate, and high metabolizing capability of melanoidins. Thus, the degradation and decolourisation of synthetic and natural melanoidins was reported by various researchers using the axenic and mixed bacterial consortium. Pioneering work on SW decolorization by bacteria was done by Kumar et al. (1997). They observed that two aerobic bacterial isolates LA-1 and D-2 brought about maximum decolorization (36.5% and 32.5%) and COD reduction (41% and 39%) under optimized conditions in eight days. Various bacterial groups, such as Bacillus sp. (Kambe et al. (1999), Pseudomonas putida, Aeromonas sp. (Ghosh et al. 2002), Lactobacillus plantarum (Tondee and Sirianutapiboon 2008), Alcaligenes faecalis (Santal et al. 2011), Pseudomonas sp. (Sankaran et al. 2015), Bacillus sp. (Krzywonos 2012), and B. badius (Mehta et al. 2014), have been reported for the degradation and decolorization of melanoidin-containing DW. Some of the bacterial species investigated for their ability for decolorization and degradation of DW are summarized in Table 6.5. Bacterial decolorization is promising and faster compared to fungal decolorization, but an individual bacterial strain usually cannot degrade melanoidins completely, and the metabolites are often more toxic compared to parental compounds, which need to be further decomposed. Therefore, the utilization of bacterial consortia offers significant advantages over the use of pure bacterial cultures in degradation and decolorization of melanoidins, as different bacterial strains may attack the melanoidin molecules at different positions or may use decomposition products produced by another strain for further decomposition. Various biological studies have been earlier carried out by a number of researchers using bacterial consortium that included Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Proteus mirabilis (Mohana et al. 2007), Klebsiella oxytoca, Serratia marcescens, Citrobacter sp., (Jiranuntipon et al. 2008), B. licheniformis, Bacillus sp., Alcaligenes sp. (Bharagava et al. 2009), and Proteus mirabilis, Bacillus sp., Raoultella planticola, and Enterobacter sakazakii (Yadav and Chandra 2012) to treat DW. In recent years, the ligninolytic system of bacteria with respect to their enzymatic potential for the bioremediation degradation of synthetic melanoidins and BMDE pollutants has been extensively studied (Pant and Adholeya 2009b). LiP, MnP, and Lac are the three major lignin-degrading enzymes with great potential in industrial applications (D’Souza et al. 2006). Out of the three, mainly Lac and MnP play a major role in the degradation of melanoidins (Kumar and Chandra 2018). Chandra and Kumar (2018) reported that MnP is profusely present at the initial phase of bacterial growth, while laccase was produced in a later phase of growth during melanoidin degradation and decolorization.
6.5.1.2.3 Cyanobacteria/Algal Treatment
Cyanobacteria are prokaryotic, gram-negative, photoautotrophic eubacteria having the ability to take up their nutrients from DW as sole carbon and nitrogen source, and thereby decolorizing the wastewater resulting in the reduction of color, BOD, and COD. Another advantage of using cyanobacteria is that, apart from the degradation of the melanoidin, it also oxygenates water bodies thereby reducing the energy need of the aerobic treatment. Kalavathi et al. (2001) reported degradation and decolorization of melanoidin in DW by the Oscillatoria boryana BDU 92181. This marine cyanobacterium degrades melanoidins due to the production of hydrogen peroxide, perhydroxyl, hydroxyl, and active oxygen radicals, resulting in the 60% decolorization of the DW. They further identified enzymes from microalgae, namely, glucose oxidase, MnP, and two MIP, involved in maximum production of hydrogen peroxide. In addition, riboflavin, manganese sulfate, methyl viologen, reduced glutathionine, and ascorbic acid could be used by O. boryana BDU 92181 for improving the degradation rate of melanoidins. A study conducted by Patel et al. (2001) examined the 26%, 81%, and 96% decolorization of DW through bio-flocculation by Synechocystis sp., Lyngbya sp., and Oscillatoria sp., respectively. Valderrama et al. (2002) used a combined treatment of Lemna minuscule and Chlorella vulgaris for color removal from DW. They reported 52% color removal from DW by this combined treatment. Solovchenko et al. (2014) have investigated the possibilities of DW bioremediation along with a new C. sorokiniana sp. cultivated in a semi-batch mode in a high-density photobioreactor. A decrease in COD of the DW from 20,000 to ca. 1500 mg L−1 was achieved over 4 days with a decline in nitrate (>95%), phosphate (77%), and sulfate (35%). Recently, Krishnamoorthy et al. (2017) have shown the treatment of anaerobically digested DW with Oscillatoria sp. This organism reduced COD up to 55% of anaerobically digested DW. Although biological methods provide an eco-friendly approach for DW treatment, these methods also have some technical difficulties as far as in situ administration of pollutant is concerned.
6.5.1.2.4 Phytoremediation Approaches
Phytoremediation is an in situ, cost-effective, and eco-friendly technique to eliminate hazardous HMs and organic pollutants from the contaminated environment (Chandra et al. 2015, Chandra and Kumar 2018; Chandra et al. 2018b, c, d). It is an emergent green technology that employs plants and their associated microbiota to remove, reduce, immobilize, and/or degrade harmful environmental pollutants (Ma et al. 2011; Glick 2010). This can reduce the health risk from contaminated water, sediments, sludge, and soil through contaminant degradation or removal (Chandra and Kumar 2015b; Alkorta et al. 2004; Rajkumar and Freitas 2008). For the removal of DW contaminants, there is some significant work done by Billore et al. (2001) for a horizontal flow gravel bed constructed wetland (CW) to treat DW. After secondary conventional treatment, the concentrations of COD and BOD5 in DW amounted to 2540 and 13,866 mg L−1, respectively, and, therefore additional treatment was essential. The CW treatment system achieved BOD5, COD, total P, and, total Kjeldahl nitrogen (TKN) reductions up to 84%, 64%, 79%, and 59%. This study recommended that CW may be a sustainable tertiary treatment technique for the remediation of contaminants present in DW. Similarly, Trivedy and Nakate (2000) used wetland plant T. latipholia for treatment of DW in a CW treatment system. This treatment system resulted in 47% and 78% decrease in BOD and COD, respectively at incubation of 10 days. Increasing concentration of DW significantly reduced the biomass of growing plants, with the highest accumulation of Fe being recorded in plants growing in 100% concentration of DW. Potamogeton pectinatus, an aquatic macrophyte, was used to accumulate Mn, Zn, Cu, and Fe and efficiently clear out the DW (Singh et al. 2005). Chaturvedi et al. (2006) reported the phytoremediation potential of P. australis grown on DW-contaminated site. She also characterized the diverse bacterial species from the rhizospheric zone of P. australis. The culturable bacterial species were helpful for the degradation and decolorization of noxious pollutants that exist in the distillery effluent. They observed a 75.5% reduction of color by the same bacterial species along with a concomitant reduction in BOD, COD, sulfate, phenol, and HMs values. Bharagava et al. (2008) studied the HMs accumulation efficiency and its physiological effects in Brassica nigra L. (Indian mustard) plants grown in soil irrigated with different concentrations (25%, 50%, 75%, 100%, v/v) of PMDE after 30, 60, and 90 days treatment. This study concluded that B. nigra L. accumulated elevated concentrations of Zn, Ni, Mn, Fe, Cu, and Cd due to the increased amount of cysteine and ascorbic acid (work as antioxidants) in root, shoot, and leaves of B. nigra L. at all the concentrations and exposure periods of PMDE except at a 90-day period. Chandra and Yadav (2010) conducted a pot culture experiment to evaluate the accumulation pattern of Cu, Pb, Ni, Fe, Mn, and Zn in T. angustifolia grown in Zn, Mn, Fe, Ni, Pb, and Cu-rich aqueous solutions of phenols and melanoidins. They concluded that T. angustifolia could be an efficient phytoremediator for HMs from melanoidin, phenol, and metal-containing industrial effluent at optimized conditions. Recently, Hatano et al. (2016) observed the chelating property of melanoidin-like product (MLP) and to assess the facilitatory influence on the phytoextraction potential of Raphanus sativus var. longipinnatus (Japanese radish). They found that MLP binds with all the tested HMs ions, and the metal ion-binding capability of MLP toward Cu2+ was found to be the maximum among them. In a separate study Hatano and Yamatsu (2018) evaluated the facilitatory effect of MLP on phytoextraction potential of three Brassica species grown in a medium containing Pb or Cd. They reported that biomass and Pb2+ uptake in the nutrient medium containing 1 mM Pb nitrate were significantly increased by the addition of MLP, and all the Pb2+ from the medium was accumulated in the root tissues. They concluded that MLP was able to detoxify Pb2+ and to improve their bioavailability in Brassica species.
6.5.1.2.5 Vermifiltration
Vermifiltration technology is an alternative DW treatment method widely used in developing countries due to its low cost and eco-friendly nature. Manyuchi et al. (2018) reported that the TDS, TSS, TKN, BOD, and COD were significantly reduced by more than 90% during the 40 h vermifiltration process. The treated DW can be used for irrigation purposes. In addition, vermicompost, a bio-fertilizer which is rich in N (1.87%), P (0.66%), and K (0.87), was produced as part of the vermifiltration process.
6.5.1.2.6 Microbial Fuel Cells
Microbial fuel cells (MFCs), which exploit living microorganism as electrode catalysts, have the potential to recover energy from biomass wastes and distillery wastewater. It has recently attracted considerable attention as green energy devices for generating electricity from various organic and inorganic materials. Simultaneous electricity generation and DW treatment were accomplished using a thermophilic MFCs. Recently, molasses DW was examined as an organic fuel for electricity production in a mesophilic MFCs (Zhang et al. 2009; Mohanakrishna et al. 2009). Ha et al. (2012) studied the treatment of DW using a bacteroidetes-dominant thermophilic MFCs. The results suggest that thermophilic MFCs, which require less energy for cooling the DW, can achieve high efficiency for electricity generation and also reduce sulfate along with oxidizing complex organic substrates. Bacterial diversity analysis by pyrosequencing of the 16S rRNA gene showed that known Deferribacteres and Firmicutes members were not dominant in the thermophilic MFCs fed with DW; instead, uncharacterized Bacteroidetes thermophiles were up to 52% of the total reads in the anode biofilm. Recently, Mohamed et al. (2018) have investigated the effect of buffers and feed pH of the DW on the overall performance of the MFCs. The results demonstrated that anolyte of MFCs at pH 8 showed to achieved a maximum power density of 168 mW/m2, which was due to the presence of microbial communities and its exoelectrogenic activity. In addition, the COD, TDS, and color elimination have achieved a maximum of 68.4%, 15.4%, and 26.4, respectively, at pH 8.
6.5.1.3 Emerging Treatment Approaches
6.5.1.3.1 Membrane Filtration
Membrane filtration (MF) is a term used to describe the removal of particulates from a feed stream (Chang et al. 1994). Tertiary treatment of aerobically treated DW by nanofiltration (NF) was carried out in a spiral wound NF membrane module, which was done by Rai et al. (2008) under different operating conditions. They obtained COD, TDS, and color removal in the range of 96–99.5%, 85–95%, 98–99.5%. respectively. The membrane-based NF and RO processes can be used to reduce the K+ COD, TDS, and the content of DW by 99.99%, 99.90, and 99.80, respectively (Nataraj et al. 2006). Nakhla et al. (2006) studied the applicability of a submerged vacuum ultrafiltration membrane technology in combination with the biological treatment system. This system achieved 99% COD and 95–96.5% BOD removal. Submerged NF for removal of melanoidins from the BMDE was evaluated by Liu et al. (2013). The melanoidins could be effectively removed from the BMDE by SNF. However, MF technology cannot be directly applied to treat DW due to its high TDS.
6.5.1.3.2 Oxidation Processes
Oxidation processes are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) pollutants in DW by the formation of highly reactive oxidant species, mainly hydroxyl radicals (•OH), a powerful, ubiquitous in nature, nonselective, electrophilic behavior, redox potential of 2.8 V, and highly effective oxidants which accelerating the oxidation and destruction of a wide range of contaminants from wastewater by abstracting hydrogen atom from aliphatic carbon, or adding hydrogen atom to the double bonds and aromatic rings.
6.5.1.3.2.1 Ozone Oxidation
Ozone oxidation, also known as ozonation, is a promising technology for the treatment of DW. Ozone is a potent oxidant for wastewater treatment; when ozone comes in contact with wastewater, it reacts with organic compounds in two different ways: (i) direct oxidation as molecular ozone and (ii) indirect reaction through the formation of secondary oxidants like free radical species, viz., the •OH radicals. Both ozone and •OH radicals are strong oxidants and are capable of oxidizing a number of compounds, and, finally, COD value is reduced (Pena et al. 2003). The color elimination from DW was most likely due to the fact that ozone is able to break down the conjugated –C=C- bonds, thus breaking the chromophore of the melanoidins (Kim et al. 1985). Oxidation by ozone could achieve 80% decolorization of PMDE with simultaneous 15–25% COD reduction (Pena et al. 2003). Benitez et al. (2003) reported the reduction of COD and total aromatic compound up to 5–25.2% and 16.8–51.4% under optimum conditions by an ozonation process. A catalyst has also enhanced the efficiency of the ozonation process. Sangave et al. (2007) have used an ultrasound (US) plus ozone treatment process to treat DW pretreated with thermal pretreatment and AD process. The result demonstrated that 13% COD reduction was attained at the end of 48 h of aerobic oxidation, while 45.6% COD reduction was obtained in ozone-treated DW. Asaithambi et al. (2012) used a hybrid technique of ozone-assisted electrocoagulation for the elimination of COD and color in the industrial effluent. They reported a maximum elimination of COD (83%) at a current density of 3 Adm−2, initial pH (6.0), and initial COD concentration 2500 ppm, and the ozone mixture flow rate was 15 L min−1, while the complete elimination of color was found within 2 h of process time. Kumar et al. (2006) have reported that COD was removed up to 95% from treated PMDE through ozone treatment. Sreethawong and Chavadej (2008) have used iron oxide to enhance the ozone oxidation process. They reported a maximum 80% COD and 50% color reduction during the process.
6.5.1.3.2.2 Hydrogen Peroxide Treatment
The chemical decolorization of model melanoidins by H2O2 treatment was studied by Hayase et al. (1984). They reported about 64% and 97% decolorization of melanoidin using H2O2 at pH 7.0 and pH 10.0, respectively. They suggested the degradation of melanoidins by active oxygen species, i.e., H2O2, which is generated by the oxidation of glucose (C6H12O6) into gluconic acid by the glucose-oxidase enzyme. They demonstrated that H2O2 reacts with hydroxyl anion (OH−) to give per hydroxyl anion (HOO-), which nucleophilically attacks carbonyl groups (COOH) of melanoidins.
6.5.1.3.3 Photocatalytic Treatment
Photocatalytic degradation is an attractive treatment process for wastewater. Charles et al. (2015) studied the degradation of OM in the form of the color of SW using nano-photocatalyst nano-Al2O3/kaolin prepared from aluminum oxide (Al2O3) nanoparticle and kaolin clay. Optimization of the process parameters using Taguchi Orthogonal Array (TOA) design resulted in a maximum of 80% SW decolorization. A vanadium-doped TiO2 (V-TiO2) photocatalyst has been used for the degradation of SW and industrial dyes (Takle et al. 2018). The degradation of colored compounds in the SW was monitored by gel permeation chromatography, which showed the degradation of high molecular weight compounds into low molecular weight fractions.
6.5.2 Physicochemical Treatment Approaches
Physicochemical treatment methods are a combination of physical and chemical technologies used for wastewater treatment by adding chemicals. Elimination of suspended solids from the DW is a physical operation, while reduction of the dissolved solid is a chemical process. Several physicochemical methods, viz., coagulation/flocculation, electrocoagulation, thermolysis, membrane filtration, oxidation by ozone, chlorine dioxide, hydrogen peroxide, and radiation, and adsorption to material such as chitosan and activated carbon, have been shown to reduce the pollutant load of DW.
6.5.2.1 Adsorption
Activated carbon (AC) is the most extensively studied adsorbent prepared from agro-waste materials such as rice husk ash, fly ash, sugarcane bagasse, wood ash, and wood sawdust. This adsorbent has been reported to adsorb a wide array of organic compounds, i.e., phenolics, heavy metals, and other various organic pollutants and bio-organisms. Therefore, the adsorbent is used for the elimination of organic and inorganic pollutants in form color, COD, BOD, and HMs from DW (Chandra and Pandey 2000; Satyawali and Balakrishnan 2007, 2009; Mane et al. 2009). Comparative studies of color removal from DW using bagasse fly ash and commercial AC showed 58% color removal with 30 g/dm3 of bagasse fly ash and 80.70% color removal with 20 g/dm3 of commercial ACs. Lalov et al. (2000) reported 98% color and 99% COD removal from DW by using natural carbohydrate polymer chitosan as an anion exchanger at 30 min contact time. ACs are not low-cost materials; hence, in spite of their good efficiency and applicability for adsorbing a wide variety of materials, their use can sometimes be restricted due to economic considerations.
6.5.2.2 Coagulation/Flocculation
Coagulation is the use of chemicals to cause pollutants to agglomerate and subsequently settle out during sedimentation. The removal of COD and color-containing compounds from DW was reported using inorganic coagulants, viz., aluminum chloride (AlCl3), FeCl3 (ferric chloride), calcium oxide (CaO), ferrous sulfate (FeSO4), and polyaluminium chloride (PAC) (Chaudhari et al. 2007). Treatment of DW using iron sulfate [Fe2(SO4)3] as a coagulant results in 40% removal of pollutants (Pikaev et al. 2001). Migo et al. (1993) studied the use of commercial inorganic flocculent [Fe(OH)n(SO4)3-n/2]m, a polymer of ferric hydroxide, for melanoidin removal from DW. Decolorization yields of 94%, 87%, and 32% were obtained for the lagoon, biodigester, and fresh slops effluents, respectively, at a flocculant dosage of 4% v/v. In addition, the reduction of TOC was 21% for fresh slops effluent and averaged to more than 73% for the biodigester and lagoon effluents. A 55% reduction in COD by using integrated Fenton-coagulant/flocculation process in distillery wastewater treatment has been reported by Beltrain de Heredia et al. (2005). Moringa oleifera seeds were also used as a coagulant for removal of color from SW (Krishna Prasad 2009). Armini et al. (2015) used manganese oxides, a strong oxidizer that oxidize aromatic amine to quinones and dimer products, for the color removal from melanoidin-rich DW. It can also oxidize the long-chain amines to nitrone, which may further dimerize to bigger compounds and precipitate out of the solution by MnOx. Different coagulants used by various researchers for the COD and decolorization of DW are shown in Table 6.6.
6.5.2.3 Electrochemical/Electrocoagulation
The electrochemical treatment methods (i.e., electrocoagulation) use electron as the main reagent as well as the presence of supporting electrolytes, to eliminate the OM from DW (Prasad and Srivastava 2009). This process involves the consumption of metals from the anode, with simultaneous formation of OH− and H2 occurring at the cathode. In the electrochemical treatment process, the pollutants are eliminated by either direct or indirect oxidation process (Kobya and Gengec 2012). In a direct oxidation process, the pollutants are first adsorbed on the anode surface and then destroyed by the anodic electron transfer reaction, while, in an indirect oxidation process, strong oxidants, viz., hypochlorite (ClO−)/chlorine (Cl), O3, and H2O2 are electrochemically generated, and these oxidants destroyed the pollutants. Among the oxidants, ClO− is cheaper, and DW has a certain amount of chloride (Cl−). The electrochemical method converts Cl− to ClO−/Cl, through the supply of electrical current. The Cl and ClO− oxidize the pollutants and are then reduced to chloride ions. Thakur et al. (2009) studied the effect of pH 3.5–9.0 on the electrochemical treatment of PMDE. The 31.5%, 43.71%, and 48.9% COD decline was obtained at pH 9.0, 6.5, and 3.5, respectively, with a current density of 117 A/m2 in 60 min. Similarly, the effect of pH on the treatment of BMDE was studied by Kumar et al. (2009). The 32.66%, 39.95%, and 44% COD reduction was obtained at pH 2.0, 5.0, and 8.0, respectively, with a current density of 133.94 A/m2 in 90 min. Prajapati and Chaudhari (2014) reported 87% color reduction and 93% COD reduction in DW using iron electrode at optimum condition.
6.5.2.4 Thermolysis
The treatment of DW by thermolysis is done due to the existence of a diverse range of compounds such as minerals, lignin, hemicellulose, proteins, lipids, and reduced carbohydrates in DW (Chaudhari et al. 2008). Lele et al. (1989) have studied the thermolysis process for treatment of SW at temperatures in the range of 160–250 °C and autogenous pressures. They observed that there was no further COD reduction after a treatment time of 2 h. The authors reported zero-order COD reduction kinetics as 6.67, 10.40, 10.8, and 14.40 kg/m3 h at 160, 200, 230, and 250 °C, respectively. The thermolysis treatment of PMDE in the absence of air at 150 °F resulting in 35% COD reduction in t = 0.6 h has also been reported by Dhale and Mahajani (2000). Chaudhari et al. (2005) reported COD reduction of PMDE at 100 °C and atmospheric pressure by using different catalysts such as Mn/Cu oxide, Mn/Ce oxide, CuSO4, and CuO. About 70%, 65%, 35%, 38%, 40%, and 36% COD reduction was obtained at pH 2.0, 2.0, 4.0, 6.0, 8.0, and 10, respectively, using CuO catalyst. Similarly, the treatment of SW with COD reduction of 58%, 60%, 51%, 36%, 30%, and 32% at pH 1, 2, 4, 6, 8, and 10, respectively at 140 °C and autogenous pressure was reported by Chaudhari et al. (2008).
6.6 Combined Biological Treatment Approaches
Since the SW contains recalcitrant highly colored pigments which cannot be separated or degraded with conventional treatment methods, there is always a lookout for advanced treatment methods. Besides, the drawbacks associated with these methods are instable decolorization efficiency, excess use of chemicals, operational difficulty, sensitivity to variable water input, and a huge amount of sludge generation with subsequent disposal problems, and occasional formation of hazardous by-products/secondary pollutants. Therefore, there are still demands to develop substitute means of decolorization and bioremediation of DW such as pioneering eco-friendly methods capable of providing a more complete cleanup of the pollutant in a more economic fashion. Investigation in implementing a hybrid method of treating the DW has gained its soundness rather than an individual treatment. In order to increase the biodegradation ability of the process, a two-stage sequential/phase separation/sequential method has demonstrated to be an efficient approach for bioremediation of DW. Numerous scientific reports indicated that the use of a hybrid technique by using bacteria, fungi, yeast, and plant or their combinatorial systems is more successful than the individual one. For instance, Ghosh et al. (2002) investigate the treatment of DW in a two-stage bioreactor by using Pseudomonas putida followed by Aeromonas sp. strain EMa. In the first stage, P. putida decreased the color and COD of DW up to 60% and 44.4, respectively, whereas in the second stage, Aeromonas sp. strain Ema reduced the effluent COD up to about 44.4%. Kaushik et al. (2010) investigated the treatment of DW in three-stage bioreactors by using fungus followed by bacteria. The potential use of fungi (Cladosporium cladosporioides) and cyanobacteria (Phormidium valdernium) for treatment of DW in a two-stage sequential step was also reported by Ravikumar and Kartik (2015). A maximum 68.5% decolorization and 81.37% COD reduction were achieved in the first-stage bioreactor during the batch experiment. Further, the SW from bioreactor was treated with cyanobacteria in the second stage and resulted in COD reduction (3652 mg L−1) of 89.5% and 92.7% decolorization, respectively. Authors recommended that sequential treatment using the combination of fungi and cyanobacteria resulted in better decolorization and degradation of SW. Combination of wetland treatment technology after bacterial degradation offers an excellent system for the elimination of color from DW and reduction of BOD, COD, TDS, and HMs for safe disposal. A two-stage sequential treatment for sugarcane molasses-based PMDE was reported by Pant and Adholeya (2009a). In the first step, DW was treated in a hydroponic-based system using two plant species (Vetiveria zizanioides and Phragmites karka) to decrease the high nitrogen content up to 84% of the wastewater. The roots of these growing plants showed profuse growth on effluent. After that, this first stage treated DW was subjected for treatment by fungal isolates; 86.33% decolorization was obtained with Pleurotus florida Eger EM1303 followed by Aspergillus flavus TERIDB9 (74.67%), with a significant reduction in COD as well. Table 6.7 summarizes the two-stage sequential results of DW decolorization by using different organisms.
6.7 Reuse and Recycling of Distillery Effluent
In India, three popular methods are employed by distilleries to handle their wastewaters:
-
(i)
Collection of DW in storage tanks, followed by irrigation.
-
(ii)
DW treatment in settling lagoons, which are placed after AD, is found to be useful to settle the solids by gravity, evaporation processes, and application of resultant sludge on land. The outcome of evaporation is the formation of concentrated sludge that can be used as biofertilizer. Besides, distillery sludge is further incinerated to generate power, and the potassium-rich ash is recovered from the combustion of sludge.
-
(iii)
The concentrated DW is used to make powders to use as raw material for power generation and mixed compound fertilizer. The overflow from settling lagoons is sent to RO plant where the permeate (water) of RO is recycled to the ethanol production unit, reducing the water requirement in distilleries. The reject of the RO plant is mixed with the press mud and marketed as a bio-compost or discharge of the effluent to a local municipal treatment facility.
However, these three methods have their associated drawback and environmental risks. Tretament of lagoons through solar evaporation requires a huge ground region and also needs to take into consideration the weather conditions prevailing in the region, because settling lagoons are also non-functional during the monsoon. Moreover, treatment of DW in lagoons generates greenhouse gas emissions, and for irrigation practices may in some cases negatively affect the structure of aquifers, soils as well as groundwater quality.
6.7.1 Composting
Composting is a sustainable approach for bioconversion of organic residue of DW into compost (manure) through microorganisms (i.e., bacteria, actinomycetes, and fungi), and this manure may be utilized as nutrients for plant growth and development in the agricultural field. This approach not only solves the pollution and disposal problems arising from the DW and adopted by several Indian distilleries associated with sugar mills but also helps in saving the cost on chemical fertilizers (Bhalerao et al. 2006; Jadhav et al. 1992). Composting of DW is carried out using pressmud, obtained from sugarcane juice before the crystallization of sugar in sugar mills. It is the best source material for microbial growth and contains dark brown-colored, amorphous, lightweight, spongy material with 74%–75% OM, 20% volatile solids, 71% moisture, and 9% ash. In composting, DW either directly or after AD is sprayed on pressmud and mixed thoroughly using an aerotiller, which makes the pressmud aerable and enhances the decomposition process. The composting process of pressmud using PMDE wash has two unique features distinguishing it from other composting processes. One is the specially developed mixed microbial culture of fungi, bacteria, and actinomycetes, selected for their ability to rapidly degrade DW. The second is the conventional blending and mixing of the refuse comprising pressmud and liquid SW using the aerotiller machine. The integrated effect of farm yard manure (FYM), bio-compost, and BMDE as a source of plant nutrients and their effect on sugarcane yield, juice quality, nutrient uptake, and soil properties were investigated by Sinha et al. (2014). The quality of juice, viz., sucrose and purity, remains unaffected. The application of BMDE and bio-compost brings remarkable changes in the properties of soil and thus enhances the fertility of soil and productivity of sugarcane significantly.
6.7.2 Ferti-Irrigation
In India, most of the distillery units have opted DW for ferti-irrigation to improve soil health and crop productivity and alleviate environmental pollution problems. Some farmers in northern and western India living in the areas adjoining distilleries often use SW and SW-containing products as a source of manure without considering its impact on the soil and groundwater quality. However, DW also contains significant amounts of phosphorus (P), nitrogen (N), sulfur (S), and potassium (K+), as well as easily biodegradable OM and micro- and macronutrients, viz., Zn, Cu, K, N, and Fe, which are essential for plant growth (Devarajan et al. 1994; Zalawadia and Raman 1994; Pathak et al. 1999; Yadav et al. 2010). Its application to soil at low concentration has been reported to be beneficial to increase mustard yield (Malaviya and Sharma 2011), wheat and rice (Pathak et al. 1998), rice (Deverajan and Oblisami 1995), sugar cane yield (Mohamed Haron and Subash Chandra Bose 2004), and physiological response of soybean and groundnut quality (Ramana et al. 2001). The use of DW in agriculture as a supplement for irrigation or soil amendment water in a judicious way to enhanced crop production as well as biological, chemical and physical properties of soil (Joshi et al. 1996; Jadhav and Savant 1975; Narain et al. 2012; Raverkar et al. 2000; Chidankumar et al. 2009; Ramana et al. 2001; Jain and Srivastava (2012). Singh et al. (1980) found that addition of SW without dilution was very effective in increasing water intake rate of the sodic calcareous soil. Zalawadia and Raman (1994) found that the application of DW in soil improved its water-retention characteristics. Ayyasamy et al. (2008) conducted a pot experiment to study the effects of different concentrations of sugar factory effluent on seed germination, seedling growth, and biochemical characteristics of green gram and maize. The higher effluent concentrations (above 60%) were found to affect plant growth, but diluted effluent (up to 60%) favored seedling growth. Previous studies revealed that the application of untreated DW to mung beans (Vigna radiata) and rice (Oryza sativa L.) suppressed seed germination and seedling growth, suggesting that pretreatment of the effluent to degrade OM before application to crops might yield better results (Arora et al. 1992; Kannan and Upreti 2008). Distillery application increased the dry matter yield and N recovery of Italian ryegrass compared to the inorganic NH4-NO3 fertilizer treatment (Douglas et al. 2003). Asano et al. (2014) reported that a large quantity of ammonium nitrogen (NH4+-N) was increased in rice-derived DW after inoculation with A. caelatus, A. oryzae, A. tamarii, and B. subtilis strain. Chauhan and Rai (2010) also indicated that irrigation with DW impaired the groundwater quality of Gajraula region, especially of agricultural zone, making it unsuitable for drinking purpose. Kumari et al. (2016) conducted field studies on Brassica compestris to assess the potential of the diluted PMDE. The results indicated that there was not much variation in pH, electrical conductivity, and nitrate of soil, whereas TDS, COD, and nitrate conductivity of the well water increased slightly but well within the permissible limit. However, there was a significant enhancement in the root hairs, the area of the leaf, diameter of the root and shoot, plant biomass, as well as number and length of pods. The application of BMDE @150 m3 ha−1 can reduce fertilizer requirement, especially N by 75%, K2O by 100%, and P2O5 by 20% (More et al. 2008). In ferti-irrigation, the yield of Tritium aestivum increased by 33% (Kumari et al. 2009) as compared to the control using diammonium phosphate and urea suggesting that the diluted DW is capable of replacing the application of chemical fertilizer when used under controlled conditions without any adverse effect on the soil and groundwater quality (Kumari et al. 2012). The use of DW as a soil amendment has generated interest in recent times. Most crops give higher yields with wastewater irrigation and decrease the need for chemical fertilizers, resulting in net cost savings to farmers. So it is an important aspect to understand the specificity of crop-effluent relationship for their appropriate application in irrigation practices. Kumar and Chopra (2012) have studied the ferti-irrigation effect of different concentrations of DW (5%, 10%, 25%, 50%, 75%, and 100%) on agronomical practices of Trigonella foenum-graecum L. (Fenugreek) along with control (bore well water). It was observed that there was a significant outcome on moisture content, PO3−4, SO2−4, NO2−3, TKN, Fe2+, Mg2+, Ca2+, K+, Na+, CO−2, HCO−3, TOC, Cl−, pH, and EC, and insignificant effect on WHC and bulk density after irrigation of soil with different DW concentrations up to 90 days. The agronomical parameters such as crop yield, LAI, chlorophyll content, dry weight, pods, flowers, number of leaves, root length, and shoot length of T. foenum-graecum were recorded to be in increasing order at low concentration of the DW, i.e., from 5% to 50%, and in decreasing order at higher DW concentration, i.e., from 75% to 100% as compared to control. The authors concluded that the long-term use of PMDE in agricultural fields may pose a serious threat to the groundwater quality.
6.8 Conclusion
The disposal of untreated or partially treated DW into the environment results in soil and water pollution leading to adverse effects on aquatic life and local vegetation. Thus, there is an urgent need to look into economically viable and easy-to-use technology for DW treatment. Several inexpensive secondary and tertiary treatment technologies including physicochemical and biological methods have been investigated for potential treatment of DW. However, these technologies are not techno-economic feasible options for mitigating the problems associated with the treatment and disposal of DW due to its complex nature of pollutants, which cannot be easily degraded by single-step treatment processes. Therefore, adequate treatment of DW by a novel two-step treatment/phase separation/sequential method by using organisms and their combinatorial systems has proven to be an effective approach for bioremediation of DW. The efficacy of the two-step treatment approach has been demonstrated under the pilot scale. This approach was found to be effective also in the field scale, and it is likely that during the next 5–10 years, its use will become widespread.
References
Acharya BK, Mohana S, Madamwar D (2008) Anaerobic treatment of distillery spent wash: a study on upflow anaerobic fixed film bioreactor. Bioresour Technol 99:4621–4626
Acharya BK, Pathak H, Mohana S, Shouche Y, Singh V, Madamwar D (2011) Kinetic modeling and microbial community assessment of anaerobic biphasic fixed film bioreactor treating distillery spent wash. Water Res 45:4248–4259
Adikane HV, Patale MB (2014) Biodegradation studies of non-sterile anaerobically treated distillery spent wash using Aspergillus nidulans isolated from irrigation site. Curr Biotechnol 3(2):174–179
Akunna JC, Clark M (2000) Performance of a granular-bed anaerobic baffled reactor (GRABBR) treating whisky distillery wastewater. Bioresour Technol 74(3):257–261
Alkorta I, Hernández-Allica J, Becerril JM, Amezaga I, Albizu I, Garbisu C (2004) Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as Zinc, Cadmium, Lead, and Arsenic. Rev Environ Sci Biotechnol 3(1):71–90
Angayarkanni J, Palaniswamy M, Swaminathan K (2003) Biotreatment of distillery effluent using Aspergillus niveus. Bull Environ Contam Toxicol 70(2):268–277
Aoshima I, Tozawa Y, Ohmomo S, Ueda K (1985) Production of decolorizing activity for molasses pigment by Coriolus versicolor Ps4a. Agric Biol Chem 49:2041–2045
Arimi MM, Zhang Y, Goetz G, Kiriamiti K, Geissen SU (2014) Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int Biodeterior Biodegr 87:34–43
Arimi MM, Zhang Y, Götz G, Geisen S-U (2015) Treatment of melanoidin wastewater by anaerobic digestion and coagulation. Environ Technol 36(19):2410–2418
Arora M, Sharma D, Behera B (1992) Upgrading of distillery effluent by Nitrosococcus oceanus for its use as a low-cost fertilizer. Resour Conserv Recycl 6:347–353
Asaithambi P, Susree M, Saravanathamizhan R, Matheswaran M (2012) Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalination 297:1–7
Asano R, Kobayashi S, Sonobe K, Shime-Hattori A, Okazaki K, Ohtomo R (2014) Plant-available inorganic nutrient levels are increased in rice-derived distillery effluents inoculated with microbes. J Appl Microbiol 117:1412–1421
Ayyasamy PM, Yasodha R, Rajakumar S, Lakshmanaperumalsamy P, Rahman PKSM, Lee S (2008) Impact of sugar factory effluent on the growth and biochemical characteristics of terrestrial and aquatic plants. Bull Environ Contam Toxicol 81:449–454
Beltrain de Heredia J, Domingues JR, Partido E (2005) Physico-chemical treatment for depuration of wine distillery wastewater (vinasses). Water Sci Technol 51(1):159–166
Benitez FJ, Real FJ, Acero JL, Garcia J, Sanchez M (2003) Kinetics of the ozonation and aerobic biodegradation of wine vinasses in discontinuous and continuous processes. J Hazard Mater B 101:203–218
Bhalerao VP, Jadhav MB, Bhoi PG (2006) Effect of spent wash, press mud and compost on soil properties, yield and quality of sugarcane. Indian Sugar 40(6):57–65
Bharagava RN, Chandra R (2010) Effect of bacteria treated and untreated post-methanated distillery effluent (PMDE) on seed germination, seedling growth and amylase activity in Phaseolus mungo L. J Hazard Mater 180:730–734
Bharagava RN, Chandra R, Rai V (2008) Phytoextraction of trace elements and physiological changes in Indian mustard plants (Brassica nigra L.) grown in post methanated distillery effluent (PMDE) irrigated soil. Bioresour Technol 99:8316–8324
Bharagava RN, Chandra R, Rai V (2009) Isolation and characterization of aerobic bacteria capable of the degradation of synthetic and natural melanoidins from distillery effluent. World J Microbiol Biotechnol 25(5):737–744
Billore SK, Singh N, Ram HK, Sharma JK, Singh VP, Nelson RM, Dass P (2001) Treatment of a molasses based distillery effluent in a constructed wetland in Central India. Water Sci Technol 44:441–448
Blonskaja V, Menert A, Vilu R (2003) Use of two-stage anaerobic treatment for distillery waste. Adv Environ Res 7:671–678
Bonugli-Santos RC, Durrant LR, Sette LD (2012) The production of ligninolytic enzymes by marine-derived basidiomycetes and their biotechnological potential in the biodegradation of recalcitrant pollutants and the treatment of textile effluents. Water Air Soil Pollut 223(5):2333–2345
Bories A, Raynal J, Bazile F (1988) Anaerobic digestion of high-strength distillery wastewater (cane molasses stillage) in a fixed-film reactor. Biol Wastes 23(4):251–267
Borja R, Martin A, Maestro R, Luque M, Durán J (1993) Enhancement of the anaerobic digestion of wine distillery wastewater by the removal of phenolic inhibitors. Bioresour Technol 45:99–104
Chandra R, Kumar V (2015a) Biotransformation and biodegradation of organophosphates and organohalides. In: Chandra R (ed) Environmental Waste Management. CRC Press, Boca Raton, pp 475–524. https://doi.org/10.1201/b19243-17.
Chandra R, Kumar V (2015b) Mechanism of wetland plant rhizosphere bacteria for bioremediation of pollutants in an aquatic ecosystem. In: Chandra R (ed) Advances in biodegradation and bioremediation of industrial waste. CRC Press, Boca Raton
Chandra R, Kumar V (2017a) Detection of androgenic-mutagenic compounds and potential autochthonous bacterial communities during in situ bioremediation of post methanated distillery sludge. Front Microbiol 8:887
Chandra R, Kumar V (2017b) Detection of Bacillus and Stenotrophomonas species growing in an organic acid and endocrine-disrupting chemicals rich environment of distillery spent wash and its phytotoxicity. Environ Monit Assess 189:26
Chandra R, Kumar V (2017c) Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilized distillery sludge as a prospective tool for in-situ phytoremediation of industrial waste. Environ Sci Pollut Res 24:2605–2619
Chandra R, Kumar V (2018) Phytoremediation: a green sustainable technology for industrial waste management. In: Chandra R, Dubey NK, Kumar V (eds) Phytoremediation of environmental pollutants. CRC Press, Boca Raton
Chandra R, Pandey PK (2000) Decolourization of anaerobically-treated distillery effluent by activated charcoal adsorption method. Indian J Environ Prot 21:134–137
Chandra R, Kumar V, Tripathi S (2018a) Evaluation of molasses-melanoidin decolorisation by potential bacterial consortium discharged in distillery effluent. 3 Biotech 8:187
Chandra R, Kumar V, Tripathi S, Sharma P (2018b) Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in situ phytoremediation. Ecol Eng 111:143–156
Chandra R, Kumar V, Tripathi S, Sharma P (2018c) Phytoremediation of industrial pollutants and life cycle assessment. In: Chandra R, Dubey NK, Kumar V (eds) Phytoremediation of environmental pollutants. CRC Press, Boca Raton
Chandra R, Kumar V, Singh K (2018d) Hyperaccumulator versus nonhyperaccumulator plants for environmental waste management. In: Chandra R, Dubey NK, Kumar V (eds) Phytoremediation of environmental pollutants. CRC Press, Boca Raton
Chandra R, Kumar V, Yadav S (2017) Extremophilic ligninolytic enzymes. In: Sani R, Krishnaraj R (eds) Extremophilic enzymatic processing of lignocellulosic feedstocks to bioenergy. Springer
Chandra R, Saxena G, Kumar V (2015) Phytoremediation of environmental pollutants: an eco-sustainable green technology to environmental management. In: Chandra R (ed) Advances in biodegradation and bioremediation of industrial waste. CRC Press, Boca Raton, pp 1–29
Chandra R, Bharagava RN, Kapley A, Purohit HJ (2012) Characterization of Phragmites cummunis rhizosphere bacterial communities and metabolic products during the two stage sequential treatment of post methanated distillery effluent by bacteria and wetland plants. Bioresour Technol 103:78–86
Chandra R, Yadav S, Bharagava RN, Murthy RC (2008) Bacterial pretreatment enhances removal of heavy metals during treatment of post-methanated distillery effluent by Typha angustata L. J Environ Manag 88:1016–1024
Chandraju S, Basavaraju HC (2007) Impact of distillery spent wash on seed germination and growth of leaves vegetables: an investigation. Sugar J (SISSTA) 38:20–50
Chandralata R, Mohandass C, Shilpa K, Shailaja MS (2004) Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzym Microb Technol 35(2–3):197–202
Chang IS, Choo KH, Lee CH, Pek UH, Koh UC, Kim SW, Koh JH (1994) Application of ceramic membrane as a pre-treatment in anaerobic digestion of alcohol-distillery wastes. J Memb Sci 90(1–2):131–139
Charles D, Arivazhagan M, Balamurali MN, Shanmugarajan D (2015) Decolorization of distillery spent wash using biopolymer synthesized by isolated from tannery effluent. Biomed Res Int 2015:1–9
Chaturvedi S, Chandra R, Rai V (2006) Isolation and characterization of Phragmites australis (L.) rhizosphere bacteria from contaminated site for bioremediation of colored distillery effluent. Ecol Eng 27:202–207
Chaudhari PK, Mishra IM, Chand S (2005) Catalytic thermal treatment (catalytic thermolysis) of a biodigester effluent of an alcohol distillery plant. Ind Eng Chem Res 44(15):5518–5525
Chaudhari PK, Mishra IM, Chand S (2007) Decolourization and removal of chemical oxygen demand (COD) with energy recovery: treatment of biodigester effluent of a molasses-based alcohol distillery using inorganic coagulants. Colloids Surf A Physicochem Eng Asp 296(1–3):238–247
Chaudhari PK, Mishra IM, Chand S (2008) Effluent treatment of alcohol distillery: catalytic thermal pretreatment (catalytic thermolysis) with energy recovery. Chem Eng J 136:14–24
Chauhan J, Rai JPN (2010) Monitoring of impact of ferti-irrigation by postmethanated distillery effluent on groundwater quality. Clean Soil, Air, Water 38(7):630–638
Chavan MN, Dandi ND, Kulkarni MV, Chaudhari AB (2013) Biotreatment of melanoidin-containing distillery spent wash effluent by free and immobilized Aspergillus oryzae MTCC 7691. Water Air Soil Pollut 224(11):5
Chidankumar CS, Chandaraju S, Nagendra Swamy R (2009) Impact of distillery spent wash irrigation on the yields of some root vegetables in untreated and spent wash treated soil. SISSTA 40:233–236
Collins G, Foy C, McHugh S, Maho T, O’Flaherty V (2005) Anaerobic biological treatment of phenolic wastewater at 15–18°C. Water Res 39:1614–1620
Dahiya J, Sing D, Nigam P (2001) Decolourisation of synthetic and spent wash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40. Bioresour Technol 78:95–98
Davamani V, Lourduraj AC, Singaram P (2006) Effect of sugar and distillery wastes on nutrient status, yield and quality of turmeric. Crop Research Hisar 32(3):563–567
Devarajan L, Rajanan G, Ramanathan G, Oblisami G (1994) Performance of field crops under distillery effluent irrigations. Kisan World 21:48–50
Deverajan L, Oblisami G (1995) Effect of distillery effluent on soil fertility status, yield and quality of rice. Madras Agric J 82:664–665
Dhale AD, Mahajani VV (2000) Treatment of distillery waste water after bio-gas generation: wet oxidation. Indian J Chem Technol 7(1):11–18
Douglas JT, Aitken MN, Smith CA (2003) Effects of five non-agricultural organic wastes on soil composition, and on the yield and nitrogen recovery of Italian ryegrass. Soil Use Manag 19(2):15–138
D’Souza DT, Tiwari R, Sah AK, Raghukumar C (2006) Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzym Microb Technol 38(3-4):504–511
Fahy V, FitzGibbon FJ, McMullan G, Singh D, Marchant R (1997) Decolourisation of molasses spent wash by Phanerochaete chrysosporium. Biotechnol Lett 19(1):97–99
Fan L, Nguyen T, Roddick FA (2011) Characterisation of the impact of coagulation and anaerobic bio-treatment on the removal of chromophores from molasses wastewater. Water Res 45(13):3933–3940
Ghosh M, Ganguli A, Tripathi AK (2002) Treatment of anaerobically digested distillery spent wash by a two stage bioreactor using Pseudomonas putida and Aeromonas sp. Process Biochem 37(8):857–862
Ghosh M, Verma SC, Mengoni A, Tripathi AK (2004) Enrichment and identification of bacteria capable of reducing chemical oxygen demand of anaerobically treated molasses spent wash. J Appl Microbiol 96(6):1278–1286
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374
Gonzalez T, Terron MC, Yague S, Zapico E, Galletti GC, Gonzalez AE (2000) Pyrolysis/gas chromatography/mass spectrometry monitoring of fungal-biotreated distillery wastewater using Trametes sp. I-62 (CECT 20197). Rapid Commun Mass Spectrom 14(15):1417–1424
González T, Terrón MC, Yagüe S, Junca H, Carbajo JM, Zapico EJ, Silva R, Arana-Cuenca A, Téllez A, González AE (2008) Melanoidincontaining wastewaters induce selective laccase gene expression in the white-rot fungus Trametes sp. I-62. Res Microbiol 159(2):103–109
Ha PT, Lee TK, Rittmann BE, Park J, Chang IS (2012) Treatment of alcohol distillery wastewater using a bacteroidetes-dominant thermophilic microbial fuel cell. Environ Sci Technol 46:3022–3030
Harada H, Uemura S, Chen A-C, Jayadevan J (1996) Anaerobic treatment of a recalcitrant distillery wastewater by a thermophilic UASB reactor. Bioresour Technol 55(3):215–221
Hatano K, Komatsu I, Aoyagi N, Takahashi K, Kubota K (2013) A study on the self-assembly behavior of dark materials from molasses. Environ Sci Pollut Res 20:4009–4017
Hatano K-I, Kanazawa K, Tomura H, Yamatsu T, Tsunoda K-i, Kubota K (2016) Molasses melanoidin promotes copper uptake for radish sprouts: the potential for an accelerator of phytoextraction. Environ Sci Pollut Res 23(17):17656–17663
Hatano K, Yamatsu T (2018) Molasses melanoidin-like products enhance phytoextraction of lead through three Brassica species. Int J Phytoremediation. https://doi.org/10.1080/15226514.2017.1393397
Hayase F, Kim SB, Kato H (1984) Decolorization and degradation products of the melanoidins by hydrogen peroxide. Agric Biol Chem 48(11):2711–2717
Inanc B, Ciner F, Ozturk I (1999) Colour removal from fermentation industry effluents. Water Sci Technol 40(1):331–338
Jadhav HD, Savant NK (1975) Influence of added spent wash (distillery waste) on chemical and physical properties of soil. Indian J Agric Chem 8:78–84
Jadhav MB, Joshi VA, Jagtap PB, Jadhav SB (1992) Effect spent wash press mud cake compost on soil physicochemical. Biological properties, yield and quality of adsali sugarcane. Ann Convention DSTA II:119–134
Jagdale HN, Sawant NK (1975) Influence of added spent wash (Distillery waste) on growth and chemical composition of immature sugarcane (Saccharum officinarum L.) cultivar CO-740. Indian Sugar 29(7):433–440
Jain R, Srivastava S (2012) Nutrient composition of spent wash and its impact on sugarcane growth and biochemical attributes. Physiol Mol Biol Plants 18(1):95–99
Jiranuntipon S, Chareonpornwattana S, Damronglerd S, Albasi C, Delia M-L (2008) Decolorization of synthetic melanoidins-containing wastewater by a bacterial consortium. J Ind Microbiol Biotechnol 35(11):1313–1321
Jiranuntipon S, Delia M-L, Albasi C, Damronglerd S, Chareonpornwattana S (2009) Decolourization of molasses based distillery wastewater using a bacterial consortium. ScienceAsia 35(4):332
Joshi HC, Kalra N, Chaudhary A, Deb DL (1994) Environmental issues related with distillery effluent utilization in agriculture in India. Asia Pacific J Environ Dev 1:92–103
Joshi HC, Pathak H, Chaudary A, Kalra N (1996) Distillery effluent as a source of plant nutrients: prospects and problems. Fert News 41:41–47
Joshi HC, Pathak H, Chaudhary A, Joshi TP, Phogat VK, Kalra N (2000) Changes in soil properties with distillery effluent irrigation. J Environ Res 6(4):153–162
Kalavathi DF, Uma L, Subramanian G (2001) Degradation and metabolization of the pigment—melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181. Enzy Microb Technol 29:246–251
Kambe TN, Shimomura M, Nomura N, Chanpornpong T, Nakahara T (1999) Decolourization of molasses wastewater by Bacillus sp. under thermophilic and anaerobic conditions. J Biosci Bioeng 87:119–121
Kannan A, Upreti R (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615
Kaushik G, Thakur IS (2009) Isolation of fungi and optimization of process parameters for decolorization of distillery mill effluent. World J Microbiol Biotechnol 25(6):955–964
Kaushik G, Thakur IS (2013) Adsorption of colored pollutants from distillery spent wash by native and treated fungus: Neurospora intermedia. Environ Sci Pollut Res 20(2):1070–1078
Kaushik G, Gopal M, Thakur IS (2010) Evaluation of performance and community dynamics of microorganisms during treatment of distillery spent wash in a three stage bioreactor. Bioresour Technol 101:4296–4305
Kaushik A, Nisha R, Jagjeeta K, Kaushik CP (2005) Impact of long and short term irrigation of a sodic soil with distillery effluent in combination with bioamendments. Bioresour Technol 96:1860–1866
Keyser M, Witthuhn RC, Ronquest LC, Britz TJ (2003) Treatment of winery effluent with upflow anaerobic sludge blanket (UASB)–granular sludges enriched with Enterobacter sakazakii. Biotechnol Lett 25(22):1893–1898
Kim SB, Hayase F, Kato H (1985) Decolourisation and degradation products of melanoidin on ozonolysis. Agric Biol Chem 49:785–792
Kim SJ, Shoda M (1999) Batch decolorization of molasses by suspended and immobilized fungus of Geotrichum candidum. J Biosci Bioeng 88(5):586–589
Kobya M, Gengec E (2012) Decolourization of melanoidins by a electrocoagulation process using aluminium electrodes. Environ Technol 33(21):2429–2438
Krishna Prasad R (2009) Color removal from distillery spent wash through coagulation using Moringa oleifera seeds: use of optimum response surface methodology. J Hazard Mater 165(1–3):804–811
Krishnamoorthy S, Premalatha M, Vijayasekaran M (2017) Characterization of distillery wastewater–an approach to retrofit existing effluent treatment plant operation with phycoremediation. J Clea Produc 148:735–750
Krzywonos M (2012) Decolorization of sugar beet distillery effluent using mixed cultures of bacteria of the genus Bacillus. Afr J Biotechnol 11(14):3464–3475
Krzywonos M, Seruga P (2012) Decolorization of sugar beet molasses vinasse, a high-strength distillery wastewater, by lactic acid bacteria. Pol J Environ Stud 21(4):943–948
Kumar P, Chandra R (2004) Detoxification of distillery effluent through Bacillus thuringiensis (MTCC 4714) enhanced phytoremediation potential of Spirodela polyrrhiza (L.) Schliden. Bull Environ Contam Toxicol 73:903–910
Kumar P, Chandra R (2006) Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour Technol 97:2096–2102
Kumar V, Chandra R (2018) Characterisation of manganese peroxidase and laccase producing bacteria capable for degradation of sucrose glutamic acid-maillard products at different nutritional and environmental conditions. World J Microbiol Biotechnol 34:32
Kumar V, Chandra R (2020) Bioremediation of melanoidins containing distillery waste for environmental safety. In: Saxena G, Bharagava RN (eds) Bioremediation of Industrial Waste for Environmental Safety. Vol II- Microbes and Methods for Industrial Waste Management. Springer
Kumar V, Chopra AK (2012) Fertigation effect of distillery effluent on agronomical practices of Trigonella foenum-graecum L. (fenugreek). Environ Monit Assess 184(3):1207–1219
Kumar S, Gopal K (2001) Impact of distillery effluent on physiological consequences in the fresh water teleost Channa punctatus. Bull Environ Contam Toxicol 66:617–622
Kumar V, Sharma DC (2019) Distillery effluent: Pollution profile, eco-friendly treatment strategies, challenges, and future prospects. In: Arora PK (eds) Microbial metabolism of xenobiotic compounds. Springer Nature. https://doi.org/10.1007/978-981-13-7462-3_17
Kumar M, Infant F, Ponselvan A, Malviya JR, Srivastava VC, Mall ID (2009) Treatment of bio-digester effluent by electrocoagulation using iron electrodes. J Hazard Mater 165:345–352
Kumar A, Saroj DP, Tare V, Bose P (2006) Treatment of distillery spent-wash by ozonation and biodegradation: Significance of pH reduction and inorganic carbon removal before ozonation. Water Environ Res 78(9):994–1004
Kumar V, Shahi SK, Singh S (2018) Bioremediation: an eco-sustainable approach for restoration of contaminated sites. In: Singh J, Sharma D, Kumar G, Sharma NR (eds) Microbial bioprospecting for sustainable development. Springer, Sharma
Kumar V, Wati L, Fitz Gibbon F, Nigan P, Banat IM, Singh D, Marchant R (1997) Bioremediation and decolorization of anaerobically digested distillery spent wash. Biotechnol Lett 19:311–313
Kumar V, Wati L, Nigam P, Banat IM, Yadav BS, Singh D, Marchant R (1998) Decolorization and biodegradation of anaerobically digested sugarcane molasses spent wash effluent from biomethanation plants bywhite-rot fungi. Process Biochem 33(1):83–88
Kumari K, Ranjan N, Ray SNC, Aggarwal PK, Sinha RC (2009) Eco-friendly utilization of diluted distillery effluent on the morphological, physiological and biochemical parameters in the wheat plant “Triticum aestivum”. Int J Bioved 20:1–8
Kumari K, Ranjan N, Sharma JP, Aggarwal PK, Sinha RC (2012) Integrated management of diluted distillery effluent and fly ash as a potential biofertilizer: a case study on the vegetative growth and chlorophyll content of the marigold plant, “Tagetespatula”. Int J Environ Tech Manag 15:275–290
Kumari K, Ranjan N, Kumar S, Sinha RC (2016) Distillery effluent as a liquid fertilizer: a win–win option for sustainable agriculture. Environ Technol 37(3):381–387
Lalov IG, Guerginov II, Krysteva A, Fartsov K (2000) Treatment of wastewater from distilleries with chitosan. Water Res 34(5):1503–1506
Lele SS, Rajadhyaksha PJ, Joshi JB (1989) Effluent treatment for alcohol distillery: thermal pretreatment with energy recovery. Environ Prog 8:245–252
Liakos TI, Lazaridis NK (2014) Melanoidins removal from simulated and real wastewaters by coagulation and electro-flotation. Chem Eng J 242:269–277
Liang Z, Wang Y, Yu Z, Liu H (2009a) Coagulation removal of melanoidins from biologically treated molasses wastewater using ferric chloride. Chem Eng J 152(1):88–94
Liang Z, Wang Y, Yu Z, Liu H, Wu Z (2009b) Variables affecting melanoidins removal from molasses wastewater by coagulation/flocculation. Sep Purif Technol 68(3):382–389
Liu J, Zhang X (2014) Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res 65:64–72
Liu M, Zhu H, Dong B, Zheng Y, Yu S, Gao C (2013) Submerged nanofiltration of biologically treated molasses fermentation wastewater for the removal of melanoidins. Chem Eng J 223:388–394
López I, Borzacconi L, Passeggi M (2018) Anaerobic treatment of sugar cane vinasse: treatability and real-scale operation. J Chem Technol Biotechnol 93:1320–1327
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Mahgoub S, Tsioptsias C, Samaras P (2016) Biodegradation and decolorization of melanoidin solutions by manganese peroxidase yeasts. Water Sci Technol 73(10):2436–2445
Malaviya P, Sharma A (2011) Effect of distillery effluent on yield attributes of Brassica napus L. J Environ Biol 32:385–389
Mane JD, Modi S, Nagawade S, Phadnis SP, Bhandari VM (2009) Treatment of Spentwash using chemically modified bagasse and color removal studies. Bioresour Technol 97:1752–1755
Manyuchi MM, Mbohwa C, Muzenda E (2018) Biological treatment of distillery wastewater by application of the vermifiltration technology. South Afr J Chem Eng 25:74–78
Mehta J, Yadav A, Dilbaghi N, Sharma P (2014) Physicochemical characterization of distillery effluent and COD reduction by using Bacillus badius and Lysinibacillus fusiformis. Int J Emerg Trends Sci Technol 1(3):340–346
Migo VP, Del Rosario EJ, Matsumura M (1997) Flocculation of melanoidins induced by inorganic ions. J Ferment Bioeng 83(3):287–291
Migo VP, Matsumura M, Delrosario EJ, Kataoka H (1993) Decolorization of molasses wastewater using an inorganic flocculent. J Ferment Bioeng:75–438
Miyata N, Iwahori K, Fujita M (1998) Manganese-independent and -dependent decolorization of melanoidin by extracellular hydrogen peroxide and peroxidases from Coriolus hirsutus pellets. J Ferment Bioeng 85(5):550–553
Miyata N, Mori T, Iwahori K, Fujita M (2000) Microbial decolorization of melanoidin-containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus. J Biosci Bioeng 89(2):145–150
Moletta R (2005) Winery and distillery wastewater treatment by anaerobic digestion. Water Sci Technol 51(1):137–144
Mohammad P, Azarmidokht H, Fatollah M, Mahboubeh B (2006) Application of response surface methodology for optimization of important parameters in decolorizing treated distillery wastewater using Aspergillus fumigatus UB2 60. Int Biodeterior Biodegrad 57(4):195–199
Mohamed SN, Karunakaran RT, Manickam M (2018) Enhancement of bioelectricity generation from treatment of distillery wastewater using microbial fuel cell. Environ Progress Sustain Energy 37(2):663–668
Mohamed Haron AR, Subash Chandra Bose M (2004) Use of distillery spent wash for alkali soil reclamation, treated distillery effluent for ferti-irrigation of crops. Indian Farming 3:48–51
Mohana S, Desai C, Madamwar D (2007) Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresour Technol 98(2):333–339
Mohanakrishna G, Venkata Mohan S, Sarma PN (2009) Bioelectrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J Hazard Mater 177(1–3):487–−494
More NB, Shinde NJ, Pol KM (2008) Utilization of distillery effluent in agriculture. Indian Sugar 58:31–61
Nakhla G, Lugowski A, Patel J, Rivest V (2006) Combined biological and membrane treatment method processing wastewater to achieve dry-ditch criteria: pilot and full scale performance. Bioresour Technol 99:1–14
Narain K, Yazdani T, Bhat MM, Yunus M (2012) Effect on physico-chemical and structural properties of soil amended with distillery effluent and ameliorated by cropping two cereal plant spp. Environ Earth Sci 66:977–984
Nataraj SK, Hosamani KM, Aminabhavi TM (2006) Distillery wastewater treatment by the membrane-based nanofiltration and reverse osmosis processes. Water Res 40(12):2349–2356
Pant D, Adholeya A (2007) Identification, ligninolytic enzyme activity and decolorization potential of two fungi isolated from a distillery effluent contaminated site. Water Air Soil Pollut 183(1–4):165–176
Pant D, Adholeya A (2009a) Nitrogen removal from biomethanated spent wash using hydroponic treatment followed by fungal decolorization. Environ Eng Sci 26:559–565
Pant D, Adholeya A (2009b) Concentration of fungal ligninolytic enzymes by ultrafiltration and their use in distillery effluent decolorization. World J Microbiol Biotechnol 25:1793–1800
Pathak H, Joshi HC, Chaudhary A, Karla N, Dwivedi MK (1998) Distillery effluent as soil amendment for wheat and rice. J Indian Soil Sci 46:155–157
Pathak H, Joshi HC, Chaudhary A, Chaudhary R, Kalra N, Dwiwedi MK (1999) Soil amendment with distillery effluent for wheat and rice cultivation. Water, Air, Soil Pollu 113:133–140
Pena M, Coca M, Gonzalez G, Rioja R, Garcia MT (2003) Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere 51:893–900
Pikaev AK, Ponomarev AV, Bludenko AV, Minin VN, Elizar’eva LM (2001) Combined electronic-beam and coagulation purification of molasses distillery slops. Features of the method, technical and economic evaluation of large scale facility. Rad Phy Chem 61(1):81–87
Prajapati A, Chaudhari P (2014) Electrochemical treatment of rice grain based distillery effluent: chemical oxygen demand and color removal. Environ Technol 35:242–248
Prajapati AK, Choudhary R, Verma K, Chaudhari PK, Dubey A (2015) Decolorization and removal of chemical oxygen demand (COD) of rice grain–based biodigester distillery effluent (BDE) using inorganic coagulants. Desalin Water Treat 53(8):2204–2214
Prasad RK, Srivastava SN (2009) Electrochemical degradation of distillery spent wash using catalytic anode: factorial design of cexperiments. Chem Eng J 146:22–29
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71(5):834–842
Raghukumar C, Rivonkar G (2001) Decolorization of molasses spent wash by the white-rot fungus Flavodon flavus, isolated from a marine habitat. Appl Microbiol Biotechnol 55(4):510–514
Rai UK, Muthukrishnan M, Guha BK (2008) Tertiary treatment of distillery wastewater by nanofiltration. Desalination 230(1–3):70–78
Ramakritinan CM, Kumaraguru AK, Balasubramanian MP (2005) Impact of distillery effluent on carbohydrate metabolism of freshwater fish, Cyprinus carpio. Ecotoxicology 14(7):693–707
Ramana S, Biswas AK, Kundu S, Saha JK, Yadava BR (2001) Effect of distillery effluent on seed germination in some vegetable crops. Bioresour Technol 82:273–275
Raverkar KP, Ramana S, Singh AB, Biswas AK, Kundu S (2000) Impact of post response of soyabean (Glycine max L.) to foliar application of distillery effluent. Ann Plant Soil Res 2:1–6
Ravikumar R, Karthik V (2015) Effective utilization and conversion of spent distillery liquid to valuable products using an intensified technology of two-stage biological sequestration. Chem Biochem Eng Q 29(4):599–608
Ravikumar R, Vasanthi NS, Saravanan K (2011) Single factorial experimental design for decolorizing anaerobically treated distillery spent wash using cladosporium cladosporioides. Int J Environ Sci Technol 8(1):97–106
Ravikumar R, Vasanthi NS, Saravanan K (2013) Biodegradation and decolorization of distillery spent wash with product release by a novel strain Cladosporium cladosporioides: optimization and Biokinetics. Chem Biochem Eng Q 27(3):373–383
Saini JK, Lohchab RK (2017) Performance of UASB reactor to treat distillery spent wash under various operating conditions. Ann Biol 33(1):10–15
Saner AB, Mungray AK, Mistry NJ (2014) Treatment of distillery wastewater in an upflow anaerobic sludge blanket (UASB) reactor. Desal WatTreat:1–17
Sangave PC, Gogate PR, Pandit AB (2007) Ultrasound and ozone assisted biological degradation of thermally pretreated and anaerobically pretreated distillery wastewater. Chemosphere 68:42–50
Sankaran K, Lakshmi P, Suriya Narayanan G, Premalatha M (2015) Bacterial assisted treatment of anaerobically digested distillery wastewater. RSC Adv 5(87):70977–70984
Santal AR, Singh NP, Saharan BS (2011) Biodegradation and detoxification of melanoidin from distillery effluent using an aerobic bacterial strain SAG of Alcaligenes faecalis. J Hazard Mater 193:319–324
Santal AR, Singh NP, Saharan BS (2016) A novel application of Paracoccus pantotrophus for the decolorization of melanoidins from distillery effluent under static conditions. J Environ Manag 169:78–83
Satyawali Y, Balakrishnan M (2007) Removal of color from biomethanated distillery spentwash by treatment with activated carbons. Bioresour Technol 98(14):2629–2653
Satyawali Y, Balakrishnan M (2009) Performance enhancement with powdered activated carbon (PAC) addition in a membrane bioreactor (MBR) treating distillery effluent. J Hazard Mater 170:457–465
Singh SS, Dikshit AK (2011) Decolourization of anaerobically digested and polyaluminium chloride treated distillery spentwash in a fungal stirred tank aerobic reactor. Biodegradation 22(6):1109–1117
Singh NK, Pandey GC, Rai UN, Tripathi RD, Singh HB, Gupta DK (2005) Metal accumulation and ecophysiological effects of distillery effluent on Potamogeton pectinatus L. Bull Environ Contam Toxicol 74:857–863
Singh R, Singh NT, Arora Y (1980) The use of spent wash for the reclamation of sodic soils. J Indian Soc Soil Sci 28:38–41
Sinha SK, Jha CK, Kumar V, Kumari G, Alam M (2014) Integrated effect of bio-methanated distillery effluent and bio-compost on soil properties, juice quality and yield of sugarcane in entisol. Sugar Tech 16(1):75–79
Sirianuntapiboon S, Zohsalam P, Ohmomo S (2004) Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process Biochem 39(8):917–924
Solovchenko A, Pogosyan S, Chivkunova O, Selyakh I, Semenova L, Voronova E, Scherbakov P, Konyukhov I, Chekanov K, Kirpichnikov M, Lobakova E (2014) Phycoremediation of alcohol distillery wastewater with a novel Chlorella sorokiniana strain cultivated in a photobioreactor monitored on-line via chlorophyll fluorescence. Algal Res 6:234–241
Sreethawong T, Chavadej S (2008) Color removal of distillery wastewater by ozonation in the absence and presence of immobilized iron oxide catalyst. J Hazard Mater 155:486–493
Srivastava S, Jai R (2010) Effect of distillery spent wash on cytomorphological behaviour of sugarcane settlings. J Environ Res 31:809–812
Takle SP, Naik SD, Khore SK, Ohwal SA, Bhujbal NM, Landge SL, Kale BB, Sonawane RS (2018) Photodegradation of spent wash, a sugar industry waste, using vanadium-doped TiO nanoparticles. RSC Adv 8:20394
Tapia-Tussell R, Pérez-Brito D, Torres-Calzada C, Cortés-Velázquez A, Alzate-Gaviria L, Chablé-Villacís R, Solís-Pereira S (2015) Laccase gene expression and vinasse biodegradation by Trametes hirsuta Strain Bm-2. Molecules 20(8):15147–15157
Tewari PK, Batra VS, Balakrishnan M (2007) Water management initiatives in sugarcane molasses based distilleries in India. Resour Conserv Recycl 52(2):351–367
Thakkar AP, Dhamankar VS, Kapadnis BP (2006) Biocatalytic decolourisation of molasses by Phanerochaete chrysosporium. Bioresour Technol 97(12):1377–1381
Thakur C, Srivastava VC, Mall ID (2009) Electrochemical treatment of a distillery wastewater: parametric and residue disposal study. Chem Eng J 148:496–505
Thiyagu R, Sivarajan P (2018) Isolation and characterization of novel bacterial strain present in a lab scale hybrid UASB reactor treating distillery spent wash. Environ Technol 15:1–7
Tiwari S, Gaur R, Singh R (2012a) Decolorization of a recalcitrant organic compound (Melanoidin) by a novel thermotolerant yeast, Candida tropicalis RG-9. BMC Biotechnol 12(1):30
Tiwari S, Gaur R, Rai P, Tripathi A (2012b) Decolorization of distillery effluent by thermotolerant Bacillus subtilis. American J Appl Sci 9(6):798–806
Tiwari S, Gaur R, Singh A (2014) Distillery Spentwash Decolorization by a Noval Consortium of Pediococcus acidilactici and Candida tropicalis under Static Condition. Pak J Biol Sci 17(6):780–791
Tondee T, Sirianutapiboon S (2008) Decolorization of molasses wastewater by Lactobacillus plantarum No. PV71-1861. Bioresour Technol 99(14):6258–6265
Tripathi DM, Tripathi S, Tripathi BD (2011) Implications of secondary treated distillery effluent irrigation on soil Cellulase and urease activities. J Environl Prot 2:655–661
Trivedy RK, Nakate SS (2000) Treatment of diluted distillery waste by constructed wetlands. Indian J Environ Prot 20:749–753
Valderrama LT, Del Campo CM, Rodriguez CM, Ede- Bashan L, Bashan Y (2002) Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula. Water Res 36(17):4185–4192
Wagh MP, Nemade PD (2018) Biodegradation of anaerobically treated distillery spent wash by Aspergillus species from a distillery effluent contaminated site. Desalin Water Treat 104:234–240
Wilkie AC, Riedesel KJ, Owens JM (2000) Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 19:63–102
Wolmarans B, De Villiers G (2002) Start up of a UASB effluent treatment plant on distillery wastewater. Water SA 28(1):63–68
Wong DWS (2009) Structure and Action Mechanism of Ligninolytic Enzymes. Applied Biochemistry and Biotechnology 157 (2):174–209
Yadav S, Chandra R (2012) Biodegradation of organic compounds of molasses melanoidin (MM) from biomethanated distillery spent wash (BMDS) during the decolourisation by a potential bacterial consortium. Biodegradation 23(4):609–620
Yadav DV, Jain R, Rai RK (2010) Impact of heavy metals on sugar cane. In: Sherameti I, Varma A (eds) Soil heavy metals: soil biology series, vol 19. Springer, Berlin, pp 339–367
Zalawadia NM, Raman S (1994) Effect of distillery wastewater with graded fertilizer level on sorghum yields and soil property. J Indian Soc Soil Sci 42:575–579
Zhang M, Wang Z, Li P, Zhang H, Li X (2017) Bio-refractory dissolved organic matter and colorants in cassava distillery wastewater: characterization, coagulation treatment and mechanisms. Chemosphere 178(2017):259–267
Zhang B, Zhao H, Zhou S, Shi C, Wang C, Ni J (2009) A novel UASB−MFC−BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour Technol (23):5687–5693
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kumar, V., Chandra, R., Thakur, I.S., Saxena, G., Shah, M.P. (2020). Recent Advances in Physicochemical and Biological Treatment Approaches for Distillery Wastewater. In: Shah, M., Banerjee, A. (eds) Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant. Springer, Singapore. https://doi.org/10.1007/978-981-15-0497-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-0497-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0496-9
Online ISBN: 978-981-15-0497-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)