Abstract
The native and physico-chemically treated fungal biomasses of Neurospora intermedia were used for adsorption of colored pollutants from distillery spent wash in batch systems. Experiments were conducted at varying color concentrations of the effluent (1,000–6,500 CU). The kinetics of effect of initial sorbate concentration, dose of biosorbent, temperature, and pH on adsorption were studied. Physical and chemical pretreatments of biomass resulted in an increase or decrease in color removal capacity. This effect was further studied by FTIR analysis of the dried fungal mycelium. The maximum color uptake on all the tested fungal biomass preparations was observed at pH 3.0 and temperature 30°C, within first 4 h. The Langmuir and Freundlich adsorption models were used for the mathematical description of the biosorption equilibrium and the data showed an optimal fit to these isotherms. Kinetic parameters indicated the dominance of Lagergren pseudo first-order kinetic model for adsorption. On the basis of maximum adsorption capacity, the color removal capacity by fungal preparations was in the order of native > heat > acid, base.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distillery effluent, a foul smelling, dark colored by-product of distillery industry is perceived as one of the serious pollution sources of the countries producing alcohol from fermentation and subsequent distillation of sugar cane molasses. It contains extremely high COD, BOD, soluble solids, inorganic solids, dark brown color, and low pH (Saha et al. 2005) besides other caramelized and recalcitrant wastes. The characteristic recalcitrance to decolorization of distillery effluent is due to the presence of brown color melanoidin polymers that are formed in Maillard reactions between amino acids and carbohydrates (Wedzicha and Kaputo 1992). Various treatment technologies including physico-chemical and biological methods have been studied by various researchers for the removal of organic–inorganic pollutants and metals from wastewaters (Goyal et al. 1996; Nandy et al. 2002; Gupta et al. 2007c, d, 2012). Among the physico-chemical treatment methods, adsorption is most widely used, as the process requires less investment in terms of initial establishment cost, efficiency and is free from generation of toxic substances (Mittal et al. 2009b; Saleh and Gupta 2012).
Activated charcoal has been found to be effective adsorbent for removal of color and synthetic dyes from waste waters. The discharge of azo dyes and their biotransformation products in water bodies is a serious concern due to their mutagenic and carcinogenic effects. Different low cost waste materials, like maize cobs, wood chips, wheat husk bottom ash, de-oiled soya, and hen feathers have been employed as potential adsorbents for the removal of various synthetic azo dyes such as Vertigo Blue 49, Orange DNA13, Reactofix golden yellow 3 RFN (Gupta et al. 2007b, e), Indigo Carmine, Amido Black 10B, Amaranth, Brilliant Yellow (Mittal et al. 2005; Mittal 2006; Mittal et al. 2007, 2012a, b), Erythrosine, Brilliant Blue FCF (Gupta et al. 2006b, c),) Congo Red, Chrysoidine Y, Triarylmethane Fast Green FCF, Triarylmethane dye, Light Green SF (Mittal et al. 2009a, b, 2010b, c) Eriochrome Black T (Mittal and Gupta 2010). The industrial waste materials like carbon columns, carbon slurry, slag, and bagasse fly ash have also been reported to be useful for remediation of hexavalent chromium, Zn, Cd, color, chlorophenols, and fluoride as well (Bernardo et al. 1997; Gupta et al. 1997, 2000; Gupta and Sharma 2003; Jain et al. 2004; Gupta et al. 2006a, 2007a, b; 2010). However, its relatively high cost restricts its usage on a large scale (Mittal et al. 2010b).
Biosorption has gained important credibility during recent years because of its ecofriendly nature, excellent performance, and low cost techniques (Gupta and Rastogi 2008a, 2009). The microbial biomasses have been widely used for removal of metals like, Pb, and Cr from aqueous solutions (Gupta and Rastogi 2008b, 2009). Biomass of Oedogonium sp. and Nostoc muscorum have been reported as a suitable biosorbent for Cd (II) and chromium removal, respectively (Gupta and Rastogi 2008a, c). Ever increasing demand for new sources of low-cost adsorbent undoubtedly make chitosan-containing biomass as one of the most attractive material for treatment of color in wastewater (Babel and Kurniawan 2003). Literature suggests that research have been carried out on the biosorption of various organic pollutants and color from wastewaters (Tsezos and Bell 1989; Fu and Viraraghavan 2001). Partial elimination of Maillard colored compounds was obtained by using microbes like Geotrichum candidum and Rhizoctonia sp. D-90. Electron microscopy revealed the presence of melanoidin-like pigments in the form of electron dense material in the cytoplasm, adsorbed by mycelia (Borja et al. 1993; Sirianuntapiboon et al. 1995). Color removal from molasses spent wash through adsorption was also studied by Miranda et al. (1996) using Aspergillus niger.
The present study was conducted to determine the ability of native and physico-chemically treated biomasses of Neurospora intermedia to adsorb colored components of distillery effluent in batch cultures. The biosorption equilibrium and kinetic data were fitted using different models and process parameters were evaluated.
Materials and methods
Sorbate
For studying the process of adsorption by fungal biomass, the effluent was collected from Modi distilleries, Modinagar, Uttar Pradesh, India. The sample was diluted to desired dilution using minimum salt media (grams per liter) (Na2HPO4.2H2O, 7.8; KH2PO4, 6.8; MgSO4, 0.2; Fe (CH3COO)3 NH4, 0.05; Ca(NO3)2 4H2O, 0.05 NaNO3, 0.085;) for adsorption studies. The pH of the sample was adjusted using 0.1 M HCl or 0.1 M NaOH.
Cultivation of fungus and preparation of biomass
The fungus “N. intermedia (MTCC-9655)” was selected for adsorption studies. It was observed in our earlier studies that the strain played significant role in decolorizing the distillery spent wash through adsorption rather than using its enzymatic system (Kaushik and Thakur 2009; Kaushik et al. 2010). Thus, it was decided to study adsorption process performed by N. intermedia. The strain was cultivated in liquid medium in PDB using shake flask method. Once inoculated, flasks were incubated on an orbital shaker at 125 rpm for 7 days at 30°C. After incubation, the biomass was harvested from medium and washed with distilled water. The washed biomass (live biomass) was used immediately thereafter. Untreated fungus was called native fungus. This native fungus was soaked in 0.1 M HCl, at room temperature, in a ratio of 1:10 w/v for 1 h at 125 rpm. The biomass was then washed with buffer saline. This fungus was called acid-treated fungus. Similarly, base-treated fungus was prepared by treating it with 0.1 M NaOH. For preparation of heat-treated fungus, biomass was heated in water bath at 100°C in buffer saline for 10 min (Bayramoglu et al. 2003).
Characterization of the fungal biomass
Changes in fungal biomass before and after adsorption were studied by SEM and FT-IR. The fungal mycelium was analyzed by scanning electron micrography at 10 kV, magnification 10KX using ZEISS Scanning Electron Microscope EVO 50. Samples were prepared as described earlier (Sirivastava and Thakur 2007). To give a qualitative and preliminary analysis of the main chemical groups present on the cell wall of biomass and its different preparations, an IR analysis in solid phase was carried out by using FT-IR spectrophotometer. For the FTIR spectra, fungal biomass (0.01 g dry weight) was mixed with KBr (0.1 g) and pressed into a tablet form by pressing the ground mixed material with the help of a bench press. Scan was recorded from 450 to 4,000 nm with Perkin-Elmer 1600 series FT-IR spectrophotometer (Nujol, KBr disks).
Adsorption studies
Batch adsorption studies were conducted by varying adsorbent (biomass), adsorbate concentration, pH, and temperature to obtain rate and equilibrium data. To determine the effect of initial concentrations of the effluent on the adsorption rate and capacity on the fungal preparations, the initial concentration of the effluent was varied between 1,000 and 6,500 CU in the biosorption medium. The distillery spent wash having 65,000 CU was served as stock solution. Different concentrations were prepared from this stock solution. In each experiment, accurately weighed 10 % fungal biomass (wet weight) was added to 100 ml of distillery spent wash solution (MSM-effluent, 6,500 CU) taken in a 250-ml conical flask along with necessary carbon (dextrose, 0.05 % w/v), nitrogen (NaNO3, 0.025 % w/v) source for the growth of fungus. The mixture was agitated at 125 rpm in an incubating shaker at constant temperature (30°C). At the end of the equilibrium period, the contents of the flasks were analyzed for residual concentration of adsorbate. The adsorption of the colored contaminants of distillery spent wash on native and heat-acid- base-treated fungal biomass were also investigated. The effects of the medium pH on the adsorption capacities of the fungal biomass preparations were later investigated with pH range of 2.0–8.0 (which was adjusted with HCl or NaOH at the beginning of the experiment) at 30°C. The effect of temperature was studied with temperature range of 20–40°C at pH 3.0.
Analysis
Before analysis of the remaining color concentration in the adsorbate, the sample was centrifuged at 5,000 rpm for 20 min. The supernatant was analyzed for estimation of color by cobalt-platinate method (APHA 2005). Coloring unit of the effluent was considered a measure of amount of chemicals present in the effluent. Such approach has also been followed in previous studies where COD was used as a measure of effluent concentration (Srivastava et al. 1995; Devi et al. 2008; Mittal et al. 2010a). The readings were taken in triplicate for each individual solution to check repeatability and the average of the values were taken.
The amount of biosorbed coloring material per unit fungal biomass (grams wet biomass) was obtained by using the following expression:
q e is the amount adsorbed at equilibrium, C 0 the initial concentration of the sorbate, C e is the equilibrium solution concentration, V the volume, and M the mass of adsorbent.
To optimize the design of a sorption system to remove the sorbates, it is important to establish the most appropriate correlation for the equilibrium curves. These equilibrium sorption capacity curves can be obtained by measuring the sorption isotherm. The experimental data of the amount of sorbate adsorbed on the sorbent were substituted into an equilibrium isotherm model to determine the best-fit model for the sorption system. Using this relationship, any variation in the concentration of sorbent with the concentration of sorbate in solution was correlated. The Freundlich and Langmuir adsorption isotherm models are widely used to analyze data for water and wastewater treatment applications. Hence, in this study, the experimental data were fitted with the Freundlich and Langmuir models.
Freundlich model
The Freundflich equation can be written as:
Where K F is a constant indicative of the relative adsorption capacity of the adsorbent (mg1−(1/n)L1/ng−1) and n is the constant indicative of the intensity of adsorption. The Freundlich expression is an exponential equation and therefore, assumes that as the adsorbate concentration increases, the concentration of adsorbate on the adsorbent surface also increases.
Langmuir model
The Langmuir model assumes uniform energies of adsorption on to the surface and no transmigration of adsorbate in the plane of the surface. The Langmuir equation may be written as
where q e is the amount of solute adsorbed per unit weight of adsorbent at equilibrium (milligrams per gram), C e is the equilibrium concentration of the solute in the solution (milligrams per liter), q m is the maximum adsorption capacity (milligrams per gram), and b is the constant related to the free energy of the adsorption. The linearized form of these models was used for plotting graphs. Langmuir was converted into five different linear equations (Hamdaoui and Naffrechoux 2007).
Result and discussion
Characteristics of fungal biomass
Scanning electron microscopy
The surface morphology of the native fungus N. intermedia mycelia is exemplified in Fig. 1. The scanning electron micrographs clearly reveal the surface texture and porosity of the sample. As shown in the scanning electron microscopy micrograph, the fungal mycelia have rough and porous surface (top) and the mycelia loses its basic structure and tear down due to the particles load after adsorption present in the effluent (bottom).
Fourier transform infrared spectroscopy
In order to confirm the existence of functional biosorption groups (i.e., amino, carboxyl and phosphate) on the fungal biomass, and its different preparations, the FTIR spectra of the finely powdered dried fungal biomasses were obtained. The spectra confirmed the presence of various functional groups in the structure of N. intermedia such as carboxylic acid, hydroxyl, amine and amido groups (Gupta and Rastogi 2008a). The broad stretching adsorption band at 3,356 cm−1could be assigned hydroxyl groups stretching vibrations. The –NH stretching of the protein and the acetamido group of chitin fraction stretching vibration bands were superimposed on the side of the hydroxyl group band at 3,500–3,200 cm−1. The band at 1,659 cm−1 was a consequence of CO stretching vibration conjugated to NH band. The peaks at around 1,411 and 1,248 cm−1 were caused by the CO stretching band of carbonyl groups. The moderately strong absorption bands at 1,030 could be assigned to the –CN stretching vibration of the chitin–chitosan and protein fractions (Gupta and Rastogi 2008b). Strong absorption bands at 2,925 cm–1 could be assigned to the –CH stretch.
The FTIR spectra bands of biosorption studies by native fungi showed that the broad overlapping peak shift to 3,391 cm−1 after the adsorption of distillery spent wash, which might be due to hydroxyl or amine groups present in the effluent itself. Additionally, the peak at 1,659 cm−1 assigned to the C O stretching shifted to 1,634 cm−1 suggesting its role in adsorption. However, the peak stretching at 1,411 and 1,248 shifted to 1,434 and 1,358, respectively, after adsorption could be attributed to the presence of additional carboxyl groups in the effluent.
Both native and physico (heat)-chemically (acid, base) treated fungal biomasses have been used to remove colored pollutant from aqueous medium (Vandevivere et al. 1998; Mahony et al. 2002; Ozer et al. 2005). FTIR spectrum of treated fungal biomasses showed that the treatments have modified surface chemistry of the fungi by the process of adsorption. As shown in Table 1, there was loss of some functional groups along with presence of some additional binding sites for the same groups, while at the same time, there was a change in the intensity for others. Thus, the mechanism of adsorption as revealed by instrumental technique like FTIR was found to be the ionic interactions and complex formation between colored pollutants and ligands contained on the surface of biomaterials (Gupta and Rastogi 2008b).
Batch adsorption studies
Effect of pH and temperature on adsorption
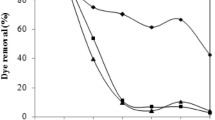
Figure 2 shows the effect of temperature on the equilibrium sorption capacity in the range of 20–40°C. The adsorption of distillery spent wash was enhanced with raising the temperature up to 30°C, and the time taken to reach the equilibrium decreases with increasing temperature; however, beyond that, there was a decrease in the further adsorption potential of the fungi with the increase in temperature. Adsorption decreases with a further increase in temperature probably due to the decreased surface activity as noted by Aksu and Tezer (2000) in the case of biosorption of Remazol Black B reactive dye by Rhizopus arrhizus. Similar results were also found by Das et al. (2006).
The pH of the aqueous solution is a controlling factor in the adsorption process. In this study, the role of hydrogen ion concentration was examined between pH values from 2 to 9. Distillery spent wash contains complex polymeric organic compounds having different aromatic rings and functional groups and thus have varying ionization potentials at different pH ultimately showing varying bioadsorption potential at different pH values. The fungal cell wall is composed of polysaccharides (i.e., chitin and chitosan), proteins, lipids, and melanin with several functional groups (such as amino, carboxyl, thiol, and phosphate groups) capable of binding various organic molecules (Yesilada et al. 2003). The ionic forms of these organic compounds in solution and the surface electrical charge of the biomass depend on the solution pH. Therefore, the interaction between a sorbate and sorbent is mainly affected by ionization states of the functional groups on both the molecule and sorbent surface (Maurya et al. 2006). The influence of pH on the bioadsorption potential of the fungi (N. intermedia) is shown in Fig. 2. The most suitable pH for sorption was 3, and there was a decrease in the extent of bioadsorption potential with increase in the pH of the solution. Hence, further studies were conducted at pH 3. Similar results were also found earlier (Hu 1992; Mahony et al. 2002) while carrying out adsorption studies of different reactive dyes.
Effect of contact time
It was found that removal of colored pollutant from distillery spent wash increases with increase in contact time to some extent. Further increase in contact time does not increase the uptake due to deposition of colored pollutants on the available adsorption sites of native fungal biomass. Preliminary investigations on the uptake of pollutants on the adsorbent material at their optimum pH and temperature values indicated that the process was quite rapid. Typically, 80 % of the total adsorption occurred within first four hours at effluent concentration of 10 % (v/v). This initial rapid adsorption subsequently paved the way to a very slow approach to equilibrium and saturation was reached in 5–6 h. For further optimization of other parameters, this contact time was considered as the equilibrium time.
Biosorption isotherms
Equilibrium data, commonly known as adsorption isotherms, are the basic requirement for the design of adsorption systems. The shape of the isotherms is the first experimental tool to diagnose the nature of a specific adsorption phenomenon. The amount of biosorbed coloring material onto the fungal biomass preparations was studied and plotted as a function of the equilibrium concentration of colored material in the biosorption medium. Several mathematical models can be used to describe experimental data of adsorption isotherms. In this study, the equilibrium data were modeled with Langmuir (five linearized expressions), Freundlich models.
The experimental values of q e and C e determined in adsorption isotherms were initially treated with linearized equations of Freundlich and Langmuir to find out the model parameters. After determining the best model, the values of q e were recalculated using linear equation of the model. The reconstitute isotherms were compared with initial isotherms to evaluate the error in the models. Linear correlation coefficients (r) showed the fit between experimental data and linearized forms of isotherm equations while the average percentage errors (APE) calculated according to given equation indicated the fit between the experimental and predicted values of isotherm curves (Hamdaoui and Naffrechoux 2007).
Where N is the number of experimental data.
Langmuir isotherm
The adsorption data obtained for colored pollutants in the distillery spent wash was analyzed by regression analysis to fit the five linearized expressions of Langmuir isotherm models. The details of different forms of linearized Langmuir equations and the method to estimate the Langmuir constants q m and b from these plots are shown in Table 2. The favorable nature of adsorption can be expressed in terms of dimensionless equilibrium parameter of Hall et al. (1966):
where b is the Langmuir constant and C 0 is the initial concentration of the adsorbate in the solution. The values of R L indicate the type of isotherm to be irreversible (R L = 0), favorable (0 < R L < 1), linear (R L = 1), or unfavorable (R L > 1) (Hamdaoui and Naffrechoux 2007).
The values for R L were 0.54, 0.81, 0.42, and 0.79 for native, acid, base, and heat, respectively. R L values were less than 1 and greater than 0 indicated favorable adsorption.
In order to check the validity of the Langmuir models, it is interesting and essential to recalculate the adsorbed amounts using the five linearized forms of the equation. Table 2 summarizes the parameters obtained using these models. Figure 3(a) shows the best fitting model for native, acid, and heat was Langmuir-1 with r values 0.8819, 0.9988 and 0.9298, respectively, and Langmuir-2 was best fitting model for base-treated fungi (r = 0.9777) (Fig. 3b). The calculated average percentage errors for Langmuir 1, 2, and 3 are shown in Table 2. It is clear from Table 2 that inspite of the lowest value of average percentage error in Langmuir-2 for acid-treated fungi, this model does not describe perfectly the equilibrium data because of the lower values of coefficients of correlation. Thus, it is not appropriate to use the coefficient of correlation of the linear regression method for comparing the best-fitting isotherms. The q m values based on Langmuir 1 model show that maximum adsorption was in the series native > heat > acid, base (Table 2).
Freundlich isotherm
The equilibrium data was further analyzed using the linearized form of Freundlich equation using the same set experimental data, by plotting lnq e versus lnC e. The calculated Freundlich isotherm constants and the corresponding coefficient of correlation values were shown in Table 2. The fitness of Freundlich model for all the four types of experimental data was in the order of acid > base > native > heat (Fig. 4). The magnitude of the exponent n gives an indication on the favorability of adsorption. The n values that were >1 for all the tested fungal biomass preparations indicated favorable adsorption process. However, heat-treated fungal biomass preparation showed poor adsorption capacity as the n value was less than 1 (0.98) along with lowest value of coefficient of correlation.
Adsorption kinetics
To investigate the mechanism of adsorption, kinetic models are used to test experimental data. Pseudo-first-order and pseudo-second-order equations can be used while assuming that the measured concentrations are equal to surface concentrations. The pseudo-first-order is expressed as described earlier by Singh et al. (1989):
where q t is the amount of solute adsorbed at time t (milligram per gram), q e the adsorption capacity in equilibrium (milligram per gram), k ad is the pseudo first-order rate constant (per minute), and t is the contact time (minutes). Linear plot of log(q e − q t) versus t was obtained and values of adsorption rate constant (k ad) were calculated from the slope of linear plots that are presented in Table 3 (figure not shown). Kinetic data were further treated with the pseudo-second order kinetic model (Ho and Mckay 1999) which is expressed as:
where k′ is the pseudo-second-order rate constant (grams per milligram per minute). For second-order kinetic model, the data was plotted between t/q t against t. The plot should be a straight line if second-order kinetic model is applicable. It was found that although the second-order equation was suitable for some of the data; it was not applicable to all the results. Therefore, no further consideration was given to it. The pseudo-first-order model, however, was applicable to all the data and the results are shown in Table 3.
Conclusions
The results of this study identified native and treated fungal biomass of N. intermedia as a suitable biosorbent for removal of colored pollutants from distillery spent wash. Batch experiments showed the strong effect of the operating variables (pH, temperature, contact time) on biosorption performance of the fungal biomass. Among the tested isotherm models, Langmuir models were more applicable. The value of constant n was greater than 1; hence, all the adsorption processes were favorable. From the model, we can say that adsorption has uniform energies on the surface and there is no transmigration of adsorbate in the plane of the surface. Overall reaction rate was best described by pseudo first-order kinetic model. The interactions between the pollutants from effluent and the functional groups on the cell wall surface of the biomass were confirmed by FTIR analysis, which indicated the participation of COOH, OH, and NH2 groups in the adsorption. This study demonstrated that the raw and treated fungal biomass of N. intermedia could be used as efficient biomass for treatment of colored wastewaters.
References
Aksu Z, Tezer S (2000) Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: effect of temperature. Process Biochem 36(5):431–439
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater B 97:219–243
Bayramoglu G, Bektas S, Arica MY (2003) Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater 101:285–300
Bernardo ECR, Egashira E, Kawasaki J (1997) Decolourization of molasses' wastewater using activated carbon prepared from cane bagasse. Carbon 35(9):1217–1221
Borja R, Martin A, Luque M, Duran MM (1993) Enhancement of the anaerobic digestion of wine distillery wastewater by the removal of phenolics inhibitors. Bioresour Technol 45:99–104
Das SK, Bhowal J, Das AR, Guha AK (2006) Adsorption behavior of rhodamine B on Rhizopus oryzae biomass. Langmuir 22:7265–7272
Devi R, Singh V, Kumar A (2008) COD and BOD reduction from coffee processing wastewater using Avacado peel carbon. Bioresour Technol 99(6):1853–1860
Fu YZ, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresour Technol 79:251–262
Goyal SK, Seth R, Handa BK (1996) Diphasic fixed-film biomethanation of distillery spentwash. Bioresour Technol 56:239–244
Gupta VK, Rastogi A (2008a) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2008b) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloid Surf B 64:170–178
Gupta VK, Rastogi A (2008c) Sorption and desorption studies of chromium (VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash—a low cost adsorbent. Ind Eng Chem Res 42(25):6619–6624
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process-development for the removal of zinc and cadmium from waste-water using slag—a blast-furnace waste material. Sep Sci Technol 32(17):2883–2912
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34(5):1543–1550
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45(4):1446–1453
Gupta VK, Mittal A, Krishanan L, Mittal J (2006b) Adsorption treatment and recovery of the hazardous dye, Brilliant Blue FCF, over bottom ash and de-oiled soya. J Colloid Interface Sci 293:16–26
Gupta VK, Mittal A, Kurup L, Mittal J (2006c) Adsorption of a hazardous dye, erythrosine, over hen feathers. J Colloid Interface Sci 304:52–57
Gupta VK, Ali I, Saini VK (2007a) Defluoridation of wastewaters using waste carbon slurry. Water Res 41:3307–3316
Gupta VK, Ali I, Saini VK (2007b) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315(1):87–93
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007c) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007d) Electrochemical removal of the hazardous dye Reactofix Red 3 BFN from industrial effluents. J Colloid Interface Sci 312(2):292–296
Gupta VK, Jain R, Varshney S (2007e) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S (2012) Chemical treatment technologies for waste-water recycling—an overview. RSC Adv. doi:10.1039/C2RA20340E
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid diffusion kinetics in fixed-bed adsorption under constant pattern conditions. Ind Eng Chem Fundam 5:212–223
Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147(1–2):381–394
Ho YS, Mckay G (1999) Pseudo-second order model for sorption process. Process Biochem 34:451–465
Hu TL (1992) Sorption of dye reactive dyes by Aeromonas biomass. Water Sci Technol 26:357–366
Jain AK, Gupta VK, Jain S, Suhas (2004) Removal of chlorophenols using industrial wastes. Environ Sci Technol 38(4):1195–1200
Kaushik G, Thakur IS (2009) Isolation of fungi and optimization of process parameters for decolorization of distillery mill effluent. World J Microbiol Biotechnol 25:955–964
Kaushik G, Gopal M, Thakur IS (2010) Evaluation of performance and community dynamics of microorganisms during treatment of distillery spent wash in sequential bioreactor. Bioresour Technol 101:4296–4305
Mahony TO, Guibal E, Tobin JM (2002) Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb Technol 31:456–463
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresour Technol 97:512–521
Miranda PM, Benito GG, Cristobal NS, Nieto CH (1996) Colour elimination from molasses wastewater by Aspergillus niger. Bioresour Technol 57:229–235
Mittal A (2006) Removal of the dye, Amaranth from waste water using hen feathers as potential adsorbent. Electron J Environ Agric Food Chem 5(2):1296–1305
Mittal A, Gupta VK (2010) Adsorptive removal and recovery of the azo dye Eriochrome Black T. Toxicol Environ Chem 92:1813–1823
Mittal A, Kurup L, Gupta VK (2005) Use of waste materials—bottom ash and de-oiled soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. J Hazard Mater 117:171–178
Mittal A, Mittal J, Kurup L (2007) Utilization of hen feathers for the adsorption of indigo carmine from simulated effluents. J Environ Prot Sci 1:92–100
Mittal A, Kaur D, Mittal J (2009a) Batch and bulk removal of a triarylmethane dye, Fast Green FCF, from wastewater by adsorption over waste materials. J Hazard Mater 163:568–577
Mittal A, Mittal J, Malviya A, Gupta VK (2009b) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340:16–26
Mittal A, Jain R, Mittal J, Varshney S, Sikarwar S (2010a) Removal of Yellow ME 7 GL from industrial effluent using electrochemical and adsorption techniques. Int J Environ Pollut 43(4):308–323
Mittal A, Mittal J, Malviya A, Gupta VK (2010b) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010c) Decoloration treatment of a hazardous triarylmethane dye, light green SF (Yellowish) by waste material adsorbents. J Colloid Interface Sci 342:518–527
Mittal A, Thakur V, Gajbe V (2012a) Adsorptive removal of toxic azo dye Amido Black 10B by hen feather. Environ Sci Pollut Res. doi:10.1007/s11356-012-0843-y
Mittal A, Thakur V, Gajbe V (2012b) Evaluation of adsorption characteristics of an anionic azo dye Brilliant Yellow onto hen feathers in aqueous solutions. Environ Sci Pollut Res. doi:10.1007/s11356-012-0756-9
Nandy T, Shastry S, Kaul SN (2002) Wastewater management in cane molasses distillery involving bioresource recovery. J Environ Manag 65:25–38
Ozer A, Akkaya G, Turabik M (2005) Biosorption of acid red 274 (AR 274) on Enteromorpha prolifera in a batch system. J Hazard Mater 126:119–127
Saha NK, Balakrishnan M, Batra VS (2005) Improving industrial water use: case study for an Indian distillery. Resour Conserv Recycl 43:163–174
Saleh TA, Gupta VK (2012) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollut Res doi:10.1007/s11356-011-0670-6
Singh DB, Prasad D, Rupainwar DC, Singh VN (1989) As(III) removal from aqueous solution by adsorption. Water Air Soil Pollut 42:376–386
Sirianuntapiboon S, Sihanonth P, Somchai P, Atthasampunna P, Hayashida S (1995) An adsorption mechanism for melanoidin decolourization by Rhizoctonia sp. Biosci Biotechnol Biochem 59:1185–1189
Sirivastava S, Thakur IS (2007) Evaluation of biosorption potency of Acinetobacter sp. for removal of hexavalent chromium from tannery effluent. Biodegradation 18:637–646
Srivastava SK, Gupta VK, Yadav IS, Mohan D (1995) Removal of 2,4, dinitrophenol using bagasse fly ash—a sugar industry waste material. Fresenius Environ Bull 4:550–557
Tsezos M, Bell JP (1989) Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass. Water Res 23:561–568
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Technol Biotechnol 72:289–302
Wedzicha BL, Kaputo MT (1992) Melanoidins from glucose and glycine: composition, characteristics and reactivity towards sulphite ion. Food Chem 43:359–367
Yesilada O, Asma D, Cing S (2003) Decolorization of textile dyes by fungal pellets. Process Biochem 38:933–938
Acknowledgements
The authors thank Modi distilleries, Modinagar, Uttar Pradesh, India for providing effluent and sludge/sediments during the course of investigation. Thanks are also to Dr. Manish Kaushik for his excellent technical assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Kaushik, G., Thakur, I.S. Adsorption of colored pollutants from distillery spent wash by native and treated fungus: Neurospora intermedia . Environ Sci Pollut Res 20, 1070–1078 (2013). https://doi.org/10.1007/s11356-012-0957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0957-2