Abstract
Five different fungi isolated from distillery mill site in which two isolates (DF3 and DF4) had higher capabilities to remove color were identified as Emericella nidulans var. lata and Neurospora intermedia, respectively. Optimization of process parameter for decolorization was initially performed to select growth factors which were further substantiated by Taguchi approach in which seven factors, %carbon, %nitrogen, duration, pH, temperature, stirring and inoculum size, at two levels applying L-8 orthogonal array were taken for both fungi. Maximum color was removed at pH 3, temperature 30°C, stirring 125 rpm, dextrose (0.05%) and sodium nitrate (0.025%) by both fungi. After optimization, there was two-fold increase in color removal from 38 to 62% (DF3) and 31 to 64% (DF4) indicating significance of Taguchi approach in decolorization of distillery mill effluent. The mechanism for decolorization was determined by enzyme analysis, laccase and glucose oxidase, and indicated significant activity in DF3 as compared to DF4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distilleries are one of the most polluting industries generating enormous amount of wastewater in which an average of 10–15 l of effluent is generated with 1 l of alcohol production. The distillery wastewater known as spent wash is characterized by its dark brown color, high temperature, low pH, and high percentage of dissolved organic and inorganic matter. It also contains nearly 2% of the dark brown recalcitrant pigment called melanoidin (Kitts et al. 1993). Putriciable organics like skatole, indole and other sulfur compounds produces obnoxious smell in the effluent and when it comes in contact with high temperature, becomes more toxic to aquatic biota (Kitts et al. 1993). A typical distillery, which uses cane molasses as raw material for the production of ethanol, generates huge amount of spent wash daily.
Molasses has very high quantity of fermentable sugars (sucrose, glucose and fructose). These sugars react with amino acids, undergo Maillard reaction (amino carbonyl complex), and then polymerize to form melanoidin, which is a major color containing compound in the distillery effluent. Effluent so generated has very high chemical oxygen demand (COD) (80,000–100,000 mg/l) and biochemical oxygen demand (BOD) (40,000–50,000 mg/l), apart from low pH, strong odor and dark brown color (Raghukumar and Rivonkar 2001). Disposal of conventionally treated distillery spent wash is harmful as it contains mostly recalcitrant compounds, which are toxic to aquatic biota. Therefore, a comprehensive treatment strategy is required for decolorization of distillery spent wash to meet the discharge safety standards.
Decolorization of melanoidin in spent wash has been studied using activated carbon prepared from sugarcane bagasse (Bernardo et al. 1997), an inorganic flocculent (Migo et al. 1993), and using various microorganisms (NakajimaKambe et al. 1999). So far various studies have been carried out using fungi such as Aspergillus fumigatus (Ohmomo et al. 1987), Phanerochaete chrysopsorium (Fahy et al. 1997), Flavodon flavus (Raghukumar and Rivonkar 2001). There are reports related to role of fungi in decolorization of melanoidin into mycelia (Ohmomo et al. 1988; Sirianuntapiboon et al. 1995; Pena et al. 1996). For effective decolorization it is essential to optimize the composition of culture media and culture conditions (Prakasham et al. 2005). In conventional methods numerous experiments have to be carried out to optimize all the parameters (factors) and to establish best possible culture condition by interrelating all the parameters. In these methods studying one variable at a time is long, cumbersome and not economical. Another approach is to use statistical tools and experimental designs (Stowe and Mayer 1999). Taguchi methods have been widely used to optimize the reaction variable by devising minimum number of experiments. This approach also facilitates to identify the impact of individual factor and find out the link between variables and operational conditions. Analysis of the experimental data using the ANOVA (analysis of variance) and factors effect gives the output that is statistically significant. This approach provides simple and efficient methodology for optimization with minimum number of experiments (Kackar 1985; Phadke and Dehnad 1988). Thus in the present study, fungi were isolated from distillery mill site and process parameters were optimized by a methodological application of Taguchi approach for optimizing the culture conditions to decolorize the distillery spent wash.
Materials and methods
Sampling site and microorganism
Fungal strains were collected from distillery effluent and sludge, Modi distilleries, Modinagar, Uttar Pradesh, India. For isolation of fungi capable of decolorizing distillery effluent, sludge and effluent were mixed and serially diluted in the order of 10−3, 10−4, 10−5 by using autoclaved double distilled water. Diluted samples (100 μl) were spread on potato dextrose agar (PDA) plates, and kept at 30°C for 4 days. Fungal colonies that appeared on PDA plates were isolated and purified by repeated culturing (Thakur 2004). The fungal isolates were identified based on morphological structures as color, texture of the mycelia, spore formation and also by amplification of internal transcribed spacer (ITS) region of genomic DNA. For the preparation of pellets, isolates were grown in potato dextrose broth with streptopenicillin (100 ppm). The flasks were incubated at 30°C for 4 days in orbital shaker. Pellets of approximately 1.5–2 mm size were used for the treatment of distillery spent wash (Thakur 2004).
Identification of fungal strains
For identification of fungi, genomic DNA was isolated from pure fungal mycelia grown in PDB at 30°C for 7 days with modifications of procedure as described by Karakousis et al. (2006). In this method, 1 mg of freshly grown mycelium was frozen in liquid nitrogen and ground to fine powder using pre-frozen pestle and mortar (pre-sterilized by baking at 200°C, overnight). Extraction buffer (5 ml, 0.1 M Tris–Cl, 1.4 M NaCl, 0.02 M EDTA, 2% CTAB) preheated at 60°C was added along with β-mercaptoethanol (0.2%). It was mixed gently and incubated at 60°C for 1 h with periodic stirring. An equal volume of chloroform:isoamyl alcohol (24:1) was added to the crude extract and mixed at room temperature for few minutes. The content was centrifuged at 10,000 rpm for 10 min and supernatant was carefully decanted. Isopropanol (0.6th volume of ice cold) was added and solution was mixed and then kept at −20°C for 1 h. The mixture was centrifuged at 10,000 rpm for 10 min and the recovered pellet was washed with equal volume ethanol (70% chilled). Finally the pellet was air dried to remove all traces of ethanol and dissolved in TE (100 μl). Internal transcribed spacer (ITS) region of the fungi were amplified using primers ITS1 having sequence 5′ TCCGTAGGTGAACCTGCGG 3′ and ITS4 having sequence 5′ TCCTCCGCTTATTGATATGC 3′ (Hortal et al. 2006). The reaction mix (25 μl) consisted of buffer containing MgCl2-2.5 μl (2.5 mM), dNTP-2.0 μl (1 mM each), ITS1 (forward)-0.7 μl (10 mM), ITS4 (reverse)-0.7 μl (10 mM), Taq polymerase-0.5 μl (5 U/μl), DNA-20–50 ng and volume was made up by water. The thermocycler program include an initial denaturation of 94°C for 5 min, followed by 35 cycles of amplification at 94°C for 1 min, 55°C for 1 min and 72°C for 1 min and a final extension of 7 min at 72°C. Amplified products were resolved on 0.7% agarose gels containing ethidium bromide (1 μg/ml). The amplified products were sequenced. The resulting sequence was entered into the BLAST nucleotide search program of the National Center for Biotechnology Information to obtain closely related phylogenetic sequences. Pairwise alignments giving the closest match were chosen. The megablast algorythm was used and the phylogeny tree was constructed using Mega 3.1 software (Yang et al. 2007).
Culture condition and decolorization of distillery effluent
The fungal strains were screened for decolorization in minimal salt medium (MSM containing (g/l): Na2HPO4·2H2O, 7.8; KH2PO4, 6.8; MgSO4, 0.2; Fe (CH3COO)3 NH4, 0.05; Ca(NO3)2·4H2O, 0.05 NaNO3, 0.085; 10% distillery spent wash, at 3 pH and incubated at 30°C (Thakur 2004). Decolorization of effluent was estimated on 3, 7, 10 and 15 days. Each experiment was performed in three replicates.
Decolorization assay
Color content was measured as described in the manual of Standard Methods for Examination of Water and Wastewater (APHA 2005). The sample was centrifuged at 10,000 rpm for 15 min to remove all the suspended matter. The supernatant was taken and absorbance was measured at 456 nm. The value of color was calculated in coloring unit (CU) by making standard curve.
Experimental design for optimization of process parameters
For the Taguchi design and analysis of results the Qualitek–4 version (7.6.0.3) software was used (Roy 2007). Traditional optimization methods involve variation of one parameter while keeping all other parameters constant and assessing the impact of that particular parameter on the process performance. In this study we have used Taguchi approach of orthogonal array and experimental designs that help to gain more information about the optimum conditions. It involves two important steps (1) the use of orthogonal arrays, (2) ANOVA for analysis. Orthogonal arrays are well-defined tables that are used to reduce the number of experiments to be conducted. Taguchi’s L-8 orthogonal array table was used to carry out decolorization experiments by choosing seven parameters at two levels using (Table 1) (Roy 2007). In the orthogonal array of L-8 type, L and subscript 8 means Latin square and the number of experiments, respectively. Full factorial approach will require 128 experiments to be conducted for optimizing a process while in fractional factorial using L-8 orthogonal array the number of experiments reduces to eight (Bakhtiari et al. 2006). After designing, the analysis of experimental data was done using ANOVA. Taguchi approach used ANOVA to evaluate which parameters were statistically significant in finding the optimum levels (Phadke and Dehnad 1988).
Screening experiments for decolorization of distillery spent wash
Screening experiments were performed to select most suitable carbon and nitrogen sources. Various carbon sources were used at an initial concentration 0.05% (w/v). MSM having distillery spent wash (5,000 C.U.) was taken as control. Batch study was conducted in an Erlenmeyer flasks containing 200 ml MSM along with 10% distillery spent wash, 5% (w/v) inoculum supplemented with different carbon sources (0.05%). Reduction in color was analyzed after 5 days. After selecting the most suitable carbon source, various nitrogen sources were screened at a concentration of 0.05% (w/v). Change in color was analyzed after 5 days. Same sets of experiments were carried out for both fungi.
Optimization of growth conditions by Taguchi approach
Once carbon and nitrogen sources were selected, the growth media was optimized for the optimum concentration of selected carbon and nitrogen sources, i.e., carbon (%) and nitrogen (%). Other process parameters such as pH, inoculum size, temperature, RPM and duration were also optimized using their lower and higher levels which were coded as 1 and 2, respectively (Table 1). All the experiments were done in triplicate.
Analysis of the data
The data obtained after the eight experiments was processed in the Qualitek-4 software with quality characteristics for deriving the optimum level for each parameter and ultimately arriving at optimal culture conditions. Besides that, the contribution of each individual factor in total decolorization and the interactions among various parameters was also studied.
Validation
In order to validate the methodology used in optimization, the culture conditions obtained after optimization was tested and compared with the values predicted by the model. Experiment was repeated three times. Qualitek-4 software (Nutek Inc., MI) for automatic design of experiments using Taguchi approach was used in this study. This software is equipped to use L-4 to L-64 orthogonal arrays along with selection of 2–63 factors (parameters) with two, three or four levels. The automatic design option in the software allows selecting the array used and assigning factors to the appropriate columns. In this study L-8 orthogonal array was used with seven parameters at two levels (Dasu et al. 2003).
Estimation of enzymes for decolorization
To measure the activities of the enzyme the culture supernatant was obtained by centrifugation at 8,000×g for 10 min. Four different enzymes, Lignin peroxidase (LiP), Manganese peroxidase (MnP), Laccase (Lac) and Glucose oxidase (GO) were estimated. LiP was measured through the oxidation of veratryl alcohol (VA) to veratryl aldehyde at 310 nm using Varian Carry 100 Bio Spectrophotometer (Tien and Kirk 1988). The reaction mixture consisted of 2 mM VA, 35 mM sodium tartrate buffer (pH 3.0), and enzyme. 1 U/ml of enzyme is defined as 1 μmol of veratryl alcohol oxidized to veratraldehyde per min. The reaction starts with the addition of 0.36 mM H2O2. MnP activity was measured through the oxidation of Mn(II) to Mn(III) at 270 nm as described (Wariishi et al. 1992). The reaction mixture consists of 0.5 mM MnSO4·H2O, 45 mM sodium malonate buffer (pH 4.5) and enzyme. The reaction started with the addition of 0.1 mM H2O2. One U/ml of MnP was defined as 1 μmol of Mn(II) oxidized to Mn(III) per min. Laccase activity was measured by the oxidation of 2, 2′-azinodi-3-ethyl-benzothiazoline-6-sulfuric acid (ABTS) at 436 nm (Niku-Paavola et al. 1988). The reaction mixture contains 10 mM ABTS, 85 mM sodium tartrate buffer (pH 3.0) and enzyme. One unit (U/ml) of enzyme activity was defined as the amount of enzyme required to oxidize 1 μmol substrates per min at 25°C. Glucose oxidase was measured by decrease in reducing sugars at 575 nm (Kona et al. 2001). In this case 0.2 ml of reducing sugar solution was incubated with 0.2 ml enzyme and citrate-phosphate buffer (1 ml, pH 5.6).
Nucleotide sequence accession number
Accession numbers for 18S rDNA partial sequences of strains DF3 and DF4 are EU741056 and EU741055, respectively.
Results and discussion
Isolation and screening of fungal strains for decolorization
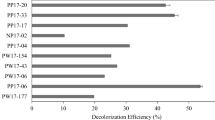
Five different types of fungal strains appeared on the plates were isolated based on morphological differentiation of individual colonies. They were observed under a microscope, Olympus and Magnus MLX-TR, at 40 and 100× attached with a camera. Fugal mycelium, spores and the spore attachment were observed. They were further purified using PDA by repeated culturing and screened on the basis of their decolorization potential. The decolorization was measured after 1, 3, 7, 10 and 15 days (Fig. 1). Maximum reduction in color was observed on 10th day by DF3 (38%), followed by DF4 (31%), DF5 (23%), DF1 (9%) and minimum by DF2 (7%). The best performing fungal isolates DF3 and DF4 were acclimatized for 3 months at pH 3 with 10% distillery spent wash. After acclimatization the time required for decolorization reduced from 10 to 3 days.
Identification and characterization of fungal strains
The two best performing strains (DF3 and DF4) were selected based on decolorizing potency of distillery mill effluent. The isolates were identified as Emericella nidulans var. lata (DF3) and Neurospora intermedia (DF4) after amplification and sequencing of ITS region of genomic DNA. The sequence of the amplified region, i.e., ITS1, 5.8S and ITS 4 of DF3 and DF4 was blasted using NCBI and the phylogeny tree was constructed using Mega 3.1 software. Figure 2 shows the phylogeny tree of DF3. The fungal strain was close to Emericella nidulans var. lata (DF3) having 98% homology. Similarly DF4 was identified as Neurospora intermedia (Fig. 3). However, this is the first report presenting the role of Neurospora intermedia (DF4) in decolorization studies.
Screening of growth factors for decolorization of distillery effluent
Among the various carbon sources (0.05%) maximum color was removed when effluent was treated by Emericella nidulans var. lata (DF3) in presence of dextrose (41%) followed by sucrose (37%), sodium citrate (14%) and sodium acetate (8%), however, Neurospora intermedia (DF4) reduced color of the effluent in presence of dextrose (43%), sucrose (36%), sodium citrate (15%) and sodium acetate (8%) (Fig. 4). Result of the study indicated dextrose was the best carbon source for both fungi. In general, high carbon levels favor the process of decolorization, however, maximum decolorization of distillery spent wash in absence of additional carbon and nitrogen sources using soil as inoculum are also reported (Sirianuntapiboon et al. 2004; Adikane et al. 2006).
Effluent was further treated in presence of inorganic and organic nitrogen source (0.05%) like urea, yeast extract, sodium nitrate and ammonium nitrate, and the results indicated that maximum decolorization was achieved with sodium nitrate (33%), followed by ammonium nitrate (16%), urea (16%) and yeast extract (13%) by Emericella nidulans var. lata (DF3). Similarly in case of Neurospora intermedia (DF4) maximum reduction in color was achieved by sodium nitrate (30%) followed by ammonium nitrate (21%), urea (15%) and yeast extract (13%) (Fig. 4). Both strains preferably used inorganic nitrogen sources as compared to organic nitrogen sources for decolorization. Sirianuntapiboon et al. (2004) reported similar observations. Inhibitory effects of nitrogen on melanoidin decolorization by Coriolus hirsutus were observed (Miyata et al. 2000). The combined effect of the study says that decolorization was improved by the addition of carbon and nitrogen sources.
Determination of optimum conditions using Taguchi method
Taguchi method was used to identify the optimum conditions and select the parameter having the most significant step in decolorization of distillery spent wash. After selecting the most suitable carbon and nitrogen source, the effect of temperature, RPM, pH, inoculum size and duration and ratio of carbon and nitrogen sources were further optimized by using Taguchi approach for both strains (Emericella nidulans var. lata (DF3) and Neurospora intermedia (DF4)).
Experiments were conducted for optimization of increased decolorization according to the L-8 orthogonal array. Total eight experiments were conducted. Same sets of experiments (Table 1) were carried out with both fungi. Results showed that maximum decolorization achieved by Emericella nidulans var. lata (DF3) (62%) when experiment conditions were as follows: temperature 30°C, pH 3, agitation 125 rpm, duration 3 days, inoculum size 10% (w/v), carbon (dextrose) 0.05% (w/v), nitrogen (sodium nitrate) (0.025%) (w/v). For Neurospora intermedia (DF4) the optimum conditions for maximum decolorization (64%) were: temperature 30°C, pH 3, agitation 125 rpm, duration 2 days, inoculum size 10% (w/v), carbon (dextrose) 0.05% (w/v), nitrogen (sodium nitrate) (0.025%) (w/v). In case of Neurospora intermedia (DF4) mycelium became dark, indicating that adsorption was the dominant phenomenon in the process of decolorization. However, in case of Emericella nidulans var. lata (DF3) the color of the mycelium remained unchanged, suggesting that the fungi might have metabolized various compounds present in the effluent in 3 days by secretion of enzymes responsible for decolorization.
Table 1 shows the results obtained after performing the experiments. The effective influence of a Taguchi L-8 orthogonal array designed experimental conditioned on maximum decolorization could be viewed and the data revealed variation in percentage decolorization of distillery spent wash (Table 1). The observed variation certainly indicated the imperative role of optimization of all factors in achieving the best possible results. The results of decolorization of all eight experiments in both cases were fed in Qualitek-4 software for analysis. The optimization software provided an opportunity to study the multi interaction effect of the factors. Among all selected factors carbon and temperature had the highest positive impact on decolorization potency in case of Emericella nidulans var. lata (DF3) and Neurospora intermedia (DF4), respectively. The contribution of individual factor is the key to control the biological processes. For each factor there is an optimum level. Figure 5 shows the increase or decrease in decolorization for each factor at different levels.
Carbon and nitrogen sources have extremely variable response in reference to decolorization. The optimum level of dextrose, most suitable carbon source, was 0.05% w/v i.e. level 1. Increasing the dextrose concentration 0.2% (level 2) reduced the decolorization drastically in both cases (Fig. 5). The percent of decolorization was increased in case of Emericella nidulans var. lata (DF3) when the factor duration was taken from level 1 (2 days) to level 2 (3 days). However, reverse effects were seen in case Neurospora intermedia (DF4). In case of pH change from lower level to higher level, a decrease in the decolorization efficiency was observed for both fungi which perhaps because of polymerization of colored compound (Shayegan et al. 2005).
An analysis of Variance (ANOVA) was conducted to determine the relative importance of each factor. The main objective of ANOVA was to conclude from the result that each factor causes how much variation relative to the total variation observed in the result. From the results of ANOVA (Table 2), it was concluded that % carbon had the largest variance; the temperature and %nitrogen indicated the second in case of Emericella nidulans var. lata (DF3). While in case of Neurospora intermedia (DF4) the highest variances were observed first for temperature, then carbon, pH and %nitrogen (Table 3). Inoculum size was pooled in both cases, as its relative variation seemed insignificant.
Validation experiments
Before optimization the average decolorization was 38 and 31% by Emericella nidulans var. lata (DF3) and Neurospora intermedia (DF4), respectively, and after optimization the expected decolorization was 66.5% (DF3) and 64% (DF4). Actual decolorization was 62% by DF3 and 64% by DF4, which showed an almost two-fold increase. Thus the decolorization was significantly improved.
Mechanism for decolorization of distillery effluent by enzymes
The mechanism of decolorization of distillery effluent was determined by lignin peroxidase, manganese peroxidase, laccase and glucose oxidase enzyme analysis. The result of the study indicated no significant increase in peroxidase and oxidase enzyme in the culture supernatant of Neurospora intermedia (DF4), however, in the case of Emericella nidulans var. lata (DF3) the laccase and glucose oxidase activity in the culture supernatant was detected (Fig. 6). A marked increase in glucose oxidase and laccase activity was detected in the Emericella nidulans var. lata (DF3) treated effluent after 1 day, which reached to maxima after 48 h. The activity of enzyme decreased after 3 days.
Glucose oxidase activity has been reported in culture supernatant of Flavodon flavus during decolorization of molasses spent wash (Raghukumar et al. 2004). There are number of reports showing role of fungi in decolorization by adsorption of melanoidin to mycelia and role of lignolytic enzymes metabolized color containing chemical compounds in the effluent (Watanabe et al. 1982; Benito et al. 1997; Vahabzadeh et al. 2004). Our results indicated production of laccase and glucose oxidase in Emericella nidulans var. lata (DF3), which were not present in Neurospora intermedia (DF4). However, results of the study indicated reduction in color of distillery effluent by Neurospora intermedia (DF4), which might be due to biosorption of coloring material in the effluent in fungal biomass, increased by carbon and nitrogen sources. These growth factors do not enhance the decolorization directly but help by increasing the growth of the microorganism, hence contributing to decolorization.
Conclusion
Fungus isolated from distillery spent wash and sludge of distillery unit was identified by amplification and sequencing of ITS1 and ITS2 region of genomic DNA as Emericella nidulans var. lata (DF3) and Neurospora intermedia (DF4), respectively. Both strains showed decolorization of distillery effluent after acclimatization and optimization. Most suitable nitrogen source was sodium nitrate and most appropriate carbon source was dextrose. Optimizing the growth factors and process parameters using Taguchi approach further enhanced the decolorization potential of strains. Before optimization the average decolorization of effluent by Emericella nidulans var. lata (DF3) was 38% and by Neurospora intermedia (DF4) was 31%. After optimization the actual decolorization increased up to 62 and 64%, respectively. Thus, the optimization technique used in this study could be widely used to enhance fermentation conditions. Enzymatic methods were employed to understand the mechanism of decolorization of distillery effluent in which the laccase and glucose oxidase activity was determined in DF3, however, no such activity was observed in the case of DF4.
References
Adikane HV, Dange MN, Selvakumari K (2006) Optimization of anaerobically digested distillery molasses spent wash decolorization using soil as inoculum in the absence of additional carbon and nitrogen source. Bioresour Technol 97:2131–2135. doi:10.1016/j.biortech.2005.10.011
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American public health association, Washington
Bakhtiari MR, Faezi MG, Fallahpour M, Noohi A, Moazami N, Amidi Z (2006) Medium optimization by orthogonal array designs for urease production by Aspergillus niger PTCC5011. Process Biochem 41:547–551. doi:10.1016/j.procbio.2005.09.002
Benito GG, Miranda MP, de los Santos DR (1997) Decolorization of wastewater from an alcoholic fermentation process with Trametes versicolor. Bioresour Technol 61(1):33–37. doi:10.1016/S0960-8524(97)84695-0
Bernardo EC, Egashira R, Kawasaki J (1997) Decolorization of molasses wastewater using activated carbon prepared from cane bagasse. Carbon 35(9):1217–1221. doi:10.1016/S0008-6223(97)00105-X
Dasu VV, Panda T, Chidambaram M (2003) Determination of significant parameters for improved griseofulvin production in a batch bioreactor by Taguchi’s method. Process Biochem 38:877–880. doi:10.1016/S0032-9592(02)00068-7
Fahy V, FitzGibbon FJ, McMullan G, Singh D, Marchant R (1997) Decolorization of molasses spent wash by Phanerochaete chrysosporium. Biotechnol Lett 19:97–99. doi:10.1023/A:1018335608459
Hortal S, Pera J, Galipienso L, Parlad’e J (2006) Molecular identification of the edible ectomycorrhizal fungus Lactarius deliciosus in the symbiotic and extraradical mycelium stages. J Biotechnol 126:123–134. doi:10.1016/j.jbiotec.2006.04.011
Kackar R (1985) Off-line quality control, parameter design and Taguchi method. J Qual Technol 17:176–188
Karakousis A, Tan L, Ellis D, Alexiou H, Wormald PJ (2006) An assessment of the efficiency of fungal DNA extraction methods for maximizing the detection of medically important fungi using PCR. J Microbiol Methods 65:38–48. doi:10.1016/j.mimet.2005.06.008
Kitts DD, Wu CH, Stich HF, Powrie WD (1993) Effect of glucose–glycine Maillard reaction products on bacterial and mammalian cells mutagenesis. J Agric Food Chem 41:2353–2358. doi:10.1021/jf00036a026
Kona RP, Qureshi N, Pai JS (2001) Production of glucose oxidase using Aspergillus niger and corn steep liquor. Bioresour Technol 78:123–126. doi:10.1016/S0960-8524(01)00014-1
Migo VP, Matsumura M, DelRosario EJ, Kataoka H (1993) Decolorization of molasses wastewater using an inorganic flocculent. J Ferment Bioeng 75(6):438–442. doi:10.1016/0922-338X(93)90092-M
Miyata N, Mori T, Iwahori K, Fujita M (2000) Microbial decolorization of melanoidin—containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus. J Biosci Bioeng 89:145–150. doi:10.1016/S1389-1723(00)88728-9
NakajimaKambe T, Shimomura M, Nomura N, Chanpornpong T, Nakahara T (1999) Decolorization of molasses wastewater by Bacillus sp. under thermophilic and anaerobic conditions. J Biosci Bioeng 87:119–121. doi:10.1016/S1389-1723(99)80021-8
Niku-Paavola ML, Karhunen E, Salola P, Raunio V (1988) Lignolytic enzymes of the white rot fungus Phlebia radiate. Biochem J 254:877–884
Ohmomo S, Kaneko Y, Sirianuntapiboon S, Somachi P, Atthasampunna P, Nakamura I (1987) Decolorization of molasses wastewater by a thermophilic strain, Aspergillus fumigatus G-2–6. Agric Biol Chem 52:3339–3346
Ohmomo S, Kainuma M, Kamimura K, Sirianuntapiboon S, Oshima I, Atthasumpunna P (1988) Adsorption of melanoidin to the mycelia of Aspergillus oryzae Y-2–32. Agric Biol Chem 52:381–386
Pena M, Gonzalez G, San N, Nieto H (1996) Color elimination from molasses wastewater by Aspergillus niger. Bioresour Technol 57:229–235. doi:10.1016/S0960-8524(96)00048-X
Phadke MS, Dehnad K (1988) Optimization of product and process design for quality and cost. Qual Reliab Eng Int 4:159–169. doi:10.1002/qre.4680040205
Prakasham RS, Rao CS, Rao S, Rajesham S, Sharma PN (2005) Optimization of alkaline protease production by Bacillus sp. using Taguchi methodology. Appl Biochem Biotechnol 120:133–144. doi:10.1385/ABAB:120:2:133
Raghukumar C, Rivonkar G (2001) Decolorization of molasses spent wash by the white rot fungus Flavadon flavus, isolated from marine habitat. Appl Microbiol Biotechnol 55:510–514. doi:10.1007/s002530000579
Raghukumar C, Mohandass C, Kamat S, Shailaja MS (2004) Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzyme Microb Technol 35:197–202. doi:10.1016/j.enzmictec.2004.04.010
Roy RK (2007) Qualitek-4, software for automatic design and analysis of Taguchi experiments, Nutek. Inc. Bloomfield Hills, Michigan, USA
Shayegan J, Pazouki M, Afshari A (2005) Continuous decolorization of anaerobically digested distillery wastewater. Process Biochem 40:1323–1329. doi:10.1016/j.procbio.2004.06.009
Sirianuntapiboon S, Sihanonth P, Somchai P, Atthasampunna P, Hayashida S (1995) An adsorption mechanism for melanoidin decolourization by Rhizoctonia sp. Biosci Biotechnol Biochem 59:1185–1189
Sirianuntapiboon S, Zohsalam P, Ohmomo S (2004) Decolorization of molasses wastewater by Citeromyces sp WR-43–6. Process Biochem 39:917–924. doi:10.1016/S0032-9592(03)00199-7
Stowe RA, Mayer RP (1999) Efficient screening of process variables. Ind Eng Chem 56:36–40
Thakur IS (2004) Screening and identification of microbial strains for removal of colour and adsorbable organic halogens in pulp and paper mill effluent. Process Biochem 39:1693–1699. doi:10.1016/S0032-9592(03)00303-0
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249. doi:10.1016/0076-6879(88)61025-1
Vahabzadeh F, Mehranian M, Saatari AR (2004) Colour removal ability of Phanerochaete chrysosporium in relation to lignin peroxidases and manganese peroxidases produced in molasses wastewaters. World J Microbiol Biotechnol 20:859–864. doi:10.1007/s11274-004-9005-9
Wariishi H, Valli K, Gold MH (1992) Manganese (II) oxidation by manganese peroxidase from the Basidiomycete Phanerochaete chrysosporium: kinetic mechanism and role of chelators. J Biol Chem 267:23688–23695
Watanabe Y, Sugi R, Tanaka Y, Hayashida S (1982) Enzymatic decolorization of melanoidin by Coriolus sp. No. 20. Agric Biol Chem 46:1623–1630
Yang Q, Jia Z, Liu R, Chen J (2007) Molecular diversity and anammox activity of novel planktomycete-like bacteria in the waste water treatment system of a full-scale alcohol manufacturing plant. Process Biochem 42:180–187. doi:10.1016/j.procbio.2006.07.032
Acknowledgments
This paper was supported by the research grants of University for potential of Excellence. The author (GK) thanks University Grants Commission, for providing Junior Research Fellowship Government of India, New Delhi, India. We thank Modi distilleries, Modinagar, Uttar Pradesh, India for providing effluent and sludge during the course of investigation. We thank R. K. Roy (Nutek Inc., USA) for providing free demo version of their Software Qulitek-4®.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushik, G., Thakur, I.S. Isolation of fungi and optimization of process parameters for decolorization of distillery mill effluent. World J Microbiol Biotechnol 25, 955–964 (2009). https://doi.org/10.1007/s11274-009-9970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-9970-0







 is Neurospora intermedi and
is Neurospora intermedi and  Emericella nidulans var. lata.
Emericella nidulans var. lata.
 is initial decolorization and
is initial decolorization and  effect of different factors
effect of different factors
 is Neurospora intermedia and
is Neurospora intermedia and  Emericella nidulans var. lata.
Emericella nidulans var. lata.