Abstract
The physico-chemical characteristics of sugar industry effluent were measured and some were found to be above those limits permissible in the Indian irrigation water standard. A pot study was initially conducted to study the effects of different concentrations (20%, 40%, 60%, 80% and 100%) of sugar factory effluent on seed germination, seedling growth and biochemical characteristics of green gram and maize. A similar study was also carried out using the aquatic plants, water hyacinth and water lettuce. The higher effluent concentrations (above 60%) were found to affect plant growth, but diluted effluent (up to 60%) favored seedling growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The sugar industry is playing an important role in the economic development of the Indian sub continent, but the effluents released produce a high degree of organic pollution in both aquatic and terrestrial ecosystems. They also alter the physico-chemical characteristics of the receiving aquatic bodies and affect aquatic flora and fauna. Sugar factory effluent, when discharged into the environment, poses a serious health hazard to the rural and semi-urban populations that uses stream and river water for agriculture and domestic purposes, with reports of fish mortality and damage to the paddy crops in these areas due to wastewaters entering agricultural land (Baruah et al. 1993). Sugar factory effluent has an obnoxious odour and unpleasant colour when released into the environment without proper treatment. Farmers have been using these effluents for irrigation, and found that the growth, yield and soil health were reduced. Contaminants, such as chloride, sulphate, phosphate, magnesium and nitrate, are discharged with the effluent of various industries, which create a nuisance due to physical appearance, odour and taste. Such harmful water is injurious to plants, animals and human beings. The effects of various industrial effluents on seed germination, growth and yield of crop plants have captivated the attention of many workers (Ozoh and Oladimeji 1984; Rahman et al. 2002; Street et al. 2007). However, no detailed experiments have been performed on the germination and plant growth using sugar factory effluent. In the present investigation, an attempt has been made to study the effects of sugar factory effluent on the seed germination, seedling growth, amino acids, proteins and chlorophyll content of green gram and maize, as well as the aquatic plants, water hyacinth and water lettuce.
Materials and Methods
The sugar factory effluent was collected in pre-cleaned, acid washed 50 L carboys from a sugar industry located in Erode district, Tamil Nadu, India and stored in a cold room until used. Temperature, colour, pH, electrical conductivity (EC), dissolved oxygen (DO), biological oxygen demand (BOD), total solids (TS), total suspended solids (TSS), total dissolved solids (TDS), chloride, alkalinity, total hardness, calcium, magnesium, sulphate, phosphate and total iron, as physico-chemical parameters, were measured using standard methods (APHA 1998).
The impact of sugar factory effluent on the growth and biochemical characteristics of the green gram (Phaseolus aureus CO-4) and maize (Zea mays CO-1) were first investigated using soil pots (30 cm height × 30 cm width). Red soil, without any contamination by sugar factory effluent, was collected and sieved (2 mm mesh). About 4 kg of soil was taken into separate pots. Five different concentrations (viz., 20%, 40%, 60%, 80% and 100%) of effluent were prepared and poured into each pot. The control was also maintained and irrigated with tap water. Five seeds of green gram and maize, pre-sterilised with 0.1% mercuric chloride, were sown separately in each pot and allowed to germinate. The pots were irrigated with 1 L of effluent at 48 h intervals. The percentage of germination was assessed (Rahman et al. 2002) and the shoot length of the plants recorded every 48 h for 20 days.
The fresh and total dry masses of green gram and maize were determined after 20 days of the experiment. The plants were uprooted, washed thoroughly with distilled water and the lengths of the roots measured. The plants were dried for 2 h under natural conditions at an open roof top garden. The fresh weights were taken, with the plants then packed in paper envelopes and oven dried for 36 h at 70°C. The dry weight of each plant was also recorded. The total amino acid, total protein and total chlorophyll, as biochemical parameters, were analyzed for each experimental plant leaf on the 20th day (Sadasivam and Manickam 1996).
For the aquatic system study, healthy water hyacinth (Eichornia crassipes) and water lettuce (Pistia stratiotes), with an average weight of 60 g, were collected from a pond and then washed thoroughly with distilled water to remove particles adhering to the plants. Further, sugar factory effluents were prepared with five different concentrations (20%, 40%, 60%, 80% and 100%) and transferred into about 1.5 L in rectangular plastic vessels (25 × 15 × 12 cm). The cleaned plants (60 g each) were introduced into the vessels, with the roots submerged in the effluent, and kept under sunlight for 20 days. The fresh weight of the plants was determined every 24 h using a physical balance after removing water by blotting. The fresh and dry masses of the plants, total amino acid, total protein and total chlorophyll were estimated at the final stage of the study (20th day). The procedures applied were similar to those for the determination of physical and biochemical properties of the plants described earlier. All the experiments were conducted in triplicate, unless otherwise stated. Data points in the tables and figures represent the means, with all error bars shown (±1 standard error of mean). Both the mean and standard deviation were performed where appropriate using the statistical package on Microsoft® Excel Version 2003.
Results and Discussion
The physico-chemical parameters of the effluent were found to be above those permissible by the Indian Standards (Table 1). The pH was relatively low due to the use of phosphoric acid and sulphur dioxide during clarification of sugar cane juice (Manivasakam 1987). Palharyal et al. (1993) reported that the pH was an essential factor in the formation of algal blooms, which makes the water unfit for irrigation, and the soil over a large area becomes acidic resulting in poor crop growth and yield. Similarly, the effluent had a very high TDS, which was in good agreement with the report by Abdul Jameel and Sirajudeen (2006), who also found a very TDS (3,950 mg/L) in sugar factory effluent.
The seeds of green gram and maize were 100% germinated in the lower concentrations (20%–80%) of sugar factory effluent; whereas, in the undiluted effluent the germinations were 73% and 80% (Table 2). Ajmal and Khan (1983) proved the effluent with a lower concentration (25%) supported 100% seed germination, with osmotic pressure associated with higher concentration of sugar factory effluent found to affect the germination in kidney bean, Phaseolus aureus and millet, Pennisetum typhoides. Rodger et al. (1957) reported that high osmotic pressures of the germination solution makes imbibitions more difficult and retards germination, while the ability of seeds to germinate under high osmotic pressure differs with variety as well as species.
The maximum shoot lengths of green gram and maize were observed in the control, followed by effluent concentrations of 20%, 40%, 60%, 80% and 100% (Table 3), with a direct relationship between shoot length and effluent concentration. Kaushik et al. (2004) reported a clear toxicity of sugar factory effluent on the growth, photo synthetic pigments and nutrient uptake in wheat seedlings in aqueous versus soil medium. The presence of calcium and magnesium cause higher osmotic pressure, resulting in the wilting of seedlings (Gomathi and Oblisami 1992). In our study, the plant growth was highly affected due to the excess amount of chloride, alkalinity, hardness, calcium, magnesium, sulphate and phosphate in the sugar factory effluent. The root length was severely affected by the higher effluent concentrations (100%) for green gram (13.5 cm) and maize (25 cm) compared to the control. Similarly, green gram and maize showed maxima fresh and dry weights in the control, with minima found in 100% effluent (Table 4).
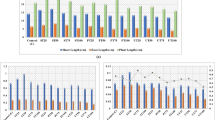
The total amino acid, protein and chlorophyll content, as biochemical parameters, were analyzed in the leaves of green gram and maize. The amounts of amino acid, protein and chlorophyll contents gradually decreased with increasing effluent concentration (Fig. 1a and b). Plants treated with higher effluent concentrations (above 20%) showed lower amounts of amino acid, protein and chlorophyll contents due to the presence of higher magnesium concentrations and the acidic pH of the effluent. Calcium and magnesium (20 mg/L) influence plant growth, biomass partitioning and fruit yield, and create symptoms of leaf chlorosis after 8 weeks in green house tomato (Hao and Athanasios 2004). Lasa et al. (2000) also reported that four different concentrations (0.1, 0.8, 5 and 10 mM) of magnesium affected the growth of sunflower plants grown with ammonium and nitrate; they also proved that the magnesium-fed plants had lower free amino acids and soluble protein contents in their leaves.
In the aquatic system, the water hyacinth plants showed gradual decreases in plant weights throughout the study with effluent concentrations of 80% and 100% (Fig. 2a). The weight of the control plant increased from 60 to 93.3 g after 20 days. In 20%, 40% and 60% effluent concentrations the plant weight gradually increased to 85.4, 84.2 and 77.6 g, respectively, after 20 days. After 10 days, the weights remained more or less constant. There was a decrease in the plant weights at 80% and 100% effluent concentrations. Similar results were observed in the aquatic plant, water lettuce (Fig. 2b). The maximum weight loss was observed in 100% effluent; whereas, in the control the growth increased from 60 to 73.1 g after 20 days. This might have been due to the presence of moderate amounts of micronutrients in the diluted effluent stimulating the plant growth. However, the excessive levels at higher concentrations could result in stunted growth.
The effects of different concentrations of sugar factory effluent on the total amino acids, protein and chlorophyll contents of the aquatic plants (water hyacinth and water lettuce) were observed, with the results given in Fig. 2c and d. The above parameters at different effluent concentrations were found to be very low compared to the control. The amino acid, protein and chlorophyll contents of the aquatic plants decreased due to the increased concentrations of sugar factory effluent. Owing to the toxic nature of the effluent, the leaves of the plants showed decreased photosynthetic rates. As a result of the higher BOD, the photosynthesis of the aquatic system was also affected (Rao et al. 1993), which reduced the plant growth parameters. The dry weights of the water hyacinth and water lettuce were higher in the control, which were 640 and 350 mg, respectively. The dry weight was significantly decreased with increasing effluent concentration (Table 5).
This study concluded that the physico-chemical parameters, such as BOD, chloride, alkalinity, hardness, calcium, magnesium, sulphate and phosphate were relatively higher in the sugar factory effluent and severely affected the plant growth. There was a gradual decrease in the shoot length, and free amino acid, protein and total chlorophyll contents in both terrestrial and aquatic plants when irrigated with various effluent concentrations compared to the control. The untreated effluent could possibly lead to soil pollution, deterioration and low productivity. Both the terrestrial and aquatic environments were affected, which could be averted by proper treatment of the effluents using suitable conventional methods.
References
Abdul Jameel A, Sirajudeen J (2006) Risk assessment of physico-chemical contaminants in ground water of Pettavaithalai area, Thiruchirappalli, Tamil Nadu, India. Environ Monit Assess 123:299–312
Ajmal M, Khan AU (1983) Effects of sugar factory effluent on soil and crop plants. Environ Pollut A 30:135–141
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Water Pollution Control Federation, Washington, DC
Baruah AK, Sharma RN, Borah GC (1993) Impact of sugar mill and distillery effluent on water quality of river Galabil, Assam. Indian J Environ Hlth 35:288–293
Gomathi V, Oblisamy G (1992) Effect of pulp and paper mill effluents on germination of the crops. Indian J Environ Hlth 34:326–328
Hao X, Athanasios P (2004) Effects of calcium and magnesium on plant growth, biomass partitioning, and fruit yield of winter greenhouse tomato. Hortscience 39:512–515
Kaushik A, Kadyan BR, Kaushik CP (2004) Sugar mill effluent effects on growth, photosynthetic pigments and nutrient uptake in wheat seedlings in aqueous vs. soil medium. Water Air Soil Poll 87:39–46
Lasa B, Frechilla S, Aleu M, Gonzalez-Moro B, Lamsfus C, Aparicio-Tejo PM (2000) Effects of low and high levels of magnesium on the response of sunflower plants grown with ammonium and nitrate. Plant soil 225:167–174
Manivasakam N (1987) Industrial effluents origin, characteristics, effects, analysis and treatment. Sakthi publications, Kovai pudur, Coimbatore, India
Ozoh PTE, Oladimeji AA (1984) Effects of Nigeria dye stuff effluent on germination latency, growth, and gross growth of Zea mays. Bull Environ Contam Toxicol 33:215–219
Palharyal JP, Siriah VK, Shobana M (1993) Environmental impact of sewage and effluent disposal on the river systems. Ashish Publishing House pp 325
Rahman KSM, Banat IM, Rahman TJ, Thayumanavan T, Lakshmanaperumalsamy P (2002) Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Biore Technol 81:25–32
Rao AV, Jain BL, Gupta IC (1993) Impact of textile industrial effluents on agricultural land – a case study. Indian J Environ Hlth 35:132–138
Rodger JBB, Williams GG, Davis RL (1957) A rapid method for determining winter hardiness of alfalfa. Agron J 49:88–92
Sadasivam S, Manickam A (1996) Biochemical methods, 2nd edn. New age International Publishers, New Delhi, India
Street RA, Kulkarni MG, Stirk WA, Southway C, Van Staden J (2007) Toxicity of metal elements on germination and seedling growth of widely used medicinal plants belonging to Hyacinthaceae. Bull Environ Contam Toxicol 79:371–376
Acknowledgements
The authors are thankful to the Head of the Department of Environmental Sciences, Bharathiar University for providing facilities for the analytical work. Also, this research was financially supported by the Korea Research Foundation, Republic of Korea (Project No. KRF-2005-070-C-00137).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayyasamy, P.M., Yasodha, R., Rajakumar, S. et al. Impact of Sugar Factory Effluent on the Growth and Biochemical Characteristics of Terrestrial and Aquatic Plants. Bull Environ Contam Toxicol 81, 449–454 (2008). https://doi.org/10.1007/s00128-008-9523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9523-5