Abstract
The presence of melanoidins in molasses wastewater leads to water pollution both due to its dark brown color and its COD contents. In this study, a bacterial consortium isolated from waterfall sediment was tested for its decolorization. The identification of culturable bacteria by 16S rDNA based approach showed that the consortium composed of Klebsiella oxytoca, Serratia mercescens, Citrobacter sp. and unknown bacterium. In the context of academic study, prevention on the difficulties of providing effluent as well as its variations in compositions, several synthetic media prepared with respect to color and COD contents based on analysis of molasses wastewater, i.e., Viandox sauce (13.5% v/v), caramel (30% w/v), beet molasses wastewater (41.5% v/v) and sugarcane molasses wastewater (20% v/v) were used for decolorization using consortium with color removal 9.5, 1.13, 8.02 and 17.5%, respectively, within 2 days. However, Viandox sauce was retained for further study. The effect of initial pH and Viandox concentration on decolorization and growth of bacterial consortium were further determined. The highest decolorization of 18.3% was achieved at pH 4 after 2 day of incubation. Experiments on fresh or used medium and used or fresh bacterial cells, led to conclusion that the limitation of decolorization was due to nutritional deficiency. The effect of aeration on decolorization was also carried out in 2 L laboratory-scale suspended cell bioreactor. The maximum decolorization was 19.3% with aeration at KLa = 2.5836 h−1 (0.1 vvm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large quantities of wastewater in Thailand are generated from the alcohol distilleries which use sugar cane molasses as raw material. There are basically two types of wastewaters; one is high strength process wastewater or concentrated wastewater that originates from distillation process during alcohol production. The high strength wastewater is high in chemical oxygen demand (COD) and high concentration of organic matters (BOD). Moreover, it has low pH and large amount of dark brown color leading to serious impact to the environment. The other is low strength process wastewater or diluted wastewater that originates from the floor washing and equipment cleaning. It has low concentration of COD, BOD and color [31, 34, 37, 48].
The presence of dark brown color is known as melanoidins which are formed by Maillard amino carbonyl reaction [23]. The release of melanoidins into environment from the effluent of alcohol distilleries has become a major concern in wastewater treatment since it can result in the reduction of photosynthetic activity and dissolved oxygen concentration in aquatic environment. Furthermore, it can also lead to eutrophication due to high organic loads. Finally, it affects aquatic plants and aquatic animals [39].
Accordingly, the method for removal of melanoidins before discharging into environment is necessary. Currently, the decolorization of melanoidins in wastewater is based mainly on physical and chemical methods such as ozonization, flocculation, chemical coagulation, precipitation, activated carbon adsorption and advanced oxidation of the wastewater [4, 17, 25, 28, 39]. However, the implement of physical and chemical methods eventually may generate significant amount of sludge, cause secondary pollution due to excessive chemical usage, and have some drawbacks such as high cost, formation of hazardous by-products and intensive energy consumption.
Several emerging technologies such as electrochemical destruction, photocatalysis and sorption are promising for molasses wastewater decolorization [21, 41]. However, these approaches often involve complicated procedures and are economically unfeasible. The conventional treatment process such as aerobic treatment and anaerobic treatment process can be accomplished as well but with only low removal of melanoidins and the COD of treated wastewater is still higher than the standard permission value of Department of Industrial Works, Ministry of Industry, Thailand [10].
Therefore, there are still demands to develop alternative means of melanoidins decolorization such as innovative biological methods capable of providing a more complete clean up of the pollutant in more economic fashion.
Over the past decade, biological treatment has been investigated [32, 35, 46]. Microbial decolorization is an environment-friendly and cost competitive alternative to chemical decomposition process [32]. Several kinds of microorganism such as fungi (Penicillium decumbens, Aspergillus sp., Aspergillus niger, Flavadon flavus) [12, 19, 38, 42], white rot fungi (Phanerochaete sp., Phanerochaete chrysosporium, Trametes versicolor, Coriolus sp.,) [6, 16, 24], yeast (Citeromyces sp.) [47] and bacteria (Bacillus sp., Pseudomonas sp., acetogenic bacteria) [9, 13, 18, 47] have been reported regarding their abilities to remove melanoidins.
There are many reports showing that white rot fungi are very effective to remove melanoidins in molasses wastewater. But for the application in large scale treatment, it has been impeded of owing to lack of an appropriate reactor system capable of coping with relatively slow fungal degradation, loss of extracellular enzymes and mediator with discharged water [33, 49].
The decolorization of molasses wastewater by bacteria has also been investigated such as Bacillus sp., Pseudomonas sp., acetogenic bacteria [9, 13, 18, 47]. Nevertheless, the application of these strains to remove melanoidins from molasses wastewater was still inconvenient from the view point of stability and maintenance of removal activity due to culture condition, nutrient supplement and growth [24].
Pure bacterial or fungal cultures have been studied in order to develop bioprocess for melanoidins decolorization in molasses wastewater. However, the performance of fungal decolorization was limited by long growth cycle and moderate decolorization rate. In contrast, the bacterial decolorization is usually faster, but it may require a mixed community to decolorize melanoidins through combined metabolic mode of individual culture [1, 22]. The mixed culture of Bacillus spp. exhibited a two- to fourfold increase in melanoidins decolorization over that showed by any individual Bacillus isolate [22]. Also, Alkane et al. [1] reported that 69% decolorization of molasses spent wash was achieved using soil samples as inoculum instead of isolated microorganisms.
Hence, the bacterial consortium seems to be more competent for molasses wastewater treatment due to maintenance of microorganism and co-metabolism to enhance the efficiency of melanoidins decolorization.
In this study, the effect of operation parameters on melanoidins decolorization and the performance of the constructed bacterial consortium for treating the melanoidin-containing wastewater were investigated.
Materials and methods
Microorganisms
In this study, a bacterial consortium was collected from waterfall sediments in Maehongsorn province, Thailand. This consortium was screened and used for melanoidin decolorization in laboratory. It showed melanoidins decolorization when cultivated in the synthetic melanoidins-containing wastewater containing 20% (v/v) of sugarcane molasses wastewater from alcoholic distillery. This consortium exhibited the highest melanoidin decolorization of 20% within 48 h under aerobic condition and this consortium was selected for further study.
Preparation of synthetic melanoidins-containing wastewater medium
Four types of synthetic melanoidins-containing wastewater media were prepared using melanoidins-containing components including sugarcane molasses wastewater, beet molasses wastewater, Viandox sauce and caramel. Other components of the each synthetic melanoidins-containing wastewater media were as follows: 0.01% NaNO3, 0.2% K2HPO4, 0.1% KH2PO4, 0.01% MgSO4•12H2O, 2% glucose and 0.1% yeast extract, and the initial pH was adjusted to 4. The characteristics of individual synthetic melanoidins-containing wastewater medium are indicated in Table 1. The sugar cane molasses wastewater was obtained from SangSom distillery, Nakhon-Pathom province, Thailand. Beet molasses wastewater, Viandox sauce and caramel were obtained from Laboratoire de Genie Chimique, Toulouse, France. Since the caramel had changed the physical property of synthetic melanoidins-containing wastewater medium as shown by high viscosity at the concentration up to 30%. Thus, this experiment could not synthesize melanoidins-containing wastewater medium using caramel with respect to color (OD475) and COD contents based on analysis of sugarcane molasses wastewater.
Decolorization of various synthetic melanoidins-containing wastewater medium by bacterial consortium
The inoculum was prepared by transferring of bacterial consortium into a flask containing 50 ml LB medium and incubated for 24 h with shaking (200 rpm) at 30 °C. The decolorization experiments were carried out by transferring of 10% inoculum into shake flasks containing 250 ml of four individual synthetic melanoidins-containing wastewater medium containing different colored substances. The consortium was incubated under shaking conditions (200 rpm) at pH 4, 30 °C. A separate set of uninoculated flasks was maintained in parallel as control. Experiments were performed in triplicate and samples were withdrawn with 8 h intervals for the determination of its growth and decolorization.
Identification of the bacterial consortium
Genomic DNAs of bacterium were amplified by PCR with the universal 16S rDNA primers 20F (5′-GAG TTT GAT CCT GGC TCA G-3′) and 802R (5′-TAC CAG GGT ATC TAA TCC-3′). The PCR products were used as template for DNA sequencing with UFUL primer (5′-GCC TAA CAC ATG CAA GTC GA-3′) [36] and Bigdye Termination v3.1 cycle sequencing kit. The sequences of 16S rDNA were compared with those available in the GenBank, EMBL and DJB databases using the gapped BLASTN 2.0.5 program through the National Center for Biotechnology Information server.
Optimal decolorization study
The bacterial consortium was transferred into 250 ml Erlenmeyer flasks containing 50 ml synthetic melanoidins-containing wastewater medium, using 2% (v/v) Viandox as a color substance, and then cultivated with shaking at 200 rpm, 30 °C for 48 h. Bacterial cells were harvested with 3 h intervals by centrifugation at 10,000 rpm, 4 °C for 10 min. Decolorization and bacterial growth were measured by optical density (OD) at 475 nm and 600 nm, respectively. In addition, the uninoculated control was also incubated under same conditions to determine the abiotic decolorization. To examine the effect of initial pH on the decolorization, synthetic melanoidins-containing wastewater medium was prepared at initial pH 4, 7 and 9.
Construction of bacterial consortia for optimal decolorization
In order to verify a bacterial composition for the effective color removal by mixed cultures, experiments were performed under various combinations of bacteria, namely Klebsiella oxytoca (T1), Serratia mercescens (T2), Citrobacter sp. (T3) and unknown bacterium DQ817737 (T4). For construction of the active bacterial consortia, a loopful of each bacterium (T1, T2, T3 and T4) from LB plate was precultured in 50 ml LB at 30 °C with shaking at 200 rpm. After 24 h, the bacterial cells of each strain were harvested by centrifugation at 10,000 rpm at 4 °C for 10 min then washed with sterile normal saline solution. Washed bacterial cells at appropriate volume were subsequenctly inoculated into fresh synthetic melanoidins-containing wastewater media to obtain an initial OD600 of 0.2. Various bacterial consortia comprising of different bacterial compositions were constructed at the same initial cell density. These consortia were incubated with shaking (200 rpm) at 30 °C for 72 h. All assays were performed in triplicate and compared with respective uninoculated control. The factorial method was designed for this experiment [5, 7, 11].
Abiotic decolorization study
This study was carried out to verify whether the decolorization observed was due to biological or non-biological activity. The living and autoclaved cells of bacterial consortium with different cell concentrations at 5–50% (v/v) were added into 250 ml Erlenmeyer flask containing 50 ml of each synthetic melanoidins-containing wastewater medium. The flasks were placed on rotary shaker (200 rpm) at room temperature for 48 h. Samples were withdrawn at respective time points and then centrifuged at 10,000 rpm for 10 min. The supernatants were read for the OD at 475 nm using spectrophotometer.
The study on limitation of decolorization
The bacterial consortium was inoculated into synthetic melanoidins-containing wastewater medium and cultivated with shaking (200 rpm) at 30 °C for 48 h. Cells were harvested by centrifugation (10,000 rpm, 10 min, 4 °C) and washed three times successively with sterile normal saline solution in order to eliminate the residual culture medium. Washed bacterial cells were resuspended in the fresh culture medium of the same volume and cultivated under condition as described above.
Meanwhile, the used culture medium was centrifuged again at 10,000 rpm for 10 min at 4 °C to completely remove the bacterial cells, then inoculated with fresh bacterial cells (10% w/v) and cultivated under the same condition as described above.
Effect of aeration on decolorization
The experiment was carried out in 2.5 L stirring bioreactor with working volume of 2 L. The agitation speed was set at 150 rpm and the temperature was maintained at 30 °C. The aeration varied at KLa = 0.3688 h−1 (0 vvm), KLa = 2.5836 h−1 (0.1 vvm), KLa = 4.6343 h−1 (0.2 vvm), and KLa = 8.9848 h−1 (0.4 vvm). Samples were withdrawn at 12 h intervals for the measurements of OD at 600 and 475 nm.
Analytical methods
Bacterial growth was determined by the OD at wavelength of 600 nm. The bacterial density (C *) was calculated using Equation; C * = C 1 − C 0; where C 1 is the OD value of culture broth; and C 0 is the OD value of supernatant obtained after centrifugation of culture broth. The color intensity in supernatant was determined by measuring OD at 475 nm. The COD content and total nitrogen, respectively, was determined by a spectrophotometric method using Hach COD reagent test kit and Hach Total nitrogen reagent test kit (HACH Company, USA).
Results and discussions
Formulation of synthetic melanoidins-containing wastewater for use as a wastewater model
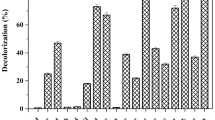
Usually, wastewater obtained from distilleries has no consistency and uniformity, rather the composition in wastewater such as COD, BOD, chemical element and color substances vary in hourly, daily or seasonal fashion [30]. For this study, the variations in wastewater would affect the result of all experiments. Moreover, molasses itself contain various amounts of melanoidins depending upon the nature of its source. Apart from melanoidins, sugarcane molasses contains other colorants such as phenolic compounds and caramel, whereas melanin is abundant in beet molasses [14]. Therefore, in order to prevent the lack of raw sugarcane molasses wastewater supply as well as variations in its composition, various kinds of synthetic melanoidins-containing wastewater media were formulated. Four kinds of melanoidins-containing substances were used in this experiment after dilution with distilled water to a concentration corresponding to color and COD contents of raw sugarcane molasses wastewater. Figure 1 shows the decolorization efficiency of bacterial consortium which was conducted with Viandox sauce, caramel, beet molasses wastewater and sugarcane molasses wastewater.
Decolorization of various synthetic melanoidins-containing wastewaters by the bacterial consortium. The melanoidins-containing substances used as a color substance for each medium was Viandox (13.5%), caramel (30%), beet molasses wastewater (41.5%) and sugarcane molasses wastewater (20%). Negative value indicated the increase in color; the data were results obtained from three independent experiments
The decolorization of 17.5, 9.5, 8.02 and 1.13% were achieved when using sugarcane molasses wastewater, Viandox sauce, beet molasses wastewater and caramel as a color substance of synthetic wastewater, respectively (Fig. 1). It was observed that the bacterial consortium could neither grow nor remove color substances in caramel. This was attributed to high processing temperatures, high acidity, high osmotic pressure and high specific gravity of caramel which were not supportive to microbial growth [3, 8]. Figure 1 shows that the decolorization of 9.5% was achieved using 13.5% (v/v) of Viandox sauce as a color substance of the synthetic melanoidins-containing wastewater.
For further investigation, the decolorization experiments were carried out in the synthetic melanoidins-containing wastewater medium containing Viandox sauce as a color substance since the variation of its compositions was lower than sugarcane molasses wastewater. However, the initial concentration of Viandox at 2% (v/v) was selected for further study due to interruption of bacterial growth by Viandox at 13.5% (v/v) (data not shown).
Identification of bacterial isolates in the consortium
The 16S rRNA gene PCR amplified from the isolates using 20F and 802R primers was subjected to DNA sequencing with UFUL internal primers [36]. The sequences were then compared using NCBI BLASTN program. Pairwise alignments giving a closest match of 99% or more were chosen. The bacteria in consortium were identified as Klebsiella oxytoca (T1), Serratia mercescens (T2), Citrobacter sp. (T3) and unknown bacterium DQ817737 (T4).
Some of bacterial strains have been reported as a molasses decolorizing bacteria. They were Pseudomonas, Acenitobacter, Bacillus and Klebsiella present in this bacterial consortium, all of which could decolorize colored components present in molasses wastewater [22, 29, 40]. Many researchers also reported the activity of Serratia mercescens on biodegradation of polycyclic aromatic hydrocarbons (PAHs) and lignin degrading activity [43].
Effect of initial pH on decolorization
In order to verify pH for the effective decolorization by bacterial consortium, experiments were performed at three different initial pH values (4, 7 and 9). The effect of initial pH on the decolorization and growth profiles of bacterial consortium is given in Fig. 2. The bacterial consortium could grow and decolorize synthetic melanoidins-containing wastewater containing 2% (v/v) Viandox at both pH 4 and 7 (Fig. 2a, b, respectively). However, this bacterial consortium gave the much lower decolorization at pH 9 (Fig. 2c). The highest decolorization of bacterial consortium was observed with an initial medium pH of 4 and the decolorization was decreased when the initial pH of medium was higher than 7 (data not shown). It appeared that the initial acidic pH has a critical effect on melanoidins decolorization. The similar pattern was also observed in another study where optimal decolorization of sugarcane molasses wastewater by soil inoculum was obtained at acidic pH [1]. Alkane et al. [1] reported that pH has a crucial role in melanoidins decolorization. An increase in pH of medium resulted in less microbial decolorization and the increase in color intensity. The increase in color may be due to the polymerization of melanoidins [1]. Hence, synthetic melanoidins-containing wastewater containing 2% (v/v) Viandox at initial pH 4 was selected for further studies since this condition gave the maximal decolorization of 18.3% under aerobic condition for 48 h (Fig. 2a). Moreover, pH 4 is closed to pH value of sugarcane molasses wastewater in Thailand.
Construction of bacterial consortium for optimal decolorization
To verify whether molasses wastewater decolorization is more effective by active mixed culture than a single bacterial isolate, the experiment was carried out by constructing feasible bacterial consortia. Totally, 16 different experiments were carried out according to the factorial method for four bacteria (T1-T4) in comparison with control. Figure 3 illustrated that the bacterial consortia were able to decolorize synthetic melanoidins-containing wastewater at a significantly higher level as compared to those achieved by individual isolates. The results showed that unknown bacterium DQ817737 (T4) accounted for the majority of decolorization of synthetic melanoidins-containing wastewater. Although strain T2 was not an effective decolorizer, its presence might still play an important role in affecting optimal color removals of bacterial consortia. In this study, the bacterial consortium namely MMP1, comprising of T1, T2 and T4 was subjected to further study since it gave the highest decolorization of 17.52%. However, decolorization did not occur in sterile cell-free medium, suggesting the absence of abiotic decolorization. The higher decolorization of MMP1 may be due to the enhanced effect of coordinated metabolic interactions on melanoidins decolorization [27, 44, 45].
Decolorization at optimal condition
The consortium was cultured in the synthetic melanoidins-containing wastewater under the optimum condition with 2% (v/v) Viandox as color substance at an initial pH 4. Figure 4 showed the typical culture profiles of the constructed bacterial consortium MMP1. Its highest decolorization approximately 22% was observed after cultivation for 72 h, while total nitrogen decreased from 390 mg/L at the beginning of this experiment to 290 mg/L after incubation for 72 h. The pH of cultured medium was not significantly changed along the experiment period (Fig. 3).
Decolorization by living and autoclaved cells
Abiotic decolorization study was carried out to verify whether the decolorization was obtained from biological activity or non-biological activity. It showed that the experiment with the different initial cell concentrations (5–50% v/v) of autoclaved cell of consortium MMP1, exhibited no melanoidins decolorization after incubation for 48 h (Fig. 5). In contrast, the melanoidins decolorization was occurred by living cell of MMP1 (Fig. 5). Additionally, to confirm that the melanoidins decolorization occurred by biological activity but not adsorption mechanism, NaOH extraction method was adopted [47]. The cell pellets of both living cell and autoclaved cell were resuspended with equal volume of NaOH 0.1 M to extract color substances adsorbed to cell surface. The extracts were centrifuged and OD was measured at 475 nm. It showed that the final fractions of NaOH-extractable color substance were negligible. These results clearly indicated that the decolorization of Viandox by consortium MMP1 was due to biological mechanisms.
The study on limitation of decolorization
Since melanoidins decolorization was dependent on bacterial growth conditions such as pH, nutrient levels, aeration and metabolites in liquid phase. Nutrient availability and metabolite accumulation might result in growth limitation and thereby consequently decreased in melanoidins decolorization. To clarify the limitation of decolorization of the bacterial consortium MMP1, the used bacterial cells and the used medium were separately subjected to further study. Used cells were inoculated into fresh medium and, meanwhile, used medium was inoculated with fresh bacterial cells. After cultivation, it was found that the used cells could decolorize of freshly prepared medium (Fig. 6). Figure 7 shows that the fresh bacterial cells could decolorize the melanoidins remaining in used culture medium during the first 24 h of incubation and its decolorization was then decreased afterward. As shown in Fig. 7, it was observed that the color of Viandox sauce could be hardly removed by fresh bacterial cells in used medium. This might be due to the effect of toxicity of melabolites, which had been formed and accumulated during decolorization, thereby repressed the decolorization ability of fresh cells [26]. Also, it was possible that the absence of nutrients markedly affected the decolorization of bacterial consortium MMP1.
Various studies on melanoidins decolorization by microorganisms have shown the similar results regarding to the effect of nutrient supplements. A melanoidins decolorization of 87% was reported after 12 days of incubation with Geotrichum candidum in the presence of 2% glucose and inorganic nutrients [20]. Removal of melanoidins from molasses waste of 84.16% using Aspergillus niger in the presence of glucose has also been reported [15]. Although higher decolorization could be achieved using additional nutrient supplement but this might lead to the addition of extra chemicals in the system [15].
Effect of aeration on decolorization
The effect of aeration on decolorization of synthetic melanoidins-containing wastewater medium by bacterial consortium was carried out in 2 L laboratory-scale suspended cell bioreactor. Figure 8 shows that the consortium could decolorize synthetic melanoidins-containing wastewater medium at aeration rate of KLa = 0.3688 h−1 (0 vvm), KLa = 2.5836 h−1 (0.1 vvm), KLa = 4.6343 h−1 (0.2 vvm), and KLa = 8.9848 h−1 (0.4 vvm) up to 18.92, 19.32, 16.94 and 8.31% within 48 h, respectively. Further increase in the aeration rate did not improve the decolorization. At the end of these experiments, the optimum aeration rate for melanoidins decolorization was found to be KLa = 2.5836 h−1 (0.1 vvm).
In general, several microorganisms that have been shown to degrade melanoidins are not best suited for treating melanoidins-containing effluent from distilleries. This is because they are depleted in oxygen, which is necessary for oxidative degradation of melanoidins [2]. However, the results presented in this study showed that color removal under low aeration conditions relatively higher than the highly aerobic condition. Hence, the decolorization mechanisms of melanoidins-containing wastewater by bacterial consortium MMP1 in this study might be due to metabolism of bacterial cell under facultative and anaerobic conditions such as fermentation and anaerobic respiration [2, 48].
Conclusions
Bacterial consortium comprising of Klebsiella oxytoca (T1), Serratia mercescens (T2) and unknown bacterium DQ817737 (T4), namely MMP1, was developed for this study. This bacterium consortium exhibited increased decolorization compared to that shown by any single isolate. This may be due to the enhanced effect of coordinated metabolic interactions on melanoidins decolorization. Also, the bacterial consortium MMP1 could be utilized for the decolorization of various kinds of melanoidins present in various industrial effluents including sugarcane and beet molasses wastewaters.
The consortium showed high growth and melanoidins decolorization at the initial pH of 4 under low aeration condition. Thus, the consortium MMP1 might be suitably applied to the acid formation phase of conventional aerobic or anaerobic treatment systems of alcoholic distillery wastewater.
The used bacterial cells inoculated in fresh medium showed that the addition of nutrients affecting the decolorization of the consortium MMP1. This suggested that the decolorization of melanoidins ran parallel with the decomposition of nutrients. Therefore, nutrients could affect the growth and melanoidins decolorization of consortium MMP1.
The comparison of decolorization of consortium MMP1 with abiotic control has proved that the color removal for synthetic melanoidins-containing wastewater medium containing 2% (v/v) Viandox was due to biotic activity of bacteria but not adsorption of color substances on cell surface.
For process application, our bacterial consortium has the potential to serve as an inoculum for decolorization of melanoidin-containing wastewaters since its highest decolorization took place under the condition similar to the real distillery wastewaters.
References
Alkane HV, Dange MN, Selvakumari K (2006) Optimization of anaerobically digested distillery molasses spent wash decolorization using soil as inoculum in the absence of additional carbon and nitrogen source. Biores Technol 97:2131–2135
Ames MJ, Wynne A, Hofmann A, Plos S, Gibson RG (1999) The effect of a model melanoidin mixture on faecal bacterial populations in vitro. British J Nutr 82:489–495
Belitz HD, Grosch W, Schieberle P (2004) Sugars, sugar alcohols, honey. In: Food Chemistry, 3rd edn. Springer, Germany, pp 862–891
Bernardo EC, Egashira R, Kawasaki J (1997) Decolorization of molasses wastewater using activated carbon prepared from cane bagasses. Carbon 35:1217–1221
Box GEP, Hunter WG, Hunter JS (1978) Statistics for experimenters. Wiley, New York
Chopra P, Singh D, Verma V, Puniya AK (2004) Bioremediation of melanoidin containing digested spentwash from cane-molasses distillery with white rot fungus Coriolus versicolor. Indian J Microbiol 44:197–200
Cox DR (1958) Planning of experiments. Wiley, New York
Chung MS, Ruan RR, Chen PL, Wang X (1999) Physical and chemical properties of caramel systems. Lebensm Wiss U Technol 32:162–166
Dahiya J, Singh D, Nigam P (2001) Decolorization of molasses wastewater by cells of Pseudomonas fluorescens immobilized on porous cellulose carrier. Biores Technol 78:111–114
Department of Industrial work (1996) The standard of industrial effluent quality due to the Ministry of Science and Technology and Environment Regulations. Department of Industrial Works, Ministry of Industry, Thailand
Duckworth WE (1968) Statistical techniques in technological research. Methuen & Co. Ltd, London
Friedrich J (2004) Bioconversion of distillery waste. In: Arora DK (ed) Fungal biotechnology in agricultural, food and environmental applications. Marcel Dekker Inc., New York, pp 431–442
Ghosh M, Ganguli A, Tripathi AK (2002) Treatment of anaerobically digested distillery spentwash in a two-stage bioreactor using Pseudomonas putida and Aeromonas sp. Process Biochem 37:857–862
Godshall MA (1999) Removal of colorants and polysaccharides and the quality of white sugar. In: Proceedings of sixth International Symposium Organized by Association Andrew van Hook (AvH). Reims, France, pp 28–35
Gomaa O, Abdel KH, Mattar Z, Hassanein H (2003) Decolorization of molasses waste water using Aspergillus niger. Egyptian J Biotechnol 13:15–28
Gonzalez T, Terron MC, Yague S, Zapico E, Galletti GC, Gonzalez AE (2000) Pyrolysis/gas chromatography/mass spectrometry monitoring of fungal-biotreated distillery wastewater using Trametes sp. I-62 (CECT 20197). Rapid Commun Mass Spec 14:1417–1424
Inanc B, Ciner F, Ozturk I (1999) Color removal from fermentation industry effluents. Water Sci Technol 40:331–338
Jain N, Minocha AK, Verma CL (2002) Degradation of predigested distillery effluent by isolated bacterial strains. Indian J Exp Biol 40:101–105
Jimenez AM, Borja R, Martin A (2003) Aerobic-anaerobic biodegradation of beet molasses alcoholic fermentation wastewater. Process Biochem 38:1275–1284
Kim SJ, Shoda M (1999) Decolorization of molasses and a dye by newly isolated strain of the fungus Geotrichum candidum Dec1. Biotechnol Bioeng 62:114–119
Kulkarni AK (1998) Solar assisted photocatalytic oxidation of distillery waste. Indian Chem Eng 40:169–172
Kumar P, Chandra R (2006) Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Biores Technol 97:2096–2102
Kumar V, Wati L, Fitzgibbon F, Nigam F, Banat IM, Singh D, Marchant R (1997) Bioremediation and decolorization of anaerobically digested distillery spent wash. Biotechnol Lett 19:285–290
Kumar V, Wati L, Nigam P, Banat IM, Yadav BS, Singh D, Marchant R (1998) Decolorization and biodegradation of anaerobically digested sugarcane molasses spentwash effluent fron biomethanation plants by white-rot fungi. Process Biochem 33:83–88
Mandal A, Ojha K, Ghosh DN (2003) Removal of color from distillery wastewater by different processes. Indian Chem Eng Sect B 45:264–267
Manjinder SK, Harvinder SS, Deepak KS, Bhupinder SC, Swapandeep SC (2005) Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res 39:5135–5141
Manjinder SK, Harvinder SS, Deepak KS, Bhupinder SC, Swapandeep SC (2005) Decolorization of various azo dyes by bacterial consortium. Dyes Pigments 67:55–61
Migo VP, Matsumara M, Rosario EJD, Kataoka H (1993) Decolorization of molasses wastewater using an inorganic flocculatant. J Ferment Bioeng 75:438–442
Mohana S, Desai C, Madamwar D (2007) Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Biores Technol 98:333–339
Mogens H (2002) Wastewater volumes and composition. In: Wastewater treatment: biological and chemical processes, 2nd edn. Springer, New York, pp 11–42
Monica C, Garcia MT, Gonzalez G, Pena G, Garcia JA (2004) Study of colored component formed in sugar beet processing. Food Chem 86:421–433
Moosvi S, Keharia H, Madamwar D (2005) Decolorization of textile dye reactive violet 5 by a newly isolated bacterial consortium RVM 11.1. World J Microbiol Biotechnol 21:667–672
Moreira MT, Palma C, Feijoo G, Lema JM (1998) Strategies for continuous production of lignolytic enzymes in fixed and fluidized bed bioreactors. J Biotechnol 66:27–39
Mutlu SH, Yetis U, Gurkan T, Yilmaz L (2002) Decolorization of wastewater of a baker’s yeast plant by membrane process. Water Res 36:609–616
Nandy T, Shastry S, Kaul SN (2002) Wastewater management in cane molasses distillery involving bioresource recovery. J Environ Manage 65:25–38
Nilsson WB, Paranjype RN, DePaola LA, Strom MS (2003) Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J Clinical Microbiol 41(1):442–446
Ohmano S, Aoshima I, Tozawa Y, Sakurada N, Ueda K (1985) Purification and some properties of melanoidin decolorizing enzymes, P-III and P-IV, from mycelia of Coriolus versicolor Ps4a. Agric Biol Chem 49:2047–2053
Patil PU, Kapadnis BP, Dhammankar VS (2003) Decolorization of synthetic melanoidin and biogas effluent by immobilized fungal isolated of Aspergillus niger UM2. All India Distiller’s Association (AIDA) Newsletter, pp 53–56
Pena M, Coca M, Gonzalez R, Rioja R, Garcia MT (2003) Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere 51:893–900
Petruccioli M, Duarte JC, Fedrerici F (2000) High rate aerobic treatment of winery wastewater using bioreactors with free and immobilized activated sludge. J Biosci Bioeng 90:381–386
Pikaev AK, Ponomarev AV, Bludenko AV, Minin VN, Elizar’eva LM (2001) Combined electronic-beam and coagulation purification of molasses distillery slops. Features of the method, technocal and economic evaluation of large scale facility. Radiat Phys Chem 61:81–87
Raghukumar C, Mohandass C, Kamat S, Shailaja MS (2004) Simultaneous detoxification and decolorization of molasses spentwash by the immobilized white-rot fungus Flavadon flavus isolated from the marine habitat. Enz Microbial Tech 35:197–202
Rhoads TL, Mikell AT, Eley MH (1995) Investigation of the lignin-degrading activity of Serratia marcescens: biochemical screening and ultrastructural evidence. Canadian J Microbiol 41:592–600
Sarayu M, Chiraya D, Datta M (2006) Biodegradation and decolourization of anaerobically treated distillery spent wash by novel bacterial consortium. Biores Technol 98:333–339
Sarayu M, Shalini S, Jyoti D, Datta M (2008) Response surface methodology for optimization of medium for decolorization of textile dye Direct Black 22 by a novel bacterial consortium. Biores Technol 99:562–569
Sennitt T (2005) Emissions and economics of biogas and power. In: 68th Annual Water Industry Engineers and Operators’ Conference, Schweppes Centre, Bendigo, 7 and 8 September 2005
Sirianuntapiboon S, Zohsalam P, Ohmomo S (2004) Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process Biochem 39:917–924
Sirianuntapiboon S, Prasertsong K (2008) Treatment of molasses wastewater by acetogenic bacteria BP103 in sequencing batch reactor (SBR) system. Biores Technol 99:1806–1815
Zhang F, Yu J (2000) Decolourisation of acid violet 7 with complex pellets of white rot fungus and activated carbon. Bioprocess Eng 23:205–301
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiranuntipon, S., Chareonpornwattana, S., Damronglerd, S. et al. Decolorization of synthetic melanoidins-containing wastewater by a bacterial consortium. J Ind Microbiol Biotechnol 35, 1313–1321 (2008). https://doi.org/10.1007/s10295-008-0413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0413-y