Abstract
Decolourization of anaerobically digested and polyaluminium chloride treated distillery spentwash was studied in a fungal stirred tank aerobic reactor without dilution of wastewater. Aspergillus niger isolate IITB-V8 was used as the fungal inoculum. The main objectives of the study were to optimize the stirrer speed for achieving maximum decolourization and to determine the kinetic parameters. A mathematical model was developed to describe the batch culture kinetics. Volumetric oxygen transfer coefficient (k L a) was obtained using dynamic method. The maximum specific growth rate and growth yield of fungus were determined using Logistic equation and using Luedeking–Piret equation. 150 rpm was found to be optimum stirrer speed for overall decolourization of 87%. At the optimum stirrer speed, volumetric oxygen transfer coefficient (k L a) was 0.4957 min−1 and the maximum specific growth rate of fungus was 0.224 h−1. The values of yield coefficient (Y x/s) and maintenance coefficient (m s) were found to be 0.48 g cells (g substrate)−1 and 0.015 g substrate (g cells)−1 h−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
About 8–15 l of spentwash is generated for every litre of alcohol produced in a typical distillery (Mohana et al. 2009). The spentwash is characterized by high chemical oxygen demand (COD) and biochemical oxygen demand (BOD), low pH, strong odour and dark brown colour (Satyawali and Balakrishnan 2007). Colour of spentwash is mainly due to melanoidins, which are natural condensation products of sugar and amino acids produced by non-enzymatic Maillard amino–carbonyl reaction taking place between the amino and carbonyl groups present in organic substances (Chandra et al. 2008). The colour imparting melanoidins are barely affected by conventional biological treatment such as biomethanation and the activated sludge process (Migo et al. 1993). However, fungal species have been reported for having potential to decolourize molasses spentwash (Pant and Adholeya 2007a). Miranda et al. (1996) used Aspergillus niger to achieve 69% colour removal and 75% COD reduction from molasses spentwash. Benito et al. (1997) got 82% decolourization and 77% COD removal in molasses wastewater using Trametes versicolor. Raghukumar et al. (2004) reported 73% decolourization of molasses spentwash by Flavodon flavus, a white-rot basidiomycete fungus isolated from a marine habitat. An Aspergillus species was selected out of 21 isolated and procured microorganisms for maximum decolourization of UASB digested and aerobically treated distillery wastewater by Shayegan et al. (2005). They found 75% decolourization with diluted wastewater which reduced to 40% on using undiluted wastewater. In another study, colour removal of 86, 50 and 47% was observed with Pleurotus florida, Penicillium pinophilum and Alternaria gaisen, respectively (Pant and Adholeya 2007b). Kaushik and Thakur (2009) isolated five different fungi from distillery mill site and the two isolates having higher capabilities to remove colour were identified as Emericella nidulans and Neurospora intermedia, respectively. Pant and Adholeya (2009) got 86% fungal decolourization after treatment with hydroponic based system. The decolourization was achieved at lower dilution and without addition of supplementary carbon sources. In many of the above mentioned studies, the dilution of molasses spentwash was necessary for effective decolourization. However, it is possible to avoid dilution of spentwash if one can use physicochemical method prior to fungal treatment. In one such study by the authors, spentwash was pretreated with polyaluminium chloride (PAC) before applying fungal treatment using Aspergillus niger to effectively decolourize undiluted anaerobically digested distillery spentwash (Singh and Dikshit 2010). In a recent study, a novel fungal consortium based bioreactor was developed for treating undiluted distillery effluent efficiently but the process optimization was not performed (Pant and Adholeya 2010). Process optimization and determination of kinetic parameters are important for design and operation of a bioreactor.
In the present research work, a fungal stirred tank aerobic reactor (FSTAR) was used in batch mode for decolourizing anaerobically digested molasses spentwash pretreated with PAC. The effect of stirrer speed on reactor performance was studied in FSTAR and the stirrer speed was optimized. Next, kinetic parameters were determined at the optimum stirrer speed. Finally, the specific growth rate of fungus and substrate uptake rate were determined using Logistic equation and Leudeking-Piret kinetic equation, respectively.
Materials and methods
Wastewater
Anaerobically digested spentwash (DSW) was obtained from a cane molasses based distillery situated near Mumbai, India. DSW was pretreated with PAC (SVS chemical corporation, Pune, India) using methodology presented in Singh and Dikshit (2010). The detailed characteristics of wastewater, before and after PAC treatment are given in Table 1. The pretreated DSW, having COD of 11,200 mg l−1 (11.2 g l−1) and colour (absolute absorbance at 475 nm) of 4.6 units, constituted the feed wastewater to fungal bioreactors.
Microorganism and inoculum
Pellets of Aspergillus niger species IITB-V8 were used in the experiments. The procedure for harvesting these pellets is described in Singh and Dikshit (2010). Initial inoculum concentration of 0.2 g l−1 was used in the FSTAR studies.
Culture medium
Culture medium fed to FSTAR consisted of PAC treated DSW supplemented with 5.5 g l−1 glucose and 1.2 g l−1 KH2PO4, which were found optimum in earlier work (Singh and Dikshit 2010). The pH of influent was maintained as 5 ± 0.01 throughout the experiments.
Bioreactor
Batch decolourization studies were carried out in fungal stirred tank aerobic reactors, named as FSTAR. The fungal bioreactors consisted of 2.5 l glass reactors of 12 cm diameter with two flat-blade stirrers of 5 cm width with speed ranging from 0 to 300 rpm. 2 l of pretreated DSW was taken in the reactor, to which required nutrients were added and the pH was adjusted as required. Air pump system was used to supply air at the rate of 2.5 l min−1 using two porous distributors placed symmetrically at the bottom of bioreactor. During the bioreactor runs, pH was measured using pH meter (Control Dynamics, India) and dissolved oxygen (DO) was monitored using DO Meter (model HQ 30d, Hach, USA). Also, 5 mL samples were drawn at required time intervals and monitored for COD and colour. The schematic of the experimental setup is shown in Fig. 1a while actual setup is shown in Fig. 1b.
Since stirrer speed has been reported to affect the fungal morphology and hence, the reactor performance (Cui et al. 1998), experiments were carried out at stirrer speeds of 50, 100, 150, 200 and 250 rpm.
Analytical methods
COD was determined based on the Standard Methods for Examination of Water and Wastewater (APHA 1998).
For colour measurement, 5 ml samples were taken from shake flasks and centrifuged at 8,000 rpm for 10 min. The supernatant after centrifugation was diluted 10 times for colour reduction measurement. The absorbance was measured at 475 nm using Thermo Spectronic visible spectrophotometer. The decolourization yield was expressed as the percentage decrease in absorbance at 475 nm related to the initial absorbance at the same wavelength.
Dry weight of mycelium mass was measured after centrifuging a known volume of sample at 8,000 rpm for 10 min and then drying the precipitant at 105°C.
Glucose was determined by the colorimetric method given by Lever (1972). The assay reagent was prepared by mixing 5% w/v solution of p-hydroxybenzoic acid hydrazide in 0.5 M HCl with 0.5 M NaOH at a ratio of 1:9 by volume (Thiruchelvam and Ramsay 2007). Sample (15 μl) was added to 4.5 ml of the reagent and the mixture was heated in a boiling water bath for 5 min. After cooling, the absorbance was read at 410 nm with Thermo Spectronic visible spectrophotometer (Model Helios Epsilon). Using a calibration curve, the concentration of glucose was determined.
Glucose was determined by the colorimetric method given by Lever (1972). The assay reagent was prepared by mixing 5% w/v solution of p-hydroxybenzoic acid hydrazide in 0.5 M HCl with 0.5 M NaOH at a ratio of 1:9 by volume (Thiruchelvam and Ramsay 2007). Sample (15 μl) was added to 4.5 ml of the reagent and the mixture was heated in a boiling water bath for 5 min. After cooling, the absorbance was read at 410 nm with Thermo Spectronic visible spectrophotometer (Model Helios Epsilon). Using a calibration curve, the concentration of glucose was determined.
Determination of kinetic parameters
Microbial growth rate
Microbial growth rate was determined using logistic equation. The logistic equation is a substrate independent model. It characterizes growth in terms of carrying capacity (Shuler and Kargi 2002).
The rate of cell growth is defined as
where x is the cell concentration (g l−1) and x m is the maximum attainable biomass concentration (g dry weight l−1) and μm is the maximum specific growth rate (h−1).
The integrated form of Eq. 1 using x = x 0 at t = 0 gives a sigmoidal variation of x as a function of t, which may represent both the exponential and the stationary phases in the following equation:
The value of kinetic coefficient μm was evaluated by fitting the Eq. 2 to the experimental data using OriginPro data analysis and graphic software version 8 (OriginLab).
kLa and oxygen uptake rate
In batch fermentation, the following mass balance equation for the dissolved oxygen (DO) concentration, containing oxygen transfer rate (OTR) and oxygen uptake rate (OUR) terms, can be established:
where C* is saturated DO concentration (mg l−1), C L is the actual DO concentration in broth (mg l−1), k L a is the volumetric oxygen transfer coefficient (min−1), \( q_{{{\text{O}}_{2} }} \) specific oxygen uptake rate (mg O2 (g cells min)−1) and x is the biomass concentration (g l−1).
The measurement of OUR using the dynamic method has been made as follows: the inlet of airflow to the reactor was shut off for a short period; then a decrease of dissolved oxygen concentration was measured using DO meter. Since there were no gas bubbles when the air was shut off, k L a was zero. Hence, OTR was also zero and Eq. 3 can be simplified to
A graph was plotted between DO concentration and the time. The slope of the descending curve gave OUR.
To measure k L a, the inlet of airflow to the reactor was opened and increase in DO was measured with time. Assuming fast re-oxygenation of the broth relative to cell growth, the DO level soon reached a steady state value, C AL. When C L is equal to C AL, dC L/dt is equal to zero 0 as there is no change in C L with time. Therefore, from Eq. 3
Substituting this result into Eq. 3 and cancelling the k L aC* term gives:
Assuming k L a as constant with the time, Eq. 6 can be integrated between limits t 1 to t 2 and resulting equation for k L a is:
ln(C AL − C L1/C AL − C L2) was plotted against (t 2 − t 1). Several values of (C L1, t 1) and C L2, t 1) were taken and k L a was calculated from the slope of the above graph. k L a and oxygen uptake rate were determined for 50, 100, 150, 200 and 250 rpm.
Substrate uptake
A carbon substrate such as glucose is assimilated for synthesis of cell, cell maintenance and synthesis of metabolites. The substrate consumption equation used is similar to Leudeking–Piret kinetic equation in which the amount of carbon substrate used for the product formation is assumed to be negligible:
where m s is the maintenance coefficient (g substrate (g cells)−1 h−1) and Y x/s is the yield factor for cells on carbon substrate (g cells (g substrate)−1).
At stationary phase, dx/dt = 0 and x = x m. Therefore, m s can be obtained using the following equation
Substituting Eq. 2 into Eq. 9 and integrating yields the following equation:
The values of kinetic coefficients Y x/s and m s were evaluated by fitting the Eq. 10 to the experimental data using OriginPro8 (OriginLab) data analysis and graphic software.
Results and discussion
Decolourization at various stirrer speeds and optimization
Optimization of stirrer speed was done by performing the FSTAR studies at stirrer speeds of 50, 100, 150, 200 and 250 rpm. DO and OUR were measured and k L a was determined by dynamic method.
DO and OUR
DO concentration was found out for various stirrer speeds at different time (Fig. 2). There was reduction in DO during the initial phase of reaction. Minimum DO for the 150, 200 and 250 rpm stirrer speed was observed at 12 h and for the 50 and 100 rpm, it was observed at 24 h. The initial phase (0–24 h) was characterized by the simultaneous conversion of glucose partly into biomass and predominantly into organic acid. The DO had an increasing trend from 12 to 24 h for 150, 200 and 250 rpm stirrer speed. After 24 h, the DO remained nearly constant. In case of 50 and 100 rpm, there was an increase in DO after 24 h and it continued up to 48 h. After 48 h, the DO was nearly constant. Shayegan et al. (2005) studied batch culture decolourization of anaerobically digested wastewater by Aspergillus-UB2 and found that DO reduced to 3 mg l−1 after 18 h. The DO increased to 4 mg l−1 after 42 h when the decolourization efficiency was maximized.
OUR in the reactor for different stirrer speed was measured using dynamic method (Fig. 3). Maximum OUR for 150, 200 and 250 rpm agitation rates was observed at 12 h and for 50 and 100 rpm, it was observed at 24 h. OUR was influenced by the microorganism growth phase. There was an increase in OUR during lag phase and exponential growth phase. Afterwards, the values of OUR decreased and maintained constant values during the stationary growth phase.
Determination of k L a
The effects of impeller speed on the k L a was investigated using the impeller speeds in the range of 50–250 rpm by maintaining constant working volume of 2 l and oxygen flow rate of 2.5 l min−1. The volumetric mass transfer coefficient, k L a, was calculated by the slope of the graph using Eq. 7. The typical graph for calculation of k L a at 150 rpm stirrer speed is represented in Fig. 4. The values of k L a for the stirrer speed 50, 100, 150, 200 and 250 were 0.2317, 0.3048, 0.4957, 0.6273 and 0.8620 min−1 respectively. It was observed that k L a increased with increase in stirrer speed.
Decolourization
Colour removal in the bioreactor was observed at different stirrer speeds. At 50 and 100 rpm stirrer speed, maximum decolourization of 54 and 59% was observed at 72 h (Fig. 5). At 150, 200 and 250 rpm, maximum colour removal was observed at 60 h. The maximum decolourization of 69% was achieved at 150 and 200 rpm, and 64% at 250 rpm stirrer speed. The reduction in decolourization at higher stirrer speed (250 rpm) may be due to greater mechanical forces or hydrodynamic shear stresses causing fungal morphological changes. From the above results, 150 rpm stirrer speed was selected as the optimum speed for all subsequent studies. Optimization of stirrer speed for decolourization of molasses by Geotrichum candidum in a jar fermenter has been reported by Kim and Shoda (1999). They varied the stirrer speed from 150 to 250 rpm at fixed air supply rate of 2 l min−1 and observed the maximum decolorizing activity for molasses at 180 rpm.
Bioreactor study at 150 rpm stirrer speed
Decolourization of wastewater was optimum at 150 rpm, so further studies were done at this stirrer speed.
Microbial growth and decolourization
The lag phase of Aspergillus niger in decolourization was short (Fig. 6). The cells entered the exponential phase instantly and microbial growth rate was very rapid during initial 24 h. The strain started to decolorize the effluent, when the cells entered the exponential phase. Taking x 0 as 0.2 g l−1, x m as 3.5 g l−1 and fitting the Logistic model, given by Eq. 2 to the experimental data as represented in Fig. 7, yielded the value of maximum specific growth rate as 0.224 h−1. The maximum specific growth rates for filamentous fungi such as Aspergillus and Penicillium have been reported in the range of 0.1–0.3 h−1, equivalent to doubling times of 2–7 h (Papagianni 2004).
Glucose consumption
Glucose was consumed for both organic acid formation and biomass growth. Glucose was possibly utilized for decolourization of melanoidin was suggested to be decolourized by the active oxygen (O2 −, H2O2) produced by the reactions with the sugar oxidases (Watanabe et al. 1982). Glucose was consumed rapidly within 12 h of reaction (Fig. 6). The initial glucose concentration was 5.5 g l−1, which was reduced to 0.96 in 12 h and 0.09 g l−1 in 72 h.
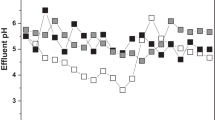
Variation of pH with time
Variation of pH with time in the bioreactor is given in Fig. 6. There was decrease in pH at initial phase from 5 to 3.15 in 36 h. The decrease in pH was caused by the fungus utilizing the glucose and it stabilized as soon as glucose got exhausted. The pH decreased due to the synthesis of various organic acids, by the fungal primary metabolism. pH started increasing after 36 h and it reached to 3.42 at 72 h. This pH increase indicated fungus utilizing organic acids after the glucose exhaustion. Shayegan et al. (2005) also studied batch culture decolourization of anaerobically digested wastewater by Aspergillus-UB2 and found that pH reduced to 3 after 18 h and then, it increased after 42 h.
COD reduction
The initial COD of the sample was 11.2 g l−1. After addition of glucose, COD increased to 16.5 g l−1. The COD reduction during initial 12 h was very high due to rapid fungal growth. In this period, the COD available in the form of glucose was used by the fungus for the growth and synthesis of organic acids. After consumption of glucose, residual COD and organic acids were utilized for the fungal growth and maintenance. The final COD reduction obtained after 72 h was 6.4 g l−1. The experimental COD values were fitted in Eq. 10 and values of yield coefficient (Y x/s) and maintenance coefficient (m s) were found out. The experimental COD values with model curve are represented in Fig. 8. The values of Y x/s and m s were found to be 0.48 g cells (g substrate)−1 and 0.015 g substrate (g cells)−1 h−1.
Conclusion
Stirrer speed had an impact on reactor (FSTAR) performance and it was optimized for maximum decolourization. Fungal decolourization is a very complex process, and it is often very difficult to obtain a complete picture of the process. However, the kinetic model gives a good description of biomass growth and substrate consumption. The kinetic parameters were calculated from the model by fitting the experimental data of the process at optimum stirrer speed. The experimental data fitted well in the model. Decolourization was found directly related to the cell growth during the process. This study is useful in designing the reactor for decolourization and for further continuous reactor study.
Abbreviations
- μm :
-
Maximum specific growth rate (h−1)
- x :
-
Cell concentration (g l−1)
- x m :
-
Maximum attainable biomass concentration (g dry weight l−1)
- k L a :
-
Volumetric oxygen transfer coefficient (min−1)
- C*:
-
Saturated DO concentration (mg l−1)
- C L :
-
Actual DO concentration in broth (mg l−1)
- \( q_{{{\text{O}}_{2} }} \) :
-
Specific oxygen uptake rate (mg O2 (g cells min)−1)
- m s :
-
Maintenance coefficient (g substrate (g cells h)−1)
- Y x/s :
-
Yield factor for cells on carbon substrate (g cells (g substrate)−1)
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, New York
Benito GG, Miranda MP, Santos DR (1997) Decolourization of wastewater from an alcoholic fermentation process with Trametes versicolor. Bioresour Technol 61:33–37
Chandra R, Bharagava N, Rai V (2008) Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour Technol 99:4648–4660
Cui YQ, Okkerse WJ, Van der Lans RGJM, Luyben KChAM (1998) Modeling and measuring of fungal growth and morphology in submerged fermentations. Biotechnol Bioeng 60:216–229
Kaushik G, Thakur IS (2009) Isolation of fungi and optimization of process parameters for decolorization of distillery mill effluent. World J Microbiol Biotechnol 25:955–964
Kim SJ, Shoda M (1999) Batch decolourization of molasses by suspended and immobilizes fungus of Geotrichum candidum Dec 1. J Biosci Bioeng 88:586–589
Lever MA (1972) New reaction for colorimetric determination of carbohydrates. Anal Biochem 47:273–279
Migo VP, Matsumara M, Rosario EJD, Kataoka H (1993) Decolorization of molasses wastewater using an inorganic flocculant. J Ferment Bioeng 75(6):438–442
Miranda PM, Benito GG, Cristobal NS, Nieto CH (1996) Colour elimination from molasses wastewater by Aspergillus niger. Bioresour Technol 57:229–235
Mohana S, Acharya BK, Madamwar D (2009) Distillery spent wash: treatment technologies and potential applications. J Hazard Mater 163:12–25
Pant D, Adholeya A (2007a) Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol 98:2321–2334
Pant D, Adholeya A (2007b) Enhanced production of ligninolytic enzymes and decolorization of molasses distillery wastewater by fungi under solid state fermentation. Biodegradation 18:647–659
Pant D, Adholeya A (2009) Nitrogen removal from biomethanated spentwash using hydroponic treatment followed by fungal decolourization. Environ Eng Sci 26:559–565
Pant D, Adholeya A (2010) Development of a novel fungal consortium for the treatment of molasses distillery wastewater. Environmentalist 30:178–182
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Raghukumar C, Mohandass C, Kamat S, Shailaja MS (2004) Simultaneous detoxification and decolourization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzyme Microb Technol 35:197–202
Satyawali Y, Balakrishnan M (2007) Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J Environ Manage 86:481–497
Shayegan J, Pazouki M, Afshari A (2005) Continuous decolourization of anaerobically digested distillery wastewater. Process Biochem 40:1323–1329
Shuler M, Kargi F (2002) Bioprocess engineering, basic concepts, 2nd edn. Prentice Hall, Inc., New Jersey
Singh SS, Dikshit AK (2010) Optimization of the parameters for decolourization by Aspergillus niger of anaerobically digested distillery spentwash pretreated with polyaluminium chloride. J Hazard Mater 176:864–869
Thiruchelvam AT, Ramsay JA (2007) Growth and laccase production kinetics of Trametes versicolor in a stirred tank reactor. Appl Microbiol Biotechnol 74:547–554
Watanabe Y, Sugi R, Tanaka Y, Hayashida S (1982) Enzymatic decolourization of melanoidin by Coriolus sp. Agric Biol Chem 46:1623–1630
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S.S., Dikshit, A.K. Decolourization of anaerobically digested and polyaluminium chloride treated distillery spentwash in a fungal stirred tank aerobic reactor. Biodegradation 22, 1109–1117 (2011). https://doi.org/10.1007/s10532-011-9467-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-011-9467-z