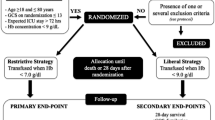
Abstract
Anemia is not safe in both acute and chronic conditions due to its association with a high risk of major organ injury. The highly oxygen-dependent brain is especially prone for hypoxic and ischemic changes which are likely to develop in neurosurgical conditions. New knowledge continues to emerge regarding the cellular mechanisms that maintain oxygen homeostasis during anemia.
The existing treatment modalities of anemia have not produced demons ratable improvement in patient outcome, and the inherent risks associated with transfusion cannot be underestimated. Currently there is no level 1 evidence by which definite transfusion guidelines for neurosurgical patients can be recommended. This chapter combines all the evidence surrounding anemia, transfusion, their effects on clinical outcome in various neurosurgical scenarios, and the possible future directions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anemia
- Blood transfusion
- Neurosurgery
- Traumatic brain injury
- Subarachnoid hemorrhage
- Delayed cerebral injury
1 Introduction: Blood and Brain
Anemia, the most common complication arising during neurosurgical practice, plays a major role in influencing outcome. The brain is especially vulnerable to decreased perfusion and hypoxia, and brain oxygenation is highly reliant on cerebral blood flow. In recent years, transfusion practice across the world has generally witnessed a shift toward adopting a more restrictive strategy for red blood cell administration. The serious risks of transfusion might dwarf the putative benefits of increased oxygen-carrying capacity. The neurosurgical patients, who belong to a different spectrum, are further complicated by lack of evidence-based guidelines. This has resulted in highly variable transfusion thresholds across different institutions worldwide. The ideal strategy between restrictive and liberal transfusion therefore remains in clinical equipoise.
2 Physiopathology of Anemia in Neurosurgical Population
The adult human brain represents about 2% of total body weight and receives 12–15% of the cardiac output to maintain its high metabolic rate. Cerebral O2 delivery (DO2) is dependent on cerebral blood flow (CBF) and the total O2 content of the blood (CaO2).
where DO2 is oxygen delivery to the brain, [Hb] is the concentration of hemoglobin, SaO2 is the oxygen saturation of hemoglobin, PaO2 is the oxygen partial pressure, and CBF is the cerebral blood flow.
Any condition affecting either of the factors is subsequently managed to some extent by the changes in the other factor to maintain the cerebral physiology. Cerebral perfusion pressure (CPP) is the difference between mean arterial pressure and cerebral venous pressure or intracranial pressure whichever is higher. CBF is normally autoregulated over a cerebral perfusion pressure range of 60–160 mm of Hg, outside which it is linearly dependent on mean arterial pressure. Autoregulation is often impaired in neurocritical care patients so that CBF becomes directly dependent on CPP, thus making the brain more vulnerable to extremes of blood flow. CBF is dependent on other factors like cerebral metabolic rate of oxygen consumption (CMRO2) and variations in partial pressure of carbon dioxide (CO2 reactivity).
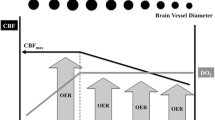
Oxygen delivery during anemia (reduced CaO2) is compensated by an increase in cardiac output, preferential distribution to cerebral circulation, cerebral vasodilatation, and increased brain tissue oxygen extraction. Upregulation of nitric oxide production by perivascular neurons is the mechanism underlying anemia-induced increase in cerebral vasodilatation. Multiple biochemical mediators like vascular endothelial growth factor, hypoxia inducible factor 1α, and erythropoietin also play a role in the cerebrovascular response to anemia. These compensatory mechanisms are however limited up to a critical cerebral DO2 value beyond which brain ischemia ensues. Many neurocritical care patients like those with aneurysmal SAH have concomitant cardiac disease or neurologically mediated cardiac dysfunction which prevents an appropriate increase in cardiac output in response to anemia. Thus, maintaining adequate Hb levels, which primarily determine DO2, is imperative to avoid hypoxic insult to the injured brain (Fig. 27.1) [1].
In the healthy brain, anemia-induced cerebral hypoxia has been shown to occur at a Hb concentration of 6–7 g/dL [2]. However, in the setting of acute brain injury, such an insult may manifest at higher Hb threshold. Coexistence of physiological stressors like cardiac dysfunction, hypotension, and anemia imposed on a central nervous system pathology with deranged regulation of CBF raises concerns regarding restrictive transfusion threshold of 7 gm/dl in neurocritical care.
2.1 Transfusion in Neurosurgery
Neurosurgical procedures are associated with higher incidence of bleeding requiring multiple transfusions. The optimal Hb concentration for transfusion in neurosurgical population is highly variable and still under a significant debate. When surveyed, neurosurgeons recommend higher threshold of Hb for transfusion as compared with the trauma surgeons and intensivists [3]. The decision to transfuse must be a balance between its presumed benefits on cerebral oxygenation and the potential complications (Fig. 27.2).
2.2 Incidence and Predictors of Transfusion
The overall rate of allogenic transfusion in neurosurgical population is 1.7–5.4% (Table 27.1) [4, 5]. The range is much wider in specific surgeries like craniosynostosis (45%) and cranial vault reconstruction (95%) in pediatric population followed by traumatic brain injury (TBI) (36%) and aneurysmal subarachnoid hemorrhage (SAH) (25%). The predictors for allogenic red blood cell transfusion in specific neurosurgeries are represented in Table 27.1.
2.3 Complications
The benefits of red blood cell transfusion to improve oxygenation are achieved only when transfused blood efficiently stores and offloads oxygen, which should be properly utilized by the compromised tissue. Numerous studies have shown that these purposes are not achieved with transfusion of stored blood due to biochemical and mechanical changes in the RBCs (termed as “storage lesion”). Depletion of 2,3-diphosphoglycerate levels shifts the oxygen hemoglobin dissociation curve to the left, reducing the amount of oxygen available for tissue consumption. Mechanical changes in the RBCs (transformed to sphero-echinocytes) result in loss of deformability and compromise the microcirculation. There is an increase in RBC aggregability and endothelial cell adhesion resulting in microvascular obstruction.
Blood transfusion has been associated with increased risk of thromboembolic events, progressive hemorrhagic injury as evidenced by clinical trials in TBI and SAH [12, 13]. A high Hb concentration results in increase in blood viscosity and reduced cerebral blood flow further exacerbating the neurological damage.
Malone et al. had identified blood transfusion as an independent risk factor for mortality and ICU stay in his study of 15,534 patients over a 3-year period at a level 1 trauma center [14]. There is enough evidence to establish that transfusion of red cells itself is associated with an increased risk of morbidity and mortality [15, 16]. The mechanism is multifactorial, including transfusion-related immunomodulation (TRIM), infectious and allergic complications, transfusion-related lung injury (TRALI), and circulatory overload (TACO). These are often translated into consequences like ARDS, respiratory failure, prolonged intubation, sepsis, adverse cardiac events, and increased length of ICU and hospital stay [17, 18].
The immunological reactions are primarily mediated by donor leucocytes which do undergo structural changes following storage. Intuitively, the use of fresh and leuco-depleted blood might minimize the transfusion risks while maximizing the physiological benefits. However, specific effects of stored blood in neurocritical care patients need trial-based evaluations.
2.4 Blood Conservation Strategies in Neurosurgery
The conflicting evidences toward allogenic blood transfusion in neurosurgical procedures emphasize the application of blood conservation strategies (Fig. 27.3). Preoperative measures include identification and correction of coagulopathy and antithrombotic reversal especially in the setting of intracranial hemorrhages (has been described below in ICH) and erythropoiesis-stimulating agents. The role of erythropoietin (EPO) as transfusion-sparing agent has been established in critically ill patients by two large randomized controlled trials. The potential benefits of erythropoietin beyond anemia include cerebral protection after ischemic injury (stroke, TBI, vasospasm) via effects on preconditioning, reducing inflammatory responses, and restoring vascular autoregulation [19]. However, caution should be exercised in patients at risk of DVT and pulmonary embolism before administration of erythropoiesis-stimulating agents. Preoperative autologous donation (PAD) has been shown to reduce allogenic transfusion in many elective surgeries, but evidence for its beneficial role in neurosurgery is limited. As per the retrospective cohort by McGirr et al., PAD does not reduce the risk of allogenic blood transfusion in neurosurgery and hence cannot be recommended as a blood conservation strategy.
Intraoperative blood conservation strategies include avoidance of NSAIDS and starch solutions, administration of antifibrinolytic agents, acute normovolemic hemodilution (ANH), and cell salvage. NSAIDS and synthetic starch solutions are known to inhibit platelet function and are associated with an increased risk of hematoma formation following intracranial procedures [20].
Antifibrinolytics are a common group of drugs which are used for blood conservation in both intraoperative and postoperative periods. Antifibrinolytic therapy prevents lysis of existing clots along the traumatized edges of the bone resulting in reduced microvascular bleeding. There are two categories of antifibrinolytics in use for blood conservation. These include the lysine analogs—tranexamic acid and epsilon-amino caproic acid (EACA)—and the serine protease inhibitor, aprotinin.
The benefits of tranexamic acid, a synthetic lysine analog which acts as a competitive inhibitor of plasmin and plasminogen, have been proven in diverse surgical procedures. In the neurosurgical population, it significantly decreases blood loss in pediatric craniosynostosis [21] surgery, spine [22] and skull base surgery [23], and intracranial brain tumors [24]. For elective intracranial meningioma surgery, use of tranexamic acid has reduced blood loss by 27% [24]. The data from CRASH-2 trial [25] (Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage) in trauma patients has shown a reduction in ICH size and mortality in patients who received tranexamic acid. In SAH it is associated with a reduction in re-bleeding albeit with an increased risk of cerebral ischemia [26]. The complications of tranexamic acid include increased risk of thromboembolism and seizures. The structural homology of tranexamic acid with GABA could be the reason for its competitive inhibition of the inhibitory receptors resulting in seizures.
The efficacy of epsilon-aminocaproic acid in reducing perioperative blood transfusion has been established in major spinal surgeries in both adult and pediatric age groups. EACA has been found to increase the levels of fibrinogen in the postoperative period predominantly [27]. A loading dose of EACA of 50 mg/kg followed by an infusion of 25 mg/kg/h is found to be associated with decreased blood loss and transfusion requirements during cranial vault reconstruction surgeries [27, 28].
Aprotinin, apart from inhibiting clot breakdown, also possesses anti-inflammatory properties. Other measures which have been tried for this purpose with little success include recombinant factor VII and aprotinin.
The evidence on the safety and efficacy of ANH as a blood conservation strategy in neurosurgery is limited. Although ANH has reduced the risk of allogenic transfusion in a small group of patients undergoing meningioma resection [29], it has failed to be of benefit in ruptured cerebral aneurysm [30]. Currently ANH can be recommended for elective neurosurgery with expected massive blood loss, in patients who are otherwise healthy. The technique may be considered if baseline hemoglobin concentration allows adequate hemodilution without hampering tissue oxygenation. Patients with poor cardiac and respiratory function are considered unsafe for this method of blood conservation.
The only study which evaluated the benefit of cell salvage in intracranial surgeries demonstrated that it was safe and decreased the amount of allogenic transfusion [31]. Cell salvage techniques are predominantly used in spine surgeries, particularly spine instrumentation and fusion, to reduce the need for intraoperative transfusion [32]. But from the health economic viewpoint, cell saver is a costly alternative. Also, reservation exists due to their concerns over tumor dissemination and infection. With the development of newer cell salvage techniques and leucocyte depletion filters, its use has been extended to metastatic spine tumors [33]. Cell salvage technique can be considered as a reasonable choice in surgeries involving massive blood loss such as spinal deformity corrections and cerebral aneurysm rupture. Firm recommendations for its use in neuro-oncological surgeries cannot be formed at present due to paucity of data.
Nonpharmacologic approaches [27] include several different surgical techniques, patient positioning, ventilatory strategies, maintenance of normothermia, and controlled hypotension in major spine surgeries. Surgical techniques for improved hemostasis include spray on collagen- thrombin, fibrin sealant, kaolin-soaked sponges, and local vasoconstrictors [34]. The various considerations during patient positioning to decrease intraoperative bleeding include avoidance of extreme rotation of the neck (leading to jugular venous engorgement) and elevation of the operative site above the right atrium (to facilitate venous drainage). In prone position excess intraabdominal pressure can increase epidural venous pressure, thereby exacerbating blood loss. Use of prone positioning devices such as Relton-Hall frame reduces the inferior vena cava pressure to one-third as compared to conventional paddings [35]. Ventilatory strategies to reduce blood loss include maintenance of low intrathoracic pressures during controlled ventilation with minimal use of positive end-expiratory pressure and low tidal volumes [36]. Controlled hypotension for reducing blood loss in elective spine surgery can be achieved with intravenous anesthetics, inhaled agents, and direct vasodilators.
3 Monitoring Blood Loss
Continuous monitoring of vital signs and estimated blood loss are commonly used to guide transfusion decisions in the intraoperative period.
3.1 Systemic Monitoring
Systemic indicators to guide transfusion include inadequate oxygen delivery, indicated by mixed (SvO2) or central venous oxygen saturation (ScvO2) and lactate. Venous oxygen saturation is a clinical measure of the relationship between whole body oxygen uptake and delivery (VO2–DO2). Central venous oxygen saturation (ScvO2) is often used as a surrogate to mixed venous oxygen saturation (SvO2) in the absence of a pulmonary artery catheter. The normal SvO2 value is in the range of 65–75% with ScVO2 being considered to be 5% above these values. When DO2 decreases, VO2 is initially maintained by an increase in oxygen extraction up to a critical DO2 value (DO2 crit) beyond which there is a state of VO2–DO2 dependency. Such a state is usually found when SvO2 falls below a critical value of 40% (SvO2 crit).
Low SvO2 or ScvO2 is predictive of bad outcome in neurosurgical practices [37]. Surve et al. in their study on acutely ill neurological patients have established that baseline ScvO2 value of <70% was a useful physiological trigger for blood transfusion in brain-injured patients [37]. However, the trend rather than absolute values of ScvO2 correlates with SvO2 during varying hemodynamic conditions [38].
Lactate is another important systemic biomarker which suggests inadequate oxygen delivery. Blood transfusion is shown to improve ScvO2 and decrease lactate levels in critically ill patients [39]. However, no specific data are available evaluating the effects of transfusion on lactate levels in neurosurgical patients.
Intraoperative coagulation abnormalities are detected by viscoelastic tests such as rotational thromboelastometry (ROTEM) and thromboelastography (TEG). Both of them have been used to guide management of perioperative coagulopathy in adults and children [40]. Early detection of coagulation disorders reduces the exposure to fresh frozen plasma (FFP) and other allogenic blood products in the pediatric population.
More accurate method to guide RBC transfusion includes continuous and noninvasive Hb monitoring (SpHb) [41]. It provides real-time trends in Hb values and has been shown to reduce blood transfusion frequency. SpHb monitoring incorporates pulse cardiac output-oximetry technology and multiwavelength sensors thus providing continuous measurements in Hb and traditional pulse oximetry [41]. SpHb monitor provides earlier detection of attainment of threshold Hb, as assessed by real-time microcirculatory value, thereby reducing unwarranted transfusion.
3.2 Regional Cerebral Monitoring
Monitoring modalities such as near-infrared spectroscopy (NIRS), brain tissue oxygen tension (PbtO2), and jugular bulb catheter sampling can be used to monitor the cerebral oxygenation and determine transfusion needs.
Near-infrared spectroscopy is an optical imaging technique used to monitor the variation of hemoglobin saturation in the tissues. It is used for continuous monitoring of regional cerebral oxygen saturation (rSO2) during various surgical procedures. A desaturation of >20% from the baseline or an absolute saturation value of <50% is associated with an increased risk of neurological damage. The usefulness of this device has been demonstrated in patients undergoing elective heart [42] and major abdominal surgeries [43] who have an otherwise healthy brain. Use of NIRS in neurosurgery is limited due to practical difficulties of maintaining probe position for longer time period, contamination from extracranial blood and the validity is questionable [44].
Jugular venous oximetry is achieved by passing a cannula into the jugular bulb allowing continuous measures of oxygen saturation in the jugular bulb (SjvO2). It is used as an indirect estimate of cerebral oxygen consumption as oxygen levels in the cerebral venous effluent correlates inversely with global brain oxygen consumption. This provides relevant information on adequacy of CBF in patients with TBI or SAH [45]. The major disadvantages of SjvO2 are question regarding side of jugular venous cannulation and poor representation of regional brain hypoxia [45].
Brain tissue oxygen monitoring involves measurement of partial pressure of oxygen at tissue level directly by insertion of catheter probes in the region of interest. In TBI patients, Hb level of less than 9 gm/dl and PbtO2 value of <20 mm of Hg were associated with poor outcome. Hence such patients should be considered as candidates for transfusion [46]. Limitations to the use of PbtO2 include relative unavailability in many centers and the information being limited to particular area of brain. Thus, PbtO2 in combination with SjvO2 better identifies all episodes of cerebral ischemia (Fig. 27.4).
4 Specific Situations
4.1 Traumatic Brain Injury
TBI is a common cause for emergency surgeries and mortality in most of the trauma centers in people less than 45 years. Traumatic brain injury is associated with almost 40–50% incidence of anemia, with patient presentation ranging from closed head injury to multiple injuries. The management of TBI patients focuses on avoiding secondary neurologic insult from reduced oxygen delivery as a result of hypoperfusion, hypoxemia, and anemia. The adaptive mechanisms to ensure an adequate cerebral oxygen delivery during anemia like cerebral vasodilation, decreased viscosity, increased cardiac output, and oxygen extraction are altered and may result in cerebral hypoxia at higher hemoglobin thresholds than in other populations [47]. But recent literature has failed to show any benefit from liberal transfusion therapy in this group of population [48].
To add upon to this complication, a scenario of coagulation disorder may coexist in TBI patients. Acute traumatic coagulopathy (ATC) is an acquired coagulation disorder that has been described in the context of isolated TBI and increases the possibility that a patient will require an RBC transfusion. In a recent meta-analysis, Epstein et al. [8] reported that ATC was uniformly associated with worse outcomes and high mortality that ranged from 17 to 86%. ATC was also associated with transfusion rates of 41%, as well as longer ICU stays, decreased ventilator-free days, and multiple organ failure.
Brain parenchyma is a rich source of tissue factor, which activates coagulation when released in TBI [9]. It has been postulated that hypoperfusion also has a role to play in coagulopathy of TBI. Brain injury has some detrimental effects on platelet function for unknown reasons. Usually patients may have associated injuries which predisposes to massive transfusion of various blood products. All these factors together contribute to the complex clinical state of coagulopathy and anemia in TBI.
It appears logical that maintaining higher hemoglobin levels might enhance cerebral oxygen delivery thereby improving outcomes in patients with TBI. But in reality, its complex decision to have a threshold hemoglobin for transfusion, as literature is lacking. Both in mild and severe TBI, transfusion is associated with poor functional outcomes. Transfusions do more harm than good in patients on both ends of the head injury severity spectrum. Carlson et al. [49] followed by Salim et al. [50] have proved that increasing transfusions have a negative impact on the Glasgow coma scale (GCS) and Glasgow outcome scale (GOS). Recent study by Warner et al. [48] concluded that in moderately anemic patients with TBI, RBC transfusions are associated with poor long-term functional outcomes. It is therefore essential to reassess transfusion recommendations in this population which have to be framed on the basis of randomized controlled trials.
Studies have shown significant variability in the response of brain tissue O2 tension to transfusions, with some patients showing improved oxygenation and others having minimal or even decreased change in brain tissue O2 tension [51]. It is unclear whether increasing brain tissue O2 tension by transfusion has translated into improved clinical outcomes [52]. The relationship between hemoglobin and brain tissue oxygenation (before and after transfusion) is not well-defined and needs further studies. Brain tissue O2 monitoring is also limited to only a few specialized trauma centers.
Preclinical and clinical data suggested erythropoietin, a glycoprotein hormone, as a promising candidate in place of transfusions in TBI. Erythropoietin has pleiotropic cytokine-like effects which ameliorated secondary brain injury after TBI [53]. But EPO TBI trial (erythropoietin in traumatic brain injury) has stated that erythropoietin did not reduce the incidence of severe neurological dysfunction at 6 months after moderate to severe TBI.
Current recommendations to maintain hematocrit levels >30% in otherwise hematologically stable patients should be reconsidered. Specific interventions aimed at reducing the demands and increasing the oxygen supply should be maximized before transfusion. Transfusion strategies should be directed toward patients with symptomatic anemia or obvious signs of physiological compromise, such as decreased brain tissue O2 tension, and transfusion volume should be minimized whenever possible. Other useful indicators like low venous hemoglobin, high lactate levels for inadequate systemic oxygen delivery and low regional hemoglobin saturation, and brain tissue oxygen tension for cerebral hypoxia can be helpful to guide transfusion [1].
4.2 Subarachnoid Hemorrhage
Anemia in SAH patients is caused by various factors like hemodilution induced as a part of the therapy for vasospasm, occult hemorrhage, surgical blood loss, aneurysm rupture and re-bleeding [54]. A growing body of evidence suggests anemia to be independently associated with poor neurological outcome and increased mortality [52] regardless of the SAH severity. Unlike other critically ill patients, those with SAH are of special concern, due to their well-defined risk of vasospasm and cerebral ischemia [55]. This makes them less tolerant to anemia and increases their likelihood to benefit from blood transfusion.
Risk factors for anemia after SAH include female sex, advanced age, worse clinical grade, lower admission Hb, and surgery. Intraoperatively, large blood loss may occur during clip reapplication or with aneurysm rupture. Even a short period of uncontrolled bleeding as during intraoperative rupture may be associated with excessive bleeding. Patients with anterior communicating artery aneurysms are most likely to receive transfusion, while those with internal carotid artery aneurysms are least prone. Other surgical factors contributing to excess blood loss include large aneurysms (>10 mm), multiple aneurysm obliteration, and intracerebral hematoma evacuation [55].
An important preventable factor associated with poor neurological outcome after SAH is delayed cerebral ischemia (DCI) [56]. DCI occurs in about 30% of patients and is often associated with arterial vasospasm which impairs CBF and cerebral DO2. Anemia may exacerbate DCI by further reducing cerebral oxygen delivery. As the risk of vasospasm continues predictably for several weeks after aneurysmal rupture (greatest risk period between days 6 and 11), anemia may be most detrimental during this period. The optimal Hb threshold for transfusion in SAH patients remains unclear although Hb > 10 g/dL is associated improved outcome [57]. Prevention of vasospasm has seldom shown to improve clinical outcome, despite reduced vessel narrowing. This lack of association between clinical outcome and vasospasm has renewed interest in intensive care strategies to prevent DCI.
A liberal use of transfusion at Hb > 10 gm/dl may offset the benefits of increased oxygen-carrying capacity by increase in blood viscosity and reduced CBF. It has also been linked with medical complications, infection, vasospasm, poor cognitive performance, and poor outcome [52, 56]. The deleterious effects of transfusion (storage lesion) like altered nitric oxide metabolism, red blood cell adhesiveness, and aggregability appear integral to vasospasm [58]. Consequently, a restrictive transfusion policy (Hb −7 g/dl) has been suggested at least in patients with normal cardiac and cerebrovascular reserves. However many SAH patients, unlike traumatic injury patients, often have associated cardiac dysfunction [59], thus posing a relative contraindication to restrictive transfusion.
Lacking concrete guidelines, presently transfusion decisions for SAH patients should focus on an individualized assessment of anemia tolerance, risk of DCI, presence of cardiac dysfunction, feasibility of blood conservation strategies, and awareness of the potential risks and benefits of blood transfusion. The results of the ongoing Aneurysmal Subarachnoid Hemorrhage: Red Blood Cell Transfusion and Outcome (SAHaRA Pilot) [60], which aims to compare RBC transfusion triggers from 10 g/dL down to 8 g/dL, will probably give us firmer guidelines in this context. Awaiting its results, the Neurocritical Care Society guidelines [61] suggest a transfusion threshold of 8 g/L in SAH patients without DCI, with a more aggressive transfusion trigger of 9–10 g/L as a tier one rescue therapy in cases of DCI unresponsive to first-line therapy.
4.3 Intracranial Tumors
Surgery for intracranial tumors is associated with higher incidence of bleeding and transfusion as compared to other neurosurgical conditions. This excessive blood loss has led to several adverse clinical outcomes including increased duration of ventilator and ICU stay. Morbidity and mortality are directly related to intraoperative blood loss especially in those who lose >500 ml [62]. Blood transfusion is usually not required in astrocytomas, low-grade gliomas, and transsphenoidal pituitary tumor excisions. Cerebellopontine tumors and meningiomas in particular are notorious for bleeding due to high vascularity from carotid and vertebral arteries and from the site of dural attachment. Recent retrospective study stated that skull base meningiomas of size greater than 4.64 cm and operative time greater than 10 h are independent factors related to excess risk of blood loss and transfusion [63]. Endovascular embolization of the tumor, particularly when complete, reduces bleeding, thereby decreasing the transfusion demand.
Tissue plasminogen activator (t-PA) is present in larger quantities in glioblastoma compared to other tumors. The t-PA-induced hyperfibrinolysis adds upon to stress-induced consumption and dilutional coagulopathy associated with protracted intracranial surgeries. This may aggravate blood loss during intracranial tumor surgeries necessitating transfusion.
Blood transfusion, however, may be an independent risk factor for cancer progression owing to its immunomodulatory effects. Aged RBCs in stored blood and allogenic leucocytes have been implicated as the possible culprits for cancer progression, favoring the use of fresh leuco-depleted blood whenever feasible [64]. A restrictive threshold of Hb as compared to liberal strategy does not appear to prolong the length of hospital stay or the risk of morbidity and mortality in intracranial tumor surgery [65].
4.4 Spine Surgeries
The incidence of blood transfusion in spine surgery is about 20–35% and is aimed at improving tissue oxygenation. Reconstructive trauma, tumor, and multilevel spine surgeries are complicated by significant intraoperative blood loss. The surgical reasons for blood loss are exposure of the spine, stripping of the muscle off the bone, and leaving exposed surfaces of the muscle and bone. In elderly patients, the periosteum is thinner, and the osteoporotic bones have wider vascular channels [66]. There is an increased bleeding from the exposed bone in osteotomies and from the epidural plexus in laminectomies. Adult deformity correction surgeries are likely to involve multiple segments as their compensatory curves become structural and may require inclusion in the surgery. They also have a higher rate of revision surgery which are associated with greater blood loss [67]. Tumors of the vertebral column tend to be highly vascular in nature and carry a risk of allogenic blood transfusion.
Intraoperative blood loss and transfusion are among the factors influencing the outcome of patients in major spine surgeries. The number of units transfused perioperatively is associated with age, comorbidities, number of levels instrumented, magnitude of arthrodesis, preoperative Hb, duration of surgery, and complexity of the operation [11]. Congenital and neuromuscular scolioses are more likely to have clinical comorbidities than in idiopathic scoliosis reflecting a lower functional reserve and hence a greater need for transfusion [68]. Patient positioning plays an important role in reducing blood loss during spinal surgeries. The benefit of controlled hypotension in spine surgery is due to decreased blood extravasation and local wound blood flow with the lowering of arterial blood pressure. However epidural venous plexus pressure and intraosseous pressure which are important determinants of blood loss are independent of arterial blood pressure. The worrisome complications of controlled hypotension in spine surgery are postoperative visual loss and decreased perfusion to end organs including the spinal cord.
Antifibrinolytics like tranexamic acid effectively decrease transfusion requirements in this population [27]. There is an increased risk of venous thromboembolism after spinal surgery, but the role of antifibrinolytics as causative is questionable [69]. Autologous blood transfusion and intraoperative cell salvage are the commonly used blood conservative method in elective spine surgery, which reduces the homologous blood exposure. Patients receiving blood transfusion in major spine surgeries have been found to have higher rates of surgical site infections and urinary tract infections [70].
Neuraxial opioids along with general anesthesia decrease intraoperative blood loss and need for transfusion in spine surgeries [71]. Unlike local anesthetics, intrathecal morphine when given alone neither causes hypotension nor interferes with neurological assessment, in addition to providing adequate pain relief. Though randomized controlled trials have proved this benefit, the mechanism still remains unknown.
4.5 Pediatric Neurosurgery
Though there have been multiple evolutions in anesthesia and surgical techniques in pediatric neurosurgery, yet there is no decrease in bleeding and allogenic blood transfusion. In intracranial surgeries the incidence of coagulation disorder is higher as compared to general pediatric surgeries. There has been reported evidence of hypercoagulable state in pediatric neurosurgery [72]. This phenomenon coupled with surgical blood loss and dilutional and consumptional coagulopathy may further amplify blood loss in this subpopulation. Children undergoing major craniofacial reconstructions, spine reconstructions, resection of vascular malformations, and tumors area at great risk for massive blood loss. Despite diligent efforts, assessment of blood loss in pediatric neurosurgery is difficult. There is an increased incidence of morbidity and mortality following transfusion in pediatric patients.
In children undergoing oncologic neurosurgeries, duration of surgery poses high risk of transfusion [72]. This is specifically attributed to the presence of large, highly vascular, inaccessible deep-seated lesions which are close to functional areas of the brain. Hemostasis disturbances are due to hyperfibrinolysis and loss of coagulation factors along with blood loss in craniotomies [72].
Craniofacial reconstructions need a special mention as they have a potential for excess blood loss ranging from 20 to 500% of patient’s circulating blood volume even in the best centers [73, 74]. In these cases, the issue continues in the postoperative period as well in the form of blood loss in the drains. The patient factors which influence transfusion in pediatric spine surgeries include neuromuscular etiology, Cobb’s angle of >50°, and a greater number of levels fused [11]. The presence of any one of these risk factors doubles the risk of transfusion as per Vitale et al. [75]. Protocol-based transfusion algorithms and blood-sparing surgical techniques have proved to reduce the transfusion of blood and products to some extent [73]. Acute normovolemic hemodilution, erythropoietin injection, acceptance of lower Hb levels, cell salvage techniques, use of antifibrinolytics like tranexamic acid, controlled hypotension, and factor administration (activated factor VII A, prothrombin complex concentrate) have been attempted with success in many studies [11].
Studies have demonstrated that lower hemoglobin levels are well tolerated by pediatric patients without adverse effects [76]. Blood conservation modalities can be safely used in pediatric neurosurgery with combined technique being more effective than any single modality.
4.6 Intracranial Hemorrhage (ICH)
Intracranial hemorrhage is a life-threatening condition, resulting from spontaneous bleed, vascular malformations, trauma, or anticoagulant therapy. An expanding intracerebral hematoma may have a rim of hypoperfusion due to mechanical compression and vasoconstriction of the surrounding vasculature producing the so-called perihematomal penumbra. However, oxygen extraction fraction is not increased in this region suggesting the hypoperfusion to be due to reduced cerebral metabolism. Thus, it remains uncertain whether transfusions help salvage the penumbral region and contribute to improve neurological recovery.
In patients with ICH, anemia is associated with larger hematoma volumes and is an independent predictor of unfavorable functional outcome [77]. Due to conflicting evidence from various studies, it still remains unclear whether treatment of anemia can improve outcomes after ICH [78, 79].
ICH related to anticoagulant use accounts for >15% of all cases. Prevention of hematoma expansion by blood pressure management and reversal of coagulopathy is an important consideration in the management of these patients. The newer oral anticoagulant drugs, which are being used in the setting of stroke, have no antagonists except for dabigatran. Hence it is very challenging for anesthesiologists to manage ICH which develop in the course of treatment with these anticoagulants. Specific recommendations have been drawn down by the Neurocritical Care Society for the reversal of these agents and they are given in Table 27.2 [80].
4.7 Neurocritical Care
The most important goal in the management of patients in the neurosurgical ICU is avoidance of secondary brain injury. Delayed cerebral injury in the ICU is the result of conglomeration of several factors which impair cerebral DO2 and include anemia, hypovolemia, hypoxemia, raised ICP, vasospasm, autoregulatory failure, and uncoupling of cerebral flow metabolism.
Anemia, common in patients admitted to ICU, and is further accelerated by frequent phlebotomy, reduced RBC survival, occasional hemorrhage, and dilution by large volume fluid resuscitation. Systemic inflammation interferes with the erythropoietin production and ability of erythroblast to incorporate iron. However, the manipulation of anemia to maintain cerebral DO2 remains debatable. The significance of anemia and optimal transfusion thresholds may not be universally applied to all neurocritical care patients.
The Stroke: RelevAnt Impact of hemoglobin, Hematocrit and Transfusion (STRAIGHT) [81] trial conducted on critical care patients with severe ischemic stroke has concluded that both low hemoglobin and red blood transfusion are associated with prolonged ICU stay. In ischemic stroke patients, the effect of hematocrit on outcome is therefore u-shaped [52] with both high and low Hb associated with poor outcome.
Presently there are no large randomized trials to guide transfusion practice in critically ill TBI and SAH patients. A recent survey conducted across 66 European trauma centers as part of the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI study [82]) found that 41% of the centers maintained target Hb of 7–9 gm/dl and 59% >9 gm/dl. Overall there was a lack of consensus across the European ICUs on blood transfusion management. In SAH patients, the consensus panel of the neurocritical care society strongly recommends blood transfusion to maintain a Hb concentration more than 8–10 gm/dl based on moderate-quality data.
5 Role of Hemoglobin-Based Oxygen Carriers (HBOC)
A lot of effort has gone into developing formulations to substitute red blood cell transfusion. Most of these formulations are Hb-based oxygen carriers (HBOCs). Blood substitutes present a promising strategy for resuscitation in patients of TBI when complicated with hemorrhagic shock. Substantial benefits on intracranial pressure, brain tissue oxygenation, and neuropathology have been suggested with the use of polymerized hemoglobin in TBI patients [69]. HBOCs possess a theoretical advantage over other fluids in neurocritical care by hemodilution-induced enhanced cerebral blood flow while simultaneously maintaining CaO2. This concept has been validated in several preclinical studies in the setting of experimental ischemic stroke, TBI, and SAH. However, reports of myocardial infarction and mortality with the use of HBOC resulted in decrease in enthusiasm and its use. Hence it is important to develop recombinant hemoglobins which would maximize the benefits of oxygen affinity targeted to specific indications and minimize the inherent toxicities. This should be followed by preclinical studies in experimental models which closely mimic complex clinical scenario in the neurologic ICU.
6 Current Consensus
Concerns regarding the safety and efficacy of blood products have led to a paradigm shift in transfusion practices. In the Transfusion Requirements in Critical Care (TRICC) trial [83], a Hb trigger of 7 gm/dl was generally agreed upon, and liberal transfusion therapy was found to be associated with poor outcome. However, in this trial, patients with primary neurological injury were excluded.
In TBI patients, there is a clear agreement that Hb < 7 gm/dl requires transfusion. However, transfusion practices between thresholds of 7 and 10 gm/dl vary widely between studies [7]. Interestingly the recently updated Brain Trauma Foundation guidelines do not mention any threshold for transfusion in severe TBI [84]. A recently conducted international survey found that most of the clinicians initiated blood transfusion at a Hb threshold of 7–8 gm/dl in their ICU for brain-injured patients. Presence of associated noncerebral factors like coronary artery disease, active bleeding, and low mixed venous oxygen saturation would shift the Hb threshold to higher limits. In geriatric population, liberal transfusion strategies produce better outcomes with a decreased risk of 30-day and 90-day mortalities [85].
In SAH, patients should receive transfusion to maintain hemoglobin concentration above 8–10 gm/dl. Higher hemoglobin concentrations might be appropriate for those who have or at high risk for DCI. Current aneurysmal SAH management guidelines recommend transfusion in anemic patients at risk for cerebral ischemia but do not suggest any particular transfusion threshold. Restrictive transfusion therapy in this group of patients, who are at risk of vasospasm and DCI, is questionable. The results of ongoing Subarachnoid Hemorrhage: Red Blood Cell Transfusion and Outcome (SAHaRA) trial [60] in adult patients with aneurysmal SAH may probably aid clinicians to arrive at a guideline for transfusion.
The common strategies employed in neurosurgical population to minimize the rate of allogenic blood transfusion include:
-
1.
Acceptance of a low transfusion trigger of Hb (<7 gm/dl)
-
2.
Initiation of EPO/iron therapy prior to surgery to improve preoperative Hb levels
-
3.
Utilization of intraoperative blood salvage techniques
-
4.
Blood and product transfusion based on standard laboratory tests for platelets, fibrinogen, TEG, ROTEM, etc.
-
5.
Recommendations for transfusion of products include:
-
(a)
Arterial blood gas, TEG, complete blood count, and fibrinogen samples must be sent with the onset of microvascular bleeding or loss of 1 estimated blood volume.
-
(b)
Initiate packed red cell transfusion (10–15 ml/kg) with PCV <27% or Hb <9 gm/dl in the presence of ongoing blood loss.
-
(c)
Initiate fresh frozen plasma (10–15 ml/kg) at a reaction time (R) >10 min in TEG.
-
(d)
Platelet transfusion (5–10 ml/kg) is indicated with platelet count of less than 1 lakh/microL.
-
(e)
Cryoprecipitate infusion (10–15 ml/kg) should be started at fibrinogen <100 mg/dl.
-
(f)
With persistent bleeding after 30 min, TEG and fibrinogen levels should be resend and managed accordingly.
-
(a)
7 Future Directions
The TRansfusion Strategies in Acute Brain Injured Patients (TRAIN) trial (ClinicalTrials.gov NCT02968654)—endorsed by European society of Intensive Care Medicine—compares liberal and restrictive transfusion strategy in TBI, SAH, and ICH patients. The HEMOglobin Transfusion Threshold in TBI OptimizatioN (HEMOTION) trial (NCT03260478) being conducted in Canada is evaluating the effects of RBC transfusion thresholds on neurological outcome in TBI patients. These along with the SAHARA study [60] should provide reliable evidence to guide transfusion therapy in most of the neurosurgical patients.
An individualized approach, intended to target physiological end points like cerebral tissue hypoxia rather than a hemoglobin cutoff, guided by multimodal neuromonitoring, has to be validated through large randomized clinical trials. Development of future guidelines based on trials in this direction would help improve outcome in most of the neurocritically ill patients.
Key Points
-
Anemia-induced cerebral hypoxia is manifested at higher threshold of hemoglobin in a setting of acute brain injury than compared with normal brain.
-
The risks of transfusion as weighed against the benefits have rewritten the transfusion threshold in neurosurgical population. The ideal strategy between restrictive and liberal transfusions therefore remains a clinical equipoise.
-
Blood conservative strategies during the perioperative period along with advanced technologies in monitoring cerebral oxygenation have reduced unwarranted transfusion to a major extent.
-
In TBI patients, there is a clear agreement that Hb < 7 gm/dl requires transfusion. However, transfusion practices between thresholds of 7–10 gm/dl vary widely between studies.
-
In SAH, patients should receive transfusion to maintain hemoglobin concentration above 8–10 gm/dl. Higher hemoglobin concentrations might be appropriate for those who have or at high risk for DCI.
References
Crippa IA, Lelubre C, Lozano-Roig A, Taccone FS. Optimizing blood transfusion practices in traumatic brain injury and subarachnoid hemorrhage. Curr Anesthesiol Rep. 2016;6(3):250–6.
Bellapart J, Boots R, Fraser J. Physiopathology of anemia and transfusion thresholds in isolated head injury. J Trauma Acute Care Surg. 2012;73(4):997–1005.
Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH. Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med. 2009;35(3):480–8.
Rolston JD, Han SJ, Lau CY, Berger MS, Parsa AT. Frequency and predictors of complications in neurological surgery: national trends from 2006 to 2011. J Neurosurg. 2014;120(3):736–45.
Linsler S, Ketter R, Eichler H, Schwerdtfeger K, Steudel WI, Oertel J. Red blood cell transfusion in neurosurgery. Acta Neurochir. 2012;154(7):1303–8.
White N, Marcus R, Dover S, Solanki G, Nishikawa H, Millar C, et al. Predictors of blood loss in fronto-orbital advancement and remodeling. J Craniofac Surg. 2009;20(2):378–81.
Boutin A, Chasse M, Shemilt M, Lauzier F, Moore L, Zarychanski R, et al. Red blood cell transfusion in patients with traumatic brain injury: a systematic review and meta-analysis. Transfus Med Rev. 2016;30(1):15–24.
Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury. 2014;45(5):819–24.
Mc Ewen J, Huttunen KH. Transfusion practice in neuroanesthesia. Curr Opin Anesthesiol. 2009;22(5):566–71.
Luostarinen T, Lehto H, Skrifvars MB, Kivisaari R, Niemela M, Hernesniemi J, et al. Transfusion frequency of red blood cells, fresh frozen plasma, and platelets during ruptured cerebral aneurysm surgery. World Neurosurg. 2015;84(2):446–50.
Oetgen ME, Litrenta J. Perioperative blood management in pediatric spine surgery. J Am Acad Orthop Surg. 2017;25(7):480–8.
Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312(1):36–47.
Vedantam A, Yamal JM, Rubin ML, Robertson CS, Gopinath SP. Progressive hemorrhagic injury after severe traumatic brain injury: effect of hemoglobin transfusion thresholds. J Neurosurg. 2016;125(5):1229–34.
Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898–905.
Shander A, Goodnough LT. Can blood transfusion be not only ineffective, but also injurious? Ann Thorac Surg. 2014;97(1):11–4.
Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667–74.
Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16(2):144–60.
Vamvakas EC, Carven JH. Allogeneic blood transfusion and postoperative duration of mechanical ventilation: effects of red cell supernatant, platelet supernatant, plasma components and total transfused fluid. Vox Sang. 2002;82(3):141–9.
Coleman T, Brines M. Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care. 2004;8(5):337–41.
Jian M, Li X, Wang A, Zhang L, Han R, Gelb AW. Flurbiprofen and hypertension but not hydroxyethyl starch are associated with post-craniotomy intracranial haematoma requiring surgery. Br J Anaesth. 2014;113(5):832–9.
Dadure MDPDC, Sauter MDM, Bringuier PDPDS, Bigorre MDM, Raux MDMSO, Rochette MDA, et al. Intraoperative Tranexamic acid reduces blood transfusion in children undergoing Craniosynostosis surgery a randomized double-blind study. Anesthesiology. 2011;114(4):856–61.
Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479–86.
Mebel D, Akagami R, Flexman AM. Use of tranexamic acid is associated with reduced blood product transfusion in complex skull base neurosurgical procedures: a retrospective cohort study. Anesth Analg. 2016;122(2):503–8.
Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017;41:132–8.
Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79.
Baharoglu MI, Germans MR, Rinkel GJ, Algra A, Vermeulen M, van Gijn J, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;8:CD001245.
Willner D, Spennati V, Stohl S, Tosti G, Aloisio S, Bilotta F. Spine surgery and blood loss: systematic review of clinical evidence. Anesth Analg. 2016;123(5):1307–15.
Thompson ME, Saadeh C, Watkins P, Nagy L, Demke J. Blood loss and transfusion requirements with epsilon-aminocaproic acid use during cranial vault reconstruction surgery. J Clin Anesth. 2017;36:153–7.
Naqash I, Draboo M, Lone A, Nengroo S, Kirmani A, Bhat A. Evaluation of acute normovolemic hemodilution and autotransfusion in neurosurgical patients undergoing excision of intracranial meningioma. J Anaesthesiol Clin Pharmacol. 2011;27(1):54–8.
Oppitz PP, Stefani MA. Acute normovolemic hemodilution is safe in neurosurgery. World Neurosurg. 2013;79(5–6):719–24.
Cataldi S, Bruder N, Dufour H, Lefevre P, Grisoli F, Francois G. Intraoperative autologous blood transfusion in intracranial surgery. Neurosurgery. 1997;40(4):765–71. discussion 71–2.
Miao YL, Ma HS, Guo WZ, Wu JG, Liu Y, Shi WZ, et al. The efficacy and cost-effectiveness of cell saver use in instrumented posterior correction and fusion surgery for scoliosis in school-aged children and adolescents. Plo S One. 2014;9(4):e92997.
Kumar N, Zaw AS, Khoo BL, Nandi S, Lai Z, Singh G, et al. Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells. Eur Spine J. 2016;25(12):4008–15.
Epstein NE. Tisseel does not reduce postoperative drainage, length of stay, and transfusion requirements for lumbar laminectomy with noninstrumented fusion versus laminectomy alone. Surg Neurol Int. 2015;6(Suppl 4):S172–S6.
Raw DA, Beattie JK, Hunter JM. Anaesthesia for spinal surgery in adults. Br J Anaesth. 2003;91(6):886–904.
Koh JC, Lee JS, Han DW, Choi S, Chang CH. Increase in airway pressure resulting from prone position patient placing may predict intraoperative surgical blood loss. Spine. 2013;38(11):E678–82.
Surve RM, Muthuchellappan R, Rao GS, Philip M. The effect of blood transfusion on central venous oxygen saturation in critically ill patients admitted to a neurointensive care unit. Transfus Med. 2016;26(5):343–8.
Dueck MH, Klimek M, Appenrodt S, Weigand C, Boerner U. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103(2):249–57.
Mazza BF, Freitas FGR, Barros MMO, Azevedo LCP, Machado FR. Blood transfusions in septic shock: is 7.0g/dL really the appropriate threshold? Rev Bras Ter Intensiv. 2015;27(1):36–43.
Haas T, Spielmann N, Mauch J, Madjdpour C, Speer O, Schmugge M, et al. Comparison of thromboelastometry (ROTEM (R)) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth. 2012;108(1):36–41.
Awada WN, Mohmoued MF, Radwan TM, Hussien GZ, Elkady HW. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015;29(6):733–40.
Cem A, Serpil UO, Fevzi T, Murat O, Umit G, Esin E, et al. Efficacy of near-infrared spectrometry for monitoring the cerebral effects of severe dilutional anemia. Heart Surg Forum. 2014;17(3):E154–9.
Green DW. A retrospective study of changes in cerebral oxygenation using a cerebral oximeter in older patients undergoing prolonged major abdominal surgery. Eur J Anaesthesiol. 2007;24(3):230–4.
Terborg C, Groschel K, Petrovitch A, Ringer T, Schnaudigel S, Witte OW, et al. Noninvasive assessment of cerebral perfusion and oxygenation in acute ischemic stroke by near-infrared spectroscopy. Eur Neurol. 2009;62(6):338–43.
Schoon P, Benito Mori L, Orlandi G, Larralde C, Radrizzani M. Incidence of intracranial hypertension related to jugular bulb oxygen saturation disturbances in severe traumatic brain injury patients. Acta Neurochir Suppl. 2002;81:285–7.
Oddo M, Levine JM, Kumar M, Iglesias K, Frangos S, Maloney-Wilensky E, et al. Anemia and brain oxygen after severe traumatic brain injury. Intensive Care Med. 2012;38(9):1497–504.
Hare GM, Tsui AK, McLaren AT, Ragoonanan TE, Yu J, Mazer CD. Anemia and cerebral outcomes: many questions, fewer answers. Anesth Analg. 2008;107(4):1356–70.
Warner MA, O’Keeffe T, Bhavsar P, Shringer R, Moore C, Harper C, et al. Transfusions and long-term functional outcomes in traumatic brain injury. J Neurosurg. 2010;113(3):539–46.
Carlson AP, Schermer CR, Lu SW. Retrospective evaluation of anemia and transfusion in traumatic brain injury. J Trauma. 2006;61(3):567–71.
Salim A, Hadjizacharia P, DuBose J, Brown C, Inaba K, Chan L, et al. Role of anemia in traumatic brain injury. J Am Coll Surg. 2008;207(3):398–406.
Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33(5):1104–8.
Kramer AH, Diringer MN, Suarez JI, Naidech AM, Macdonald LR, Le Roux PD. Red blood cell transfusion in patients with subarachnoid hemorrhage: a multidisciplinary north American survey. Crit Care. 2011;15(1):R30.
Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. 2015;386(10012):2499–506.
Festic E, Rabinstein AA, Freeman WD, Mauricio EA, Robinson MT, Mandrekar J, et al. Blood transfusion is an important predictor of hospital mortality among patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2013;18(2):209–15.
Rosenberg NF, Koht A, Naidech AM. Anemia and transfusion after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2013;25(1):66–74.
Le Roux PD. Anemia and transfusion after subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):342–53.
Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35(10):2383–9.
Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31(2):151–8.
Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30(4):780–6.
English SW, Fergusson D, Chassé M, Turgeon AF, Lauzier F, Griesdale D, et al. Aneurysmal sub arachnoid hemorrhage—red blood cell transfusion and outcome (SAHaRA): a pilot randomised controlled trial protocol. BMJ Open. 2016;6(12):e012623.
Diringer MN, Bleck TP, Claude Hemphill J 3rd, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical care Society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15(2):211–40.
Wu W-C, Trivedi A, Friedmann PD, Henderson WG, Smith TS, Poses RM, et al. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg. 2012;255(4):708–14.
Rogers L, Zhang P, Vogelbaum M, Perry A, Ashby LS, Modi J, et al. Mngi-08. High-risk meningioma: initial outcomes from Nrg oncology/Rtog-0539. Neuro-Oncology. 2017;19(suppl_6):vi133.
Atzil S, Arad M, Glasner A, Abiri N, Avraham R, Greenfeld K, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109(6):989–97.
Alkhalid Y, Lagman C, Sheppard JP, Nguyen T, Prashant GN, Ziman AF, et al. Restrictive transfusion threshold is safe in high-risk patients undergoing brain tumor surgery. Clin Neurol Neurosurg. 2017;163:103–7.
Hu SS. Blood loss in adult spinal surgery. Eur Spine J. 2004;13(Suppl 1):S3–5.
Zheng F, Cammisa FP Jr, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine. 2002;27(8):818–24.
Master DL, Son-Hing JP, Poe-Kochert C, Armstrong DG, Thompson GH. Risk factors for major complications after surgery for neuromuscular scoliosis. Spine. 2011;36(7):564–71.
Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, et al. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock (Augusta, Ga). 2009;31(1):64–79.
Janssen SJ, Braun Y, Wood KB, Cha TD, Schwab JH. Allogeneic blood transfusions and postoperative infections after lumbar spine surgery. Spine J. 2015;15(5):901–9.
Guay J. The effect of neuraxial blocks on surgical blood loss and blood transfusion requirements: a meta-analysis. J Clin Anesth. 2006;18(2):124–8.
Vassal O, Desgranges FP, Tosetti S, Burgal S, Dailler F, Javouhey E, et al. Risk factors for intraoperative allogeneic blood transfusion during craniotomy for brain tumor removal in children. Paediatr Anaesth. 2016;26(2):199–206.
Nguyen TT, Hill S, Austin TM, Whitney GM, Wellons JC 3rd, Lam HV. Use of blood-sparing surgical techniques and transfusion algorithms: association with decreased blood administration in children undergoing primary open craniosynostosis repair. J Neurosurg Pediatr. 2015;31:1–8.
Vega RA, Lyon C, Kierce JF, Tye GW, Ritter AM, Rhodes JL. Minimizing transfusion requirements for children undergoing craniosynostosis repair: the CHoR protocol. J Neurosurg Pediatr. 2014;14(2):190–5.
Vitale MG, Levy DE, Park MC, Choi H, Choe JC, Roye DP Jr. Quantifying risk of transfusion in children undergoing spine surgery. Spine J. 2002;2(3):166–72.
Lavoie J. Blood transfusion risks and alternative strategies in pediatric patients. Paediatr Anaesth. 2011;21(1):14–24.
Kuramatsu JB, Gerner ST, Lücking H, Kloska SP, Schellinger PD, Köhrmann M, et al. Anemia is an independent prognostic factor in intracerebral hemorrhage: an observational cohort study. Crit Care. 2013;17(4):R148.
Diedler J, Sykora M, Hahn P, Heerlein K, Schölzke MN, Kellert L, et al. Low hemoglobin is associated with poor functional outcome after non-traumatic, supratentorial intracerebral hemorrhage. Crit Care. 2010;14(2):R63.
Sheth KN, Gilson AJ, Chang Y, Kumar MA, Rahman RM, Rost NS, et al. Packed red blood cell transfusion and decreased mortality in intracerebral hemorrhage. Neurosurgery. 2011;68(5):1286–92.
Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the neurocritical care society and society of critical care medicine. Neurocrit Care. 2016;24(1):6–46.
Kellert L, Martin E, Sykora M, Bauer H, Gussmann P, Diedler J, et al. Cerebral oxygen transport failure?: decreasing hemoglobin and hematocrit levels after ischemic stroke predict poor outcome and mortality: STroke: RelevAnt impact of hemoGlobin, hematocrit and transfusion (STRAIGHT)--an observational study. Stroke. 2011;42(10):2832–7.
Maas AI, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80.
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409–17.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury. Fourth edition. Neurosurgery. 2017;80(1):6–15.
Murphy MF, Estcourt L, Goodnough LT. Blood transfusion strategies in elderly patients. Lancet Haematol. 2017;4(10):e453–e4.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jayaram, K., Padhy, S. (2019). Blood Transfusion in Neurosurgery. In: Prabhakar, H., Ali, Z. (eds) Textbook of Neuroanesthesia and Neurocritical Care. Springer, Singapore. https://doi.org/10.1007/978-981-13-3387-3_27
Download citation
DOI: https://doi.org/10.1007/978-981-13-3387-3_27
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3386-6
Online ISBN: 978-981-13-3387-3
eBook Packages: MedicineMedicine (R0)