Abstract
Background
The necessity of red blood cell (RBC) transfusions in neurosurgical procedures is under debate. Although detailed recommendations exist for many other surgical disciplines, there are very limited data on the probability of transfusions during neurosurgical procedures.
Methods
Three-thousand and twenty-six consecutive adult patients undergoing neurosurgical procedures at Saarland University Hospital from December 2006 to June 2008 were retrospectively analyzed for administration of RBCs. The patients were grouped into 11 main diagnostic categories for analysis. The transfusion probability and cross-match to transfusion ratio (C/T ratio) were calculated.
Results
Overall, the transfusion probability for neurosurgical procedures was 1.7 % (52/3,026). The probability was 6.5 % for acute subdural hematoma (7/108), 6.2 % for spinal tumors (5/80), 4.6 % for intracerebral hemorrhage (ICH, 4/98), 2.8 % for abscess (3/108), 2.4 % for traumatic brain injury (4/162), 2.3 % for cerebral ischemia (1/44), 1.9 % for subarachnoid hemorrhage (SAH) /aneurysms (4/206), 1.4 % for brain tumors (10/718), 0.8 % for hydrocephalus (2/196), 0.4 % for degenerative diseases of the spine (5/1290), including 3.6 % (3/82) for posterior lumbar interbody fusion (PLIF) and 0 % for epidural hematoma (0/15). The transfusion probabilities for clipping and coiling of SAH were 2.9 % (2/68) and 1.7 % (2/120) respectively.
Conclusions
The probability of blood transfusion during neurosurgical procedures is well below the 10 % level which is generally defined as the limit for preoperative appropriation of RBCs. Patients with spinal tumors, acute subdural hematomas or ICH, i.e., patients undergoing large decompressive procedures of bone or soft tissue, had a higher probability of transfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is one of the most common medical complications arising during surgical procedures [4]. Traditionally, red blood cell (RBC) transfusions were ubiquitous practice in most countries. However, in recent years, transfusion practice across the world has generally become more restrictive due to clinical trials which have demonstrated significant transfusion-associated risks and also due to increased cost awareness [7, 12, 13, 18, 23, 24, 28].
The exact level at which anemia threatens tissue oxygenation is unknown and very likely varies by tissue type and pathology. Hopf et al. [17] demonstrated that acute hemodilution down to 5 g/dl in healthy volunteers does not compromise subcutaneous tissue oxygen tension due to compensation with increasing blood flow. However, because reduced oxygen delivery contributes to ‘secondary’ cerebral injury, anemia may not be as well tolerated among neurosurgical patients. In addition, the possibility of worsening brain injury has been demonstrated at hemoglobin levels above the commonly accepted threshold of 7.0 g/dl [15, 19, 35, 36]. Decisions about whether to transfuse neurosurgical patients must further weigh up the putative benefits of increasing oxygen-carrying capacity and delivery against the potentially serious risks associated with the transfusion of blood products. These risks include infection, hemolysis, transfusion-related acute lung injury, alloimmunization, and immunosuppression [16], as well as concerns about whether or not transfused red cell products effectively augment oxygen delivery [29, 30]. In spite of the fact that transfusion may confer important benefit or significant harm, current transfusion strategies for RBCs, as well as coagulation factors and platelets as they relate to neurological pathology and procedures, are primarily based on expert opinion and tradition as opposed to high quality evidence. The little evidence existing is at times conflicting or applicable only to certain limited patient populations [20]. Given this situation, it is not surprising that the pattern of practice of practitioners from different disciplines or different centers may vary considerably [27].
In neurosurgical procedures, there is only a low level of evidence about the probability of transfusions [22], although a high probability of transfusions is postulated in many cases. Generally, the number of RBC units ordered before surgery has been based on the physician’s transfusion experiences with neurosurgical patients. Until recently in our institution, 2–4 RBC units were kept ready in the operating room depending upon the experience of the surgeon and anesthetist for procedures such as spinal tumors and acute subdural hematoma and 2–6 RBC units for intracranial aneurysm surgery.
Hence, a retrospective analysis of more than 3,000 consecutive patients undergoing neurosurgical procedures at the University of Saarland was conducted to determine the probability of blood transfusions, depending on the disease and procedure. The aim of the study was to investigate the efficiency of blood usage in our department.
Material and methods
Patient population
This analysis was performed at the Department of Neurosurgery, Saarland University in Homburg/Saar, Germany. Procedures performed in this study were approved by an independent ethics committee.
All consecutive procedures performed in the neurosurgical department between December 2006 and June 2008 were retrospectively analyzed for administration of RBCs intraoperatively or during the next 2 days after operation.
All patients aged 18 years or over who underwent surgical treatment in our neurosurgical department were included in this retrospective analysis. A balanced coagulation (international normalized ratio [INR] less than 1.15) and hemoglobin (more than 9 g/dl) in the preoperative standard laboratory testing at the beginning of the surgical treatment were assumed. Exclusion criteria were: age under 18 years, an INR over 1.15, hemoglobin lower than 9 g/dl or outpatient procedures. Patients with a hemoglobin concentration less than 9 g/dl received RBCs until the hemoglobin concentration reached 9 g/dl. In general, cell saving procedures were not used.
Data acquisition
All ordered RBCs and all finally applied RBCs were documented with the corresponding name of the patient in a central blood-product documentation system. Hence, the number of units of cross-matched and ordered RBCs and number of units applied was available for each individual patient. The corresponding patient data were collected in the electronic chart in the hospital documentation system.
The patients were divided into 11 diagnosis groups for the analysis: traumatic brain injury (TBI), epidural hematoma, acute subdural hematoma, intracerebral hemorrhage (ICH), cerebral ischemia, subarachnoid hemorrhage (SAH)/aneurysms, spinal tumors, degenerative diseases of the spine, intracranial and spinal abscesses, brain tumors, and hydrocephalus. Additionally, the necessity of RBC transfusion in patients subjected to posterior lumbar interbody fusion (PLIF) and in SAH patients with clipped and coiled aneurysms were separately analyzed.
Statistics
The transfusion probability (number of patients transfused with RBCs × 100/number of patients cross-matched and RBCs ordered) was calculated for each diagnosis group separately. The number of units of RBCs cross-matched and ordered for surgery and the number of units finally transfused were characterized as the cross-matched to transfused ratio (C/T ratio) of RBCs. The C/T ratio was determined for each group.
The illustration and analysis of data were performed using Excel (Microsoft Corp., version 2003, Redmond, USA).
Results
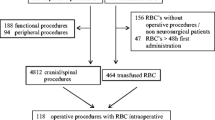
During the analyzed time period, 3,331 consecutive patients were operated on in the neurosurgical department of our hospital. Of these, 305 were outpatients and underwent operation procedures on peripheral nerves or had CT interventions and were excluded from the analysis. Hence, a total of 3,026 patients were included in the analysis.
The overall transfusion probability was 1.7 % (52/3,026). Table 1 gives the transfusion probabilities for the diagnostic groups. The transfusion probabilities were low for all diagnoses/surgical procedures and ranged from 0 % (0/15) for epidural hematoma to 6.5 % (7/108) for acute subdural hematoma. In the subgroup analyses, patients with clipped aneurysms had a transfusion probability of 2.9 % compared with 1.7 % for patients with coiled aneurysms, and patients subjected to PLIF had a probability of 3.6 %.
Analysis of the number of patients with ordered and finally applied RBCs demonstrate that most patients did not require the ordered blood products (see Fig. 1). For example, only one-third of the ordered RBC concentrates were given to patients with TBI. In most cases (ICH, tumors of the spine, insults, and abscess), only a quarter of the ordered blood products was needed and in all other cases much less was required.
The C/T ratios for the various procedures in this study are listed in Table 2 and are an indicator of transfusion efficiency. The number of units or RBCs cross-matched and ordered for surgery was always greater than the number of units actually transfused, as indicated by the high C/T ratios. TBI, spinal tumors and ICH had a very high C/T ratio (≥25) indicating that a large amount of cross-matched and ordered blood was not needed. With degenerative spinal diseases (C/T ratio 8.8) and SAH/aneurysms (C/T ratio 7.5), the over ordering of blood was less pronounced.
Discussion
Per year, 220,000 neurosurgical procedures were performed in Germany [25]. There is only a low level of evidence about the probability of RBC transfusions in neurosurgical procedures [23] although a high probability of transfusions is postulated in many cases. Generally, the number of RBC units ordered before surgery has been based on the physician’s transfusion experiences with neurosurgical patients and also in all other patients.
Given the growing concerns about the safety and effectiveness of blood transfusions, much effort has focused on examining the blood ordering and transfusion probability in various types of surgery, and several reviews have been published [2, 3, 8–10, 14, 18, 23, 26, 31–33].
With the ongoing health care reforms, the costs of transfusion and the adequacy of the blood supply are becoming more relevant in all countries. For example, an ordered unit of RBCs costs 80 EUR at the Saarland University Hospital and a cross-match test costs 10 EUR per case.
In addition, although the blood supply is now safer than ever, there are still risks for transmission of diseases such as HIV and hepatitis (about 1 in 1,000,000) as well as a risk of a fatal hemolytic transfusion reaction (1 in 300,000) or transfusion acquired lung injury (1 in 5,000) [7]. This has led to an increased use of autologous blood transfusion, although this is considerably more expensive than allogeneic blood transfusion.
Therefore, knowledge of the transfusion probability for each surgical procedure is essential for an adequate preparation of the patient before surgery.
In our study, the probability of blood transfusion during neurosurgical procedures was well below 10 %, which is generally defined as the limit for preoperative appropriation of RBCs [1]. In other studies, the incidence of intraoperative transfusion for cerebrovascular procedures such as aneurysm clipping and carotid endarterectomy has also been described as relatively low, with some published reports of intraoperative transfusion rates under 10 % [7]. These reported results correlate well with the demonstrated data.
The highest probability of transfusion exists for patients with spinal tumors, acute subdural hematomas or ICH, i.e., patients needing large decompressive procedures of bone or tissue—often as a case of emergency.
The main finding of this retrospective observational study is that the department of our hospital overestimated the potential loss of blood involved with neurosurgical procedures during the 18 month period to June 2008. This study indicates that the number of RBC units ordered routinely for neurosurgical procedures can be reduced.
Generally, the number of cross-matched or ordered units of RBCs for surgery is always greater than the number of units actually transfused. Several studies have examined the problem of excessive cross-matching [23, 24, 26]. The cross-matched to transfused ratio (C/T ratio) demonstrates the efficiency of blood usage: a high C/T ratio means that the blood bank must keep more blood, which increases hospital costs and also the likelihood of having outdated blood products. An ideal C/T ratio would be 1.0, although a ratio below 2.5 is generally accepted to indicate efficient blood usage. The C/T ratios were high for all neurosurgical procedures conducted in the study period with TBI, intracerebral hemorrhage and cerebral ischemia having the greatest number of unused cross matched blood units.
Problems with the blood supply can occur when products with a short shelf life are prepared and sent to the operating room but are not used. When blood is cross-matched for surgery, it is unavailable for others for 24–48 h, and the chance of the RBCs becoming outdated is increased. A recent study has also shown that relatively older RBCs can potentiate transfusion-related toxicity in trauma patients [34].
Several new strategies that have been shown to effectively reduce the perioperative transfusion of blood products are being implemented [11, 21]. There is no evidence that mild to moderate anemia contributes to perioperative morbidity. The timing and indication of RBC transfusion has changed in the last years.
In neurosurgical—especially in neurovascular—procedures, there is a possibility that blood will be needed urgently. If this is the case, an immediate-spin cross-match can be performed before transfusion to eliminate reactions that may result from human errors in ABO-Rh typing. Blood given in this manner is very safe [5].
Notable questions for the future include the exact role of transfusion-sparing practices such as cell salvage or acute normovolemic hemodilution [6], and whether or not they may be associated with improved neurological outcomes compared with allogeneic transfusion. It cannot be stressed strongly enough, however, that these targets may need to be modified or revised in the context of significant comorbidities such as coronary artery disease or hypoxemia. We must continue to examine these issues to identify which patient groups in the heterogeneous neurosurgical population will benefit from the interventions that we might offer. The role of strategies to address anemia preoperatively prior to elective neurosurgical procedures also remains to be addressed with regard to outcome.
References
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies (2006) Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 105:198-208
Argov S, Shechter Y (1981) Is routine crossmatching for two units of blood necessary in elective surgery? Am J Surg 142:370–371
Barr PJ, Donnelly M, Cardwell C, Alam SS, Morris K, Parker M, Bailie KE (2011) Drivers of transfusion decision making and quality of the evidence in orthopedic surgery: a systematic review of the literature. Transfus Med Rev 25(4):304–316
Beattie WS, Karkouti K, Wijeysundera DN, Tait G (2009) Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 110:574–581
Boyd PR, Sheedy KC, Henry JB (1980) Type and screen. Use and effectiveness in elective surgery. Am J Clin Pathol 73:694–699
Cataldi S, Bruder N, Dufour H, Lefevre P, Grisoli F, Francois G (1997) Intraoperative autologous blood transfusion in intracranial surgery. Neurosurgery 40:765–771, discussion 771-762
Couture DE, Ellegala DB, Dumont AS, Mintz PD, Kassell NF (2002) Blood use in cerebrovascular neurosurgery. Stroke 33:994–997
Diaz MQ, Casado MS, Leal Noval SR, Garcia de Lorenzo YMA (2009) Results of a national survey on transfusion practice in Intensive Care Units. Med Intensiva 33:8–15
Friedman BA (1979) An analysis of surgical blood use in United States hospitals with application to the maximum surgical blood order schedule. Transfusion 19:268–278
Friedman BA (1979) Estimation of blood use. Transfusion 19:788–789
Garcia-Erce JA, Cuenca J, Leal-Noval SR, Munoz M (2007) Preoperative autologous blood donation in Spain (1994-2004). Vox Sang 93:89–90
Goodnough LT (1999) What is a transfusion medicine specialist? Transfusion 39:1031–1033
Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP (1999) Transfusion medicine. First of two parts–blood transfusion. N Engl J Med 340:438–447
Grzeskiewicz JL, Hall RA, Anderson G, Mintz PD (1989) Preoperative crossmatch guidelines for total hip arthroplasty. Orthopedics 12:549–553
Hare GM, Tsui AK, McLaren AT, Ragoonanan TE, Yu J, Mazer CD (2008) Anemia and cerebral outcomes: many questions, fewer answers. Anesth Analg 107:1356–1370
Hendrickson JE, Hillyer CD (2009) Noninfectious serious hazards of transfusion. Anesth Analg 108:759–769
Hopf HW, Viele M, Watson JJ, Feiner J, Weiskopf R, Hunt TK, Noorani M, Yeap H, Ho R, Toy P (2000) Subcutaneous perfusion and oxygen during acute severe isovolemic hemodilution in healthy volunteers. Arch Surg 135:1443–1449
Jenkins AD, Mintz PD (1981) Optimal blood use in genitourinary surgery. J Urol 126:497–499
Leal-Noval SR, Munoz-Gomez M, Murillo-Cabezas F (2008) Optimal hemoglobin concentration in patients with subarachnoid hemorrhage, acute ischemic stroke and traumatic brain injury. Curr Opin Crit Care 14:156–162
Leal-Noval SR, Rincon-Ferrari MD, Marin-Niebla A, Cayuela A, Arellano-Orden V, Marin-Caballos A, Amaya-Villar R, Ferrandiz-Millon C, Murillo-Cabeza F (2006) Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med 32:1733–1740
Liang H, Zhao Y, Wang D, Wang B (2009) Evaluation of the quality of processed blood salvaged during craniotomy. Surg Neurol 71:74–80
McEwen J, Huttunen KH (2009) Transfusion practice in neuroanesthesia. Curr Opin Anaesthesiol 22:566–571
Mintz PD, Lauenstein K, Hume J, Henry JB (1978) Expected hemotherapy in elective surgery. A follow-up. JAMA 239:623–625
Mintz PD, Nordine RB, Henry JB, Webb WR (1976) Expected hemotherapy in elective surgery. N Y State J Med 76:532–537
Reulen H-J, Messing-Jünger M, Steiger H-J, Steudel W-I (2011) Entwicklung der OP-Zahlen in der Neurochirurgie 2005-09: Auswertung nach den DRG-Daten. Sonderausgabe Mitteilungen DGNC Ausgabe April 2011
Sarma DP (1980) Use of blood in elective surgery. JAMA 243:1536–1538
Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH (2009) Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med 35:480–488
Sharma S, Sharma P, Tyler LN (2011) Transfusion of blood and blood products: indications and complications. Am Fam Physician 83:719–724
Tinmouth A, Fergusson D, Yee IC, Hebert PC (2006) Clinical consequences of red cell storage in the critically ill. Transfusion 46:2014–2027
Tinmouth AT (2006) The value of a clinical prediction rule for allogeneic transfusion in cardiac surgery. Transfusion 46:1072–1074
Turgeon AF, Fergusson DA, Doucette S, Khanna MP, Tinmouth A, Aziz A, Hebert PC (2006) Red blood cell transfusion practices amongst Canadian anesthesiologists: a survey. Can J Anaesth 53:344–352
Verlicchi F, Desalvo F, Zanotti G, Morotti L, Tomasini I (2011) Red cell transfusion in orthopaedic surgery: a benchmark study performed combining data from different data sources. Blood Transfus:1-5
Verlicchi F, Facco G, Macri M, Antoncecchi S, Bonomo P (2011) Blood transfusion practice: a nationwide survey in Italy. Blood Transfus 9:430-435
Weinberg JA, Barnum SR, Patel RP (2011) Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion 51:867–873
Weiskopf RB, Feiner J, Hopf HW, Viele MK, Watson JJ, Kramer JH, Ho R, Toy P (2002) Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology 96:871–877
Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, Watson JJ, Hopf HW, Toy P (2000) Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 92:1646–1652
Acknowledgements
The authors thank the stuff of the neurosurgical ICU of the Saarland University/Homburg for the documentation of the ordered blood products in all cases and to Rosemary collier who provided editing support..
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linsler, S., Ketter, R., Eichler, H. et al. Red blood cell transfusion in neurosurgery. Acta Neurochir 154, 1303–1308 (2012). https://doi.org/10.1007/s00701-012-1373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1373-6