Abstract
Purpose
This study aimed at evaluating our hypothesis that tumour cells, which pass through the intraoperative cell salvage (IOCS) machine, lose viability due to possible injury to the cell membrane during centrifugation and filtration, enabling safe reinfusion even without filtration.
Methods
Thirteen patients who underwent metastatic spine tumour surgery (MSTS) at our institution were recruited. Blood samples (5 ml each) were collected at five different stages during surgery, namely, stage A and B: from patients’ vein during induction and at the time of maximum tumour manipulation; stage C, D and E: from the operative blood prior to IOCS processing, after IOCS processing and after IOCS-LDF (leucocyte depletion filter) processing, respectively. The samples were then analysed for viability of tumour cells using microwell-based culture.
Results
The median age of the patients was 65 years (range 37–77 years). The most common primary tumour was lung, followed by breast, hepatocellular and renal cell carcinoma. The median blood loss was 680 ml (range 300–1500 ml). Analysis of cultured blood samples showed that CTC-containing clusters were developed from some samples before IOCS-LDF processing (stage A: three patients, stage B: three patients and stage C: one patient). None of the samples from stages D and E generated clusters after culture, suggesting the absence of viable cancer cells after IOCS processing.
Conclusions
The salvaged blood may contain some tumour cells after processing with IOCS machine, but these cells are damaged and hence unable to replicate and unlikely to metastasise. The results of this study support the hypothesis that salvaged blood in MSTS is safe for transfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal column is the third most frequently involved organ by metastases, following the lung and liver [1]. Metastatic involvement of the spinal column forms stage IV disease in all cancer patients. Metastasis to the spinal column results from cancer cells which extravasate into the peripheral bloodstream from the primary cancer site in these patients. These cancer cells, termed as circulating tumour cells (CTCs), can be found in all patients with advanced metastatic disease.
Alleviation of pain, decompression of the neural elements and mechanical stabilisation of the spine in conjunction with preservation of quality of life are the objectives of surgical intervention for metastatic spine disease (MSD). Surgical interventions in MSD, especially when considering anterior corpectomy, are fraught with significant intraoperative blood loss. Blood loss can be as high as 2180 ml [2] or higher, up to 5 L which is nearly equivalent to one circulating blood volume of an average young adult [3]. Currently, allogeneic blood transfusion (ABT) remains the gold standard for blood replenishment. However, it still has associated risks of infection transmission, immunosuppression and transfusion reactions despite better improvement in screening of allogeneic blood [4, 5].
Intraoperative cell salvage (IOCS) is a novel alternative for addressing the above concerns related to ABT [6, 7]. However, it has not been widely adopted in oncological surgery due to the hypothetical concern of reinfusing cancer cells. Despite the emerging evidence of the safety of IOCS, especially in combination with leucocyte depletion filter (LDF) [6, 8–16], most surgeons are still doubtful about using IOCS during oncological surgery.
We previously presented studies on the feasibility of IOCS-LDF in removing cancer cells from blood salvaged during metastatic spine tumour surgery (MSTS) [7, 14]. Qualitative and quantitative analyses were carried out using immunohistochemistry cell block [6, 7] and flow cytometry [14] techniques, respectively, to detect cancer cells present in the samples taken from different stages during operation. In the qualitative study using cell-block technique [6, 7], we were unable to detect any viable cancer cells in the salvaged blood after filtration with both IOCS and LDF. We then validated our findings with flow cytometry technique with which we could quantitatively analyse cancer cells in the filtered salvaged blood. In that particular study [14], we found that the remnant cancer cells in the filtered salvaged blood or salvaged blood were significantly less than the circulating tumour cell load in each patient.
In this study, we aimed to further evaluate our hypothesis that cancer cells, which pass through the surgical suction system and processed with the cell saver system, were either removed or if left behind, would lose viability due to possible injury to the cell membrane during centrifugation and filtration. Hence, any residual cancer cells in the salvaged blood may not be viable, enabling safe reinfusion even without filtration using LDF. We, therefore, conducted a study using a microwell-based culture method for the expansion of primary CTCs to evaluate our hypothesis and validate the safety of IOCS processed blood in MSTS.
Methods
Study design and study population
This prospective observational study included patients undergoing surgery for MSD from known primary tumours operated between August 2014 and July 2015. Ethics approval for the study was sought from Institutional Review Board prior to commencement of the study. Patients who presented with spinal metastases from known epithelial primary tumours (lung, breast, prostate, renal, colorectal, hepatocellular, cervix, etc.), and requiring spinal surgery for management of MSD, were recruited. They had to be physically fit for surgery and above 18 years of age to be able to provide informed consent for surgery. The exclusion criteria were patients with spinal metastasis from unknown primary, patients medically unfit or lacking mental capacity to provide informed consent for surgery and pregnant patients. Detailed informed consent was obtained from recruited patients who were explained that salvaged blood would not be transfused back to them.
Sample collection
During surgery, IOCS devices (OrthoPAT® Orthopedic Perioperative Autotransfusion System, Haemonetics, USA or Dideco, Sorin Group, Italy) were used to collect blood lost from the operative field. The anticoagulant used was heparin 30000 units diluted in 1 L of normal saline.
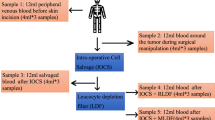
To optimise the safety of salvaged blood, Pall RS leucocyte depletion filter (RS1VAE, Pall Corporation, Portsmouth, UK) was used as a final filtration after the blood had been processed by IOCS. Blood samples were taken at five stages (Fig. 1): (1) Stage A: venous blood from the patient during induction by anaesthetist; (2) Stage B: venous blood from the patient during maximum tumour manipulation by the surgeons; (3) Stage C: blood from the operative field at the time of maximum tumour manipulation and prior to IOCS cell saver processing; (4) Stage D: salvaged blood after being processed by the cell saver prior to LDF filtration; and (5) Stage E: salvaged blood after being processed by both IOCS-LDF.
At each stage, 5 ml samples were drawn and sent to the lab for microwell-based culture. All blood samples were stored in sterile Ethylenediaminetetraacetic acid (EDTA)-coated vacutainer tubes (Becton–Dickinson, NJ, USA) and kept at 4° till processing.
Evaluation of cancer cells viability
To evaluate the presence of viable and proliferative cancer cells in salvaged blood, we utilised a microwell-based culture assay previously established for the expansion of human clinical CTCs in blood [17]. CTCs are primary cancer cells present in the blood of cancer patients and were shed from either primary or secondary tumours [18].
In addition to determining the presence of CTC-containing clusters after culture, the proportion of cytokeratin positive (CK+)/CD45 negative (CD45−) cancer cells were also evaluated. CK is an epithelial marker to identify cancer cells originating from epithelial tumours; CD45 is a leucocyte marker and Hoechst dye will enable the labelling of cell nuclei. The current standard of CTC definition is ‘pan-CK+/CD45−/Hoechst+ with a high nuclear/cytoplasmic (N/C) ratio’ [19]. High N/C ratio is a phenotype associated with malignancy. The proportion of CK+ cells serve as an estimate for the amount of cancer cells after culture. Recent reports confirmed the presence of mesenchymal-like CTCs, which demonstrated reduced expression of CK, can also be detected [20].
Preparation of microwell-based culture assay for primary CTCs
A dense array of microwells was generated using 60 mm plastic non-coated petri dishes (Becton–Dickinson, Franklin Lakes, NJ), which were patterned with a laser engraving machine (VLS-2.30, Universal Laser System Inc., Scottsdale, AZ). Details of the fabrication and microwell dimensions were reported in a previous paper [17].
Culture preparation and conditions
To ensure viability of the cells, blood samples were processed within 10 h after collection. Blood samples were mixed gently with red blood cell (RBC) lysis buffer (Life Technologies, Carlsbad, CA) for 5 min, followed by decanting of the supernatant. The resultant cell pellet consisted of nucleated cells and CTCs. These were washed once with sterile phosphate-buffered saline (PBS) before final re-suspension in fresh high-glucose Dulbecco’s modified Eagle’s medium (DMEM). The media was supplemented with 10 % foetal bovine serum (FBS) and 1 % penicillin–streptomycin (Invitrogen, Carlsbad, CA).
Patterned dishes containing the samples were stored in humidified incubators maintained at 37 °C in 5 % (v/v) CO2 and 1 % O2. Resultant clusters were dissociated with manual pipetting aided by trypsinisation with 0.01 % trypsin and 5.3 mM EDTA (Lonza, Basel, Switzerland) solution in PBS for 3 min at 37 °C. Dishes were imaged using phase contrast microscopy after 2 weeks to determine positivity of culture (Fig. 2). Images were processed with image processing software (ImageJ, NIH, Bethesda, MD).
Immunostaining of cultured cells (Fig. 3)
Cultured cells were harvested and fixed with fresh 4 % paraformaldehyde (PFA) (Sigma-Aldrich, St Louis, MO). Fixed cells were incubated with conjugated antibodies, namely pan-cytokeratin (CK)-Fluorescein isothiocyanate (FITC), CD45-Allophycocyanin (APC), both 1:100 (Miltenyi Biotec Asia Pacific, Singapore) and Hoechst 33342 dye (Sigma-Aldrich). The above three antibodies were mixed as “antibody cocktail” and prepared in PBS with 5 % bovine serum albumin (BSA).
Statistical analysis
Statistical analysis was performed using STATA statistical software (version 12, StataCorp, College Station, Texas). The primary outcome was the formation of tumour clusters in the samples from various stages. In order to obtain a meaningful comparison in the analysis, we collapsed stage A and B together and called it “baseline CTC group”. This group would include the minimum number of patients detected with circulating tumour cells in our study cohort. Using two-sample proportion test, the proportion of patients with tumour clusters formed in the samples in baseline CTC group was compared to that in stage D as well as stage E to assess the difference in the viability of any possible tumour cells in these samples. P value <0.05 was considered to be statistically significant.
Results
Patient cohort
We recruited 13 patients, of which 12 were included in final analysis. One patient was excluded because her blood samples could not be processed within 10 h after collection due to a delay in delivery. Table 1 shows demographic characteristics, details of primary and secondary tumours and results of microwell-based culture for each recruited patient. There were 7 females and 5 males. The median age of the patients was 65 years (range 37–77 years). The most common primary tumour in this cohort was lung, followed by breast, hepatocellular and renal cell carcinoma. The median blood loss was 680 ml (range 300–1500 ml). Ten patients underwent posterior instrumentation and decompression while two underwent anterior corpectomy. Haemonectics cell saver was used in ten patients and Dideco was used in two patients.
Absence of viable CTCs after cell saver processing
Blood samples obtained were processed in the laboratory using the methods mentioned above. Analysis of cultured blood samples showed that CTC-containing clusters were developed from some samples before IOCS-LDF processing (stage A: three patients, stage B: three patients and stage C: one patient) (Fig. 2). None of the samples from stages D and E generated clusters after culture, suggesting the absence of viable cancer cells after IOCS or IOCS-LDF processing (Fig. 2). The percentage of pan-CK+/CD45-/Hoechst+ cells in the samples that formed clusters is also presented in Table 1.
Proportion test revealed that there was a significant difference between the proportion of patients with tumour clusters in their samples in combined stage A and B (i.e., baseline CTC group) as against either stage D or stage E (33 vs 0 %, P = 0.02).
Discussion
Success of surgical intervention in MSD is supported by evidence of adequate decompression and appropriate stabilisation. Longevity of decompression can be marred by local recurrence at the site of decompression. Patient’s longevity, however, can also be affected by the appearance of newer secondaries and distant metastases. Appearance of newer metastasis is secondary to spread from primary tumour or spread from residual spinal metastases post-operation or from other metastases in any organ of the body (Fig. 3).
Transfusion of salvaged blood has been implicated to be responsible for the appearance of newer metastases by reinfusing tumour cells and disseminating them. In our previous study [14], using flow cytometry, we found that the detectable count of tumour cells in the filtered salvaged blood samples or even salvaged blood samples was significantly less than the number of CTCs in the samples from corresponding patient’s circulation. Hence, it was concluded that for a particular patient, the number of CTCs in the salvaged blood was always significantly lower than the CTC load in the patient’s circulation at any time point. The safety of transfusion of salvaged blood was supported by this crucial fact.
Despite demonstrating a reduced tumour cell count in the salvaged blood, some skeptical surgeons remain concerned about the presence of even a single cancer cell in the salvaged blood which may be capable of seeding, replicating and presenting as newer secondary metastases. Concerns about the presence of trace counts of CTCs may be due to several literature which suggested that the presence of one or more CTCs per 7.5 ml of blood is an independent predictor of relapse and death in chemonaïve patients with non-metastatic cancer [21]. Other studies also identified that both progression-free and overall survival were worse in patients with one or more CTCs [22, 23]. Lucci et al. suggested that assessment of CTCs might provide important prognostic information in these patients [21].
Our present study provided the evidence which showed the absence of significant counts of viable CTCs in the samples taken from salvaged blood as well as salvaged and filtered blood (i.e., stage D and E, respectively). Several samples obtained from patients’ circulation at two different time points (baseline CTC group) yielded positive cultures in 4 out of 12 patients (33 %). The statistical analysis also revealed that the difference between the proportion of patients with formation of tumour clusters in their samples in the baseline CTC group was significantly different from that in either stage D or E (the samples from salvaged blood or salvaged and filtered blood). These findings proved our hypothesis to be correct. We envisaged that the cancer cells, which leave the surgical field to be processed by the IOCS machine, can get physically damaged and would lose viability due to possible injury to the cell membrane during centrifugation and filtration. Hence, we can conclude that any residual cancer cells, which are present in the salvaged blood, may be non-viable, thereby incapable of seeding and replicating. This will support safe reinfusion of salvaged blood even without filtration using LDF.
Our findings were in line with those from a previous study on gynae-oncology conducted by Catling et al. In that study, the authors noted that only fragmented cytoplasmic debris from labelled cells was detectable in the reservoir, pre-filter and post-filter samples, indicating destruction of the labelled cells during the cell salvage process. This also matched with our observation from previous cell block study [7] where the majority of cell blocks generated from salvaged and filtered blood had cytoplasmic debris with no viable nuclei.
It is well known that the metastatic process is complex, requiring not only adequate number of cells but also viability of CTCs in a good milieu. The pathogenesis of metastasis involves a series of steps dependent on both intrinsic properties of tumour cells and host response [24]. It also requires congenial organs supporting the suggested philosophy of “dependence of the seed upon the soil” [25]. For cancer cells to metastasise, they must successfully complete the sequential steps to give rise to a metastatic tumour. These steps comprise dissemination of cancer cells in the blood stream, survival in the circulation, extravasation into the metastatic site, and the expression of the appropriate cell surface receptors to form clinically detectable metastases [26–30]. As indicated by the results of our present study, it is unlikely that the tumour cells that have passed through the cell saver will be capable of completing these steps since they would have encountered several physical barriers during processing which affects their viability. These non-viable cells may not have appropriate morphological features to effectually form metastatic disease. In addition, they would not be in sufficient numbers to result in effective metastasis. Karczewski and colleagues [31] indicated that after processing with IOCS device, 62 % of tumour cells in the blood suffered lethal trauma and the remaining tumour cells had morphological changes. The above may explain why the patients who received salvaged blood had no significant difference in distant metastatic rate as compared to those who received other forms of blood transfusion in previous clinical studies [8, 10–12, 32–35] where the safety of salvaged blood was evaluated in actual clinical application.
In our study, microwell-based culture assay was used to evaluate the presence of viable and proliferative cancer cells in salvaged blood. In a previous study [17], it was demonstrated that the microwell-based assay could establish positive cultures in >60 % of all samples (advanced metastatic to early stage; n = 226) taken at various time points in treatment and the ability of CTC-containing cluster formation was reduced in blood samples from patients under a longer period of systemic therapy (pre-treatment: 39/44 (88.6 %); >5 weeks post-treatment: 26/61 (42.6 %), P < 0.001). Hence, CTC cluster formation can be affected by the presence and duration of systemic therapy, and its persistence may reflect therapeutic resistance.
Our study is the first prospective observational study looking at viability of cancer cells in salvaged blood using an innovative microwell-based culture technique despite having a small sample size. Presently, there is no prospective randomised trial on evaluating the reinfusion of salvaged blood in patients undergoing MSTS. There has been a randomised controlled trial in scoliosis surgery where the authors found that the use of salvaged blood significantly reduced the need for allogeneic blood in spine deformity surgery [36]. Our study strongly suggests that a prospective clinical reinfusion study will provide more warranted results, which might lead to newer approaches in blood management during MSTS and further in musculoskeletal oncological surgeries.
Conclusions
Our study showed that none of the samples from salvaged blood as well as salvaged and filtered blood generated clusters after culture, suggesting the absence of viable cancer cells after IOCS or IOCS-LDF processing. This supports the hypothesis that these cells have lost their metastatic capability making allowance for safety of salvaged blood transfusion in metastatic spine tumour surgery.
References
Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL (2006) Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2:87–94. doi:10.1038/ncpneuro0116 (quiz 116)
Chen Y, Tai BC, Nayak D, Kumar N, Lim JW, Goy RWL, Wong HK (2013) Blood loss in spinal tumour surgery and surgery for metastatic spinal disease: a meta-analysis. Bone Joint J 95-B:683–688
Kawai A, Kadota H, Yamaguchi U, Morimoto Y, Ozaki T, Beppu Y (2005) Blood loss and transfusion associated with musculoskeletal tumor surgery. J Surg Oncol 92:52–58. doi:10.1002/jso.20375
Blajchman MA, Bordin JO (1995) The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest 24:311–317
Blumberg N (1997) Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol 34:34–40
Kumar N, Ahmed Q, Lee VK, Chen Y, Zaw AS, Goy R, Agrawal RV, Dhewar AN, Wong HK (2014) Can there be a place for intraoperative salvaged blood in spine tumor surgery? Ann Surg Oncol 21:2436–2443. doi:10.1245/s10434-014-3569-x
Kumar N, Ahmed Q, Lee VK, Zaw AS, Goy R, Wong HK (2015) Are we ready for the use of intraoperative salvaged blood in metastatic spine tumour surgery? Eur Spine J. doi:10.1007/s00586-015-4112-x
Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC (2011) Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol 18:166–173. doi:10.1245/s10434-010-1228-4
Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L (2008) Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia 63:1332–1338. doi:10.1111/j.1365-2044.2008.05637.x (ANA5637 [pii])
Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE (1995) Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol 86:373–378. doi:10.1016/0029-7844(95)00183-R
Ford BS, Sharma S, Rezaishiraz H, Huben RS, Mohler JL (2008) Effect of perioperative blood transfusion on prostate cancer recurrence. Urol Oncol 26:364–367. doi:10.1016/j.urolonc.2007.06.004
Kim JM, Kim GS, Joh JW, Suh KS, Park JB, Ko JS, Kwon CH, Yi NJ, Gwak MS, Lee KW, Kim SJ, Lee SK (2012) Long-term results for living donor liver transplant recipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl Int 26:84–89. doi:10.1111/tri.12001
Kumar N, Chen Y, Zaw AS, Nayak D, Ahmed Q, Soong R, Wong HK (2014) Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol 15:e33–e41. doi:10.1016/S1470-2045(13)70245-6
Kumar N, Lam R, Zaw AS, Malhotra R, Tan J, Tan G, Setiobudi T (2014) Flow cytometric evaluation of the safety of intraoperative salvaged blood filtered with leucocyte depletion filter in spine tumour surgery. Ann Surg Oncol 21:4330–4335. doi:10.1245/s10434-014-3950-9
Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM (2005) Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol 12:1017–1024. doi:10.1245/ASO.2005.12.018
Perseghin P, Vigano M, Rocco G, Della Pona C, Buscemi A, Rizzi A (1997) Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang 72:221–224
Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SG, Nandi S, Lim CT, Thiery JP (2015) Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget 6:15578–15593
Alix-Panabieres C, Pantel K (2013) Circulating tumor cells: liquid biopsy of cancer. Clin Chem 59:110–118. doi:10.1373/clinchem.2012.194258
Marrinucci D, Bethel K, Lazar D, Fisher J, Huynh E, Clark P, Bruce R, Nieva J, Kuhn P (2010) Cytomorphology of circulating colorectal tumor cells: a small case series. J Oncol 2010:861341. doi:10.1155/2010/861341
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339:580–584. doi:10.1126/science.1228522
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13:688–695. doi:10.1016/S1470-2045(12)70209-7
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, Marty M, Pierga JY (2010) Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 21:729–733. doi:10.1093/annonc/mdp391
Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M (2008) Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res: Off J Am Assoc Cancer Res 14:7004–7010. doi:10.1158/1078-0432.CCR-08-0030
Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573
Allan AL, Keeney M (2010) Circulating tumor cell analysis: technical and statistical considerations for application to the clinic. J Oncol 2010:426218. doi:10.1155/2010/426218
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572. doi:10.1038/nrc865
Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC (2001) Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin North Am 10:243–255 (vii)
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4:448–456. doi:10.1038/nrc1370
Woodhouse EC, Chuaqui RF, Liotta LA (1997) General mechanisms of metastasis. Cancer 80:1529–1537
Karczewski DM, Lema MJ, Glaves D (1994) The efficiency of an autotransfusion system for tumor cell removal from blood salvaged during cancer surgery. Anesth Analg 78:1131–1135
Gorin MA, Eldefrawy A, Manoharan M, Soloway MS (2012) Oncologic outcomes following radical prostatectomy with intraoperative cell salvage. World J Urol 30:379–383. doi:10.1007/s00345-011-0746-4
Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, Lavayssiere L, Rostaing L, Fourtanier G (2005) Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int 18:1236–1239. doi:10.1111/j.1432-2277.2005.00207.x
Nieder AM, Manoharan M, Yang Y, Soloway MS (2007) Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology 69:881–884. doi:10.1016/j.urology.2007.01.060
Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Choi WH (2012) Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J Korean Surg Soc 82:356–364. doi:10.4174/jkss.2012.82.6.356
Liang J, Shen J, Chua S, Fan Y, Zhai J, Feng B, Cai S, Li Z, Xue X (2015) Does intraoperative cell salvage system effectively decrease the need for allogeneic transfusions in scoliotic patients undergoing posterior spinal fusion? A prospective randomized study. Eur Spine J 24:270–275. doi:10.1007/s00586-014-3282-2
Acknowledgments
The authors thank AOSpine for granting AOSpine East Asia Research Award 2015 [AOSEA(R)2015-03]. 2. National University Health System for granting Bridging grant 2014 (NUHSRO/2014/013/Bridging/09) for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kumar, N., Zaw, A.S., Khoo, B.L. et al. Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells. Eur Spine J 25, 4008–4015 (2016). https://doi.org/10.1007/s00586-016-4478-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4478-4