Abstract
Currently, microbiological proteases, along with lipases, are the most significant enzymes for biotechnology. They are produced in great quantity and since they are qualitatively diversified, they can be successfully applied in various branches of industry, including medicine. For several decades, the number as well as the significance of studies on extremophilic proteases have been growing. Extremophilic proteases are isolated from extremophiles which, given their unique kinetic and structural adaptations, can be used at low and high temperatures and in extreme environments (alkaline, acidic, saline). These enzymes have already enriched the range of commercial proteases and the studies on their properties in relation to their structural features stimulated a rational engineering of conventional proteases, aimed at enhancing their ability to adapt to specific conditions. In the chapter below, we characterized selected representatives of this most significant, in terms of economy, group of extremophilic proteases and discussed possible directions for their application in biotechnology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction: General Characteristics and Classification of Proteases
Proteases (proteolytic enzymes, peptidases) were the first enzymes to be introduced into industrial practice a few decades ago. At first, they found application in the laundry detergent industry (Rao et al. 1998), which has remained the biggest recipient of protease preparations till this day (Kumar et al. 2008; Khan 2013). Currently, proteolytic enzymes, especially those of microbiological origin, are used on a wide scale in food, feed, textile, leather and pharmaceutical industries. They are also more and more widely applied in medical therapy and cosmetology. In terms of production, proteases represent the heart of the global market for enzymes which has been growing at a high rate for several decades now (Sarethy et al. 2011).
Proteases occur in various variants in all living organisms, from viruses and bacteria to a human being. The genes encoding proteases represent approx. 2 % of the total human genome (Li et al. 2013). They are involved not only in the process of dietary protein digestion and the cellular protein turnover, but also in numerous metabolic and regulatory processes that are significant for the proper functioning of an organism. Proteases are among the best-characterized group of enzymes and the studies devoted to them have handsomely contributed to the current state of knowledge on the relations between the structure and function of proteins.
In a formal sense, proteases were classified by the Nomenclature Committee of IUBMB (International Union of Biochemistry and Molecular Biology) as hydrolases (EC 3) that act on peptide bonds (subclass 4, EC 3.4), otherwise known as peptidases (Enzyme Nomenclature, http://www.chem.qmul.c.uk/iubmb/enzyme). The classification of this group of enzymes kept changing until the second half of the 1950s, when the first list of enzymes was officially presented. New proteins that hydrolyze peptide bonds were being described and molecular studies on highly diversified mechanisms, used by the enzymes in the process of catalysis, were being undertaken. Currently, the EC 3.4 class consists of 15 subclasses which include exo- (amino-, carboxy- and dipeptidases) and endopeptidases (aspartic, cysteine, serine, threonine, metallopeptidases and proteases of unknown catalytic mechanism; EC 3.4.21-25 and EC 3.4.99). At the same time, owing to the studies on the primary and secondary structure of proteolytic enzymes, we expand our knowledge on the evolutionary relatedness between these proteins, on the basis of which they were divided into aspartic, cysteine, glutamyl, serine, threonine proteases, metallopeptidases and proteases of mixed and unknown catalytic mechanism, including, along with hydrolases, asparagine peptide lyases. Each group is further divided into families, which include enzymes that display a great sequence homology, and clans, which include families with a similar 3D structure or the same catalytic amino acid sequence in a molecule. Currently, the MEROPS database (http://merops.sanger.ac.uk), which groups peptidases into families and clans and provides descriptions of thousands of individual peptidases, itemizes 353 families, 106 subfamilies and 49 clans of proteolytic enzymes, which shows how structurally diversified these proteins are. Therefore, despite the fact that they all hydrolyze peptide bonds, they can participate in various metabolic processes.
Our knowledge of proteolytic enzymes has considerably expanded owing to the studies on proteases derived from extremophilic microorganisms and, to a lesser extent, from extreme and moderate metagenomes, where genes of proteases with extremophilic properties might be present. In the following chapter we would like to present the current state of the research conducted in this field, including the actual use of extremophilic proteases in economy and their possible application in biotechnology.
2 Sources, Properties and Structural Adaptations of Extremophilic Proteases
2.1 Thermophilic Proteases
Thermophilic microorganisms constitute the greatest source of proteases, adapted to thrive at high temperatures. They can be classified into facultative thermophiles, which grow at temperatures of both 60–65 °C and 37 °C; obligate thermophiles, which grow at high temperatures of 65–70 °C, but do not grow at a temperature below 40 °C; extreme thermophiles, which thrive at temperatures of 40–70 °C and whose optimum growth temperature is 65 °C; and finally, hyperthermophiles, which require a temperature of 80–115 °C to grow (Kikani et al. 2010). Thermophilic microorganisms which produce proteolytic enzymes can be found in various biotopes, such as tropical soils (De Azeredo et al. 2004; Jaouadi et al. 2010a), marine geothermal springs (Klingeberg et al. 1995), including hydrothermal vents located 2500 m below the sea level at a pressure exceeding 250 atm., geysers (Matsuzawa et al. 1988; van den Burg et al. 1991), geothermal sediments, geothermal hot streams (Jang et al. 2002), solfataras (Morikawa et al. 1994; Chavez Croocker et al. 1999), volcanoes as well as fermenting compost (Hasbay Ifrij and Ogel 2002), boiling outflows of geothermal power plants, hot water pipeline systems (Dib et al. 1998), thermophilic digester fed with tannery waste and cattle manure (Majeed et al. 2013) and many other environments. Proteases adapted to high temperatures are isolated from hyperthermophilic and thermophilic archaea, bacteria and filamentous fungi. They are stable and active at temperatures above 60–70 °C or, in some cases, even above 100 °C (Sako et al. 1997; Morikawa et al. 1994). In comparison to their mesophilic counterparts they are more resistant to organic solvents, detergents (Jaouadi et al. 2010a; Lagzian and Asoodeh 2012), extreme pH values and other denaturing factors (Synowiecki 2010). They are used under high temperatures due to, for instance, a low solubility of substrates at a moderate temperature or endoergic character of the reaction catalyzed by enzymes. Higher temperature enhances the solubility of substrates as well as accelerates the diffusion thanks to a lower viscosity of the environment. It also decreases the possibility of contamination caused by meso- and psychrophilic microflora, which helps control the sterility of the process.
Increased thermoactivity and thermostability are determined by numerous structural adaptations. Even minor changes in the amino acid sequence can boost high temperature resistance. The difference in only three amino acid positions can cause that a neutral protease from Bacillus caldolyticus maintains 50 % of its activity after 30 min of incubation at a 8.2 °C higher temperature than Nrp protease from Bacillus stearothermophilus in a parallel experiment (van den Burg et al. 1991). Stability at higher temperatures depends on the level of molecular packing. A rise in temperature causes the structure to loose and increases the molecular flexibility, which can eventually lead to the loss of native properties as a result of irreversible conformational changes. Thermophilic proteases are characterized by a more closed-packed and rigid structure than their mesophilic and psychrophilic homologs, which helps them maintain their catalytic properties despite a high temperature. The molecular rigidity is influenced by additional ionic bonds, disulfide bridges, hydrogen bonds and hydrophobic interactions.
Furthermore, thermophilic enzymes that bind metal ions , have fewer empty inter-spaces as well as hydrophile regions on the surface and possess shorter loops and a lower number of residues, prone to deamination and oxidization (Li et al. 2005). In the case of most metals, especially Ca2+, but also Mg2+, Mn2+, Zn2+ or Sr2+, which bind either specifically or non-specifically with the molecule of an enzyme, the ionic bonding is essential to stabilize the structure and prevent unfolding at a higher temperature (De Azeredo et al. 2004; Li et al. 2007).
Electrostatic interactions in the molecules of thermophilic proteases are not only more numerous, but also more stable. In comparison with meso- and psychrophilic subtilisins, thermitase, a serine protease from Thermoactinomyces vulgaris (Kleine 1982), which displays the highest activity at a temperature of 85–90 °C and the highest stability at 70 °C, has a very complex and stable network of salt bridges, often formed by arginine residues (Tiberti and Papaleo 2011). If a salt bridge is removed from Thermus aquaticus YT-1 aqualysin I after an appropriate mutation, the thermostability of this protein is significantly reduced—there is a decrease in its half-life from 120 to 40 min at 80 °C (Matsuzawa et al. 1988).
Disulfide bridges are also significant when it comes to the stability and catalytic activity of aqualysin I at high temperatures. Their degradation through suitable mutations leads to a sharp decrease in thermostability. Moreover, it has been proved that the presence of proline residues in the surface loops of the protein contributes to its hyperstability (Sakaguchi et al. 2008). All these modifications ensure increased activity at high temperatures, but significantly reduce the activity at lower temperatures, which facilitates the inactivation of thermophilic proteases through cooling after the process is completed, so expensive inhibitors do not have to be used.
So far, it was possible to isolate and describe numerous thermophilic proteases (Table 14.1), active within the pH range of 2.0–12.0, whose optimum temperature of activity ranges from 45 to 115 °C. Those produced by Archaea proved to be the most thermophilic. They include serine proteases, cysteine proteases, metalloproteases and aspartic proteases. Strains of hyperthermophilic archaeon Pyrococcus sp. produce serine proteases with optimum temperature of activity above 100 °C. The S66 enzyme, isolated from Pyrococcus furiosus, shows maximum activity at 105 °C and pH 7.0. Its measured half-life at 98 °C is 33 h (Blumentals et al. 1990). Pyrolysin has an even higher optimum temperature of activity (115 °C), but it is less thermostable (t1/2 only 9 h at 95 °C) than S66 (Eggen et al. 1990; Voorhorst et al. 1996). Serine proteases produced by Aeropyrum pernix K1 are equally active and thermostable. One of them, pernisine with an optimum temperature of 90 °C and optimum pH of 8.0–9.0 is the most thermostable serine protease of all the proteases described so far. It remains active for 4 h at 90 °C and its t1/2 is 30 min at 120 °C. The presence of 1 mM Ca2+ further increases its thermostability to 4 h at 120 °C, without loss of its activity (Catara et al. 2003). Sulfolobus solfataricus produces SsMTP, a protease which shows optimum activity at 70 °C and pH 2.0 and is characterized by high thermostability (its half-life is 20 h at 80 °C).
Thermophilic bacterial proteases constitute an even more numerous group. So far, more than 40 proteases, active at temperatures between 50 and 95 °C, have been isolated and described. The highest optimum temperature of activity, 95 °C, has been reported in the case of a subtilisin-like protease from Bacillus sp. MLA64 (Lagzian and Asoodeh 2012). In comparison with other bacterial proteases, the subtilisin-like protease proves extremely thermostable as its measured half-life is 150 and 10 min at 100 °C and 120 °C, respectively. It remains active in the presence of Tween 80, SDS and Triton X-100, similarly to an alkaline serine protease from Coprothermobacter proteolyticus, which is additionally resistant to H2O2 and whose activity is further enhanced by SDS (Majeed et al. 2013). Another alkaline protease from C. proteolyticus, proteolysin, whose optimum temperature of protein hydrolysis is 85 °C at pH 9.5, tolerates the presence of organic solvents far better than commercially available subtilisin A (Toplak et al. 2013). A serine protease from B. laterosporus AK1 is another enzyme which remains stable in the presence of detergents. It reaches optimum activity at 75 °C and pH 9.0 and retains from 75 to 38 % of its activity after 1 h of incubation with commercial detergents. It also remains stable at 80 °C, in the pH range of 7.0–11.0, which indicates its possible use as an ingredient of the new generation detergents (Arulmani et al. 2007). Some bacterial thermophilic proteases show high resistance to denaturing substances like urea and guanidine hydrochloride. Aqualysin I from T. aquaticus YT-1, with optimum pH of 10.0 at 80 °C, remains active in the presence of 7 M urea, 6 M guanidine hydrochloride and 1 % SDS (Matsuzawa et al. 1988), similarly to caldolysin from T. aquaticus T-351 (Cowan and Daniel 1982).
Proteases produced by thermophilic fungi and actinomycetes are not so numerous. In case of fungal enzymes , they include PRO33 and PRO66 from Chaetomium thermophilum (Li et al. 2007), thermomycolase from Malbranchea pulchella var. sulfurea (Ong and Gaucher 1976) and protease I from Thermoascus aurantiacus var. levisporus (Marcy et al. 1984). Among actinomycetes , we can differentiate between KERAB from Streptomyces sp. AB1 (Jaouadi et al. 2010a), keratinase from Thermoactinomyces candidus (Ignatova et al. 1999) and TfrA from Thermomonospora fusca YX (Kim and Lei 2005). Their optimum temperature of catalysis is lower than in the case of enzymes from archaea and bacteria, ranging from 45 to 70 °C. A keratinolytic alkaline proteinase from Chrysosporium keratinophilum, which is optimally active at 90 °C and pH 9.0, is one of the two exceptions (Dozie et al. 1994). The second one is a serine protease, TfpA from T. fusca YX, which is optimally active at 80 °C and pH 8.5. It remains stable under reaction conditions and its half-life at 85 °C is 15 min (Kim and Lei 2005).
2.2 Psychrophilic Proteases
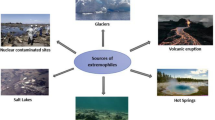
Cold-adapted proteases are produced by psychrophilic and psychrotolerant microorganisms. Both these groups are capable of growth at 0 °C. In case of true psychrophiles, the optimum temperature of growth does not exceed 15 °C, whereas the maximum temperature is 20 °C. For psychrotolerant microorganisms, optimum growth temperatures are higher and range approx. from 20 °C to 35 °C (Morita 1975). Both of these groups inhabit permanently cold areas and environments with periodic temperature drops. They can be found in sea waters, arctic soils, glaciers, alpine soils and permafrost. They constitute a microflora of plants and animals living in cold areas as well as contaminations detected in cooled and frozen food.
One of the most important adaptations of cold-loving microorganisms, enabling them to sustain in the cold environment, is the production of enzymes that are kinetically and structurally adapted to thrive at low temperatures. Kinetic adaptations involve a relatively low optimum temperature of activity, which is usually 20–30 °C lower than their mesophilic counterparts and is in the range of 30–40 °C. Next characteristic features of this group of enzymes are higher values of catalytic constant kcat and catalytic efficiency kcat/Km for a temperature range of 0–30 °C as well as low thermostability. The enzymes synthesized by psychrophiles exhibit much greater flexibility of the structure in comparison with their mesophilic and thermophilic counterparts, caused by weaker intramolecular interactions, especially in the active site, and the presence of surface loops that interact with the polar environment surrounding the enzyme. These proteins contain fewer proline residues, which increase the rigidity of a native conformation, and more glycine residues whose presence, especially in clusters, has a favorable influence on flexibility. A decrease in the concentration of basic amino acid residues, like arginine and lysine, may be observed. At appropriate pH of environment, these amino acids can form ionic bonds that add rigidity to protein molecules. An important feature of the structure is a decreased number of hydrophobic interactions, thanks to which the core of a molecule occupies less space than in the case of homologous mesophilic proteins. When it comes to cold-active enzymes, there is an increase in the number of hydrophilic interactions between a molecule and a solvent owing to the presence of an increased number of polar amino acid residues on the surface. Furthermore, in such a case, there is usually a better access to the active site, which offsets the decreased diffusivity of substrates (Feller 2003, 2013).
Among cold-active proteases-producing bacteria that have been described so far, the most dominant bacterial groups are Pseudomonas, Pseudoalteromonas, Bacillus, Clostridium, Colwellia, Serratia, Shewanella, Sulfitobacter, Halomonas and Marinomonas. Much less attention has been devoted to proteases from psychrophilic yeast and filamentous fungi (Table 14.2). One of the most interesting enzymes from this eukaryotic group is a psychropiezophilic Aspergillus ustus serine protease from deep-sea sediments of the Central Indian Basin, which maintains its activity even at 300 bar pressure (Damare et al. 2006). There are also other strains of proteolytic bacteria isolated from deep-sea sediments, for instance Pseudoalteromonas sp. SM9913 (Chen et al. 2003), Halobacillus sp. SCSIO 20089 (Yang et al. 2013), Pseudomonas lundensis (Yang et al. 2010) and Colwellia psychrerythraea (Huston et al. 2004). Often, these strains are also present in sea waters. Yuan et al. (2009), for instance, isolated a strain from the Sea of Japan known as Enterococcus faecalis TN-9, which produces psychrophilic metalloprotease. Another strain which secretes serine protease, Pseudomonas aeruginosa HY1215, was found in the Yellow Sea (Hao and Sun 2015). A similar enzyme is produced from a strain identified as Penicillium chrysogenum FS010, isolated from the Huanghai Sea (Zhu et al. 2009). Another example of a cold environment abundant in proteolytic strains is Antarctic soil from where yeast identified as Cryptococcus humicola (formerly Candida humicola) (Ray et al. 1992) has been isolated. It secretes the only one psychrophilic extracellular aspartyl protease from yeast that has been described in literature so far. Serine protease-producing bacteria such as Stenotrophomonas maltophilia (Vazquez et al. 2005) and Clostridium sp. (Alam et al. 2005) were also found there. Another important source of psychrophilic proteolytic microorganisms are highlands, where bacteria such as Serratia marcescens TS1 (Tariq et al. 2011), S. maltophilia MTCC 7528 (Kuddus and Ramteke 2009) and Pedobacter cryoconitis (Margesin et al. 2005) can be found.
The majority of psychrophilic proteases described in literature achieve a maximum activity at temperatures ranging from 30 to 40 °C (Table 14.2). Nevertheless, there are some enzymes that have a lower optimum temperature, for instance an alkaline protease from Stenotrophomonas sp. IIIM-ST045, isolated from a soil sample collected from the Thajiwas glacier of Kashmir, India, which displays significant activity in casein hydrolysis within a temperature range of 4–37 °C with maximum activity at 15 °C and at pH 10.0 (Saba et al. 2012). In turn, a metalloprotease from S. maltophilia MTCC 7528, isolated from the Gangotri glacier in the Western Himalaya, exhibits maximal activity at 20 °C and pH 10.0 during the hydrolysis of azocasein. The enzyme remains stable in commercially available detergents (maintains from 65 to 80 % of its activity after a 3 h incubation) and is an excellent remover of protein-containing stains at low temperatures. It is also resistant to repeated freezing and thawing which may be related with the habitat of S. maltophilia MTCC 7528 (Kuddus and Ramteke 2009). Similar features are showed by a metalloprotease from P. cryoconitis, isolated from an alpine glacier cryoconite (Margesin et al. 2005), and a metalloprotease produced by P. lundensis HW08 isolated from the Yellow Sea (Yang et al. 2010). Serine proteases from P. aeruginosa HY1215 (Hao and Sun 2015) and Glaciozyma antarctica (formerly Leucosporidium antarcticum) (Turkiewicz et al. 2003) are two other enzymes that can thrive at relatively low temperatures, with their optimum temperature of activity being 25 °C. They maintain 30 and 20 % of their maximum activity at 0 °C, respectively. Another important feature of cold-adapted enzymes is their thermolability, related with high catalytic efficiency at a temperature that is optimal for the existence of an enzyme producer. The majority of cold-adapted proteases described in literature possess a low thermostability, undergoing a partial or complete inactivation at temperatures ranging from 40 to 50 °C. In this context, it is worth mentioning three serine proteases produced by the following strains: Clostridium sp. (Alam et al. 2005), Clostridium sp. LP3 (Alam et al. 2006) and Planomicrobium sp. 547 (Yang et al. 2011). The first one maintains 40 % of its maximum activity after a 1 h preincubation at 60 °C, the second one—80 % of its activity after 30 min exposure to 50 °C, and the third one—40 % of its maximum activity after 2 h at 50 °C.
It is also important to mention the role of calcium ions , which stabilize the structure of particular proteases. The presence of these ions usually leads to an increase in thermostability, like in the case of metalloproteases from P. lundensis HW08 and Flavobacterium psychrophilum. In the absence of calcium ions, the first enzyme loses 20 % of its maximum activity, observed in the presence of 5 mM Ca2+ at temperatures between 20 and 35 °C, and 50 % at 40 °C (Yang et al. 2010). Regarding the second strain, the presence of 5 mM CaCl2 causes the optimum temperature of metalloprotease Fpp2 to shift from 24 °C to 33–37 °C and leads to an increase in the thermostability of the enzyme, which after a 5 min exposure to 40 °C maintains 95 % of its activity that was otherwise completely lost in the absence of calcium ions (Secades et al. 2003).
A vast majority of psychrophilic proteases described in literature are enzymes showing maximum activity in a neutral or alkaline environment. So far, only one extracellular aspartic protease, with pH optimum of 1.0, has been described (Ray et al. 1992). In the context of detergent industry, proteases active at high pH values seem especially interesting. These include serine enzymes from such strains as Pseudomonas sp. strain DY-A (Zeng et al. 2003), Bacillus amyloliquefaciens S94 (Son and Kim 2003), Bacillus TA39 (Narinx et al. 1997) and metalloproteases from P. lundensis (Yang et al. 2010) and S. maltophilia MTCC 7528 (Kuddus and Ramteke 2009).
Metalloprotease Ps5 from P. lundensis (Yang et al. 2010) has a potential for the use in the laundry detergent industry as it maintains maximum activity during the hydrolysis of casein at 30 °C and pH 10.4. Moreover, it shows a significant stability in H2O2 solutions. In 1 % H2O2 solution Ps5 displays a 24 % higher stability than in a control sample, whereas in a 10 % solution its activity decreases only by 27 %. In addition, this enzyme remains stable in 1 % non-ionic detergent solutions (Tween 20 and Tween 80) and decreases its stability by half only in a 5 % solution. A serine protease from P. aeruginosa HY1215, a strain of bacteria isolated from the Yellow Sea (Hao and Sun 2015), has a similar stability. At concentrations from 0.2 to 1.2 % of H2O2, it has a 45 % higher activity than in a control sample, but in a 2 % H2O2 solution, the activity falls by 60 %. Furthermore, the enzyme exhibits a high stability in non-ionic surfactants, like Tween 40, Tween 80 and Triton X-100, retaining its total activity in solutions with a concentration of less than 1.8 % for 1 h.
2.3 Alkaliphilic Proteases
Alkaliphilic proteases show activity and stability in alkaline environments. They are produced by alkaliphiles (including thermo-, psychro- and haloalkaliphiles), which are organisms that grow optimally or very well at pH values above 9.0 but cannot grow or grow slowly at neutral pH values (Horikoshi 1999; Sarethy et al. 2011). These enzymes are also produced by other groups of extremophiles, which do not require high pH values to grow.
Alkaliphilic microorganisms secreting alkaliphilic proteases, were isolated from mud, soils from various environments, alkaline soda lakes, salt lakes, mine-water containment dam 3.2 km below the earth’s surface in an ultra-deep gold mine, compost, tile-joint of a bathroom, feather samples collected at the shore of lakes and seawater (Gessesse et al. 2003; Saeki et al. 2002; Kobayashi et al. 2007; Karan et al. 2011; Dastager et al. 2008; Deng et al. 2010; Mitsuiki et al. 2002; Bakhtiar et al. 2002; Raval et al. 2014). These are mainly bacteria, including those classified as actinomycetes (Fig. 14.1) and haloalkaliphilic Archaea described in Sect. 2.4.
Phylogenetic tree showing relationship among the representatives of alkaliphilic microorganisms described in Sect. 2.3 prepared on the basis http://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi (Sayers et al. 2009; Benson et al. 2009)
Alkaliphilic proteases are mainly extracellular serine enzymes (Table 14.3) inhibited by PMSF (phenylmethanesulfonyl fluoride) or 3,4-DCl (3,4-dichlorocoumarin), as in the case of PMSF-insensitive protease AL-20 from Nesterenkonia sp. (Gessesse et al. 2003). However, Kuddus and Ramteke (2009) described a unique metalloprotease which can be classified as alkaliphilic since it shows maximum activity in the hydrolysis of azocasein at pH 10.0, retains approx. 84 % of its maximum activity in pH range of 8.0–11.0 and remains stable in pH range of 8.0–10.0 for 1 h at 20 °C. Considering the fact that the producers of alkaliphilic proteases are isolated from various biotopes, the enzymes display a broad range of optimal temperatures, from 15 °C in the case of a protease from Stenotrophomonas sp. IIIM-ST045 (Saba et al. 2012) to 70 °C in the case of a protease AL-20 from Nesterenkonia sp. AL-20 (Gessesse et al. 2003). The most important factor influencing the activity and stability of the enzymes is the alkalinity of the environment. Most often, the optimum pH value varies between 9.0 and 11.0, but Kobayashi et al. (2007) described a high-alkaline protease ALTP, isolated from strictly anaerobic and extremely alkaliphilic Alkaliphilus transvaalensis, which showed maximum activity in the hydrolysis of casein at pH 12.6 and 40 °C. The enzymes retain stability in a pH range of 5.0–12.0, as in the case of protease BCAP from Bacillus clausii I-52, which remained stable under such conditions for 72 h (Joo et al. 2003). It seems that the enzymes are able to thrive in high pH values thanks to some subtle structural differences that distinguish them from those with lower optimum pH values. Strongly alkaliphilic enzymes (opt. pH 10.0–11.0) showed increased number of alkaline Arg and His residues in a molecule and decreased number of acidic Asp and Glu residues. The increased concentration of arginine residues leads to the formation of more hydrogen bonds and ionic pairs (mainly Arg-Asp), which stabilize the structure of a protein in alkaline environments. Such amino acid composition results in higher isoelectric point values of these proteins (Fujinami and Fujisawa 2010).
Among alkaliphilic peptide hydrolases, there is a group identified as oxidatively stable serine proteases (OSPs) which is resistant to oxidizing agents (Saeki et al. 2002). It includes protease SAPB from Bacillus pumilus CBS, whose activity increased by 28 % in comparison to a control sample in the presence of 5 % H2O2 after a 24 h preincubation at 40 °C (Jaouadi et al. 2008), and a protease from B. clausii I-52, which retains its activity during a 72 h preincubation with 2.5 % sodium perborate at room temperature and increases its activity by 16 % in the presence of 5 % H2O2 in comparison with a control sample (Joo et al. 2003). Some enzymes remain stable also in the presence of detergents used under laboratory conditions (SDS, Triton X-100, Tween 20, cetrimonium bromide) and commercially available washing powders, a good example can be protease SABP. It remains active for 15 min at 65 °C and does not lose its stability for 24 h at 40 °C in the presence of 5 % Tween 20, Tween 80, Triton X-100 and 1 % SDS (Jaouadi et al. 2008) and for 1 h at 40 °C in the presence of commercially available liquid detergents, such as Dinol, Lav+, Nadhif, and solid detergents, like OMO, Dixan or Det (Jaouadi et al. 2009). A serine protease from Bacillus subtilis VSG-4 is another example of an enzyme which did not lose its activity for 1 h at 37 °C in 1 % solution of ionic detergents (Tween 80 and Triton X-100), while in the presence of 5 mM SDS lost only 25 % of its activity. Under the same conditions of preincubation, the enzyme retained from 68 to 100 % of its activity in the presence of 1 % solid detergents (Surf excel, Rin, Tide and Ariel) (Giri et al. 2011).
Most usually, alkaliphilic proteases effectively hydrolyze casein, azocasein or hemoglobin (Gupta et al. 2005; Jaouadi et al. 2008; Kobayashi et al. 2007; Deng et al. 2010), but some of them also demonstrate keratinolytic properties, which are very much desired, like in the case of a protease from Nocardiopsis sp. SD5 that degraded native feather keratin at temperatures between 45 and 50 °C for 96–120 h (Saha et al. 2013). On the other hand, bacterial strains identified as Nesterenkonia sp. AL-20 and Bacillus pseudofirmus AL-89, isolated and described by Gessesse et al. (2003), utilize feathers, which did not undergo grinding or any other mechanical pretreatment. It was the only source of nitrogen in a medium, enabling the growth of these strains and production of extracellular proteases. In this context, the strain AL-89 demonstrated outstanding properties with a high-level production of proteases and a quick and efficient hydrolysis of feathers, with no other supplements added to the medium. Alkaliphilic proteases displaying keratinolytic activity are able to bind and hydrolyze keratin substrates in solid form. This is an important feature of enzymes used in detergents that should act upon protein substrates bound to a solid surface, such as keratin stains on shirt collars which constitute a huge problem in case of enzymes currently used in detergents (Gessesse et al. 2003).
2.4 Halophilic Proteases
Halophilic proteases are proteins produced by halophilic organisms that usually require the presence of NaCl for their catalytic activity. When it comes to halotolerant proteins, they do not always derive from halophiles, but they remain active in a broad range of NaCl concentrations, without any specific dependence on NaCl (Graziano and Merlino 2014).
Microorganisms producing salt-dependent extracellular proteases belong to archaea, bacteria and eukaryotes (Fig. 14.2) which are isolated from saline and hypersaline lakes, salt pans, salt marshes, playas, brine springs from underground salt deposits, solar salterns, salt maines, soda lakes, deep-sea sediments, sea water, fermented fish sauces, pastes and others (Yin et al. 2014; Setati 2010; Sinha and Khare 2013). In case of Archaea, these are usually extreme halophiles which, depending on a given genus, require 3.4–5.1 M of NaCl in a medium for growth (Graziano and Merlino 2014). Bacterial strains include halophiles, haloalkalophiles as well as bacteria that do not require an increased concentration of NaCl in a growth medium. Halophilic bacteria can be divided into slight halophiles, which show optimum growth at 0.5 M NaCl, moderate halophiles, which grow best at 0.5–2.6 M NaCl, and few examples of extreme halophiles, which require 4.3 M NaCl to grow (Setati 2010).
Phylogenetic tree showing relationship among the representatives of halophilic microorganisms described in Sect. 2.4 prepared on the basis http://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi (Sayers et al. 2009; Benson et al. 2009). *—strains belong to Archaea
All extracellular proteases from haloarchaea that have been described so far (Table 14.4) are serine enzymes , inhibited by PMSF. In case of bacterial enzymes, serine enzymes represent the largest group although several metalloproteases have also been described, e.g. proteases from Pseudoalteromonas sp. (Sánchez-Porro et al. 2003; Xiong et al. 2007). Both groups include enzymes whose activity is inhibited by β-mercaptoethanol and dithiothreitol or by p-chloromercuribenzoic acid (for instance, proteases from Pseudoalteromonas sp. (Sánchez-Porro et al. 2003), Geomicrobium sp. EMB2 (Karan and Khare 2010), Natrialba magadii (Giménez et al. 2000), Haloferax lucentensis VKMM 007 (Manikandan et al. 2009)), which indicates the significance of disulfide bridges and/or cysteine residues in maintaining biologically active conformations of the proteins. The optimum temperature for halophilic proteases at a proper NaCl concentration varies from 35 °C, as in the case of a cold-adapted metalloprotease from Pseudoalteromonas sp., isolated from sediment samples found in the area of the Aleutian margin in the Gulf of Alaska, Pacific Ocean (Xiong et al. 2007), to 75 °C, as in the case of a serine protease from Chromohalobacter sp. TVSP101 (Vidyasagar et al. 2009). The optimum pH range is quite broad, though alkaline (7.0–11.0) and it is even broader when it comes to stability—from 5.0 up to 12.0.
The most important factor influencing the activity and stability of these enzymes is the salinity of the environment in which a given reaction takes place. It is undoubtedly connected with habitats of halophilic microorganisms which, in order to survive in environments with a high osmotic pressure, must remain isosmotic. Therefore, they retain high concentrations of NaCl intracellularly (Manikandan et al. 2009). Halophilic proteins have an altered amino acid sequence when compared with other enzymes. Frequently, they have even 20 % more acidic amino acid residues (Asp and Glu), localized as clusters on the surface of a protein, in comparison with non-halophilic enzymes. At the same time, they have fewer Lys residues and an increased number of small hydrophobic residues, Ala and Gly, as well as polar residues, Ser and Thr, at the expense of large non-polar residues, Leu, Ile, Met and Phe. Owing to an increased negative charge, halophilic proteins may bind more hydrogen ions, which reduces the hydrophobicity of the molecular surface and reduces their tendency towards aggregation at high NaCl concentrations. This is a basic halophilic adaptation to a decreased amount of water that is bound by salts present in the environment at high concentrations (Graziano and Merlino 2014; Gomes and Steiner 2004). While proteases from Halobacterium sp. TuA4 (Schmitt et al. 1990) and unidentified haloalkaliphilic strain A2 (Yu 1991) need no more than 0.3 M NaCl (De Castro et al. 2005), the majority of enzymes show maximum activity at higher NaCl concentrations—from 1.0 to 5.1 M. Still, the number is even higher when synthetic substrate is being hydrolyzed, for instance in the case of protease Fll from Natrialba asiatica (Kamekura and Seno 1990), which breaks down N-succ-AAPF-pNa most quickly at 5.1 M NaCl concentration while azocasein at 1.7–2.4 M. It might be explained by the fact that at higher NaCl concentrations, azocasein becomes less hydrophobic and loses its proper conformation, which makes the hydrolysis more difficult. On the other hand, oligopeptides do not change their conformation under the same conditions and thus, can be effectively hydrolyzed by proteases. Several authors (Nordberg and von Hofsten 1969; Studdert et al. 2001; Shi et al. 2006) compared the efficiency of proteases in the presence of NaCl and KCl, receiving higher values of activity with NaCl , which is understandable considering the fact that producers of these enzymes live in environments rich in NaCl. A relatively high level of salinity, from 1.0 M for protease Nep (Giménez et al. 2000) up to 4.5 M for protease from Haloferax mediterranei strain 1583 (Stepanow et al. 1992), is also required to maintain the stability of these proteins. In case of several enzymes, such as halolysin R4 (Kamekura et al. 1996), a protease from Halobacterium halobium (Izotova et al. 1983) and a protease from Hfx. mediterranei strain 1583 (Stepanow et al. 1992), studies have shown that the removal of NaCl from the environment leads to the irreversible inactivation of these proteases, caused by their accelerated autodegradation in environments with low ionic strength. Therefore, it is worth noted that protease SptA (Shi et al. 2006) retains 20 % of its activity in the absence of NaCl, only to regain 60 % of its initial activity once the NaCl concentration is increased to 2.5 M. According to the authors, this indicates that in the absence of NaCl, the enzyme undergoes only a reversible denaturation. On the other hand, a protease from Halogeometricum borinquense TSS101 (Vidyasagar et al. 2006) maintains its total activity in the absence of NaCl, provided that the concentration of sucrose and betaine in the environment is 10 and 20 %, respectively. This shows that in order to maintain its activity, the enzyme needs an adequate osmotic pressure or reduced water activity (aw) in the environment rather than the presence of Na+ or Cl− (Vidyasagar et al. 2006; Litchfield 2011).
Halophilic and halotolerant bacterial proteases described so far show a greater salinity tolerance than proteins from Archaea. The optimal salinity conditions for enzymes from Bacillus sp. EMB9 (Sinha and Khare 2013), Pseudoalteromonas sp. CP76 (Sánchez-Porro et al. 2003), Geomicrobium sp. EMB2 (Karan and Khare 2010) or B. subtilis FP-133 (Setyorini et al. 2006) vary from 0 to 1.0 M NaCl. In turn, a serine protease from Virgibacillus sp. SK33 shows maximum activity at NaCl concentration between 1.7 and 4.3 M (Sinsuwan et al. 2010). For a serine protease from Chromohalobacter sp. TVSP101, the optimal salinity is 4.5 M NaCl (Vidyasagar et al. 2009). Purohit and Singh (2011) described an interesting property of an enzyme isolated from Oceanobacillus iheyensis O.M.A18, which changes its optimal temperature depending on the salinity of the environment. At 0.25–0.5 M NaCl, its optimal temperature was 60 °C, but it increased by 20 °C, when the concentration of NaCl rose to 2.0–3.0 M. Although these enzymes can hydrolyze substrates without the presence of salt in the environment, they tend to require some small amounts of NaCl (e.g. 90 mM) in order to maintain stability. Still, the majority of proteins described so far remain stable in solutions with 2.0–4.5 M of NaCl (Table 14.4). In the case of a protease from Chromohalobacter sp. TVSP101, one of the few bacterial strains belonging to the group of extreme halophiles, the activity is completely and irreversibly lost at salinity below 1.0 M. However, the enzyme maintains its total activity if NaCl is replaced by glycine, sucrose (10 %) or glycerol (20 %) (Vidyasagar et al. 2009). Another protease from moderately haloalkaliphilic bacterium, Geomicrobium sp. EMB2, which requires only 0.17 M NaCl to maintain its activity, loses more than half of it once NaCl is removed from the environment. Nevertheless, when NaCl or other osmolytes (mannitol, sucrose, glycerol or betaine) are re-added, so that the final concentration is 5 %, the enzyme regains most of its initial activity (Karan and Khare 2010).
3 Application of Extremophilic Proteases
Currently, the global market for enzymes is estimated at approx. 5.1 billion USD. It is one of the best developing markets in the world, with an annual growth rate of 7 % (Sarethy et al. 2011). Proteases, as a dominating class of enzymes, occupy a pivotal position in the market, accounting for approx. 60 % of all commercially available enzymes. Their main sources are animals (e.g. calf stomach), plants (e.g. pineapple, fig and papaya) and microorganisms (e.g. Bacillus sp., Pseudomonas spp.). For several decades, proteases, especially microbiological proteases, have occupied a dominant position in the market and have been used for industrial purposes. When it comes to plant proteases, their production is to a large extent hindered by climate conditions, whereas the production of animal enzymes stirs up ethical debates. Nevertheless, regardless of their origin, proteases are used on an industrial scale mainly for their ability to hydrolyze peptide bonds. Numerous proteases can also efficiently catalyze synthesis reactions in micro-aqueous environments. This property is mainly used for pharmaceutical and nutritional purposes.
At the very beginning, the market for proteases was dominated by mesophilic enzymes (most often alkalitolerant), but an intensive development of research on extremophilic microorganisms proved that it is possible to improve the efficiency of biocatalytic technologies and lower their costs by replacing currently used enzymes by extremozymes. Depending on their origin, extremophilic proteases are naturally adapted to thrive at low or high temperatures and in acidic, alkaline or extremely saline environments. They can either show an exceptional resistance to thermal denaturation or, quite the contrary, be extremely thermolabile. Thus, they can be selectively inactivated under process conditions. Examples of proteases which achieved the biggest commercial success include subtilisin (for industrial purposes, it is produced mainly from alkaliphilic or alkalitolerant strains) and other alkaline proteases which constitute approx. 50 % of all proteolytic enzymes (Table 14.5). The enzymes have a wide range of applications thanks to their properties, analyzed in details in Sect. 2.3. The list of commercially available enzymes used in detergents, silk degumming, food and feed industry, leather dehairing, cosmetics and pharmaceuticals can be found in Table 14.5. The majority of alkaliphilic proteases were isolated from Bacillus strains. The main recipient of these enzymes is the laundry detergent industry which uses them as additives to washing powders and automatic dishwashing detergents. Their function is to degrade proteinaceous stains, which typically include blood, milk, egg, grass and sauces.
Once the success of detergent enzymes was recognized, numerous detergent proteases with specific uses were discovered. One of them was Alkazym (Novodan, Copenhagen, Denmark), an enzyme which plays a significant role in the process of membrane cleaning. Tergazyme (Alconox, New York, USA), Ultrasil (Henkel, Dusseldorf, Germany) and P3-paradigm (Henkel-Ecolab, Dusseldorf, Germany) are other enzymes used for this very purpose. Another example is Pronod 153L, an enzyme-based cleaner that is used to remove blood proteins from surgical instruments. A good example of other commercial protease is subtilopeptidase A used in optical cleaner in India (Gupta et al. 2002).
3.1 Detergent Industry
The first detergent containing a serine protease isolated from bacteria was introduced in 1956 as BIO-40. Seven years later, a Danish company Novo Industry A/S introduced Alcalase from alkalitolerant strain, identified as Bacillus licheniformis, under the trade name of Biotex (Maurer 2004). It is a serine endoprotease which is resistant to anionic or non-ionic surfactants and displays maximal activity at 60 °C and pH 8.3.
As previously mentioned, alkaline serine proteases, known as subtilisins and produced by Bacillus strains, account for almost half of all proteolytic enzymes produced in the market; in 2002, their production in EU amounted to 900 tons in terms of pure protein (Maurer 2004). The commercial success of this enzyme was guaranteed thanks to its properties, which include high alkalinity, stability in alkaline environment, broad specificity and its secretion by cells that make production of the enzyme preparation easier. Since 1984, numerous attempts have been undertaken to improve subtilisins and other proteases. Still, out of hundreds of different variants, only few entered industrial practice, the first one in 1990. These were solely subtilisin variants with improved characteristics. In 2004, eight such subtilisins, improved through site-directed mutagenesis or directed evolution, were available in the market. The improved characteristics included increased thermostability and activity in organic solvents, changed substrate specificity and pH optimum (Table 14.6). In the first engineered subtilisin Met222 (an easily oxidized amino acid, located in the neighborhood of catalytic Ser221) was replaced by non-oxidizable amino acids, Ala, Ser or Leu, in order to obtain a protein with increased oxidation tolerance.
The subtilisins from Bacillus strains, currently used in detergents are the subject of numerous patents with broadly-defined claims and therefore constantly classical screening is performed in order to isolate a new form of alkaline proteases from mesophilic and extremophilic microorganisms. For this purpose also genomes and metagenomic libraries are searched. For instance, Rai et al. (2010) described a potential alternative for Bacillus sp. proteases, widely used in the laundry detergent industry, which is a laundry-detergent stable serine protease, isolated from thermophilic bacterial strain, Paenibacillus tezpurensis sp. nov. The enzyme is Ca2+ independent and displays maximal activity at pH 9.5 and in temperature range of 45–50 °C. It also exhibited a significant stability and compatibility with surfactants and the majority of tested commercial laundry detergents at room temperature. Proteases from halophilic strains, for instance Geomicrobium sp. EMB2 (Karan and Khare 2010) or Virgibacillus sp. SK33 (Sinsuwan et al. 2010), also have a great potential for application in the detergent industry. It should be emphasized that frequently, extremophilic proteases are obtained during the selection of mesophilic strains. For instance, an alkaliphilic protease from a mesophilic filamentous fungus, Conidiobolus coronatus, is an enzyme that is compatible with commercial detergents used in India and retains 43 % of its activity at 50 °C for 56 min in the presence of Ca2+ (25 mM) and glycine (1 M) (Phadatare et al. 1993). Also, as reported by Khan (2013), an alkaline serine protease from Aspergillus clavatus ES1 can be used in the detergent industry because this enzyme is salt, solvent, detergent and bleach tolerant.
During the metagenomic research , Neveu et al. (2011) isolated two serine proteases, DV1 and M30, from metagenomic libraries derived from samples of surface sand from the Gobi and Death Valley deserts, respectively. Both enzymes are active in alkaline environments (pH optimum for DV1—8.0, pH optimum for M30—11.0) and remain stable in the presence of non-ionic detergents and SDS, which renders them useful for household chemistry. Often, metagenomic screening of conventional environments results in the discovery of proteases that are capable of thriving in extreme conditions, for instance in extremely alkaline environments, which is one of the determining factors when it comes to the application of particular proteins in detergents. Examples of such enzymes include an alkaline protease KP-43, isolated from the A horizon of a Belgian deciduous forest (Biver et al. 2013), or a serine protease (AS-protease) isolated from the goat skin surface metagenome (Pushpam et al. 2011).
Another approach towards searching for subtilisin-like proteases, to be used mainly in detergents, was suggested by Toplak et al. (2013). They looked for new variants of subtilisin through genome mining, with regard to the presence of genes encoding these proteolytic enzymes. In this way, the authors selected genes that encode potential homologues of subtilisin E in the genomes of thermophilic bacteria and archaea. They looked for sequences that were at least 30 % identical in sequence to the gene of subtilisin. They found 24 putative homologous genes of subtilisin E and isolated sixteen of them from selected strains via PCR, using primers designed on the basis of preprosubtilisin-coding genes. Next, they cloned them into pBAD vectors and expressed them in E. coli. Through the selection of clones on agar plates with selective growth compounds (skim milk, antibiotic, inducer), six different functional clones with genes of proteases isolated from Thermus aquaticus, Pseudomonas mendocina, Geobacillus thermodenitrificans, Deinococcus geothermalis and Coprothermobacter proteolyticus were obtained. A clone that contained a gene of subtilase from the latter strain, C. proteolyticus, showed the highest activity with an ability to grow at 80 °C. This protease, named as proteolysin , was expressed in E. coli with efficiency of 20 mg/L. The enzyme proved thermostable and it shows optimum activity at 85 °C and pH 8.0. After 20 h of incubation at 70 °C, it retains 35 % of its activity. Another characteristic feature of the proteolysin is its great tolerance to DMSO, DMF, ethanol and guanidine hydrochloride. This thermophilic enzyme is also activated by a 10 % non-ionic detergent Tween 20 (80 % increase in activity).
The detergent industry is also the recipient of psychroalkaliphilic proteases which show stability in organic solvents and which are able to thrive at low temperatures. This is significant especially when it comes to cleaning applications in a wide range of industries, including laundry, dishwashing, food, dairy and brewing, medical devices and water treatment (Cavicchioli et al. 2011). For these purposes, thermolabile character of these proteins is used along with their high efficiency in catalyzing reactions at low temperatures. Their thermolability leads to a selective inactivation of proteins after the process is complete through a subtle increase in temperature. In this way, the risk of enzymatic degradation of the final product is reduced.
It is generally thought that detergents containing cold-adapted proteases are much more effective in comparison with non-enzyme detergents and they remove protein contamination from clothes stained with blood, milk, grass or sweat much more efficiently (Kuddus and Ramteke 2011). A cold-adapted enzyme , designed for washing clothes at lower temperatures, is produced by Novozymes under the trade name of Polarzyme. The estimations concerning potential energy savings, made on the assumption that in every household, the temperature of washing is lowered from 40 to 30 °C, show how important the availability of such products is. In this way, we could reduce the energy consumption by 30 % and decrease the CO2 emission by 100 g per one laundry (Cavicchioli et al. 2011). Apart from economic and environmental advantages, the use of cold-adapted proteases in detergents may lead to the improvement in the quality of textile materials, especially those containing synthetic fibers, which do not tolerate temperatures above 50–60 °C (Joshi and Satyanarayana 2013a). Consequently, more and more attention is devoted to the production of such proteases. For instance, Baghel et al. (2005) described a cold-active protease from B. subtilis, which shows stability in SDS solutions as well as increased activity in Tween 80 and Wheel detergents. Recently, a cold-active serine protease (CP70) from Flavobacterium balustinum was patented. Its optimum temperature is lower by 20 °C compared to a protease Savinase that has been traditionally used in the detergent industry. The enzyme remains stable for 1 h at 30 °C and its optimum pH values range from 6.5 to 10.0. Moreover, surface-active components/bleaching agents do not affect its activity (Hasan and Tamiya 2001). Another alkaline cold-active protease, isolated from Stenotrophomonas maltophilia, shows maximum activity at 20 °C and pH 10.0. Its other properties include great stability and compatibility with commercial detergents, resulting in its high efficiency in removing various types of protein stains at low temperatures (Kuddus and Ramteke 2009). According to Kuddus and Ramteke (2011), a cold-adapted protease from S. maltophilia can completely remove blood and grass stains and increases their reflectance by 26 and 23 %, respectively. According to the authors, the protease can be used in mild detergents, designed for washing delicate clothes, and in the textile industry to remove gums from raw silk, as their proteinaceous content adds rigidity and dullness to materials. Doddapaneni et al. (2007) also see a potential in the use of low-temperature detergents. They mention two proteases from Serratia rubidaea which remain active at 30–40 °C and in the pH range of 8.0–10.0. Pawar et al. (2009) isolated and described a thermolabile protease from Bacillus sp. 158 (stable at 30–40 °C and pH 6.0–7.0) which could be used in contact lens clearing to increase the transmittance of lenses.
Halophilic enzymes also show some potential as laundry detergents. Apart from their ability to thrive in extremely saline environments, they remain stable in the presence of detergents and organic solvents. Examples of such enzymes are proteases isolated from halophilic strains: Bacillus sp. EMB9 (Sinha and Khare 2013), Geomicrobium sp. EMB2 (Karan and Khare 2010) or Virgibacillus sp. EMB13 (Sinha and Khare 2012). The latter was tested in terms of its compatibility with various detergents (after a thermal denaturation of enzymes present in commercial washing powders) and its ability to remove blood stains from fabrics in combination with these detergents. The results were extremely encouraging.
3.2 Food Industry
Microbiological proteases are broadly applied in the food industry , especially the extremophilic proteases, which are often used to increase the efficiency of a process and enhance the quality of the final product. Their main advantage is higher specificity of enzyme, lower risk of adverse reactions, lower risk of contamination with mesophilic microflora and, in the case of psychro- and thermophilic enzymes, the possibility of selective thermal inactivation. Without a doubt, in the context of food processing, alkaliphilic proteases come to the front, as it was the case with household chemicals. The primary function of alkaliphilic proteases is the hydrolysis of naturally-occurring protein substrates. For more than 40 years, protein hydrolysates of high nutritional value have been obtained thanks to alkaliphilic proteases. Commercial protein hydrolysates can be derived from the decomposition of such substances as casein (Miprodan; MD Foods, Germany), whey (Lacprodan; MD Foods, Germany), soy (Proup; Novo Nordisk, Denmark) and meat (Flavourzyme, Novo Nordisk, Denmark) (Gupta et al. 2002). They play a significant role in blood pressure regulation and are used in infant food formulations, specific therapeutic dietary products and as the fortification of fruit juices and soft drinks. Alkaline proteases also find their use in meat processing, e.g. a commercially available protease named SEB Tender 70. The enzyme is extensively used in meat tenderization for breaking down collagen and making the meat more tasty for consumers (Singhal et al. 2012). One of the alkaliphilic proteases, often discussed in the context of its application in the food industry, is Alcalase from B. licheniformis. It is used in the processing of soy meal to obtain a soluble, non-bitter hydrolysate, used as component of protein-fortified soft drinks and dietetic food. Alcalase is also helpful for the recovery of proteins from by-products of fish and meat industries and from crustacean shell waste during chitin production (Synowiecki 2010). Cold-active proteases could be used for similar purposes, but this would necessitate the use of highly thermolabile substrates or products. These enzymes are efficient and highly specific at low temperatures and thus, they reduce the amount of by-products, facilitating the selective inactivation of labile proteins throughout the process, or after its completion, without a huge input of energy. They are used for the treatment of beer, in bakeries and for the accelerated maturation of cheese. Similarly to alkalozymes, cold-adapted proteases, e.g. cold-adapted collagenolytic protease MCP-01, can be used to tenderize meat or improve the taste of refrigerated meat. This enzyme decreases the meat shear force by 23 % and enhances the relative myofibrillar fragment at ion index of the meat by 91.7 % at 4 °C. MCP-01 also helps maintain the fresh color of the meat as well as its moisture because it showed a unique tenderization mechanism and had a strong selectivity for the degradation of collagen at 4 °C in comparison to papain and bromelain as the commercially used tenderizers (Zhao et al. 2012). Otherwise, psychrophilic proteases facilitate the removal of the membrane from the fish roe, the evaporation of fish/meat stickwater and the rendering of fat. Cold-active proteases may be used in the production of digest, which is either coated onto or mixed into dry pet food to improve its tastiness (Kuddus and Ramteke 2012).
Halophilic and halotolerant bacteria also produce proteases that will be more suitable for the application in food industry, performed under saline conditions or in saline-free systems. Some examples may include saline fermentation processes, involved in the production of protein-rich food, such as processing of fish and meat-based products, and the production of soy sauce (Setati 2010). Enzymes from Halobacillus sp. SR5-3 (Namwong et al. 2006) and Halobacterium constitute good examples of halophilic proteases, used for the production of fish sauces (Akolkar et al. 2010).
3.3 Leather and Textile Industry
Alkaliphilic properties of proteases isolated from extremophiles have been used in the leather industry for a long time. These enzymes may serve an important role in the treatment of leather by substituting harmful substances used in soaking, dehairing and bating (e.g. sodium chloride). Currently, alkaline proteases with hydrated lime and sodium chloride are involved in a selective hydrolysis of non-collagenous components of the skin as well as for the removal of globular proteins, such as albumins and globulins. The increased usage of enzymes for dehairing and bating not only prevents pollution, but is also time-saving and enhances the quality of leather. At the moment, pancreatic proteases are used for the treatment of leather, but their alkaliphilic substitutes also constitute some alternative. For instance, Varela et al. (1997) used an alkaline protease from B. subtilis HQDB32, to unhair sheep skin. George et al. (1995) used a similar enzyme from B. amyloliquefaciens to unhair hides and skins, while Hameed et al. (1999) for bating and leather processing. Over the last few years, several new extremophilic proteolytic enzymes have been described as showing dehairing activity and, at the same time, displaying no collagen and keratin degrading properties that destroy the collagen structure of hide and hinder keratin recovery. These include proteases isolated from an alkaliphilic bacterium, B. pumilus CBS (Jaouadi et al. 2009), and from a mesophilic bacterium, P. aeruginosa PD 100 (Najafi et al. 2005). The latter enzyme, despite being isolated from a mesophilic strain is a unique protein since given its numerous properties, like alkali- and thermostability or resistance to organic solvents and detergents. It can be considered a polyextremozyme. Furthermore, it has a broad substrate specificity and therefore, can be used not only in dehairing, but also in the animal food industry, clearing of beverages, skin bating, production of amino acids and peptides and in molecular biology for the purification of DNA during its isolation. Another detergent-stable serine alkaline protease from B. pumilus CBS, which can efficiently remove hair from the skin with minimal damage of the collagen, effectively degraded feather-meal, chicken feather, goat hair and bovine hair (Jaouadi et al. 2009).
Alkaliphilic cold-adapted proteases can be also applied in the textile industry for improving production methods and fabric finishing. They prove effective in degumming threads of raw silk to remove sericin, a proteinaceous substance that covers silk fiber. Traditionally, degumming is done in an alkaline solution containing soap. This is a harsh process because not only sericin but also the fiber is attacked. On the contrary, proteolytic enzymes remove sericin without attacking silk fiber which is not damaged. Therefore, silk threads are stronger than in the case of traditional treatments. The surface of the wool and silk fibers treated by cold-active protease can provide new and unique finishes (Najafi et al. 2005). Example of this enzyme is an alkaline protease from Bacillus sp. RGR-14 described by Puri (2001). It is characterized by the silk-degumming efficiency. The scanning electron microscopy (SEM) of the fibers revealed that some clusters had broken apart, whereas the treated fiber maintained its smooth and compact structure.
3.4 Poultry Industry
Recently, much thought has been given to the possible application of thermostable proteases with keratinolytic activity in the decomposition and disposal of various types of waste (e.g. meat industry by-products or feathers from the poultry industry ), whose present production amounts to approx. 10,000 tons per year (Suzuki et al. 2006). Currently, such raw materials are degraded through nonenzymatic alkaline hydrolysis and steam pressure cooking, during which particular amino acids are broken down and non-nutritive lysinoalanine and lantionine are produced. Enzymatic degradation of wastes from the poultry industry leads to the formation of hydrolysates, which can be used as fertilizers and dietary protein supplement for animals. Moreover, keratinases can be used as dehairing agents in the leather and cosmetic industry and as a component of detergents and edible films. The majority of keratinases described so far are produced by mesophilic microorganisms, despite their high thermostability. For instance, a mesophilic strain B. licheniformis K-19, cultivated at 37 °C, produces keratinase which is active in the temperature range of 30–90 °C. It shows optimum activity at 60 °C and at pH 7.5–8.0 (Xu et al. 2009). On the contrary, a protease isolated from a thermophilic bacterial strain identified as Fervidobacterium islandicum AW-1, shows keratinolytic activity at 100 °C and pH 9.0, whereas its half-life at optimum temperature is 90 min (Nam et al. 2002). Another keratinolytic protease from a thermoalkalitolerant halophilic strain, Bacillus tequilensis hsTKB2, which was described by Paul et al. (2014), is alkaliphilic (pH 9.0–10.5), thermostable (50–80 °C) and halophilic (0–30 % NaCl).
3.5 Medical and Pharmaceutical Industry
Currently, there is little information on the application of microbiological proteases from extremophiles in medicine. In the 1990s, there was some discussion concerning the use of elastolytic activity of B. subtilis 316 M for the preparation of elastoterase, which is applied in the treatment of burns, purulent wounds, carbuncles, furuncles and deep abscesses (Kudrya and Simonenko 1994). Another idea was to use an alkaline protease from Bacillus sp. strain CK 11-4, which shows fibrinolytic activity, as a thrombolytic agent (Kim et al. 1996). Still, if we share the opinion expressed by Feller (2013), who said that coldwater fish and crustaceans may be classified as extremophiles, we cannot forget about the trypsin from Atlantic cod (Gadus morhua) which is commercially available under the trade name of ColdZyme®. Trypsin is used in mouthwashes and it prevents the formation of dental plaque, which is a biofilm of caries-associated bacteria, mainly Streptococcus mutans and Lactobacillus acidophilus. Another product used to prevent tooth decay and periodontal diseases is known as Krillase®. It contains proteolytic enzymes isolated from the Antarctic krill, Euphausia superba Dana, with trypsin- and chymotrypsin-like proteases and carboxypeptidases A and B. The product is also used for wound dressing, in the treatment of hard-to-heal wounds and ulcerations. It not only removes necrotic tissue, but also accelerates the granulation of a healthy tissue (Fornbacke and Clarsund 2013).
In recent years, there have appeared new possibilities for the use of peptide hydrolases, especially thermophilic proteases with keratinolytic activity, in the degradation of infectious isoforms of prion proteins, PrPSc, which tend to aggregate and form anomalous structures known as amyloids. The deposition of amyloids may lead to such disorders as bovine spongiform encephalopathy (BSE) and Creutzfeldt-Jakob disease in a human organism. Numerous studies proved that prion aggregates are extremely resistant to conventional proteases and do not undergo degradation even during sterilization at 121 °C (Langeveld et al. 2003). First reports on the degradation of PrPSc without the use of detergents and in a non-alkaline environment were related with the application of keratinolytic proteases isolated from Streptomyces, Thermoanaerobacter and Thermococcus (Hui et al. 2004). Proteolytic potential of the last two extremophiles was used for the hydrolysis of a thermally pre-denatured prion aggregate; the possibility of using these two strains in the disinfection of animal wastes was also mentioned (Suzuki et al. 2006).
3.6 Protease Applications in Non-Conventional Media
Kinetic and thermodynamic studies on the reaction of proteolysis have already shown that it is possible to shift its equilibrium towards the formation of peptide bonds through synthesis (Glass 1981; Bongers and Heimer 1994; Elmore 2002). The kinetic control of the reaction consists in maintaining the concentration of a condensation product below the equilibrium concentration. Only then it is possible to conduct a protease-catalyzed peptide synthesis in the presence of water (Morihara and Oka 1981), but given the ionization constant of the substrates (thermodynamic control), the majority of enzymes require an environment with lower polarity. In general, three ty pes of such environments are currently used: (1) water-organic solvent systems (water + water-miscible organic solvent), (2) two-phase systems (water + water-immiscible organic solvent system) (Doukyu and Ogino 2010) and (3) microaqueous solvent systems with fixed water activity (aw) (Halling 1994). Nevertheless, the conditions which are favorable for the reaction of synthesis may negatively affect the activity of an enzyme. Therefore, in order to use a given protease for the catalysis in a water-restricted system, the enzyme should display high activity and stability in such environments (Deetz and Rozzell 1988). The analysis of these parameters is essential to properly assess the usefulness of an enzyme in the reaction of synthesis performed in non-conventional media.
3.6.1 Properties of Extremophilic Proteases in Low-Polarity Media
There are numerous reasons for which enzymes lose their activity in non-polar environments. It may be due to conformational changes in proteins, decreased conformational flexibility, loss of crucial water or interfacial inactivation (Doukyu and Ogino 2010). These changes may be prevented via genetic and chemical enzyme engineering, immobilization of enzymes and optimization of the mixing during a two-phase reaction (Kumar and Bhalla 2005). Another approach is the use of extremophilic proteases, mainly psychrophilic and halophilic, which are naturally predisposed to catalysis in non-polar environments thanks to their structural adaptations (see Sects. 2.2 and 2.4).
Extremely valuable tools for syntheses in low-polarity media are extracellular proteases from halophilic microorganisms, since they are adapted to environments of low water activity due to hygroscopic properties of salts, which are usually present at high concentrations. A serine protease from γ-Proteobacterium is an example of such an enzyme as it maintains approx. 80 % of its initial activity after 18 h incubation at 35 % NaCl. When tested in a water-organic solvent system (ethylene glycol, ethanol, butanol, acetone, dimethyl sulfoxide, xylene, perchloroethylene), both one-phase and two-phase, the enzyme shows higher activity (with even a twofold increase) and stability (up to 10 days) than without a solvent (Sana et al. 2006).
Unlike the protease from γ-Proteobacterium, some other enzymes, like a haloalkaliphilic protease from archaeon Natrialba magadii, require higher salt concentration to act in an organic medium. The enzyme was tested in media with various logP values (octanol/water partition coefficient; Laane et al. 1987) ranging from −1.76 (glycerol) to 0.5 (isopropanol), with 0.5 or 1.5 M NaCl. The protease showed higher activity in all the solvents with higher NaCl concentrations (Ruiz and De Castro 2007). Studies on the stability of an extremely halophilic protease from Halobacterium halobium (Kim and Dordick 1997) revealed it was 40-fold more stable in 40 % dimethyl sulfoxide (DMSO) containing 0.2 M NaCl than in 0.2 M NaCl. Its stability increased proportionally with NaCl concentration in 40 % DMSO and was 150-fold higher for 2 M NaCl. However, when DMSO was replaced by 40 % 1,4-dioxane, the stability of the protein significantly decreased, despite the fact that the concentration of NaCl was maintained. Also tetrahydrofuran (THF) containing 0.2 M NaCl increased the stability of this protease, however, only in the concentration range up to 40 %. When THF concentration reached 80 %, the stability was lower than in the absence of the solvent. These significant differences in the Hbt. halobium protease stability in various organic solvents could stem from their salting-in-out nature (Kim and Dordick 1997). Sellek and Chaudhuri (1999) who studied properties of the same enzyme found that it showed maximum synthetic activity in 32 % dimethylformamide (DMF), in which the efficiency of hydrolysis catalyzed by this protein, expressed as catalytic efficiency (kcat/Km), was threefold lower. For comparison, this constant was not changed in case of subtilisin Carlsberg. The differences between these two proteins suggest that halophilic enzymes are particularly adapted to carry out synthesis in organic media.
Despite limited usage, psychrophilic proteases are attractive catalysts of peptide synthesis in low-polarity media. Firstly, a decrease in temperature leads to a shift in the equilibrium from hydrolysis towards synthesis as the formation of acyl-enzyme intermediate is slowed down. Secondly, a decrease in temperature leads to lower energy consumption. Finally, psychrophilic enzymes are more stable in organic media as the number of ion-pairs that reduce the hydrophobic effect on protein folding increases. Moreover, owing to a greater flexibility of psychrophilic proteins, it is possible to use them with more hydrophobic solvents which as such, decrease the conformational mobility of a protein and thus, its catalytic efficiency (Sellek and Chaudhuri 1999).
Apart from halophiles and psychrophiles, many other thermophilic microorganisms produce proteases that are stable in organic solvents. A protease from Thermus sp. Rt4A2 is a good example of such an enzyme. It loses only 25 % of its activity at 4 °C, in the presence of 90 % acetonitrile. However, when acetonitrile is replaced by butanol at the same concentration, the decrease in activity is twice higher (59 %) (Freeman et al. 1993). Different stability of proteases from P. aeruginosa PST-01 and Thermus sp. Rt4A2, in the presence of butanol, prove that there is no universal rule which can be applied while choosing a proper solvent for the reaction of synthesis catalyzed by these enzymes. Subtle and very often hardly noticeable differences in the structure of enzymes may result in their different stability and suitable solvents for a particular reaction catalyzed by a given enzyme must be selected experimentally. One of thermostable proteases that might be useful in peptide synthesis is a proteolysin from Coprothermobacter proteolyticus (Toplak et al. 2013). The enzyme shows great tolerance to such solvents as DMSO, DMF and ethanol, although it is not stabilized by them. The research on the stability of immobilized thermolysin from B. thermoproteolyticus, which is one of the enzymes that are most frequently used to catalyze peptide synthesis, confirm that although thermophilic proteases are not stabilized by organic solvents, they retain much of their activity in the presence of such substances as acetonitrile (thermolysin—72 % of its activity after a 5 h incubation), tert-butyl alcohol (93 %) or tert-amyl alcohol (98 %).
Noteworthy, not only enzymatic proteins may be more stable in organic solvents. There are also solvent-tolerant strains which can be classified as another group of extremophilic microorganisms. They embrace not only halophiles, such as archaeon Halobacterium ssp. (Akolkar et al. 2008), but also some non-halophilic strains, like P. aeruginosa PST-01. PST-01 protease shows significantly greater stability (five to tenfold) in the presence of organic solvents (25 %), such as ethylene glycol, 1,4-butanediol, 1,5-pentanediol, ethanol, n-hexanol, methanol, butanol, DMSO and others, than in the aqueous environment. Its stability is much greater than that of subtilisin Carlsberg, which is commonly applied in biotransformations (Ogino et al. 1999a, b).
3.6.2 Synthesis Using Proteases
Only few proteases from extremophiles have been applied in peptide synthesis processes. Their examples are presented in Table 14.7. It is to note, that in hydrophobic environments with low water content, proteases synthesize mainly di- and tripeptides. Therefore, several proteases with different substrate specificities are needed to synthesize longer peptides. For example, Kimura et al. (1990b, Table 14.7) needed three steps to synthesize Z-L-Tyr-Gly-Gly-L-Phe-L-Leu-OEt. In the first step they received Z-Gly-Gly-OBut and Z-L-Tyr-Gly-Gly-OBut using papain and α-chymotrypsin. The latter peptide was converted to Z-L-Phe-L-Leu-OEt, and that to the target peptide using thermolysin. Kullman (1982, Table 14.7) used another method, in which the synthesis of cholecystokinin was conducted via chemical condensation of peptides, obtained using several enzymes: papain, α-chymotrypsin, arylsulfatase (EC 3.1.6.1; for desulfation of tyrosine-O-sulfate), thermolysin and aminopeptidase M.
In kinetically controlled peptide synthesis, an important role is played by subtilisins from alkaliphilic Bacillus strains that show optimum activity in alkaline pH. For these enzymes, the rate of peptide bond synthesis was found to be greater than the rate of its hydrolysis (Stepanov 1996). One of such enzymes, most commonly used in organic synthesis, is subtilisin Carlsberg, whose great potential was reported by Klein et al. (2000). In the medium with pH 9.5, containing 50 % (v/v) DMF and strongly basic piperidine (added to neutralize acid groups in the amine substrate) subtilisin catalyzed synthesis of peptide bonds between the acyl donor (Z-Val-Trp-OMe) and (1) amino acid amides as nucleophiles (with more than 70 % yield for Gly-NH2) and (2) dipeptides Gly-Xaa (where Xaa = Gly, Ala, Phe, Gln, Ser, Val, Lys, Trp). The reaction of hydrolysis was not observed.
Subtilisins are known for their broad substrate specificity in the reaction of hydrolysis. In synthesis reactions, their specificity can be altered through a change in the medium. For instance, Klein et al. (2000) who studied subtilisin Carlsberg, observed that addition of acetonitrile (acetonitrile/DMF/H2O = 8/1/1) increased the synthesis yield for single amino-acid nucleophiles (with the exception of Gly-NH2) and the highest yield (at least 70 %) was achieved in the condensation reaction of Z-Val-Trp-OMe and Lys-NH2 and Ala-NH2. For dipeptide nucleophiles Xaa-Gly, the addition of acetonitrile resulted in a threefold increase in the yield of the synthesis for Xaa = Ser, Lys, Thr, Asn, Gln, Met, His, Val, Glu, Asp, and Arg. Still, for Gly-Xaa, the change in the yield was significantly lower. A marked increase in the yield was observed only for Xaa = Asp and Pro.
Some extremophilic proteases are capable of synthesizing non-protein compounds, such as esters via transesterification. An alkaline protease from B. pseudofirmus AL-89 (opt pH 10.0) is a good example. It catalyzes the synthesis of sucrose laurate (Pedersen et al. 2003) in an optimal solvent system (DMF-DMSO). Subtilisin Carlsberg is another example of a protease used in the synthesis of esters. It can be applied for the acylation of di- and oligosaccharides containing d-fructose moiety (Riva et al. 1998). Riva et al. (1998) received 1-O-butanoyl-derivatives of lactulose, maltulose, stachyose, and sucrose with 50 % yield in anhydrous DMF with activated ester—trifluoroethyl butanoate. Such sugar esters, produced via selective esterification, are extremely valuable surfactants.
4 Concluding Remarks and Challenges
Proteases, particularly these of microbial origin, play a leading role in industrial processes which use enzymatic catalysis for fabrication of a wide range of high value-added products. A unique diversity of these enzymes provides the basis, and undoubtedly will provide the basis also in future, for their newer and newer applications in various branches of industry and in medicine. Some of these uses have been currently even difficult to define owing to a small number of known proteases synthesized by microbial species. However, studies on proteolytic enzymes from extremophilic microorganisms caused that over the last decades the number of available commercial preparations of proteases has been increasing and will increase in future, as may be predicted based on the current tendency. Notwithstanding this progress, only a small part of microflora populating extreme biotopes has been known so far, and it means that the majority of catalytic proteins, synthesized by this microflora, including most valuable, unique enzymes, have not been available for industry. Analyses of metagenomes and metatranscriptomes derived from extreme environments, which already enabled isolation of genes encoding unique proteases are a promising approach to synthesis of a greater number of such enzymes, in particular those from microorganisms that have hitherto been unculturable under laboratory conditions. Such studies should be intensified. However, also classical prospecting for novel extremophilic microorganisms, synthesizing proteolytic enzymes should be continued. Application of improved, quick techniques of DNA sequencing caused a rapid growth in a number of available complete microbial genomes in databases. Mining of these genomes may give rise to finding new variants of proteolytic enzymes. Also the studies on subtle molecular adaptations of extremophilic proteases are very promising. Their results may facilitate rational engineering of already known proteases, and in further perspective, may be used to design novel variants of these enzymes that will be tailored to specific applications and industrial conditions. There is also an urgent need to establish novel expression systems, dedicated to enzymes from extremophilic microorganisms because expression of genes encoding these enzymes in mesophilic hosts is either insufficiently efficient or often, even impossible, that generally hinders broader application of many proteases with properties interesting for industry and medicine.
References
Akolkar AV, Desai AJ (2010) Catalytic and thermodynamic characterization of protease from Halobacterium sp. SP1(1). Res Microbiol 161(5):355–362
Akolkar AV, Deshpande GM, Raval KN, Durai D, Nerurkar AS, Desai AJ (2008) Organic solvent tolerance of Halobacterium sp. SP1(1) and its extracellular protease. J Basic Microbiol 48(5):421–425
Akolkar AV, Durai D, Desai AJ (2010) Halobacterium sp. SP1(1) as a starter culture for accelerating fish sauce fermentation. J Appl Microbiol 109(1):44–53
Alam SI, Dube S, Reddy GSN, Bhattacharya BK, Shivaji S, Singh L (2005) Purification and characterization of extracellular protease produced by Clostridium sp. from Schirmacher oasis, Antarctica. Enzyme Microb Technol 36(5–6):824–831
Alam S, Dube S, Agarwal M, Singh L (2006) Purification and characterization of an extracellular protease produced by psychrotolerant Clostridium sp. LP3 from lake sediment of Leh, India. Can J Microbiol 52(12):1238–1246
Amoozegar MA, Fatemi ZA, Karbalaei-Heidari HR, Razavi MR (2007) Production of an extracellular alkaline metalloprotease from a newly isolated, moderately halophile, Salinivibrio sp. strain AF-2004. Microbiol Res 162(4):369–377
Aqel H, Al-Quadan F, Yousef TK (2012) A novel neutral protease from thermophilic Bacillus strain HUTBS62. J Biosci Biotechnol 1(2):117–123
Arulmani M, Aparanjini K, Vasanthi K, Arumugam P, Arivuchelvi M, Kalaichelvan PT (2007) Purification and partial characterization of serine protease from thermostable alkalophilic Bacillus laterosporus-AK1. World J Microbiol Biotechnol 23(4):475–481
Baghel VS, Tripathi RD, Ramteke RW, Gopal K, Dwivedi S, Jain RK, Rai UN, Singh SN (2005) Psychrotrophic proteolytic bacteria from cold environments of Gangotri glacier, Western Himalaya, India. Enzyme Microb Technol 36(5–6):654–659
Bakhtiar S, Andersson MM, Gessesse A, Mattiasson B, Hatti-Kaul R (2002) Stability characteristics of a calcium-independent alkaline protease from Nesterenkonia sp. Enzyme Microb Technol 32(5):525–531
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2009) GenBank. Nucleic Acids Res 37:D26–D31
Biver S, Portetelle D, Vandenbol M (2013) Characterization of a new oxidant-stable serine protease isolated by functional metagenomics. SpringerPlus 2:410
Blumentals II, Robinson AS, Kelly RM (1990) Characterization of sodium dodecyl sulfate-resistant proteolytic activity in the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56(7):1992–1998
Bongers J, Heimer EP (1994) Recent applications of enzymatic peptide synthesis. Peptides 15(1):183–193
Cannio R, Catara G, Fiume I, Balestrieri M, Rossi M, Palmieri G (2010) Identification of a cell-bound extracellular protease overproduced by Sulfolobus solfataricus in peptide-rich media. Protein Pept Lett 17(1):78–85
Capiralla H, Hiroi T, Hirokawa T, Maeda S (2002) Purification and characterization of a hydrophobic amino acid-specific endopeptidase from Halobacterium halobium S9 with potential application in debittering of protein hydrolysates. Process Biochem 38(4):571–579
Carter P, Nilsson B, Burnier JP, Burdick D, Wells JA (1989) Engineering subtilisin BPN’ for site-specific proteolysis. Proteins: Struct Funct Bioinf 6(3):240–248
Catara G, Ruggiero G, La Cara F, Digilio FA, Capasso A, Rossi M (2003) A novel extracellular subtilisin-like protease from the hyperthermophile Aeropyrum pernix K1: biochemical properties, cloning, and expression. Extremophiles 7(5):391–399
Cavicchioli R, Charlton T, Ertan H, Mohd Omar S, Siddiqui KS, Williams TJ (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4(4):449–460
Chavez Croocker P, Sako Y, Uchida A (1999) Purification and characterization of an intracellular heat-stable proteinase (pernilase) from the marine hyperthermophilic archaeon Aeropyrum pernix K1. Extremophiles 3(1):3–9
Chen K, Arnold FH (1993) Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide. Proc Natl Acad Sci U S A 90(12):5618–5622
Chen XL, Zhang YZ, Gao PJ, Luan XW (2003) Two different proteases produced by a deep-sea psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913. Mar Biol 143(5):989–993
Choi IG, Bang WG, Kim SH, Yu YG (1999) Extremely thermostable serine-type protease from Aquifex pyrophilus. Molecular cloning, expression, and characterization. J Biol Chem 274(2):881–888
Cowan DA, Daniel RM (1982) Purification and some properties of an extracellular protease (caldolysin) from an extreme thermophile. Biochim Biophys Acta 705(3):293–305
Damare S, Raghukumar C, Muraleedharan U, Raghukumar S (2006) Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzyme Microb Technol 39(2):172–181
Dastager SG, Dayanand A, Li WJ, Kim CJ, Lee JC, Park DJ, Tian XP, Raziuddin QS (2008) Proteolytic activity from an alkali-thermotolerant Streptomyces gulbargensis sp. nov. Curr Microbiol 57(6):638–642
Davail S, Feller G, Narinx E, Gerday C (1994) Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA41. J Biol Chem 269(26):17448–17453
De Azeredo L, Freire D, Soares R, Leite S, Coelho R (2004) Production and partial characterization of thermophilic proteases from Streptomyces sp. isolated from Brazilian cerrado soil. Enzyme Microb Technol 34(3–4):354–358
De Castro RE, Maupin-Furlow JA, Giménez MI, Herrera Seitz MK, Sánchez JJ (2005) Haloarchaeal proteases and proteolytic systems. FEMS Microbiol Rev 30(1):17–35
Deetz JS, Rozzell JD (1988) Enzyme-catalysed reactions in non-aqueous media. Trends Biotechnol 6(1):15–19
Deng A, Wu J, Zhang Y, Zhang G, Wen T (2010) Purification and characterization of a surfactant-stable high-alkaline protease from Bacillus sp. B001. Bioresour Technol 101(18):7100–7106
Dib R, Chobert JM, Dalgalarrondo M, Barbier G, Haertlé T (1998) Purification, molecular properties and specificity of a thermoactive and thermostable proteinase from Pyrococcus abyssi, strain st 549, hyperthermophilic archaea from deep-sea hydrothermal ecosystem. FEBS Lett 431(2):279–284
Dixit VS, Pant A (2000) Comparative characterization of two serine endopeptidases from Nocardiopsis sp. NCIM 5124. Biochim Biophys Acta 1523(2–3):261–268
Doddapaneni KK, Tatineni R, Vellanki RV, Gandu B, Panyala NR, Chakali B, Mangamoori LN (2007) Purification and characterization of two novel extra cellular proteases from Serratia rubidaea. Process Biochem 42(8):1229–1236
Dodia MS, Rawal CM, Bhimani HG, Joshi RH, Khare SK, Singh SP (2008) Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6. J Ind Microbiol Biotechnol 35(2):121–131
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48(13):270–282. doi:10.1016/j.bej.2009.09.009
Dozie IN, Okeke CN, Unaeze NC (1994) A thermostable, alkaline-active, keratinolytic proteinase from Chrysosporium keratinophilum. World J Microbiol Biotechnol 10(5):563–567
Eggen R, Geerling A, Watts J, de Vos WM (1990) Characterization of pyrolysin, a hyperthermoactive serine protease from the archaebacterium Pyrococcus furiosus. FEMS Microbiol Lett 71(1–2):17–20
Elmore DT (2002) Peptide synthesis. In: Barret GC, Davies JS (eds) Amino acids, peptides and proteins: Volume 33. RSC Publishing, UK, pp. 83–134
Estell DA, Graycar TP, Wells JA (1985) Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J Biol Chem 260(11):6518–6521
Feller G (2003) Molecular adaptations to cold in psychrophilic enzymes. Cell Mol Life Sci 60(4):648–662
Feller G (2013) Psychrophilic enzymes: from folding to function and biotechnology. Scientifica. Article ID 512840
Fornbacke M, Clarsund M (2013) Cold-adapted proteases as an emerging class of therapeutics. Infect Dis Ther 2(1):15–26
Freeman SA, Peek K, Prescott M, Daniel R (1993) Characterization of a chelator-resistant protease from Thermus strain Rt4A2. Biochem J 295(Pt 2):463–469
Fujinami S, Fujisawa M (2010) Industrial applications of alkaliphiles and their enzymes – past, present and future. Environ Technol 31(8–9):845–856. doi:10.1080/09593331003762807
Fusek M, Lin XL, Tang J (1990) Enzymic properties of thermopsin. J Biol Chem 265(3):1496–1501
George S, Raju V, Krishnan MRV, Subramanian TV, Jayaraman K (1995) Production of protease by Bacillus amyloliquefaciens in solid-state fermentation and its application in the unhairing of hides and skins. Process Biochem 30(5):457–462
Gessesse A, Hatti-Kaul R, Gashe BA, Mattiasson B (2003) Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzym Microb Technol 32(5):519–524
Giménez MI, Studdert CA, Sánchez JJ, De Castro RE (2000) Extracellular protease of Natrialba magadii: purification and biochemical characterization. Extremophiles 4(3):181–188
Giri SS, Sukumaran V, Sen SS, Oviya M, Banu BN, Jena PK (2011) Purification and partial characterization of a detergent and oxidizing agent stable alkaline protease from a newly isolated Bacillus subtilis VSG-4 of tropical soil. J Microbiol 49(3):455–461
Glass JD (1981) Enzymes as reagent in the synthesis of peptides. Enzyme Microb Technol 3(1):2–8
Gohel SD, Singh SP (2012) Purification strategies, characteristics and thermodynamic analysis of a highly thermostable alkaline protease from a salt-tolerant alkaliphilic actinomycete, Nocardiopsis alba OK-5. J Chromatogr B Analyt Technol Biomed Life Sci 889–890:61–68
Gohel SD, Singh SP (2013) Characteristics and thermodynamics of a thermostable protease from a salt-tolerant alkaliphilic actinomycete. Int J Biol Macromol 56:20–27
Gomes J, Steiner W (2004) The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol 42(4):223–235
Graziano G, Merlino A (2014) Molecular bases of protein halotolerance. Biochim Biophys Acta 1844(4):850–858
Grøn H, Bech LM, Branner S, Breddam K (1990) A highly active and oxidation-resistant subtilisin-like enzyme produced by a combination of side-directed mutagenesis and chemical modification. Eur J Biochem 194(3):897–901
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial application. Appl Microbiol Biotechnol 59(1):15–32
Gupta A, Roy I, Patel RK, Singh SP, Khare SK, Gupta MN (2005) One-step purification and characterization of an alkaline protease from haloalkaliphilic Bacillus sp. J Chromatogr A 1075(1–2):103–108
Halling PJ (1994) Thermodynamic prediction for biocatalysis in nonconventional media: theory, tests, and recommendations for experimental design and analysis. Enzyme Microb Technol 16(3):178–206
Hameed A, Keshavarz T, Evans CS (1999) Effect of dissolved oxygen tension and pH on the production of extracellular protease from a new isolate of Bacillus subtilis K2, for use in leather processing. J Chem Technol Biotechnol 74(1):5–8
Hao JH, Sun M (2015) Purification and characterization of a cold alkaline protease from a psychrophilic Pseudomonas aeruginosa HY1215. Appl Biochem Biotechnol 175(2):715–722
Hasan AKMQ, Tamiya E (2001) Cold-active protease CP70. Patent US 6200793
Hasbay Ifrij I, Ogel ZB (2002) Production of neutral and alkaline extracellular proteases by the thermophilic fungus, Scytalidium thermophilum, grown on microcrystalline cellulose. Biotechnol Lett 24(13):1107–1110
Hiraga K, Nishikata Y, Namwong S, Tanasupawat S, Takada K, Oda K (2005) Purification and characterization of serine proteinase from a halophilic bacterium, Filobacillus sp. RF2-5. Biosci Biotechnol Biochem 69(1):38–44
Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63(4):735–750
Hui Z, Doi H, Kanouchi H, Matsuura Y, Mohri S, Nonomura Y, Oka T (2004) Alkaline serine protease produced by Streptomyces sp. degrades PrP(Sc). Biochem Biophys Res Commun 321(1):45–50
Huston A, Methe B, Deming J (2004) Purification, characterization, and sequencing of an extracellular cold-active aminopeptidase produced by marine psychrophile Colwellia psychrerythraea strain 34H. Appl Environ Microbiol 70(6):3321–3328
Ignatova Z, Gousterova A, Spassov G, Nedkov P (1999) Isolation and partial characterization of extracellular keratinase from a wool degrading thermophilic actinomycete strain Thermoactinomyces candidus. Can J Microbiol 45(3):217–222
Iqbal I, Aftab MN, Afzal M, Ur-Rehman A, Aftab S, Zafat A, Ud-Din Z, Khuharo AR, Iqbal J, Ul-Haq I (2015) Purification and characterization of cloned alkaline protease gene of Geobacillus stearothermophilus. J Basic Microbiol 55(2):160–171
Izotova LS, Strongin AY, Chekulaeva LN, Sterkin VE, Ostoslavskaya VI, Lyublinskaya LA, Timokhina EA, Stepanov VM (1983) Purification and properties of serine protease from Halobacterium halobium. J Bacteriol 155(2):826–830
Jang HJ, Kim BC, Pyun YR, Kim YS (2002) A novel subtilisin-like serine protease from Thermoanaerobacter yonseiensis KB-1: its cloning, expression, and biochemical properties. Extremophiles 6(3):233–243
Jaouadi B, Ellouz-Chaabouni S, Rhimi M, Bejar S (2008) Biochemical and molecular characterization of a detergent stable serine alkaline protease from Bacillus pumilus CBS with high catalytic efficiency. Biochimie 90(9):1291–1305
Jaouadi B, Ellouz-Chaabouni S, Ben Ali M, Ben Messaoud E, Naili B, Dhouib A, Bejar S (2009) Excellent laundry detergent compatibility and high dehairing ability of the Bacillus pumilus CBS alkaline proteinase (SAPB). Biotechnol Bioprocess Eng 14:503–512
Jaouadi B, Abdelmalek B, Fodil D, Ferradji FZ, Rekik H, Zarai N, Bejar S (2010a) Purification and characterization of a thermostable keratinolytic serine alkaline proteinase from Streptomyces sp. strain AB1 with high stability in organic solvents. Bioresour Technol 101(21):8361–8369
Jaouadi B, Aghajari N, Haser R, Bejar S (2010b) Enhancement of the thermostability and the catalytic efficiency of Bacillus pumilus CBS protease by site-directed mutagenesis. Biochimie 92(4):360–369
Joo HS, Chang CS (2005) Oxidant and SDS-stable alkaline protease from a halo-tolerant Bacillus clausii I-52: enhanced production and simple purification. J Appl Microbiol 98(2):491–497
Joo HS, Kumar CG, Park GC, Paik SR, Chang CS (2003) Oxidant and SDS-stable alkaline protease from Bacillus clausii I-52: production and some properties. J Appl Microbiol 95(2):267–272
Joshi S, Satyanarayana T (2013a) Biotechnology of cold-active proteases. Biology 2(2):755–783
Joshi S, Satyanarayana T (2013b) Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Bioresour Technol 131:76–85
Kamekura M, Dyall-Smith ML (1995) Taxonomy of the family Halobacteriaceae and the description of two new genera Halorubrobacterium and Natrialba. J Gen Appl Microbiol 41(4):333–350
Kamekura M, Seno Y (1990) A halophilic extracellular protease from a halophilic archaebacterium strain 172 P1. Biochem Cell Biol 68(1):352–359
Kamekura M, Seno Y, Dyall-Smith ML (1996) Halolysin R4, a serine proteinase from the halophilic archaeon Haloferax mediterranei; gene cloning, expression and structural studies. Biochim Biophys Acta 1294(2):159–167
Karan R, Khare SK (2010) Purification and characterization of a solvent-stable protease from Geomicrobium sp. EMB2. Environ Technol 31(10):1061–1072
Karan R, Singh SP, Kapoor S, Khare SK (2011) A novel organic solvent tolerant protease from a newly isolated Geomicrobium sp. EMB2 (MTCC 10310): production optimization by response surface methodology. New Biotechnol 28(2):138–145
Karbalaei-Heidari HR, Shahbazi M, Absalan G (2013) Characterization of a novel organic solvent tolerant protease from a moderately halophilic bacterium and its behavior in ionic liquids. Appl Biochem Biotechnol 170(3):573–586
Khan F (2013) New microbial proteases in leather and detergent industries. Innov Res Chem 1(1):1–6
Kikani BA, Shukla RJ, Singh SP (2010) Biocatalytic potential of thermophilic bacteria and actinomycetes. In: Mendez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Research Center, Badajoz, Spain, pp 1000–1007
Kim J, Dordick JS (1997) Unusual salt and solvent dependence of a protease from an extreme halophile. Biotechnol Bioeng 55(3):471–479
Kim T, Lei XG (2005) Expression and characterization of a thermostable serine protease (TfpA) from Thermomonospora fusca YX in Pichia pastoris. Appl Microbiol Biotechnol 68(3):355–359
Kim W, Choi K, Kim Y, Park H, Choi J, Lee Y, Oh H, Kwon I, Lee S (1996) Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol 62(7):2482–2488
Kimura Y, Muraya K, Araki Y, Matsuoka H, Nakanishi K, Matsuno R (1990a) Synthesis peptides consisting of essential amino acids by a reactor system using three proteinases and an organic solvent. Agric Biol Chem 54(12):3331–3333
Kimura Y, Nakanishi K, Matsuno R (1990b) Enzymatic synthesis of the precursor of Leu-enkephalin in water-immiscible organic solvent systems. Enzyme Microb Technol 12(4):273–280
Kimura Y, Yoshida T, Muraya K, Nakanishi K, Matsuno R (1990c) Continuous synthesis of a tripeptide by successive condensation and transesterification catalyzed by two immobilized proteinases in organic solvent. Agric Biol Chem 54(6):1433–1440
Klein UJ, Prykhodzka A, Cerovsky V (2000) The applicability of subtilisin Carlsberg in peptide synthesis. J Pept Sci 6(11):541–549
Kleine R (1982) Properties of thermitase, a thermostable serine protease from Thermoactinomyces vulgaris. Acta Biol Med Ger 41(1):89–102
Klingeberg M, Galunsky B, Sjoholm C, Kasche V, Antranikian G (1995) Purification and properties of a highly thermostable, sodium dodecyl sulfate-resistant and stereospecific proteinase from the extremely thermophilic archaeon Thermococcus stetteri. Appl Environ Microbiol 61(8):3098–3104
Knight ZA, Garrison JL, Chan K, King DS, Shokat KM (2007) A remodelled protease that cleaves phosphotyrosine substrates. J Am Chem Soc 129(38):11672–11673
Kobayashi T, Hakamada Y, Adachi S, Hitomi J, Yoshimatsu T, Koike K, Kawai S, Ito S (1995) Purification and properties of an alkaline protease from alkalophilic Bacillus sp. KSM-K16. Appl Microbiol Biotechnol 43(3):473–481
Kobayashi T, Lu J, Li Z, Hung VS, Kurata A, Hatada Y, Takai K, Ito S, Horikoshi K (2007) Extremely high alkaline protease from a deep-subsurface bacterium, Alkaliphilus transvaalensis. Appl Microbiol Biotechnol 75(1):71–80
Kuddus M, Ramteke PW (2009) Cold-active extracellular alkaline protease from an alkaliphilic Stenotrophomonas maltophilia: production of enzyme and its industrial applications. Can J Microbiol 55(11):1294–1301
Kuddus M, Ramteke PW (2011) Production optimization of an extracellular cold-active alkaline protease from Stenotrophomonas maltophilia MTCC 7528 and its application in detergent industry. Afr J Microbiol Res 5(7):809–816
Kuddus M, Ramteke PW (2012) Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol 38(4):330–338
Kudrya VA, Simonenko IA (1994) Alkaline serine proteinase and lectin isolation from the culture fluid of Bacillus subtilis. Appl Microbiol Biotechnol 41(5):505–509
Kühn D, Dürrschmidt P, Mansfeld J, Ulbrich-Hofmann R (2002) Boilysin and thermolysin in dipeptide synthesis: a comparative study. Biotechnol Appl Biochem 36(Pt 1):71–76
Kullmann W (1982) Protease-catalyzed peptide bond formation: application to synthesis of the COOH-terminal octapeptide of cholecystokinin. Proc Natl Acad Sci U S A 79(9):2840–2844
Kumar D, Bhalla TC (2005) Microbial proteases in peptide synthesis: approaches and applications. Appl Microbiol Biotechnol 68(6):726–736
Kumar D, Savitri, Thakur N, Verma R, Bhalla TC (2008) Microbial proteases and application as laundry detergent additive. Res J Microbiol 3(12):661–672
Kumar CG, Tiwari MP, Jany KD (1999) Novel alkaline serine proteases from alkalophilic Bacillus spp.: purification and some properties. Process Biochem 34(5):441–449
Laane C, Boeren S, Vos K, Veeger C (1987) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 30(1):81–87
Lagzian M, Asoodeh A (2012) An extremely thermotolerant, alkaliphilic subtilisin-like protease from hyperthermophilic Bacillus sp. MLA64. Int J Biol Macromol 51(5):960–967
Lama L, Romano I, Calandrelli V, Nicolaus B, Gambacorta A (2005) Purification and characterization of a protease produced by an aerobic haloalkaliphilic species belonging to the Salinivibrio genus. Res Microbiol 156(4):478–484
Langeveld JP, Wang JJ, Van de Wiel DF, Shih GC, Garssen GJ, Bossers A, Shih JC (2003) Enzymatic degradation of prion protein in brain team stem from infected cattle and sheep. J Infect Dis 188(11):1782–1789
Li AN, Li DC (2009) Cloning, expression and characterization of the serine protease gene from Chaetomium thermophilum. J Appl Microbiol 106(2):369–380
Li WF, Zhou XX, Lu P (2005) Structural features of thermozymes. Biotechnol Adv 23(4):271–281
Li AN, Ding AY, Chen J, Liu SA, Zhang M, Li DC (2007) Purification and characterization of two thermostable proteases from the thermophilic fungus Chaetomium thermophilum. J Microbiol Biotechnol 17(4):624–631
Li Q, Yi L, Marek P, Iverson BL (2013) Commercial proteases:present and future. FEBS Lett 587(8):1155–1163
Lin X, Tang J (1990) Purification, characterization, and gene cloning of thermopsin, a thermostable acid protease from Sulfolobus acidocaldarius. J Biol Chem 265(3):1490–1495
Litchfield CD (2011) Potential for industrial products from the halophilic Archaea. J Ind Microbiol Biotechnol 38(10):1635–1647
Lü J, Wu X, Jiang Y, Cai X, Huang L, Yang Y, Wang H, Zeng A, Li A (2014) An extremophile Microbacterium strain and its protease production under alkaline conditions. J Basic Microbiol 54(5):378–385
Majeed T, Tabassum R, Orts WJ, Lee CC (2013) Expression and characterization of Coprothermobacter proteolyticus alkaline serine protease. Sci World J. Article ID 396156
Manikandan M, Pašić L, Kannan V (2009) Purification and biological characterization of a halophilic thermostable protease from Haloferax lucentensis VKMM 007. World J Microbiol Biotechnol 25(12):2247–2256
Marcy RM, Engelhardt TC, Upadhyay JM (1984) Isolation, partial characterization, and some properties of protease I from a thermophilic mold Thermoascus aurantiacus var. levisporus. Mycopathologia 87(1–2):57–65
Margesin R, Dieplinger H, Hofmann J, Sarg B, Lindner H (2005) A cold-active extracellular metalloprotease from Pedobacter cryoconitis - production and properties. Res Microbiol 156(4):499–505
Martinez R, Jakob F, Tu R, Siegert P, Maurer KH, Schwaneberg U (2013) Increasing activity and thermal resistance of Bacillus gibsonii alkaline protease (BgAP) by directed evolution. Biotechnol Bioeng 110(3):711–720
Matsuzawa H, Tokugawa K, Hamaoki M, Mizoguchi M, Taguchi H, Terada I, Kwon ST, Ohta T (1988) Purification and characterization of aqualysin I (a thermophilic alkaline serine protease) produced by Thermus aquaticus YT-1. Eur J Biochem 171(3):441–447
Maurer KH (2004) Detergent proteases. Curr Opin Biotechnol 15(4):330–334
Mei HC, Liaw YC, Li YC, Wang DC, Takagi H, Tsai YC (1998) Engineering subtilisin YaB: restriction of substrate specificity by the substitution of Gly124 and Gly151 with Ala. Protein Eng 11(2):109–117
Mitsuiki S, Sakai M, Moriyama Y, Goto M, Furukawa K (2002) Purification and some properties of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Biosci Biotechnol Biochem 66(1):164–167
Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH (2000) Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol 297(4):1015–1026
Moreno ML, Garcia MT, Ventosa A, Mellado E (2009) Characterization of Salicola sp. IC10, a lipase- and protease-producing extreme halophile. FEMS Microbiol Ecol 68(1):59–71
Morihara K, Oka T (1981) Peptide bond synthesis catalyzed by subtilisin, papain, and pepsin. J Biochem 89(2):385–395
Morikawa M, Izawa Y, Rashid N, Hoaki T, Imanaka T (1994) Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl Environ Microbiol 60(12):4559–4566
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39(2):144–167
Najafi MF, Deobagkar D, Deobagkar D (2005) Potential application of protease isolated from Pseudomonas aeruginosa PD 100. Electron J Biotechnol 8(2):197–203
Naki D, Paech C, Ganshaw G, Schellenberger V (1998) Selection of a subtilisin-hyperproducing Bacillus in a highly structured environment. Appl Microbiol Biotechnol 49(3):290–294
Nam GW, Lee DW, Lee HS, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT, Pyun YR (2002) Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol 178(6):538–547
Namwong S, Hiraga K, Takada K, Tsunemi M, Tanasupawat S, Oda K (2006) A halophilic serine proteinase from Halobacillus sp. SR5-3 isolated from fish sauce: purification and characterization. Biosci Biotechnol Biochem 70(6):1395–1401
Narhi LO, Stabinsky Y, Miller L, Sachdev R, Finley S, Park S, Kolvenbach C, Arakawa T, Zukowski M (1991) Enhanced stability of subtilisin by three point mutations. Biotechnol Appl Biochem 13(1):12–24
Narinx E, Baise E, Gerday C (1997) Subtilisin from psychrophilic antarctic bacteria: characterization and site-directed mutagenesis of residues possibly involved in the adaptation to cold. Protein Eng 10(11):1271–1279
Neveu J, Regeard C, DuBow MS (2011) Isolation and characterization of two serine proteases from metagenomic libraries of the Gobi and Death Valley deserts. Appl Microbiol Biotechnol 91(3):635–644
Nordberg P, von Hofsten B (1969) Proteolytic enzymes from extremely halophilic bacteria. J Gen Microbiol 55(2):251–256
Ogino H, Watanabe F, Yamada M, Nakagawa S, Hirose T, Noguchi A, Yasuda M, Ishikawa H (1999a) Purification and characterization of organic solvent-stable protease from organic solvent-tolerant Pseudomonas aeruginosa PST-01. J Biosci Bioeng 87(1):61–68
Ogino H, Yamada M, Watanabe F, Ichinose H, Yasuda M, Ishikawa H (1999b) Peptide synthesis catalyzed by organic-solvent-stable protease from Pseudomonas aeruginosa PST-01 in monophasic aqueous-organic solvent systems. J Biosci Bioeng 88(5):513–518
Ong PS, Gaucher GM (1976) Production, purification and characterization of thermomycolase, the extracellular serine protease of the thermophilic fungus Malbranchea pulchella var. sulfurea. Can J Microbiol 22(2):165–176
Pandey S, Rakholiya KD, Raval VH, Singh SP (2012) Catalysis and stability of an alkaline protease from a haloalkaliphilic bacterium under non-aqueous conditions as a function of pH, salt and temperature. J Biosci Bioeng 114(3):251–256
Pantoliano MW, Ladner RC, Bryan PN, Rollence ML, Wood JF, Poulos TL (1987) Protein engineering of subtilisin BPN’: enhanced stabilization through the introduction of two cysteines to form a disulfide bond. Biochemistry 26(8):2077–2082
Pantoliano MW, Whitlow M, Wood JF, Rollence ML, Finzel BC, Gillialand GL, Poulos TL, Bryan PN (1988) The engineering of binding affinity at metal ion binding sites for the stabilization of proteins: subtilisin as a test case. Biochemistry 27(22):8311–8317
Paul T, Das A, Mandal A, Jana A, Halder SK, Das Mohapatra PK, Pati BR, Mondal KC (2014) Smart cleaning properties of a multi tolerance keratinolytic protease from an extremophilic Bacillus tequilensis hsTKB2: prediction of enzyme modification site. Waste Biomass Valoriz 5(6):931–945
Pawar R, Zambare V, Barve S, Paratkar G (2009) Application of protease isolated from Bacillus sp. 158 in enzymatic cleansing of contact lenses. Biotechnology 8(2):276–280
Pedersen NR, Wimmer R, Matthiesen R, Pedersen LH, Gessesse A (2003) Synthesis of sucrose laurate using a new alkaline protease. Tetrahedron Asymmetry 14(6):667–673
Peek K, Daniel RM, Monk C, Parker L, Coolbear T (1992) Purification and characterization of a thermostable proteinase isolated from Thermus sp. strain Rt41A. Eur J Biochem 207(3):1035–1044
Phadatare SU, Deshpande VV, Srinivasan MC (1993) High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): enzyme production and compatibility with commercial detergents. Enzyme Microb Technol 15(1):72–76
Phrommao E, Yongsawatdigul J, Rodtong S, Yamabhai M (2011) A novel subtilase with NaCl-activated and oxidant-stable activity from Virgibacillus sp. SK37. BMC Biotechnol 11:65
Puri S (2001) An alkaline protease from a Bacillus sp.: production and potential applications in detergent formulation and degumming of silk. MSc thesis, University of Delhi, New Delhi
Purohit MK, Singh SP (2011) Comparative analysis of enzymatic stability and amino acid sequences of thermostable alkaline proteases from two haloalkaliphilic bacteria isolated from Coastal region of Gujarat, India. Int J Biol Macromol 49(1):103–112
Pushpam PL, Rajesh T, Gunasekaran P (2011) Identification and characterization of alkaline serine protease from goat skin surface metagenome. AMB Express 1(1):3
Qua D, Simidu U, Taga N (1981) Purification and some properties of halophilic protease produced by a moderately halophilic marine Pseudomonas sp. Can J Microbiol 27(5):505–510
Rai SK, Roy JK, Mukherjee AK (2010) Characterization of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Appl Microbiol Biotechnol 85(5):1437–1450
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62(3):597–635
Raval VH, Pillai S, Rawal CM, Singh SP (2014) Biochemical and structural characterization of a detergent-stable serine alkaline protease from seawater haloalkaliphilic bacteria. Process Biochem 49(6):955–962
Ray MK, Devi KU, Kumar GS, Shivaji S (1992) Extracellular protease from the Antarctic yeast Candida humicola. Appl Environ Microbiol 58(6):1918–1923
Rheinnecker M, Baker G, Eder J, Fersht AR (1993) Engineering a novel specificity in subtilisin BPN’. Biochemistry 32(5):1199–1203
Rheinnecker M, Eder J, Pandey PS, Fersht AR (1994) Variants of subtilisin BPN’ with altered specificity profiles. Biochemistry 33(1):221–225
Riva S, Nonini M, Ottolina G, Danieli B (1998) Subtilisin-catalyzed esterification of di- and oligosaccharides containing a D-fructose moiety. Carbohydr Res 314(3–4):259–266
Ruiz DM, De Castro RE (2007) Effect of organic solvents on the activity and stability of an extracellular protease secreted by the haloalkaliphilic archaeon Natrialba magadii. J Ind Microbiol Biotechnol 34(2):111–115. doi:10.1007/s10295-006-0174-4
Ruiz DM, Iannuci NB, Cascone O, De Castro RE (2010) Peptide synthesis catalysed by a haloalkaliphilic serine protease from the archaeon Natrialba magadii (Nep). Lett Appl Microbiol 51(6):691–696
Saba I, Qazi PH, Rather SA, Dar RA, Qadri QA, Ahmad N, Johri S, Taneja SC, Shawl S (2012) Purification and characterization of a cold active alkaline protease from Stenotrophomonas sp., isolated from Kashmir, India. World J Microbiol Biotechnol 28(3):1071–1079
Saeki K, Hitomi J, Okuda M, Hatada Y, Kageyama Y, Takaiwa M, Kubota H, Hagihara H, Kobayashi T, Kawai S, Ito S (2002) A novel species of alkaliphilic Bacillus that produces an oxidatively stable alkaline serine protease. Extremophiles 6(1):65–72
Saha S, Dhanasekaran D, Shanmugapriya S, Latha S (2013) Nocardiopsis sp. SD5: a potent feather degrading rare actinobacterium isolated from feather waste in Tamil Nadu, India. J Basic Microbiol 53(7):608–616
Sakaguchi M, Takezawa M, Nakazawa R, Nozawa K, Kusakawa T, Nagasawa T, Sugahara Y, Kawakita M (2008) Role of disulphide bonds in a thermophilic serine protease Aqualysin I from Thermus aquaticus YT-1. J Biochem 143(5):625–632
Sako Y, Croocker PC, Ishida Y (1997) An extremely heat-stable extracellular proteinase (aeropyrolysin) from the hyperthermophilic archaeon Aeropyrum pernix K1. FEBS Lett 415(3):329–334
Sana B, Ghosh D, Saha M, Mukherjee J (2006) Purification and characterization of a salt, solvent, detergent and bleach tolerant protease from a new gamma-Proteobacterium isolated from marine environment of the Sundarbans. Process Biochem 41(1):208–215
Sánchez-Porro C, Mellado E, Bertoldo C, Antranikian G, Ventosa A (2003) Screening and characterization of the protease CP1 produced by the moderately halophilic bacterium Pseudoalteromonas sp. strain CP76. Extremophiles 7(3):221–228
Santos AF, Valle RS, Pacheco CA, Alvarez VM, Seldin L, Santos ALS (2013) Extracellular proteases of Halobacillus blutaparonensis strain M9, a new moderately halophilic bacterium. Braz J Microbiol 44(4):1299–1304
Sarethy IP, Saxena Y, Kapoor A, Sharma M, Sharma SK, Gupta V, Gupta S (2011) Alkaliphilic bacteria: applications in industrial biotechnology. J Ind Microbiol Biotechnol 38(7):769–790
Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, Feolo M, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Madden TL, Maglott DR, Miller V, Mizrachi I, Ostell J, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Yaschenko E, Ye J (2009) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 37:D5–D15
Schmitt W, Rdest U, Goebel W (1990) Efficient high-performance liquid chromatographic system for the purification of halobacterial serine protease. J Chromatogr A 521(2):211–220
Secades P, Alvarez B, Guijarro J (2003) Purification and properties of a new psychrophilic metalloprotease (Fpp2) in the fish pathogen Flavobacterium psychrophilum. FEMS Microbiol Lett 226(2):273–279
Selim S, Hagagy N, Aziz MA, El-Meleigy ES, Pessione E (2014) Thermostable alkaline halophilic-protease production by Natronolimnobius innermongolicus WN18. Nat Prod Res 28(18):1476–1479
Sellek GA, Chaudhuri JB (1999) Biocatalysis in organic media using enzymes from extremophiles. Enzyme Microb Technol 25(6):471–482
Setati ME (2010) Diversity and industrial potential of hydrolase-producing halophilic/halotolerant eubacteria. Afr J Biotechnol 9(11):1555–1560
Setyorini E, Takenaka S, Murakami S, Aoki K (2006) Purification and characterization of two novel halotolerant extracellular protease from Bacillus subtilis strain FP-133. Biosci Biotechnol Biochem 70(2):433–444
Shao Z, Zhao H, Giver L, Arnold FH (1998) Random-priming in vitro recombination: an effective tool for directed evolution. Nucleic Acids Res 26(2):681–683
Shi W, Tang XF, Huang Y, Gan F, Tang B, Shen P (2006) An extracellular halophilic protease SptA from a halophilic archaeon Natrinema sp. J7: gene cloning, expression and characterization. Extremophiles 10(6):599–606
Shi WL, Zhong CQ, Tang B, Shen P (2007) Purification and characterization of extracellular halophilic protease from haloarchaea Natrinema sp. R6-5. Acta Microbiol Sin 47(1):161–163
Singh J, Batra N, Sobti RC (2001) Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Process Biochem 36(8–9):781–785
Singhal P, Nigam VK, Vidyarthi AS (2012) Studies on production, characterization and applications of microbial alkaline proteases. Int J Adv Biotechnol Res 3(3):653–669
Sinha R, Khare SK (2012) Isolation of a halophilic Virgibacillus sp. EMB13: characterization of its protease for detergent application. Indian J Biotechnol 11:416–426
Sinha R, Khare SK (2013) Characterization of detergent compatible protease of a halophilic Bacillus sp. EMB9: differential role of metal ions in stability and activity. Bioresour Technol 145:357–361
Sinsuwan S, Rodtong S, Yongsawatdigul J (2010) A NaCl-stable serine proteinase from Virgibacillus sp. SK33 isolated from Thai fish sauce. Food Chem 119(2):573–579
Son E, Kim JI (2003) Multicatalytic alkaline serine protease from psychrotrophic Bacillus amyloliquefaciens S94. J Microbiol 41(1):58–62
Stepanov VM, Rudenskaya GN, Revina LP, Gryaznova YB, Lysogorskaya EN, Filippova IY, Ivanova II (1992) A serine proteinase of an archaebacterium, Halobacterium mediterranei. A homologue of eubacterial subtilisins. Biochem J 285(Pt 1):281–286
Stepanov VM (1996) Proteinases as catalysts in peptide synthesis. Pure Appl Chem 68(6):1335–1339
Strausberg SL, Alexander PA, Gallagher DT, Gillialand GL, Barnett BL, Bryan PN (1995) Directed evolution of a subtilisin with calcium-independent stability. Nat Biotechnol 13:669–673
Studdert CA, Herrera Seitz MK, Plasencia Gil MI, Sanchez JJ, De Castro RE (2001) Purification and biochemical characterization of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease. J Basic Microbiol 41(6):375–383
Suzuki Y, Tsujimoto Y, Matsui H, Watanabe K (2006) Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. J Biosci Bioeng 102(2):73–81
Synowiecki J (2010) Some application of thermophiles and their enzymes for protein processing. Afr J Biotechnol 9(42):7020–7025
Takagi H, Takahashi T, Momose H, Inouye M, Maeda Y, Matsuzawa H, Ohta T (1990) Enhancement of the thermostability of subtilisin E by introduction of a disulfide bond engineered on the basis of structural comparison with thermophilic serine protease. J Biol Chem 265(12):6874–6878
Takai H, Sakato K, Nakamizo N, Isowa Y (1981) Enzymatic synthesis of caerulein peptide. In: Oyama K, Nikimura S (eds) Peptide chemistry. Protein Research Foundation, Osaka, Japan, pp 213–241
Tariq AL, Reyaz AL, Prabakaran J (2011) Purification and characterization of 56 kDa cold active protease from Serratia marcescens. Afr J Microbiol Res 5(32):5841–5847
Thangam EB, Rajkumar GS (2002) Purification and characterization of alkaline protease from Alcaligenes faecalis. Biotechnol Appl Biochem 35(2):149–154
Thumar J, Singh SP (2007) Two-step purification of a highly thermostable alkaline protease from salt-tolerant alkaliphilic Streptomyces clavuligerus strain Mit-1. J Chromatogr B 854(1–2):198–203
Thumar JT, Singh SP (2009) Organic solvent tolerance of an alkaline protease from salt-tolerant alkaliphilic Streptomyces clavuligerus strain Mit-1. J Ind Microbiol Biotechnol 26(2):211–218
Tiberti M, Papaleo E (2011) Dynamic properties of extremophilic subtilisin-like serine-proteases. J Struct Biol 174(1):69–83
Toplak A, Wu B, Fusetti F, Quaedflieg PJ, Janssen DB (2013) Proteolysin, a novel highly thermostable and cosolvent-compatible protease from the thermophilic bacterium Coprothermobacter proteolyticus. Appl Environ Microbiol 79(18):5625–5632
Turkiewicz M, Pazgier M, Kalinowska H, Bielecki S (2003) A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 7(6):435–442
van den Burg B, Enequist HG, van den Haar ME, Eijsink VG, Stulp BK, Venema G (1991) A highly thermostable neutral protease from Bacillus caldolyticus: cloning and expression of the gene in Bacillus subtilis and characterization of the gene product. J Bacteriol 173(13):4107–4115
Varela H, Ferrari MD, Belobrajdic L, Vazquez A, Loperena ML (1997) Skin unhairing proteases of Bacillus subtilis: production and partial characterization. Biotechnol Lett 19(8):755–758
Vazquez S, Ruberto L, Mac Cormak W (2005) Properties of extracellular proteases from three psychrotolerant Stenotrophomonas maltophilia isolated from Antarctic soil. Polar Biol 28(4):319–325
Vidyasagar M, Prakash S, Litchfield C, Sreeramulu K (2006) Purification and characterization of a thermostable, haloalkaliphilic extracellular serine protease from the extreme halophilic archaeon Halogeometricum borinquense strain TSS101. Archaea 2:51–57
Vidyasagar M, Prakash S, Mahajan V, Shouche YS, Sreeramulu K (2009) Purification and characterization of an extreme halothermophilic protease from a halophilic bacterium Chromohalobacter sp. TVSP101. Braz J Microbiol 40(1):12–19
VijayAnand S, Hemaprita J, Selvin J, Kiran S (2010) Production and optimization of haloalkaliphilic protease by an extremophile – Halobacterium sp. Js1, isolated from thalassohaline environment. Glob J Biotechnol Biochem 5(1):44–49
Voorhorst WGB, Eggen RIL, Geerling ACM, Platteeuw C, Siezen RJ, de Vos WM (1996) Isolation and characterization of the hyperthermostable serine protease, pyrolysin, and its gene from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 271(34):20426–20431
Waghmare SR, Gurav AA, Mali SA, Nadaf NH, Jadhav DB, Sonawane KD (2015) Purification and characterization of novel organic solvent tolerant 98 kDa alkaline protease from isolated Stenotrophomonas maltophilia strain SK. Protein Expr Purif 107:1–6
Wang QF, Miao JL, Hou YH, Ding Y, Wang GD, Li GY (2005) Purification and characterization of an extracellular cold-active serine protease from the psychrophilic bacterium Colwellia sp. NJ341. Biotechnol Lett 27(16):1195–1198
Wang QF, Hou HY, Xu Z, Miao JL, Li GY (2008) Purification and properties of an extracellular cold-active protease from the psychrophilic bacterium Pseudoalteromonas sp. NJ276. Biochem Eng J 38(3):362–368
Wilson SA, Daniel RM, Peek K (1994) Peptide synthesis with a proteinase from the extremely thermophilic organism Thermus Rt41A. Biotechnol Bioeng 44(3):337–346
Xiong H, Song L, Xu Y, Tsoi MY, Dobretsov S, Qian PY (2007) Characterization of proteolytic bacteria from the Aleutian deep-sea and their proteases. J Ind Microbiol Biotechnol 34(1):63–71
Xu B, Zhong Q, Tang X, Yang Y, Huang Z (2009) Isolation and characterization of a new keratinolytic bacterium that exhibits significant feather-degrading capability. Afr J Biotechnol 8(18):4590–4596
Yang C, Wang F, Hao J, Zhang K, Yuan N, Sun M (2010) Identification of a proteolytic bacterium, HW08, and characterization of its extracellular cold-active alkaline metalloprotease Ps5. Biosci Biotechnol Biochem 74(6):1220–1225
Yang XS, Chen XL, Xu XZ, Zeng RY (2011) Cold-adaptive alkaline protease from the psychrophilic Planomicrobium sp. 547: enzyme characterization and gene cloning. Adv Polar Sci 22(1):49–54
Yang J, Li J, Mai Z, Tian X, Zhang S (2013) Purification, characterization, and gene cloning of a cold-adapted thermolysin-like protease from Halobacillus sp. SCSIO 20089. J Biosci Bioeng 115(6):628–632
Yin J, Chen JC, Wu Q, Chen GQ (2014) Halophiles, coming stars for industrial biotechnology. Biotechnol Adv 33(7):1433–1442
Yu TX (1991) Proteases of haloalkaliphiles. In: Horikoshi K, Grand WD (eds) Superbugs: microorganisms in extreme environments. Japan Scientific Societies Press/Springer-Verlag, Tokyo/Berlin, pp 76–83
Yuan Q, Hayashi A, Kitamura Y, Shimada T, Na R, Jin X (2009) Purification and characterization of cold-adapted metalloprotease from deep sea water lactic acid bacteria Enterococcus faecalis TN-9. Int J Biol 1(2):12–21
Zeng R, Zhang R, Zhao J, Lin N (2003) Cold-active serine alkaline protease from the psychrophilic bacterium Pseudomonas strain DY-A: enzyme purification and characterization. Extremophiles 7(4):335–337
Zhao H, Arnold FH (1999) Directed evolution converts subtilisin E into a functional equivalent of thermitase. Protein Eng 12(1):47–53
Zhao GY, Zhou MY, Zhao HL, Chen XL, Xie BB, Zhang XY, He HL, Zhou BC, Zhang YZ (2012) Tenderization effect of cold-adapted collagenolytic protease MCP-01 on beef meat at low temperature and its mechanism. Food Chem 134(4):1738–1744
Zhu HY, Tian Y, Hou YH, Wang TH (2009) Purification and characterization of the cold-active alkaline protease from marine cold-adaptive Penicillium chrysogenum FS010. Mol Biol Rep 36(8):2169–2174
Internet Website
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Aneta Białkowska, Ewa Gromek, Tomasz Florczak, Joanna Krysiak, Katarzyna Szulczewska, and Marianna Turkiewicz declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Białkowska, A., Gromek, E., Florczak, T., Krysiak, J., Szulczewska, K., Turkiewicz, M. (2016). Extremophilic Proteases: Developments of Their Special Functions, Potential Resources and Biotechnological Applications. In: Rampelotto, P. (eds) Biotechnology of Extremophiles:. Grand Challenges in Biology and Biotechnology, vol 1. Springer, Cham. https://doi.org/10.1007/978-3-319-13521-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-13521-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13520-5
Online ISBN: 978-3-319-13521-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)