Abstract
A psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913, was isolated from deep-sea sediment collected at 1,855 m depth. Two proteases produced by Pseudoaltermonas sp. SM9913 were purified, MPC-01 and MCP-02. MCP-01 is a serine protease with a molecular weight of 60.7 kDa. It is cold-adapted with an optimum temperature of 30–35°C. Its K m and E a for the hydrolysis of casein were 0.18% and 39.1 kJ mol−1, respectively. It had low thermostability, and its activity was reduced by 73% after incubation at 40°C for 10 min. MCP-02 is a mesophilic metalloprotease with a molecular weight of 36 kDa. Its optimum temperature for the hydrolysis of casein was 50–55°C. The K m and E a of MCP-02 for the hydrolysis of casein were 0.36% and 59.3 kJ mol−1, respectively. MCP-02 had high thermostability, and its activity was reduced by only 30.5% after incubation at 60°C for 10 min. At low temperatures, Pseudoaltermonas sp. SM9913 mainly produced the psychrophilic protease MCP-01.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oceans constitute >70% of the earth's surface, of which about 60% is covered by water >2,000 m deep. Paradoxically, the oceans represent the earth's last environment to be explored with regards to microbiology, especially the abyssal and hadal oceans (depths >2,000 and 6,000 m, respectively). Most of the deep-sea environment is subject to high pressure, high salt concentration and low temperature, and an ecosystem exists including various animals and microorganisms adapted to these conditions. More and more attention is paid to the study of deep-sea creature diversity in order to discover new creatures, new types of medicine and new enzymes. Much of the interest in marine enzymes is related to their activity and stability under extreme reaction conditions. The psychrotrophic amylases produced by a deep-sea psychrophilic bacterial strain showed 35% and 40% of their maximum activity at 10°C (Hamamoto and Horikoshi 1991, 1993). The activity of alkaline serine protease produced by Sporosarcina sp. (isolated from the Japan Trench at 6,500 m) was nearly doubled at 60 MPa compared with the activity under atmospheric pressure (Kato et al. 1995).
We isolated a protease-excreting psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913, from deep-sea sediment collected at 1,855 m depth (Chen et al. 2001). Here, two different proteases produced by Pseudoaltermonas sp. SM9913 were purified, and their characters were compared.
Materials and methods
Bacterial strain
The psychrotrophic strain Pseudoaltermonas sp. SM9913 was isolated from deep-sea sediment collected at 1,855 m depth. Its isolation, identification and culture were described in a previous article (Chen et al. 2001). The GenBank accession number for the 16SrDNA sequence of this strain is AY305857.
Purification of proteases
Pseudoaltermonas sp. SM9913 was grown at 12°C in a seawater liquid medium composed of 2.0% corn powder, 2.0% bean powder, 1.0% wheat bran, 0.4% Na2PO4, 0.03% KH2PO4 and 0.1% CaCl2 (pH 7.5), mixed at 200 rpm for 72 h. The culture was centrifuged at 10,000 g for 12 min, and the supernatant was precipitated by slowly adding 55% solid ammonium sulfate powder. The precipitate was collected by centrifugation (8,500 g, 10 min) and dissolved in 50 mmol l−1 Tris-HCl buffer (pH 8.5). The sample was put on a column of Sephadex G25 to remove salt and pigment. Fractions showing protease activity were pooled and then concentrated. One-half of the concentrated sample was then put on a column of Sephadex G100. Fractions of protease activity peak top were pooled. The other half was put on a column of Sephadex DEAE A50, which had been equilibrated with 50 mmol l−1 Tris-HCl (pH 8.5). The bound proteins were eluted with the same buffer containing a linear NaCl gradient (0–0.8 mol l−1). Fractions showing protease activity were pooled and then put on a Sephadex G100 column. Fractions of the top of the protease activity peak were pooled. All purification procedures were carried out below 5°C.
Protease assay
Protease activity was measured by the digestion of casein; 1 ml diluted enzyme solution was mixed with 1 ml of 2.0% casein in 50 mM Tris-HCl (pH 8.5) and incubated for 10 min at a given temperature. The usual incubation temperatures were 30°C for MCP-01 and 50°C for MCP-02, except in experiments performed to determine the effect of temperature on enzyme activity. After incubation, the reaction was stopped by the addition of 2 ml of 0.4 M trichloroacetic acid. Then, the precipitate was removed by centrifugation (10,000 g), and 1 ml of supernatant was neutralized with 5 ml of 0.4 M sodium carbonate and incubated with 1 ml of 1 N Folin–Ciocalteu's reagent solution at 40°C for 20 min. Subsequently the absorbance at 660 nm was measured. One unit of activity was defined as the amount of enzyme that liberated 1 μg of tyrosine per milliliter of reaction mixture per minute.
The buffer used to determine the optimal pH of MCP-01 and MCP-02 covered a wide range of pH values from 2.6 to 12, and contained 6.008 g citrate acid, 3.893 g potassium phosphate monobasic, 1.769 g boric acid and 5.266 g barbital in 1 l; the pH was adjusted with 0.2 M NaOH.
Protein analysis
Protein concentration was measured by the method of Lowry et al. (1951). Bovine serum albumin was used as the standard. Protein content in column fractions was monitored by absorbance at 280 nm.
Electrophoresis
SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) of the purified proteases was done by the method of Laemmli (1970) using 12.5% polyacrylamide gel. The following proteins were used as standards: rabbit phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), ovalbumin (43 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (20.1 kDa) and lysozyme (14 kDa). After electrophoresis, the gel was stained with Coomassie brilliant blue R-250. Native-PAGE was done according to the same method as SDS-PAGE without SDS. Percent content of the protease in the gel was calculated by the software in "Gel Documentation and Analysis System of Gene Genius".
Results
Purification of two proteases produced by Pseudoaltermonas sp. SM9913
The proteases produced by Pseudoaltermonas sp. SM9913 were purified from the culture supernatant by the procedure described in "Materials and methods". After using Sephadex G25 and Sephadex G100 columns, one protease activity peak was obtained, and fractions of the top of the peak were pooled, with the protease activity yield of 16.6%. The purified protease showed a single protein band after SDS-PAGE, with a molecular weight of 60.7 kDa, which was named MCP-01 (Fig. 1). On a Sephadex DEAE A50 column, the protease was eluted with 0.4 M NaCl as a single protein peak that coincided with the peak of protease activity. The other protein peaks that eluted showed no proteolytic activity. After running on a Sephadex DEAE A50 column the protease was purified further by using a Sephadex G100 column, and the activity yield was 0.8%. The purified protease showed a single protein band after SDS-PAGE, with a molecular weight of 36 kDa, which was named MCP-02 (Fig. 2).
Character comparison of proteases MCP-01 and MCP-02
Effects of protease inhibitors
The effects of various protease inhibitors on the activities of the proteases MCP-01 and MCP-02 are summarized in Table 1. The activity of protease MCP-01 was inhibited by PMSF (phenylmethylsulfonyl fluoride), EDTA (ethylenediaminetetraacetic acid), sodium oxalate and tri-sodium citrate. PMSF is a special inhibitor of serine proteases, and oxalate and citrate are known to be Ca specific (Margesin and Schinner 1992). Therefore, the protease MCP-01 is a serine protease containing Ca2+. The protease MCP-02 was inhibited by o-phenanthroline, a special inhibitor of metalloproteinases with Zn2+ in their active center, which suggested that MCP-02 is a metalloproteinase containing Zn2+ (Table 1).
Optimum pH
The activities of the proteases MCP-01 and MCP-02 were measured from pH 5.0 to pH 10.0 in the buffer described in "Materials and methods". MCP-01 had the highest activity at pH 6.5–7.0, and MCP-02 had the highest activity at pH 8.0 (Fig. 3).
Optimum temperature
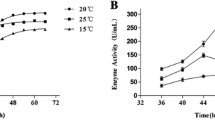
The effects of temperature on protease activity were examined by incubating the enzyme at 0–65°C for 10 min, using casein as the substrate. The optimum temperature for casein hydrolysis of the protease MCP-01 was 30–35°C and that of MCP-02 was 50–55°C. The protease MCP-01 at 0°C still showed 12.3% of maximum activity, and MCP-02 at 0°C only showed 0.86% of maximum activity (Fig. 4).
Thermostability
The effect of temperature on protease stability was examined by incubating the enzyme at different temperatures, respectively, for different time periods, and then the residual activity was measured. The protease MCP-01 was stable at 30°C for 20 min but its stability at 40°C was very low. The activity was reduced by 73% after incubation at 40°C for 10 min (Fig. 5). The protease MCP-02 was stable below 50°C. Its activity was reduced by 30.5% after incubation at 60°C for 10 min (Fig. 5). Therefore, MCP-02 had a higher thermostability than MCP-01.
Kinetic parameters
The activities of the proteases MCP-01 and MCP-02 were measured at 30°C and 50°C, respectively, using various concentrations of casein as a substrate, and Lineweaver–Burk plots were made (Fig. 6). The K m and V m of MCP-01 were, respectively, 0.18% and 67.6 μmol l−1 min−1 and the values of MCP-02 were 0.36% and 105.3 μmol l−1 min−1, respectively. Because casein has no standard molecular weight, percent concentration was used here according to common practice. These results showed that MCP-01 had greater affinity to the substrate than MCP-02. The activities of MCP-01 at 15°C and 25°C and the activities of MCP-02 at 35°C and 45°C were measured, respectively, using casein as a substrate. The activation energy E a of the protease for the hydrolysis of casein was calculated using the Arrhenius formula:
The E a of MCP-01 was 39.1 kJ mol−1 and that of MCP-02 was 59.3 kJ mol−1. The E a of MCP-01 was similar to that of its psychrophilic homologue, and the E a of MCP-02 was similar to that of its mesophilic homologue (Margesin and Schinner 1992).
Quantities of the proteases MCP-01 and MCP-02 produced by the bacterial strain Pseudoaltermonas sp. SM9913
The culture supernatant of Pseudoaltermonas sp. SM9913 included two proteases, MCP-01 and MCP-02. The optimum temperature for casein hydrolysis of MCP-01 was 30–35°C and that of MCP-02 was 50–55°C (Fig. 4). The optimum temperature for casein hydrolysis of the crude proteases in the culture supernatant was 35–40°C (Chen et al. 2001). These results indicated that the amount of MCP-01 in the culture supernatant was probably much more than that of MCP-02; this was confirmed by the results of native-PAGE of the proteases after ammonium sulfate precipitation (Fig. 7). The optimum temperature for casein hydrolysis of the proteases after ammonium sulfate precipitation was still 35–40°C (data not shown). The protein amount of MCP-01 was 25.2% and that of MCP-02 was only 3.1%, according to results of native-PAGE of the proteases after ammonium sulfate precipitation. Therefore, the bacterial strain Pseudoaltermonas sp. SM9913 mainly produced the protease MCP-01 at low temperatures.
Quantities of MCP-01 and MCP-02 after ammonium sulfate precipitation. In order to show the position of MCP-01 and MCP-02 in lane B, the purified MCP-01 was put in lane A and the purified MCP-02 in lane C. The protein amount of MCP-01 was 25.2% and that of MCP-02 was 3.1% in lane B, which was calculated by the software in "Gel Documentation and Analysis System of Gene Genius" (lane A purified MCP-01; lane B protein after ammonium sulfate precipitation; lane C purified MCP-02)
Discussion
Cold-adapted enzymes, or psychrophilic enzymes, are produced by psychrophiles and psychrotrophs. Compared with mesophilic enzymes, cold-adapted enzymes have higher catalysis efficiency at low temperatures, lower thermostability and more flexible structure. They are usually characterized by low optimum temperatures, low K m and low E a (Feller and Gerday 1997; Gerday et al. 2000). The optimum temperature of mesophilic proteases is usually 50–60°C, and the optimum temperature of most psychrophilic proteases has been reported to be 30–45°C. The psychrophilic protease produced by Pseudomonas PL-4 showed maximum activity at 25°C, and the activity was reduced by 50% when incubated at 32°C for 1 h (Hoshino et al. 1997). An extremely psychrophilic bacterial isolate, strain 34H, isolated from the Arctic, yielded an extract of cell-free protease with activity optimized at 20°C, the lowest optimum yet reported for extracellular protease (Huston et al. 2000). The reduction of K m and E a values may indicate strategies involving psychrophilic enzymes to keep high catalysis efficiency at low temperatures (Feller and Gerday 1997). K m and E a for three psychrophilic proteases reported by Margesin and Schinner (1992) were all much lower than the values for mesophilic proteases, under the same reaction conditions. Pseudoaltermonas sp. SM9913 produced two proteases, MCP-01 and MCP-02. The protease MCP-01 had characters similar to those reported for cold-adapted proteases, such as low optimum temperature, low thermostability and low K m and E a values. Therefore, MCP-01 was considered a cold-adapted protease. However, the characters of the protease MCP-02, such as optimum temperature, thermostability and E a value, were similar to those of the reported mesophilic proteases (Margesin and Schinner 1992). And so MCP-02 was considered a mesophilic protease.
The enzymes excreted by psychrophiles are not all typical psychrophilic enzymes. The proteases excreted by psychrotrophic bacteria isolated from glaciers had optimum temperatures from 30°C to 60°C. The better the thermostability was, the higher the optimum temperature (Margesin et al. 1991). The protease excreted by Xanthomonas maltophilia, isolated from high mountain soil, almost had the same characters as the mesophilic proteases (Margesin and Schinner 1991). The trypsin isolated from Atlantic cold-adapted salmon did not possess biophysical features common to psychrophilic enzymes (Outzen et al. 1994). Most of deep-sea environments are permanently cold. Many microorganisms live in the cold deep-sea environments; they are cold-adapted and barophilic. The bacterial strain Pseudoaltermonas sp. SM9913 was isolated from deep-sea sediment collected at 1,855 m depth. Its optimum and highest growth temperatures were 15°C and 35°C, respectively (Chen et al. 2001). It was a psychrotrophilic bacterium according to Morita's definition of cold-adapted bacteria (Morita 1975). In the permanently cold environment, Pseudoaltermonas sp. SM9913 mainly produced psychrophilic enzymes such as protease MCP-01 to adapt to the cold environment.
References
Chen XL, Zhang YZ, Wang YT, Gao PJ, Luan XW (2001) Psychrotrophilic protease from a deep sea psychrotrophilic strain Pseudoaltermonas sp. SM9913. Mar Sci (China) 25:4–8
Feller G, Gerday C (1997) Psychrophilic enzymes: molecular basis of cold adaptation. Cell Mol Life Sci 53:830–841
Gerday C, Aittaleb M, Bentahir M, Chessa JP, Claverie P, et al (2000) Cold-adapted enzymes: from fundamentals to biotechnology Tibtech March 18:103–107
Hamamoto T, Horikoshi K (1991) Characterisation of amylase from a psychrotrophic Vibrio isolated from a deep-sea mud sample. FEMS Microbiol Lett 84:79–84
Hamamoto T, Horikoshi K (1993) Deep-sea microbiology research within the Deepstar program. J Mar Biotechnol 1:119–124
Hoshino T, Ishizaki K, Sakamoto T, et al (1997) Isolation of a Pseudomonas species from fish intestine that produces a protease active at low temperature. Lett Appl Microbiol 25:70–72
Huston AL, Krieger-Brockett BB, Deming JW (2000) Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environ Microbiol 2:383–388
Kato C, Suzuki S, Hata S, Ito T, Horikoshi K (1995) The properties of a protease activated by high pressure from Sporosarcina sp. strain DSK25 isolated from deep-sea sediment. JAMSTEC J Deep Sea Res 32:7–13
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol regent. J Biol Chem 193:265–275
Margesin R, Schinner F (1991) Characterization of a metalloprotease from psychrophilic Xanthomonas maltophilia. FEMS Microbiol Lett. 79:257–262
Margesin R, Schinner F (1992) A comparison of extracellular proteases from three psychrotrophic strains of Pseudomonas fluorescens. J Gen Appl Microbiol 38:209–225
Margesin R, Palma N, Knauseder F, Schinner F (1991) Proteases of psychrotrophic bacteria isolated from glaciers. J Basic Microbiol 31:377–383
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167
Outzen H, Berglund GI, Smalas AO, Willassen NP (1994) Temperature and pH sensitivity of trypsins from Atlantic salmon (Salmo salar) in comparison with bovine and porcine trypsins. Comp Biochem Physiol B 115:33–45
Acknowledgements
The work was supported by the Hi-Tech Research and Development Program of China (No. 2002AA628100) and the National Natural Science Foundation of China (No. 40276047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Chen, XL., Zhang, YZ., Gao, PJ. et al. Two different proteases produced by a deep-sea psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913. Marine Biology 143, 989–993 (2003). https://doi.org/10.1007/s00227-003-1128-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1128-2