Abstract
Hypertension is common among both peritoneal and hemodialysis patients. It is an important modifiable condition, and one of the most important contributors to the excess morbidity and mortality in this population. Accurate diagnosis with appropriate blood pressure measurement, especially with the use of ambulatory blood pressure monitoring, is crucial in order to achieve optimal blood pressure control. Achievement of dry weight during dialysis and avoidance of excessive interdialytic weight gain are the most important therapeutic strategies. When hypertension persists despite the achievement of euvolemia, antihypertensive medications may be required, and in some patients, native kidney nephrectomies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Children
- Adolescents
- Hypertension
- Sodium intake
- Fluid overload
- Dry weight
- Peritoneal dialysis
- Hemodialysis
- Antihypertensive treatment
Introduction
Patients on maintenance dialysis therapy have an excessively increased all-cause and cardiovascular morbidity and mortality compared with the general population. Adolescents and young adults may already have symptomatic cardiovascular disease, including ischemic heart disease and stroke, and at least every second child on dialysis presents with early signs of cardiovascular end-organ damage such as left ventricular hypertrophy (LVH) or alterations of vascular morphology and function. One of the main risk factors for the high cardiovascular morbidity and mortality is arterial hypertension. The percentage of hypertensive patients on maintenance dialysis is up to 80%, and while hypertension in mild-to-moderate chronic kidney disease (CKD) is mainly caused by underlying renal parenchymal disease, in dialysis patients the most important factor influencing blood pressure (BP) is fluid and salt overload.
The aim of this chapter is to review the prevalence and etiology of hypertension and associated cardiovascular morbidity and mortality in children on dialysis, as well as treatment strategies and targets.
Prevalence of Hypertension in Pediatric Dialysis Patients
Hypertension is highly prevalent in the pediatric dialysis population. Almost 4 out of 5 children and adolescents requiring dialysis are hypertensive or have been prescribed antihypertensive medication.
In a survey of the European ERA/EDTA registry, comprising more than 1300 pediatric dialysis patients from 15 European countries, the prevalence of hypertension was 69.7% in hemodialysis (HD) and 68.2% in peritoneal dialysis (PD) patients. Forty-five percent of HD and 35% of PD patients had uncontrolled hypertension [72]. Similar findings have been seen in data from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry . In an analysis including almost 3500 children, 67.9% of patients were found to be hypertensive 6 months after initiation of dialysis [49]. In another study in long-term HD patients, hypertension was present in 79% of patients. Sixty-two percent of patients were on antihypertensive medication; however, hypertension was uncontrolled in 74% of treated patients. [22].
It should be noted that these epidemiologic data were derived from casual/office BP measurements with single BP recordings per patient reported to the registries. Hypertension was commonly defined as either systolic or diastolic BP above the 95th percentile for sex, age and height. For the interpretation of these data, consideration of the time of BP measurement is important. Pre-dialysis measurements are usually higher compared with post-dialysis measurements, resulting in a higher probability to be classified as hypertensive when only pre-dialysis measurements are available. In HD patients, the median (or mean) interdialytic BP measured by ambulatory BP monitoring (ABPM) is usually lower compared with casual pre-dialysis measurements, resulting in a lower number of patients being classified as hypertensive by ABPM [51]. When the ABPM measurement duration has been extended from the conventional 24 h to 44 h, covering a complete midweek interdialytic period, a higher percentage of patients were diagnosed with hypertension and all BP indexes and loads were significantly higher on interdialytic day 2 compared to day 1 [51]. Volume fluctuations and fluid overload are probably the most important factors responsible for the poor diagnostic value of pre- and post-dialytic BP measurements to predict hypertension in the interdialytic period [3, 125].
It should also be noted that ABPM may identify patients with nocturnal or masked hypertension [21] and patients with reversed nocturnal dipping or altered circadian and ultradian BP rhythms. Unfortunately, data on hypertension prevalence according to interdialytic ABPM are scarce [21, 77].
Etiology of Hypertension in Pediatric Dialysis Patients
The dominant factor contributing to hypertension in dialysis patients is volume overload; other contributing factors include activation of the renin-angiotensin-aldosterone system and the sympathetic nervous system, endothelial dysfunction, increased arterial stiffness, hyperparathyroidism, and exposure to BP elevating drugs.
Additionally, registry studies have identified young age, being on HD, having glomerulopathies as the primary renal disease, and shorter duration of renal replacement therapy as risk factors for dialysis-associated pediatric hypertension [22, 49, 72].
Volume overload plays a pivotal role in the development of hypertension in dialysis patients. Several studies in humans have demonstrated a direct effect of extracellular volume on BP in HD patients [4, 61, 133], and interdialytic weight gain has been shown to correlate with higher systolic BP load in 44-h ABPM profiles on the second day of the BP recording [51]. As might be expected, attainment of dry weight and normalization of sodium balance were able to normalize BP without the need for antihypertensive medication [16].
In dialysis patients, extracellular volume, cardiac output, and BP are increased by impaired or absent ability of the kidneys to excrete sodium and water. These alterations are worsened by insufficient intradialytic removal of fluid and salt. Therefore, in addition to an adequate dialysis prescription, interdialytic fluid restriction and limited salt intake are therapeutic cornerstones for the attainment of dry weight as part of the management of hypertension in dialysis patients. However, efforts to compensate for decreasing residual renal function and diuresis by increasing intradialytic sodium and water removal are often insufficient, as seen in one recent study, in which 25% of dialysis-associated hypertension was felt to be related to factors other than volume overload [32].
Loss of residual renal function is another risk factor for the development of hypertension. BP is inversely correlated to residual renal function and hypertensive children on dialysis have less residual urine output compared to normotensive children [130].
Fluid balance is inextricably linked to serum sodium concentration. However, the hypertensive effects of sodium are exerted by mechanisms both related and unrelated to extracellular volume expansion; elevated sodium concentration may also induce vasoconstriction by altering endothelial cell responses and further contribute to the development of hypertension [99].
It has been demonstrated that intradialytic salt exposure (i.e., the sodium content of the dialysate) has a direct impact on BP. HD patients set to time-averaged dialysate sodium concentrations of 147 mEq/L were found to have higher 24-h systolic BP levels compared to patients set to a sodium concentration of 138 mEq/L [124]. Additionally, a higher dialysate-to-plasma-sodium gradient may increase thirst and interdialytic weight gain, impeding attainment and maintenance of dry weight [115].
Contrary to the physiologically expected suppression of the renin-angiotensin-aldosterone system (RAAS) in a state of salt or fluid overload, plasma renin activity was found to be significantly higher in a study comparing hypertensive to normotensive dialysis patients. The study results strongly suggested that the RAAS is an important factor involved in the pathogenesis of hypertension in end-stage renal disease (ESRD), when sodium balance is adequately controlled [71]. In addition, the significant decline in BP that occurs following bilateral nephrectomy [138] points to volume-independent mechanisms of hypertension in dialysis patients.
Children with end-stage renal disease showed a 25-fold increase in angiotensin (1–7) compared to control values. These marked changes in plasma angiotensin (1–7) were associated with the presence of hypertension and progression of kidney dysfunction [121], while angiotensin II levels were similar and plasma renin activity was lower compared to hypertensive patients with non-ESRD CKD. In dialysis patients, angiotensin II was only poorly suppressed by angiotensin converting enzyme inhibitor (ACEi) treatment . The significance of the elevated angiotensin (1–7) levels is still not clear, but might be a consequence of the altered RAAS pathway in pediatric ESRD patients.
Dialysis patients also have higher sympathetic nervous system (SNS) activity and vascular resistance than healthy controls or ESRD patients after bilateral nephrectomies [26]. An early manifestation of abnormal activation of the SNS activity is the absence of the physiological nocturnal BP dipping in 24 h ambulatory BP monitoring [79].
Endothelial dysfunction , which participates in accelerated atherosclerosis, is a hallmark of CKD. Patients with ESRD display impaired endothelium-dependent vasodilatation, elevated soluble biomarkers of endothelial dysfunction, and increased oxidative stress. Several uremic toxins, mostly protein-bound, have been shown to have specific endothelial toxicity: e.g., asymmetric dimethylarginine (ADMA), homocysteine, and advanced glycosylation end-products (AGEs). These toxins are insufficiently or not removed by dialysis, promote pro-oxidative and pro-inflammatory response, and inhibit endothelial repair, thereby inducing endothelial damage [64].
The most important vasodilatory substance is nitric oxide (NO). The disturbed balance between decreased NO (mediator of vasodilatation) and increased endothelin-1 (ET-1; mediator of vasoconstriction) in dialysis patients results in endothelial cell dysfunction with increased vasoconstriction. NO release is reduced by CKD-induced elevation of ADMA, an endogenous inhibitor of endothelial NO synthase. Increased levels of ADMA have been found to be directly associated with increased cardiovascular and all-cause mortality in the ESRD population [12]. Oxidative stress with increased reactive oxygen species (ROS) can also interfere with NO synthesis and availability.
As a result, arterial stiffness , usually a problem of vascular aging and arteriosclerosis, is accentuated in the presence of end-stage renal disease and hypertension. The stiffened, non-compliant arteries transmit each ejected pulse wave so quickly that the reflected pressure wave, coming backwards from the peripheral circulation, coincides with the still ongoing systole. The consequence is increased systolic BP and pulse pressure resulting in LVH [80]. Higher pulse wave velocity (PWV) due to increased vascular stiffness is also present in pediatric ESRD. PWV is elevated compared to age-, height-, and weight-matched controls [68]. However, the elevated PWV in pediatric ESRD patients was not clearly correlated with the BP level and was found to be persistently elevated despite the use of pharmacological vasodilatation.
Another study in pediatric ESRD patients showed that aortic distensibility, another measure of arterial stiffness, was lower (i.e., higher arterial stiffness) in both HD and PD patients compared to healthy controls. Children on HD had more severe impairment than PD patients [110].
Plasma levels of renalase, a protein released by the kidneys and responsible for the degradation of catecholamines, are markedly decreased in ESRD. Renalase deficiency and the resulting increase of circulating catecholamine levels may also contribute to hypertension and cardiovascular disease in ESRD [30, 137].
Secondary hyperparathyroidism, a complication of CKD, may be yet another contributor to the high prevalence of hypertension. A retrospective study in adults with pre-dialysis CKD demonstrated that systolic and diastolic BP were significantly increased in patients with elevated parathyroid hormone (PTH) levels [108]. A possible mechanism might be increased platelet cytosolic calcium in patients with elevated PTH. Mean BP correlated highly with cytosolic calcium and PTH. In contrast, treatment with vitamin D lowered cytosolic calcium , PTH, and mean BP significantly.
Therapy with erythropoiesis stimulating agents , i.e., erythropoietin, is also associated with an increase of the BP level and development of hypertension. The prevalence of BP increase in adults on erythropoietin therapy is given as high as 10–75%. In a study in 23 pediatric dialysis patients, hypertension developed or worsened in 67% of CAPD patients and 36% of HD patients after initiation of erythropoietin, while no differences were observed in plasma level of aldosterone or plasma renin activity [69].
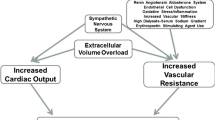
Mechanisms involved in the development of hypertension and cardiovascular end-organ damage in pediatric dialysis patients are summarized in Fig. 31.1.
Mechanisms involved in the development of hypertension in pediatric dialysis patients. BMI body mass index, CKD chronic kidney disease, EPO erythropoietin, ESRD end-stage renal disease, GFR glomerular filtration rate, ROS reactive oxygen species, RRT renal replacement therapy, SNS sympathetic nervous system
While most pediatric dialysis patients lack the cardiovascular and metabolic comorbidities that lead to hypertension in adults with ESRD, underlying renal disease is another important factor influencing the BP level in children with ESRD.
In glomerulopathies, activation of the RAAS, present from the earliest stages of glomerular disease through ESRD, may complicate BP control. Patients with glomerular disease are also less likely to be normotensive compared to patients with congenital anomalies of the kidney and the urinary tract (CAKUT; 12% vs. 31%) [49] and to have an approximately two-fold higher risk of uncontrolled hypertension [49, 72].
Patients with autosomal-recessive polycystic kidney disease may have very severe or therapy refractory hypertension, necessitating bilateral nephrectomy in some cases. In contrast, patients suffering from CAKUT are less prone to renal hypertension, and attainment of dry weight often succeeds in achieving BP control without the need of additional antihypertensive medication .
Short- and Long-Term Consequences of Hypertension
Hospitalization
Fluid overload and hypertension are a frequent cause for morbidity, accounting for 41% of hospitalizations in children on HD at the Texas Children’s Hospital [44]. The risk of hospitalization correlated with the duration of the interdialytic interval. Children receiving chronic HD were more likely to be hospitalized for hypertension, fluid overload, or electrolyte abnormalities following a longer interdialytic interval. Accordingly, the odds ratio of hospital admission was 2.6 on Monday versus other days of the week, while the odds ratio of admission among PD patients was not significantly different on Mondays [126]. Thus, changes to the frequency and intensity of the dialysis treatment may effect admissions in this high-risk population.
Alterations of Vascular Morphology and Function
Increased arterial stiffness is a risk factor for mortality in adults with ESRD. A long-term outcome study including all living adult Dutch patients with childhood onset of ESRD between 1972 and 1992 at age 0–14 years showed a similar intima media thickness, but a reduced mean arterial wall distensibility and increased arterial stiffness compared to healthy controls. Systolic hypertension was the main determinant of these arterial wall changes [46].
The ESCAPE Trial group was able to provide clear evidence that CKD is associated with morphologic alterations of both muscular- and elastic-type arteries as early as in the second decade of life. The degree of pathology depended on the degree of renal dysfunction, correlated with systolic BP, and was most marked in patients on dialysis [78]. In another study including 39 children and adolescents on dialysis (15 HD, 24 PD), indexed diastolic BP was a significant predictor if cIMT [24].
Left Ventricular Hypertrophy
LVH is a common complication in dialysis patients. Forty-eight percent of PD patients were noted to have LVH and 75% had abnormal left ventricular geometry according to a registry analysis of the International Pediatric Peritoneal Dialysis Network (IPPN) [15]. In this analysis, hypertension, high body mass index, fluid overload, renal disease other than hypo/dysplasia, and hyperparathyroidism were predictors of LVH. The lower prevalence of LVH in patients with renal hypo/dysplasia is likely the result of lower BP and polyuria in these patients [15].
In HD patients, the prevalence of LVH was even higher at eighty-five percent, and abnormal left ventricular geometry was found in 80% of patients [91]. The impact of different BP parameters on LVH was analyzed in 25 PD patients, of whom 52% had LVH. Left ventricular mass index (LVMI) was significantly correlated with casual BP measurements and the majority of ABPM parameters [102]. In contrast, in 17 HD patients studied by casual BP measurements and 44-h ABPM, casual BP measurements did not correlate well with measures of cardiovascular end-organ damage, while nighttime BP during 44-h interdialytic ABPM most strongly predicted increased LVMI and LVH [66].
Forty-four-hour ABPM BP load was also correlated with a higher left ventricular mass index. Children with LVH had higher daytime and nighttime systolic BP loads, significantly higher daytime and nighttime diastolic BP loads, and a lesser degree of nocturnal dipping of systolic BP compared to children without LVH [51].
Cardiovascular Mortality
Twenty years ago, it was shown that overall mortality in children on dialysis was increased 1000-fold compared to the normal pediatric population [104] and 40–50% of deaths were from cardiovascular and cerebrovascular causes [47, 87, 101].
Encouragingly, over the past several decades the risk of death has decreased significantly in this population. For example, in the USRDS registry, cardiovascular mortality in pediatric dialysis patients has decreased significantly over the last 20 years, from 33.5/1000 patient-years in patients <5 years of age and 16.2/1000 patient-years in patients >5 years to 22.6 and 9.3/1000 patient-years, respectively [92].
In a European review, overall mortality was 28/1000 patient-years in children and adolescents who started dialysis between 2000 and 2013. Overall mortality risk was highest (36.0/1000) during the first year of dialysis and in the 0- to 5-year age group (49.4/1000), and cardiovascular events accounted for 18.3% of death. Children selected to start on HD had an increased mortality risk compared with those on PD, especially during the first year of dialysis [23].
Improved implementation of clinical practice guidelines, associated with better control of anemia, hyperparathyroidism, and BP, might have contributed to this reduction in mortality as recently shown by a NAPRTCS registry analysis [135]. Similarly, in a systematic review and meta-analysis of 8 trials including 1679 adult patients on dialysis and 495 cardiovascular events, BP lowering was associated with a lower risk of cardiovascular events , all cause-mortality and cardiovascular mortality [55].
Diagnosis of HTN in Dialysis Patients
Current European and American guidelines for evaluation and management of hypertension in children and adolescents [38, 82] do not specify different thresholds for diagnosing hypertension when it is known that the patient has a specific underlying diagnosis, such as renal disease; one would still make the diagnosis of hypertension once the BP had exceeded the specific age, sex, and height threshold. Given the close association between CKD and hypertension in children and adolescents [119], it is likely that a pediatric dialysis patient would be hypertensive at the initiation of dialysis. Thus, the problem under consideration herein is more likely to be an issue of recognition of hypertension, as opposed to making an initial diagnosis of hypertension. Specifically, the problem is how to best diagnose hypertension in a dialysis patient when their measured BP in the clinic or dialysis unit does NOT exceed the thresholds found in the guidelines, but does at other times, a condition known as masked hypertension. Masked hypertension is particularly common in children and adolescents with pre-dialysis CKD [114].
Role of Ambulatory Blood Pressure Monitoring (ABPM)
24-h ABPM is a procedure whereby repeated BP measurements can be obtained outside of a clinical setting, including during sleep. A detailed discussion of ABPM is beyond the scope of this chapter; interested readers should consult other references [37, 83]. There are several distinct hypertension phenotypes that can be identified using the combination of clinic and ambulatory BP values (Table 31.1). All four phenotypes have been identified in adult HD patients [6]. While masked hypertension (and its opposite, white coat hypertension) can be diagnosed using resting BPs obtained in a non-clinical setting, ABPM is generally agreed to be the gold standard approach for identifying patients with these BP patterns [37]. As will be discussed in more detail below, widespread application of ABPM in patients undergoing dialysis is absolutely essential for optimal BP management in this high-risk population.
ABPM in Hemodialysis Patients
The assessment of BP in HD patients is challenging for many reasons, not the least of which is the timing of when BP is measured [74]. It is clear that pre- and post-dialysis BPs provide an inaccurate estimate of the interdialytic BP burden compared to assessment by ABPM [1]. Additionally, BPs obtained surrounding dialysis do not correlate with end organ damage such as elevated left ventricular mass index [2, 93, 94]. Forty-four-hour ABPM has demonstrated increased accuracy in detecting hypertension as compared to a 24-h assessment, likely due to the higher BPs seen in the day following dialysis (the second portion of 44-h ABPM). BP loads >25% on 44-h ABPM have been associated with higher left ventricular mass index in children on chronic HD as compared to assessment with 24-h ABPM [51]. Given these advantages, 44-h ABPM is felt to be the gold standard for BP assessment in HD patients [7].
ABPM in Peritoneal Dialysis Patients
Abnormal circadian BP patterns are common in adult PD patients, and blunted nocturnal dipping and higher BP loads on ABPM correlate with higher left ventricular mass index [13]. Similarly, among 47 children on PD, systolic BP loads on 24 h ABPM were associated with an increased risk of elevated left ventricular mass index [19]. In another study, ABPM was more sensitive in diagnosing hypertension as compared to clinic BPs among 25 pediatric PD patients (56 vs. 32%, p < 0.05) [102]. As with HD patients, these data support the routine use of ABPM in assessing BP in patients receiving PD.
Treatment of Hypertension
Adjustment of Dry Weight/Optimization of Dialysis
Dry Weight
Dry weight is defined as the lowest body weight at the end of dialysis at which the patient can remain normotensive without antihypertensive medication, despite fluid accumulation, until the next dialysis treatment. Stated differently, dry weight is the lowest weight a patient can tolerate without having symptoms of hypotension [62]. When a patient is at their dry weight, it is thought that they are less likely to have hypertension from volume overload.
However, determination of dry weight is difficult. Typically, dry weight is often achieved by trial and error; dry weight is thought to have been achieved when the patient develops signs of hypotension, such as drop in BP, cramping, yawning, headache, abdominal pain, etc. Common clinical methods to assess dry weight include monitoring weight pre- and post-dialysis, examination for the presence of edema, jugular venous distension or crackles on lung auscultation, or detection of hypotension in those with intravascular volume depletion. Clinical assessment can be inaccurate in states of subtler volume excess/depletion. Markers such as change in weight are further confounded in a growing child. Due to the limitations of relying on clinical assessment to determine dry weight, different techniques have been studied to aid in the assessment and achievement of dry weight.
Biochemical markers of volume status include atrial natriuretic peptide, cyclic guanidine monophosphate, brain natriuretic peptide, and troponin T [31, 139]. Most of the biomarkers can be affected by various factors other than volume status, thus limiting their clinical utility. Ultrasound measurement of the inferior vena cava diameter and its collapsibility is a simple and noninvasive way to assess intravascular volume status. Challenges that prevent the broad use of this parameter include interoperator error and patient variability in diameter measurements [31, 62]. Bioelectrical impedance analysis, or bioimpedance, is a method that determines the electrical opposition (impedance) to the flow of an electric current through the body. Bioimpedance can be applied to both HD and PD patients [28]. In adults, bioimpedance analysis has shown that extracellular volume change correlated with the ultrafiltration volume [81]. Other studies in adults using bioimpedance have demonstrated the underestimation of ultrafiltration volumes by 30% based on ECF volumes pre and post HD [62].
Pediatric studies of bioimpedance have demonstrated the utility of this technique, showing good correlation of measured blood volume change to percentage body weight change [100], and serial clinical use to assess dry weight at a single center led to improvement in left ventricular mass index and reduction in LVH [103]. In one recent study, the assessment of dry weight by bioimpedance was compared to clinical assessment in 30 children with stage 5 CKD, 20 of whom were on dialysis (10 HD, 10 PD). Assessment by bioimpedance was felt to more accurately determine hydration status, and correlated with biomarkers of volume overload such as plasma N-terminal pro-B natriuretic peptide and cardiovascular markers such as LVH [32]. The technology does have limitations. Temperature and ion changes that occur during dialysis may effect electrical impedance, as may patient factors such as electrolyte imbalance, hematocrit values, and protein levels [62].
Relative plasma volume monitoring during HD provides insight into the relative rate of ultrafiltration compared to the rate of refilling of plasma volume from the extravascular space. Photo-optical technology measures hematocrit or protein values. An increase in hematocrit or protein concentration is inversely proportional to the change in plasma volume. The use of this technology in adults has led to mixed results, with some reporting improvement in determining and achieving dry weight [111, 127] and some reporting improvement in casual BPs [27] and lower systolic BP as measured by 44 h ABPM [122]. Several pediatric studies have studied the use of plasma volume monitoring [20, 63, 90, 105]. In a multicenter prospective study of 20 pediatric patients, plasma volume monitoring was used to target the 100% ultrafiltration goal, with 50% to be removed in the first hour (max plasma volume change of 8–12% per hour) and the remaining 50% over the subsequent time (max plasma volume change of 5% per hour). They demonstrated a decrease in dialysis-associated morbidity, reduction in antihypertensive medication usage, and improved ABPM profiles. There was no change in weight or left ventricular mass index at the end of the 6-month study, which the authors attributed to somatic growth in their young patients [105]. In 9 pediatric HD patients, systematic use of plasma volume monitoring to challenge dry weight and reduce antihypertensive use resulted in mean dry weight reduction, decreased BP measured both casually and by ABPM, and a reduction in antihypertensive burden [20].
Lung ultrasound has also been used to assess volume status. In the setting of extracellular fluid excess, hydrostatic forces will create a transudative effusion that leads to a decrease in the acoustic mismatch between lung and surrounding tissues. This creates a partial reflection and discrete hyper-echogenic reverberation of the ultrasound beam arising from the pleural line known as “B-lines” [11]. In adults, lung ultrasound findings including B-lines correlated with other markers of fluid overload including: clinical parameters [98, 131], B type natriuretic peptide, inferior vena cava diameter, and bioimpedance [18, 134]. In a single-center study of 96 patients on HD where lung ultrasound, bioimpedance, and echocardiography were prospectively studied for their ability to predict mortality, pre-dialysis B-line score and left ventricular mass index were significantly associated with survival [123]. A recent pediatric study that included patients with ESRD treated with both modalities of dialysis and patients with acute kidney injury demonstrated a significant correlation between B-lines and volume excess as determined by target weight [11]. Among 13 children on dialysis in which objective parameters of volume excess were studied including lung ultrasound, bioimpedance, clinical parameters, and inferior vena cava parameter, only lung ultrasound correlated significantly with volume overload [10, 11].
Clearly, each of these approaches to determining dry weight has advantages and disadvantages, many of which will depend on local expertise as well as the availability of each technique. While the utilization of a combination of techniques may be ideal [112], each dialysis center should follow a standardized approach that allows for longitudinal evaluation of each patient.
Optimization of Dialysis
For both HD and PD, optimization of dialysis with respect to control of BP means utilizing different approaches to reduce volume overload during the dialysis treatment. Just as important is avoidance of intradialytic hypotension, which may be associated with myocardial stunning [57, 58, 88], and prevention of excessive interdialytic weight gain.
Adjusting the duration of therapy and/or the concentration of dialysate sodium is the main strategy used in HD to improve fluid removal. Currently, there is increasing evidence that reduction in dialysate sodium at or slightly below the patient’s pre-dialysis serum concentration leads to reduction in thirst, interdialytic weight gain, and hypertension [17, 97, 129]. A small pediatric study consisting of 5 patients demonstrated a reduction in interdialytic weight gain and pre-dialysis BP when dialysate sodium was reduced from 140 to 138 mEq/L [85]. A systematic review of 23 studies comparing high vs. low dialysate sodium concentration in chronic adult HD patients demonstrated that while BP was unaffected by the concentration of dialysate sodium, there was an increase in interdialytic weight gain in the higher dialysate sodium group and increased intradialytic hypotension in the low dialysate sodium group [17]. It is, in turn, important not to reduce the dialysate sodium too far. Mortality was assessed in 3 observational studies and demonstrated reduced mortality overall with higher dialysate sodium concentrations, but was confounded by patients’ serum sodium concentrations, which demonstrated an inverse relationship between serum sodium concentration and death [17, 53, 54]. Specifically, Hecking et al. demonstrated lower serum sodium (<137 mEq/L) was associated with the highest risk of death, while dialyzing against a bath >140 mEq/L was protective [54].
Increasing dialysis treatment time is another factor associated with improved outcomes. Adult and pediatric studies have demonstrated improved control of BP, faster achievement of dry weight, and reduction in medication burden including antihypertensive medications with increased dialysis time in both adults [40, 42, 128] and children [45, 56]. Increasing time also allows for a reduction in the ultrafiltration rate, which reduces the risk of myocardial stunning [88]. The current recommendation in adult HD patients is to reduce the ultrafiltration rate to <13 ml/kg/h, although even rates <10 ml/kg/h have been associated with increased morbidity and mortality [95, 116].
It should be emphasized that the phenomenon of myocardial stunning is not limited to adult dialysis patients. Work by Hothi and colleagues has shown that excessive intradialytic BP reduction was associated with myocardial stunning in pediatric HD patients [57, 58]. However, no “ideal” rate of ultrafiltration has been determined for pediatric HD patients. In the absence of data, many pediatric dialysis centers, at least in the United States, have been following the recommendation for adults mentioned above. Further discussion on avoidance of intradialytic hypotension can be found in a recent review by Raina et al., in which the lack of evidence-based approaches to this issue in pediatric HD patients is emphasized [107].
Optimization of sodium and water removal in PD can be achieved by managing osmotic potential (dialysate dextrose concentration, dwell time) and surface area recruitment and hydrostatic pressure (fill volume). The 3 pore model theory of peritoneal transport [109] describes 3 various sized pores of the peritoneal endothelium through which transport of water and solutes occurs. The smallest pores are the aquaporin channels, via which only water can be transported; these are activated by intraperitoneal hyperosmolarity created by dextrose-based solutions. There are also small pores that allow transport of both small solutes and water, and large pores that transport macromolecules. Water removal is optimized by short dwell times to maintain the higher osmotic potential of the dialysate, and lower fill volumes to reduce hydrostatic pressure that would counteract the osmotic potential. In contrast, solute removal (including sodium) is optimized by increased fill volumes that increase the recruitment of peritoneal surface area, and longer dwell time [36].
The drawback of using higher dialysate dextrose concentrations is the production of glucose degradation products that are toxic to the peritoneum [33]. This can be avoided in part by the use of icodextrin, a maltodextrin polymer produced by the metabolism of cornstarch. Icodextrin is absorbed from the peritoneal space much more slowly via the lymphatics and thus maintains the osmotic potential longer. It further exerts its effect via colloid osmosis, and therefore exerts its effects via the small pores and not the aquaporin channels, thus leading to less sodium sieving [39]. However, icodextrin is only meant to be used for the long dwell, as metabolism over time increases its colloid potential. Studies in adults have demonstrated equivalent ultrafiltration of icodextrin over 10 h and superior ultrafiltration beyond that time as compared to 4.25% dextrose solutions [25, 96]. A recent retrospective study of 50 pediatric patients who used icodextrin for a long daytime dwell demonstrated improved ultrafiltration overall and improved ultrafiltration with increasing patient age [113].
Finally, adapted automated PD, where the PD cycler alternates between short dwells with low fill volumes to enhance ultrafiltration and long dwells with large fill volumes to enhance solute clearance [9, 34, 35], can be used to improve BP control. In a prospective, crossover study in adults, adapted PD resulted in increased sodium and water removal and improved BPs as compared to conventional PD [35]. To date, no studies of this approach to PD in children have been reported.
Dietary Intervention: Fluid and Salt Intake
The observation that dietary sodium restriction and ultrafiltration led to improved BP management was noted by Belding Scribner when treating the first patient to receive chronic dialysis, who suffered from malignant hypertension [118]. Controlling dietary sodium intake facilitates achievement of dry weight [70], and is associated with decreased thirst, lower interdialytic weight gain, improved BP control, lower LVMI, and decreased mortality in adults [67, 84, 86]. It is important to recognize that fluid restriction will not be possible if sodium intake is not reduced, as increased sodium intake will inexorably increase thirst, which leads to greater interdialytic weight gain [76]. While most studies of sodium intake and dialysis have focused on HD patients, a limited number of studies in in adults undergoing PD have shown that a reduction in sodium intake reduces fluid overload and reduces BP in this population as well [60].
Restriction of sodium intake, although ideal, is difficult to achieve given the high sodium intake of many children, including those with CKD. Despite guidelines recommending limiting daily sodium intake in children with kidney disease and hypertension to between 1500 mg and 2300 mg [65], data from a registry of children with CKD stage 2–4 demonstrated that sodium intake was greater than 3000 mg daily, with 25% of adolescents consuming more than 5000 mg of sodium per day [59]. A study examining sodium intake among school-aged children found that the top ten food categories that contributed to 48% of the salt intake are from processed foods, with the exception of cow’s milk, which naturally has sodium [106]. Similar studies in American adults demonstrated that 70.9% of the salt consumed was sodium added to food outside the home [50]. Renal dieticians are key members of the treatment team because of their role educating the patient and their family on low sodium food with high nutritional content. The social worker can also play a role by providing better access to these often more expensive foods .
Pharmacological Treatment
All classes of antihypertensive medications are useful for BP control in the dialysis population, although the choice of agent needs to be individualized [43]. Dosing of many agents may need to be adjusted in dialysis patients, as summarized in Table 31.2. However, it should be noted that antihypertensive medications are ineffective when volume excess is the etiology of hypertension, and studies have demonstrated that reliance on antihypertensive medications instead of correction of volume overload leads to persistent hypertension [5].
Antihypertensive medication use in dialysis patients has been shown to not only reduce BP, but to also improve intermediate markers of cardiovascular disease. In a recent randomized, controlled trial in hypertensive chronic adult HD patients with LVH, lisinopril or atenolol given three times a week after dialysis lowered BP on 44 h ABPM and led to regression of LVH. However, when monthly home BPs were assessed, the lisinopril group had higher BPs despite a greater number of antihypertensive agents and reduction in dry weight; this and other events in the study suggested that atenolol was overall superior to lisinopril [8].
In our experience in children, beta-adrenergic blockers and agents affecting the RAAS are the most effective classes of antihypertensive agents once volume overload has been corrected. Long-acting vasodilating medications (i.e., amlodipine, minoxidil) are best avoided as they may impair the ability to correct volume overload with fluid removal during dialysis. Clonidine may also have a role given the activation of the sympathetic nervous system in ESRD [117].
There has been an increased interest in the use of diuretics in dialysis patients who still have residual renal function [73, 132]. In patients with preserved residual renal function, loop diuretics may enhance urine output and limit interdialytic weight gain [75]. A recent study comparing patients who continued loop diuretics after HD initiation to those who did not showed that those who continued diuretics had lower rates of hospitalization and intradialytic hypotension, as well as lower interdialytic weight gain over the first year of dialysis, but there was no difference in mortality [120]. In PD, one small study showed that the use of oral loop diuretics led to better volume control in the first year after dialysis initiation [89]. There have also been studies showing that the use of potassium-sparing diuretics in PD patients is useful for correction of hypokalemia [41]. There is one study of pediatric PD patients in which diuretic use was retrospectively studied [48]. Children who received diuretics from the initiation of PD were 80% less likely to develop oligoanuria compared to those who did not receive diuretics; other outcomes were not examined.
Native Nephrectomy
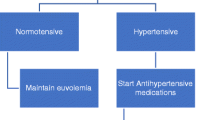
Native kidney nephrectomy is typically considered the last resort in the treatment of hypertension in dialysis patients, reserved for those who remain hypertensive despite the measures discussed above (Fig. 31.2). The procedure has been shown to be effective in treating hypertension in children with ESRD [14], and newer surgical techniques may allow quick resumption of dialysis in children on PD who require this procedure [29].
There are two possible explanations for how nephrectomy can improve hypertension in patients on dialysis. As discussed earlier, the RAAS is a well-established cause of hypertension in CKD and in ESRD, and this may be related to the presence of native, diseased kidneys. Among 51 HD patients, plasma renin activity was higher among patients who had uncontrolled hypertension as compared to those whose BP was controlled by ultrafiltration and sodium restriction. Among the 18 who had uncontrolled hypertension, 17 had significant improvement in BPs after native nephrectomies [136]. In another study, treating patients with CKD with angiotensin converting enzyme inhibitors (ACEi) resulted in increased angiotensin 1–7 and decreased angiotensin II, whereas ESRD patients with ACEi therapy did not have a decrease in angiotensin II levels [121]. This may help explain why refractory hypertensive ESRD patients may benefit from native nephrectomies.
ESRD patients are also known to have increased sympathetic nervous system activity [26, 117]. The origin of the increased sympathetic nervous system activity may also be from the diseased native kidney. This was determined in an elegant study in transplant recipients who had continued activation of the sympathetic nervous system until they underwent native nephrectomies [52].
Summary
Hypertension is common among both PD and HD patients. It is an important modifiable condition, and one of the most important contributors to excess morbidity and mortality in this population. Accurate diagnosis with appropriate BP measurement, especially the use of ambulatory BP monitoring, is crucial in order to achieve optimal BP control. Management begins with the achievement of dry weight and avoidance of excessive interdialytic weight gain. When hypertension persists despite the achievement of euvolemia, antihypertensive medications may be required, and in some patients, native nephrectomies.
Abbreviations
- ABPM:
-
ambulatory blood pressure monitoring
- ACEi:
-
angiotensin converting enzyme inhibitor
- BP:
-
blood pressure
- CAKUT:
-
congenital anomalies of kidney and urinary tract
- ESRD:
-
end-stage renal disease
- HD:
-
hemodialysis
- LVH:
-
left ventricular hypertrophy
- NO:
-
nitric oxide
- PD:
-
peritoneal dialysis
- PTH:
-
parathyroid hormone
- PWV:
-
pulse wave velocity
- RAAS:
-
renin-angiotensin-aldosterone-system
References
Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006a;47(1):62–8.
Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006b;1(3):389–98.
Agarwal R, Saha C. Dialysis dose and the diagnosis of hypertension in hemodialysis patients. Blood Press Monit. 2007;12(5):281–7.
Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53(3):500–7.
Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(7):1255–60.
Agarwal R, Sinha AD, Light RP. Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(8):2003–8.
Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014a;25(8):1630–46.
Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014b;29(3):672–81.
Akonur A, Guest S, Sloand JA, Leypoldt JK. Automated peritoneal dialysis prescriptions for enhancing sodium and fluid removal: a predictive analysis of optimized, patient-specific dwell times for the day period. Perit Dial Int. 2013;33(6):646–54.
Allinovi M, Saleem MA, Burgess O, Armstrong C, Hayes W. Finding covert fluid: methods for detecting volume overload in children on dialysis. Pediatr Nephrol. 2016;31(12):2327–35.
Allinovi M, Saleem M, Romagnani P, Nazerian P, Hayes W. Lung ultrasound: a novel technique for detecting fluid overload in children on dialysis. Nephrol Dial Transplant. 2017;32(3):541–7.
Anderstam B, Katzarski K, Bergstrîm J. Serum levels of NO, NG-dimethyl-L-Arginine, a potential endogenous nitic oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–42.
Atas N, Erten Y, Okyay GU, Inal S, Topal S, Onec K, et al. Left ventricular hypertrophy and blood pressure control in automated and continuous ambulatory peritoneal dialysis patients. Ther Apher Dial. 2014;18(3):297–304.
Baez-Trinidad LG, Lendvay TS, Broecker BH, Smith EA, Warshaw BL, Hymes L, et al. Efficacy of nephrectomy for the treatment of nephrogenic hypertension in a pediatric population. J Urol. 2003;170(4 Pt 2):1655–7. discussion 8
Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Buscher R, Salas P, et al. Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol. 2011;6(8):1926–33.
Bakris GL, Burkart JM, Weinhandl ED, McCullough PA, Kraus MA. Intensive hemodialysis, blood pressure, and antihypertensive medication use. Am J Kidney Dis. 2016;68(5S1):S15–23.
Basile C, Pisano A, Lisi P, Rossi L, Lomonte C, Bolignano D. High versus low dialysate sodium concentration in chronic haemodialysis patients: a systematic review of 23 studies. Nephrol Dial Transplant. 2016;31(4):548–63.
Basso F, Milan Manani S, Cruz DN, Teixeira C, Brendolan A, Nalesso F, et al. Comparison and reproducibility of techniques for fluid status assessment in chronic hemodialysis patients. Cardiorenal Med. 2013;3(2):104–12.
Bircan Z, Duzova A, Cakar N, Bayazit AK, Elhan A, Tutar E, et al. Predictors of left ventricular hypertrophy in children on chronic peritoneal dialysis. Pediatr Nephrol. 2010;25(7):1311–8.
Candan C, Sever L, Civilibal M, Caliskan S, Arisoy N. Blood volume monitoring to adjust dry weight in hypertensive pediatric hemodialysis patients. Pediatr Nephrol. 2009;24(3):581–7.
Chaudhuri A, Sutherland SM, Begin B, Salsbery K, McCabe L, Potter D, et al. Role of twenty-four-hour ambulatory blood pressure monitoring in children on dialysis. Clin J Am Soc Nephrol. 2011;6(4):870–6.
Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, et al. Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol. 2009;4(8):1363–9.
Chesnaye NC, Schaefer F, Groothoff JW, Bonthuis M, Reusz G, Heaf JG, et al. Mortality risk in European children with end-stage renal disease on dialysis. Kidney Int. 2016;89(6):1355–62.
Civilibal M, Caliskan S, Oflaz H, Sever L, Candan C, Canpolat N, et al. Traditional and ‘new’ cardiovascular risk markers and factors in pediatric dialysis patients. Pediatr Nephrol. 2007;22:1021–9.
Collins A, Mujais S. Advancing fluid management in peritoneal dialysis. Kidney Int Suppl. 2002;81:S1–2.
Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327(27):1912–8.
Dasselaar JJ, Huisman RM, de Jong PE, Burgerhof JG, Franssen CF. Effects of relative blood volume-controlled hemodialysis on blood pressure and volume status in hypertensive patients. ASAIO J. 2007;53(3):357–64.
Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86(3):489–96.
De Carli C, Guerra LA. Simultaneous bilateral laparoscopic nephrectomy in a child with peritoneal catheter dialysis using a 4-port trans-abdominal technique. Can Urol Assoc J. 2015;9(1–2):59–61.
Desir GV. Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr Opin Nephrol Hypertens. 2008;17(2):181–5.
Dou Y, Zhu F, Kotanko P. Assessment of extracellular fluid volume and fluid status in hemodialysis patients: current status and technical advances. Semin Dial. 2012;25(4):377–87.
Eng CSY, Bhowruth D, Mayes M, Stronach L, Blaauw M, Barber A, et al. Assessing the hydration status of children with chronic kidney disease and on dialysis: a comparison of techniques. Nephrol Dial Transplant. 2018;33(5):847–55.
Erixon M, Wieslander A, Linden T, Carlsson O, Forsback G, Svensson E, et al. How to avoid glucose degradation products in peritoneal dialysis fluids. Perit Dial Int. 2006;26(4):490–7.
Fischbach M, Issad B, Dubois V, Taamma R. The beneficial influence on the effectiveness of automated peritoneal dialysis of varying the dwell time (short/long) and fill volume (small/large): a randomized controlled trial. Perit Dial Int. 2011;31(4):450–8.
Fischbach M, Zaloszyc A, Schaefer B, Schmitt C. Adapted automated peritoneal dialysis. Adv Perit Dial. 2014;30:94–7.
Fischbach M, Schmitt CP, Shroff R, Zaloszyc A, Warady BA. Increasing sodium removal on peritoneal dialysis: applying dialysis mechanics to the peritoneal dialysis prescription. Kidney Int. 2016;89(4):761–6.
Flynn JT, Urbina EM. Pediatric ambulatory blood pressure monitoring: indications and interpretations. J Clin Hypertens (Greenwich). 2012;14(6):372–82.
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904.
Frampton JE, Plosker GL. Icodextrin: a review of its use in peritoneal dialysis. Drugs. 2003;63(19):2079–105.
Frequent Hemodialysis Network (FHN) Trial Group, Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–300.
Fulop T, Zsom L, Rodriguez B, Afshan S, Davidson JV, Szarvas T, et al. Clinical utility of potassium-sparing diuretics to maintain normal serum potassium in peritoneal dialysis patients. Perit Dial Int. 2017;37(1):63–9.
Georgianos PI, Sarafidis PA, Sinha AD, Agarwal R. Adverse effects of conventional thrice-weekly hemodialysis: is it time to avoid 3-day interdialytic intervals? Am J Nephrol. 2015;41(4–5):400–8.
Georgianos PI, Agarwal R. Pharmacotherapy of hypertension in chronic dialysis patients. Clin J Am Soc Nephrol. 2016;11(11):2062–75.
Goldstein SL, Smith CM, Currier H. Noninvasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol. 2003;14(8):2127–31.
Goldstein SL, Silverstein DM, Leung JC, Feig DI, Soletsky B, Knight C, et al. Frequent hemodialysis with NxStage system in pediatric patients receiving maintenance hemodialysis. Pediatr Nephrol. 2008;23(1):129–35.
Groothoff JW, Gruppen MP, Offringa M, De Groot E, Stok W, Bos WJ, et al. Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol. 2002a;13:2953–61.
Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, et al. Mortality and cause of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002b;61:621–9.
Ha IS, Yap HK, Munarriz RL, Zambrano PH, Flynn JT, Bilge I, et al. Risk factors for loss of residual renal function in children treated with chronic peritoneal dialysis. Kidney Int. 2015;88(3):605–13.
Halbach SM, Martz K, Mattoo T, Flynn J. Predictors of blood pressure and its control in pediatric patients receiving dialysis. J Pediatr. 2012;160(4):621–5. e1
Harnack LJ, Cogswell ME, Shikany JM, Gardner CD, Gillespie C, Loria CM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135(19):1775–83.
Haskin O, Wong CJ, McCabe L, Begin B, Sutherland SM, Chaudhuri A. 44-h ambulatory blood pressure monitoring: revealing the true burden of hypertension in pediatric hemodialysis patients. Pediatr Nephrol. 2015;30(4):653–60.
Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, et al. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106(15):1974–9.
Hecking M, Karaboyas A, Saran R, Sen A, Horl WH, Pisoni RL, et al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2012a;59(2):238–48.
Hecking M, Karaboyas A, Saran R, Sen A, Inaba M, Rayner H, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012b;7(1):92–100.
Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373(9668):1009–15.
Hoppe A, von Puttkamer C, Linke U, Kahler C, Booss M, Braunauer-Kolberg R, et al. A hospital-based intermittent nocturnal hemodialysis program for children and adolescents. J Pediatr. 2011;158(1):95–9, e1.
Hothi DK, Rees L, Marek J, Burton J, McIntyre CW. Pediatric myocardial stunning underscores the cardiac toxicity of conventional hemodialysis treatments. Clin J Am Soc Nephrol. 2009;4(4):790–7.
Hothi DK, Rees L, McIntyre CW, Marek J. Hemodialysis-induced acute myocardial dyssynchronous impairment in children. Nephron Clin Pract. 2013;123(1–2):83–92.
Hui WF, Betoko A, Savant JD, Abraham AG, Greenbaum LA, Warady B, et al. Assessment of dietary intake of children with chronic kidney disease. Pediatr Nephrol. 2017;32(3):485–94.
Inal S, Erten Y, Tek N, Ulusal Okyay G, Onec K, Akbulut G, et al. The effect of dietary salt restriction on hypertension in peritoneal dialysis patients. Turk J Med Sci. 2014;44(5):814–9.
Inrig JK, Patel UD, Gillespie BS, Hasselblad V, Himmelfarb J, Reddan D, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50(1):108–18, e1–4.
Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10(2):392–403.
Jain SR, Smith L, Brewer ED, Goldstein SL. Non-invasive intravascular monitoring in the pediatric hemodialysis population. Pediatr Nephrol. 2001;16(1):15–8.
Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24(3):327–37.
Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290.
Katsoufis CP, Seeherunvong W, Sasaki N, Abitbol CL, Chandar J, Freundlich M, et al. Forty-four-hour interdialytic ambulatory blood pressure monitoring and cardiovascular risk in pediatric hemodialysis patients. Clin Kidney J. 2014;7(1):33–9.
Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24(3):956–62.
Kis E, Cseprekal O, Horvath Z, Katona G, Fekete BC, Hrapka E, et al. Pulse wave velocity in end-stage renal disease: influence of age and body dimensions. Pediatr Res. 2008;63(1):95–8.
Komatsu Y, Ito K. Erythropoietin associated hypertension among pediatric dialysis patients. Adv Perit Dial. 1992;8:448–52.
Kooman JP, van der Sande F, Leunissen K, Locatelli F. Sodium balance in hemodialysis therapy. Semin Dial. 2003;16(5):351–5.
Kornerup HJ. Hypertension in end-stage renal disease. The relationship between blood pressure, plasma renin, plasma renin substrate and exchangeable sodium in chronic hemodialysis patients. Acta Med Scand. 1976;200(4):257–61.
Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, et al. Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int. 2011;80:1092–8.
Kumra R, Bargman JM. A review of diuretic use in dialysis patients. Adv Perit Dial. 2014;30:115–9.
Lazar AE, Smith MC, Rahman M. Blood pressure measurement in hemodialysis patients. Semin Dial. 2004;17(4):250–4.
Lemes HP, Araujo S, Nascimento D, Cunha D, Garcia C, Queiroz V, et al. Use of small doses of furosemide in chronic kidney disease patients with residual renal function undergoing hemodialysis. Clin Exp Nephrol. 2011;15(4):554–9.
Lindley EJ. Reducing sodium intake in hemodialysis patients. Semin Dial. 2009;22(3):260–3.
Lingens N, Soergel M, Loirat C, Busch C, Lemmer B, SchÑrer K. Ambulatory blood pressure monitoring in paediatric patients treated by regular hemodialysis and peritoneal dialysis. Pediatr Nephrol. 1995;9:167–72.
Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, et al. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16:1494–500.
Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, et al. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18:563–9.
London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end-stage renal disease. Adv Chronic Kidney Dis. 2004;11(2):202–9.
Lukaski HC, Bolonchuk WW. Estimation of body fluid volumes using tetrapolar bioelectrical impedance measurements. Aviat Space Environ Med. 1988;59(12):1163–9.
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34(10):1887–920.
Macumber I, Flynn JT. Ambulatory blood pressure monitoring in children and adolescents. In: White WB, editor. Blood pressure monitoring in cardiovascular medicine and therapeutics. 3rd ed. New York: Humana Press; 2016. p. 227–52.
Maduell F, Navarro V. Dietary salt intake and blood pressure control in haemodialysis patients. Nephrol Dial Transplant. 2000;15(12):2063.
Marsenic O, Anderson M, Couloures KG, Hong WS, Kevin Hall E, Dahl N. Effect of the decrease in dialysate sodium in pediatric patients on chronic hemodialysis. Hemodial Int. 2016;20(2):277–85.
Mc Causland FR, Waikar SS, Brunelli SM. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney Int. 2012;82(2):204–11.
McDonald SP, Craig JC. Australian, New Zealand paediatric nephrology a. long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350(26):2654–62.
McIntyre CW. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76(4):371–5.
Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59(3):1128–33.
Michael M, Brewer ED, Goldstein SL. Blood volume monitoring to achieve target weight in pediatric hemodialysis patients. Pediatr Nephrol. 2004;19(4):432–7.
Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF. Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol. 2001;16(4):318–23.
Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA. 2013;309(18):1921–9.
Moriya H, Ohtake T, Kobayashi S. Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant. 2007;22(4):1198–204.
Moriya H, Oka M, Maesato K, Mano T, Ikee R, Ohtake T, et al. Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):416–22.
Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, et al. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant. 2007;22(12):3547–52.
Mujais S, Vonesh E. Profiling of peritoneal ultrafiltration. Kidney Int Suppl. 2002;81:S17–22.
Munoz Mendoza J, Arramreddy R, Schiller B. Dialysate sodium: choosing the optimal hemodialysis bath. Am J Kidney Dis. 2015;66(4):710–20.
Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJR, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135(6):1433–9.
Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104(41):16281–6.
Oh G, Wong C, Begin B, Salsbery K, Sutherland S, Chaudhuri A. Whole-body single-frequency bioimpedance analysis in pediatric hemodialysis patients. Pediatr Nephrol. 2014;29(8):1417–23.
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–5.
Ozcakar ZB, Yalcinkaya F, Tutar E, Cakar N, Ucar T, Elhan A, et al. Hypertension and left ventricular hypertrophy in pediatric peritoneal dialysis patients: ambulatory blood pressure monitoring and echocardiographic evaluation. Nephron Clin Pract. 2006;104(2):c101–6.
Paglialonga F, Ardissino G, Galli MA, Scarfia RV, Testa S, Edefonti A. Bioimpedance analysis and cardiovascular status in pediatric patients on chronic hemodialysis. Hemodial Int. 2012;16(Suppl 1):S20–5.
Parekh RS, Caroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–7.
Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, et al. A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(2):252–7.
Quader ZS, Gillespie C, Sliwa SA, Ahuja JK, Burdg JP, Moshfegh A, et al. Sodium intake among US school-aged children: national health and nutrition examination survey, 2011–2012. J Acad Nutr Diet. 2017;117(1):39–47, e5.
Raina R, Lam S, Raheja H, Krishnappa V, Hothi D, Davenport A, et al. Pediatric intradialytic hypotension: recommendations from the Pediatric Continuous Renal Replacement Therapy (PCRRT) Workgroup. Pediatr Nephrol. 2019;34(5):925–41.
Raine AE, Bedford L, Simpson AW, Ashley CC, Brown R, Woodhead JS, et al. Hyperparathyroidism, platelet intracellular free calcium and hypertension in chronic renal failure. Kidney Int. 1993;43:700–5.
Rippe B. A three-pore model of peritoneal transport. Perit Dial Int. 1993;13(Suppl 2):S35–8.
Robinson RF, Nahata MC, Sparks E, Daniels C, Batisky DL, Hayes JR, et al. Abnormal left ventricular mass and aortic distensibility in pediatric dialysis patients. Pediatr Nephrol. 2005;20(1):64–8.
Rodriguez HJ, Domenici R, Diroll A, Goykhman I. Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int. 2005;68(2):854–61.
Ronco C, Kaushik M, Valle R, Aspromonte N, Peacock WF. Diagnosis and management of fluid overload in heart failure and cardio-renal syndrome: the “5B” approach. Semin Nephrol. 2012;32(1):129–41.
Rousso S, Banh TM, Ackerman S, Piva E, Licht C, Harvey EA. Impact of fill volume on ultrafiltration with icodextrin in children on chronic peritoneal dialysis. Pediatr Nephrol. 2016;31(10):1673–9.
Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60(1):43–50.
Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(2):522–30.
Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–8.
Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the sympathetic nervous system and its modulation in renal hypertension. Front Med (Lausanne). 2018;5:82.
Scribner BH, Buri R, Caner JE, Hegstrom R, Burnell JM. The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs. 1960;6:114–22.
Shatat IF, Flynn JT. Hypertension in children with chronic kidney disease. Adv Chronic Kidney Dis. 2005;12(4):378–84.
Sibbel S, Walker AG, Colson C, Tentori F, Brunelli SM, Flythe J. Association of continuation of loop diuretics at hemodialysis initiation with clinical outcomes. Clin J Am Soc Nephrol. 2019;14(1):95–102.
Simoes e Silva AC, Diniz JS, Pereira RM, Pinheiro SV, Santos RA. Circulating renin Angiotensin system in childhood chronic renal failure: marked increase of Angiotensin-(1-7) in end-stage renal disease. Pediatr Res. 2006;60(6):734–9.
Sinha AD, Light RP, Agarwal R. Relative plasma volume monitoring during hemodialysis AIDS the assessment of dry weight. Hypertension. 2010;55(2):305–11.
Siriopol D, Hogas S, Voroneanu L, Onofriescu M, Apetrii M, Oleniuc M, et al. Predicting mortality in haemodialysis patients: a comparison between lung ultrasonography, bioimpedance data and echocardiography parameters. Nephrol Dial Transplant. 2013;28(11):2851–9.
Song JH, Lee SW, Suh CK, Kim MJ. Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis. 2002;40(2):291–301.
Sorof JM, Brewer ED, Portman RJ. Ambulatory blood pressure monitoring and interdialytic weight gain in children receiving chronic hemodialysis. Am J Kidney Dis. 1999;33(4):667–74.
Springel T, Laskin B, Shults J, Keren R, Furth S. Longer interdialytic interval and cause-specific hospitalization in children receiving chronic dialysis. Nephrol Dial Transplant. 2013;28(10):2628–36.
Steuer RR, Germain MJ, Leypoldt JK, Cheung AK. Enhanced fluid removal guided by blood volume monitoring during chronic hemodialysis. Artif Organs. 1998;22(8):627–32.
Tandon T, Sinha AD, Agarwal R. Shorter delivered dialysis times associate with a higher and more difficult to treat blood pressure. Nephrol Dial Transplant. 2013;28(6):1562–8.
Thein H, Haloob I, Marshall MR. Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrol Dial Transplant. 2007;22(9):2630–9.
Tkaczyk M, Nowicki M, Balasz-Chmielewska I, Boguszewska-Baczkowska H, Drozdz D, Kollataj B, et al. Hypertension in dialysed children: the prevalence and therapeutic approach in Poland–a nationwide survey. Nephrol Dial Transplant. 2006;21:736–42.
Trezzi M, Torzillo D, Ceriani E, Costantino G, Caruso S, Damavandi PT, et al. Lung ultrasonography for the assessment of rapid extravascular water variation: evidence from hemodialysis patients. Intern Emerg Med. 2013;8(5):409–15.
Trinh E, Bargman JM. Are diuretics underutilized in dialysis patients? Semin Dial. 2016;29(5):338–41.
Van Buren PN, Inrig JK. Hypertension and hemodialysis: pathophysiology and outcomes in adult and pediatric populations. Pediatr Nephrol. 2012;27(3):339–50.
Vitturi N, Dugo M, Soattin M, Simoni F, Maresca L, Zagatti R, et al. Lung ultrasound during hemodialysis: the role in the assessment of volume status. Int Urol Nephrol. 2014;46(1):169–74.
Weaver DJ Jr, Somers MJG, Martz K, Mitsnefes MM. Clinical outcomes and survival in pediatric patients initiating chronic dialysis: a report of the NAPRTCS registry. Pediatr Nephrol. 2017;32(12):2319–30.
Weidmann P, Maxwell MH, Lupu AN, Lewin AJ, Massry SG. Plasma renin activity and blood pressure in terminal renal failure. N Engl J Med. 1971;285(14):757–62.
Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115(5):1275–80.
Zazgornik J, Biesenbach G, Janko O, Gross C, Mair R, Brucke P, et al. Bilateral nephrectomy: the best, but often overlooked, treatment for refractory hypertension in hemodialysis patients. Am J Hypertens. 1998;11(11 Pt 1):1364–70.
Zhu F, Rosales L, Kotanko P. Techniques for assessing fluids status in patients with kidney disease. Curr Opin Nephrol Hypertens. 2016;25(6):473–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wühl, E., Flynn, J.T. (2021). Management of Hypertension in Pediatric Dialysis Patients. In: Warady, B.A., Alexander, S.R., Schaefer, F. (eds) Pediatric Dialysis. Springer, Cham. https://doi.org/10.1007/978-3-030-66861-7_31
Download citation
DOI: https://doi.org/10.1007/978-3-030-66861-7_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66860-0
Online ISBN: 978-3-030-66861-7
eBook Packages: MedicineMedicine (R0)