Abstract
The aim of this study was to adjust dry weight by short-term blood volume monitoring (BVM)-guided ultrafiltration and evaluate the effects of optimizing dry weight on blood pressure (BP) control and intradialytic symptoms (IDS) in a group of hypertensive hemodialysis (HD) patients. The study was performed in four sequential phases, each of which lasted for 1 week, on nine hypertensive HD patients (six girls, age 16.9 ± 3.1 years). In phase I, patients were observed by BVM. In phase II, BVM was used to guide ultrafiltration to adjust dry weight. Antihypertensive drugs were gradually tapered or withheld in phase III, when the patients were hypotensive and/or their IDS increased. In phase IV, this particular weight was maintained without any intervention. Pre- and post-HD body weight, pre-HD, post-HD, 30 min after HD casual BP values, and IDS in each HD session were recorded. The BP was also assessed by 44-h ambulatory BP monitoring (ABPM), which is an ideal method to determine BP changes throughout the interdialytic period at the beginning of phase I and at the end of phase IV. There was a decrease in mean dry weight, all casual systolic BPs, and systolic/diastolic ABPM at the end of the study (all p ≤ 0.05). Antihypertensive drugs were stopped in five patients and reduced in two during phase III of the study. The IDS was more frequent (36%) in phase IV than in phase I (16%); however, this increase did not reach statistical significance. The results of this study suggest that short-term BVM guided-ultrafiltration may be a useful tool to diagnose volume overload and to adjust dry weight and, consequently, to achieve a better control of BP in pediatric HD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a risk factor for cardiovascular morbidity and mortality in children undergoing hemodialysis (HD), and it remains widely prevalent in these patients despite antihypertensive medication [1, 2]. A recent multicenter study reported that chronic fluid overload can be a significant cause of poor blood pressure (BP) control in pediatric HD patients and that circumspect achievement of dry weight may be the most important factor in controlling hypertension [3].
Although high BP can largely be controlled by adequate ultrafiltration (UF) and the maintenance of dry weight, an accurate estimation of this latter parameter is a difficult task in clinical practice [4]. An underestimation of dry weight leads to hypovolemia-induced symptoms, whereas overestimation results in hypertension and left ventricular hypertrophy. Since the value of a physical examination is limited, more sensitive methods are needed to assess ideal dry weight [5, 6]. Of the methods currently available, blood volume monitoring (BVM) is one of the less laborious when assessing hydration variables and is relatively more suitable for routine patient care. This methodology can be performed using an on-line optical reflection method; hence, changes in blood volume can be measured continuously and noninvasively during a HD session [7–9].
The results of earlier studies on adult cohorts support the use of BVM in chronic HD patients as a useful tool for both preventing intradialytic hypotension [10, 11] and identifying patients with volume overload who could benefit from intensified UF [12–14]. Studies on children suggest that achieving target weight by means of BVM helps to reduce the need for antihypertensive medication and intradialytic symptoms (IDS) as compared to more traditional control methods [15, 16]. Goldstein et al. [17] reported that the use of BVM to assess patients’ target weight can significantly decrease hospitalization rates and related costs due to fluid overload/hypertension in hemodialyzed children. Patel et al. [18] showed that a BVM-guided UF algorithm resulted in improved BP control in pediatric HD patients after 6 months. The aim of the prospective study reported here was to optimize, within a relatively short time, dry weight by BVM-guided UF and to evaluate the effect of this intervention on BP control and IDS in all hypertensive of our HD patients.
Materials and methods
Subjects
Nine (six female, three male) hypertensive HD patients with a stable BP for more than at least 2 months prior to the study were included in the study. Inclusion criteria were: (1) age 6–20 years, (2) absence of myocardial disease, (3) absence of any acute illness during the 2 months preceding the study, (4) serum albumin level of more than 3 g/dL, and (5) urine output of less than 400 mL/m2/day. The subjects had not been on BVM before the study.
The mean age and mean weight of the patients was 16.9 ± 3.1 (range 11.7–20.0) years and 31.2 ± 7.8 (range 19.0–39.5) kg, respectively. The patients had been on HD for 15.8 ± 17.9 (range 2.2–56.9) months. Prior to the study, the single pool fractional clearance index for urea (spKt/Vurea) of all patients was above 1.2. Causes of end-stage renal disease in the nine patients were, respectively, systemic lupus eritematosus, cystinosis, galactosialydosis, chronic diffuse proliferative glomerulonephritis, chronic pyelonephritis, reflux nephropathy, renal dysplasia, bladder extrophia, and Hinman syndrome. None of the patients had undergone native kidney nephrectomy. Five patients were anuric, one of whom had a failed renal transplant, which had been surgically removed due to resistant hypertension. For this study, hypertension was defined by whether a patient was receiving at least one antihypertensive medication. Thus, all patients were taking antihypertensive medications at the start of the study. The distribution of the various classes of antihypertensives among the patients were as follows: calcium channel blockers (nine patients), angiotensin converting enzyme inhibitors (four), alpha blockers (two), and a beta blocker (one).
The Ethical Committee of the Cerrahpasa Medical Faculty approved the study. Informed consent was obtained from the patients and/or their parents.
Hemodialysis procedure
All patients underwent HD for 4 h three times a week on volumetric HD machines (Fresenius 4008; Fresenius Medical Care, Bad Homburg, Germany). Low flux dialyzer membranes (Asahi Kasai Medical, Tokyo, Japan) and bicarbonate-based dialysate were used. The dialysate temperature (37°C) and sodium concentration (140 mmol/L) were kept constant. Blood and dialysate flow rates were 5–7 ml/kg per minute and 500 ml/min, respectively. The patients were not allowed to eat and drink during the dialysis sessions. Neither micturition nor stooling occurred during dialysis sessions.
Blood volume monitoring
Plasma volume changes were monitored noninvasively and continuously using the Crit-Line III TQA Instrument (Hemametrics,Kaysville, UT). This instrument uses a transmissive photometric technique to determine the hematocrit on the basis of both the absorption properties of hemoglobin and the scattering properties of red blood cells passing through the blood chamber [8, 9]. It has been proven to be reliable [19].
Before each HD session, a sterile, disposable blood chamber was clipped onto the arterial blood line of the patient, and monitoring of hematocrit and blood volume changes was performed by the same investigator during each HD session throughout the study period.
Study protocol
The study was performed in four sequential phases, each of which lasted 1 week.
In phase I, UF volume to be removed during the HD session was kept similar with that in previous HD treatments based on clinical assessment of the estimated dry weight (the lowest post-dialysis weight that the patient can tolerate without the appearance of intradialytic symptoms or hypotension in the absence of overt volume overload). The UF rate was maintained constant throughout each HD session, and neither testing of sodium nor UF profiling was performed. The BMV of patients occurred without any intervention. Data from phase I served as the control for phase IV of the study.
In the second phase, BVM was used to guide UF in reaching the ideal dry weight, as recommended by Goldstein et al. [17]. All patients received half of their prescribed UF in the first hour of HD treatment, while not being allowed to exceed a maximum blood volume change of 8–10%. The UF rates were then adjusted to attain the second half of their target UF over the remaining 3 h of the HD session, up to a blood volume reduction that did not exceed 4% per hour and 18% throughout the HD session. In the last hour of the HD treatment, the patients received UF until they had hypotension or a blood volume change of more than 4% per hour, at which point UF was discontinued for 5 min. If the monitor demonstrated refilling of the intravascular compartment, UF was resumed until the patient was symptomatic or a blood volume change of more than 4% per hour was noted again. If no intravascular refilling was seen after 5 min, then the patient was determined to be at the target dry weight [17]. If needed, UF volume was deliberately increased 250–500 ml at each session until the plasma volume curves were at the desired levels. In this study, the average of two or three dry weights obtained during phase II was accepted as the ideal target weight. In phases III and IV, this particular weight was kept constant.
During phase III, one class of antihypertensive drug was gradually reduced when BP levels at 30 min after HD became less than the age-, sex-, and height-specific 95 percentiles; it was stopped when the BP became less than the age-, sex-, and height-specific BP, even after dose reduction.
In phase IV, patients were observed by BVM without any change being made in the therapy protocol. The caregivers were not blinded in terms of BVM curve in phase IV, and they were able to stop the UF if they observed a more than 4% per hour change in blood volume. However, this intervention was never needed throughout the study period.
Blood pressure measurements
Blood pressure was measured immediately before and after dialysis and at 30 min after each dialysis treatment using a standard automatic BP device (Dinamap XL; Critikon, Tampa, FL), with the patient in a sitting position. The definition of hypertension for casual BP at 30 min after HD was based on the 2004 report of the National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents [20].
Blood pressure was also assessed by 44-h ambulatory BP monitoring (ABPM) in phase I and after the end of phase IV. The measurement of the 44-h BP was obtained using the Spacelabs 90207 monitor (Spacelabs Medical, Redmond, WA). The monitor was attached onto the patient’s nonfistula arm 30 min after dialysis during the midweek HD days. It was set up to record BP every 20 min from 0700 to 2300 hours, and hourly from 2300 to 0700 hours during the interdialytic period. The results were evaluated on the basis of previously published ABPM normative data [21].
Weight measurements
Pre- and post-dialysis weights of barefoot patients in underclothes were measured using same scale during consecutive dialysis treatments over the course of 4 weeks. Intradialytic weight decrease (IDWD) was expressed as the difference between pre- and post-dialysis weight, and the IDWD percentage was obtained using the ratio between the IDWD and the patient’s post-dialysis weight.
Intradialytic symptoms
Intradialytic symptoms were recorded during each HD session throughout the whole study period. These symptoms included hypotension and/or other morbid symptoms (muscle cramps, headache, dizziness) requiring intervention (turning off UF, placing the patient in the Trandelenburg position, and/or infusing with a bolus saline).
Statistical analysis
Values are presented as mean ± standard deviation (SD). The values analyzed for comparison between phases I and IV were obtained from group means of the mean values for each of the nine patients. The significance of the differences between phase I and phase IV was determined by using the Wilcoxon t-test. The Fisher exact test was used to compare IDS prevalence among the study phases. The calculations were made using the SPSS ver. 13.0 Statistical Package Program (SPSS, Chicago, IL). A p value of less than 0.05 was considered to be statistically significant.
Results
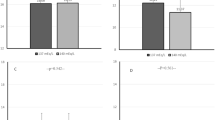
Data from 108 treatments of nine patients were obtained during our study period. Seven of these treatments were excluded from analysis for technical reasons, resulting in 101 treatments being subjected to analysis. Only 50 of these treatments, 25 in phase I and 25 in phase IV, were used to compare data between phase I and phase IV. Data on pre- and post-dialysis weights, intradialytic weight decreases, and plasma volume changes in the patients during phase I and phase IV are provided in Table 1. The mean pre- and post-dialysis weights of the patients had decreased significantly by the end of the study, but the intradialytic weight decrease in patients during phase IV was not different from that during phase I. The difference in the blood volume changes did not achieve statistical significance.
Mean values for various casual BP measurements are shown in Table 2. All values significantly decreased during phase IV as compared to phase I. Antihypertensive drugs were stopped in five patients and reduced in two others during phase III; the remaining two patients, using two and three agents, respectively, continued to receive the same antihypertensive medications (Table 3).
The mean ABPM results for the 44-h measurement and separately for daytime and nighttime are given in Table 4. The mean 44-h systolic and diastolic ABPM readings were lower at the end of the study than during phase I. When daytime and nighttime BP readings were considered separately, only daytime mean diastolic and nighttime mean systolic BPs were significantly lower at the end of the study. The 44-h systolic and nighttime diastolic BP loads were also lower at the end of the study. However, five patients had a 44-h systolic BP load and eight patients had a diastolic BP load greater than 50% at the end of study. There were no differences between study phases in terms of dipping status.
Intradialytic symptoms occurred in four of 25 treatments (16%) during phase I, in 13 of 24 treatments (56%) during phase II, in 11 of 27 treatments (40%) during phase III, and in nine of 25 treatments (36%) during phase IV. There was an increase in the frequency of IDS in phase IV as compared to phase I. However, the difference between phase I and phase IV did not reach statistical significance (p = 0.098).
Discussion
The use of continuous BVM during HD treatments to correctly estimate the patient’s dry body weight has shown considerable promise as a monitoring tool to date [17, 22]. Although blood volume monitors cannot determine dry weight directly, they can detect fluid overload. The results of our study demonstrate that BVM can be a reliable approach for adjusting the dry weight in pediatric HD patients and thus achieving better BP control.
The intradialytic decreases in blood volume in our patients were surprisingly high during phase I; consequently, it can be assumed that their dry weight was optimal. However, patients who have relatively high intradialytic plasma reduction during HD may still be overhydrated. Using Crit-Line, Rodriguez et al. [14] observed that some adult HD patients who experienced significant intradialytic reductions in blood volume had high postdialytic refilling rates after the end of dialysis. These authors interpreted this finding to be a marker of chronic fluid overload; the dry weight of these patients were successfully decreased in their study. Similarly, based on our results we considered that the hypertensive patients were in a chronic state of fluid overload that was not recognized clinically but which became apparent using BVM.
Cohen [23] suggested that the initial step for the management of hypertension in adult HD patients should be to reduce the extracellular volume by 0.5–1 kg at each treatment until the predialysis BP begins to fall; at that particular stage, the antihypertensive medications can be reduced. However, the ideal method for measuring the BP in HD patients as well as the timing of the BP measurement are open to controversy among clinicians [24]. Mitra et al. [25] showed that the best representation of interdialytic BP was the 20-min post-dialysis reading, as compared to ABPM. In our study, 30-min post-dialysis BP measurements along with BVM values were used to assess fluid overload, with higher values still indicating volume overload.
In our study, after adjustment of the target weight with online BVM in phase II, five patients no longer needed antihypertensive medications and the doses were greatly reduced in two others, leaving only two patients still requiring the same medications at the end of the study. Although volume overload is the most important factor in hypertension of HD patients, the role of other factors should not be overlooked in the pathogenesis. In these two patients still requiring the same medications, however, it remains debatable whether the etiology of the hypertension may have been related to causes other than fluid overload or to inadequate achievement of dry weight. The underlying etiology of their end-stage renal diseases were galactosialydosis and chronic diffuse proliferative glomerulonephritis; the latter patient presented with intractable hypertension at the onset of the disease and so may have already had an important element of renin-mediated hypertension.
Ambulatory BP monitoring has an important role in evaluating persistent poor BP control in HD patients [25]. In our study, the 44-h ABPM results for both systolic and diastolic BP were significantly lower at the end of the study than during phase I. When daytime and nighttime BP readings were considered as separate data sets, only daytime diastolic and nighttime systolic BP values were significantly lower at the end of the study. In a recent multicenter prospective study, Patel et al. [18], using 24-h ABPM, demonstrated that daytime BP was statistically significantly improved and nighttime BP also improved, although it did not reach statistical significance, after 6 months of BVM-guided UF in a group of normotensive and hypertensive children undergoing HD. Patel et al. [18] may not have been able to document significantly improved nighttime BP and daytime BP as was the case in our study because they only used 24-h rather than 44-h ABPM measurements and so did not fully assess the entire interdialytic period as we did in our study.
The sleep BP decrease was attenuated in our study. It has previously been shown that blunted nocturnal dipping is a prevalent finding in children with chronic renal insufficiency [26] and pediatric HD patients [18]. In our study, the SBP and DBP loads on ABPM after phase IV were still elevated, although they were lower than the values found during phase I. These findings can be explained in two ways—either the patients had not achieved true dry weight post-HD, or antihypertensive medications had been prematurely discontinued. During phases II and III, we observed that the adjustment of dry weight in the five patients using one antihypertensive was easier than in those patients who suffered from more serious hypertension and using multiple antihypertensives. Mailloux and Haley [27] suggested withdrawing antihypertensive medications as slowly as possible in the process of establishing a rational dry weight and setting a realistic timetable for this intervention, e.g. 3–6 weeks for young adult HD patients. In another study, Sorof et al. [28] found that casual BP measurements correlated poorly with ABPM during the interdialytic period in normotensive children receiving chronic HD. These authors concluded that children undergoing chronic HD with normal postdialysis casual BP may be significantly hypertensive [28]. The ABPM data in our study supports the conclusion of Sorof et al. [28]. Therefore, we believe that improving the BP measurement techniques, such as implementing a post-dialysis 30-min reading in the dialysis unit, can be useful in a qualitative sense. However, there is no substitute for ABPM in the interdialytic period, if an accurate level of BP in HD patients is needed to correctly identify hypertension and evaluate cardiovascular morbidity.
Michael et al. [16] suggested that the recommended BVM-guided UF algorithm [15, 17] may be an aggressive approach. When the algorithms with different UF rates were evaluated, they demonstrated a twofold increase in IDS with the 100% UF model as compared to the 80% and 90% models despite the lack of any significant difference in BP [16]. Indeed, we also observed an increase in IDS in phase IV as compared to that in phase I using the recommended BVM algorithm, but our BP values were significantly lower in phase IV than in phase I, despite a revision of antihypertensive medication. We suggest that a strict control of extracellular volume by BVM, indirectly, is useful even if intradialytic symptoms are higher during the early stage. Özkahya et al. [29] demonstrated that the incidence of hypotension and cramps also decreased during the follow-up period while they were reducing the BP of adult hypertensive hemodialysed patients with strict volume control. Patel et al. [18] also showed that both BP and IDS were improved in pediatric HD patients after 6 months of recommended BVM-guided UF.
Intradialytic symptoms may occur when the clinician is attempting to adjust the dry weight, especially in patients with large interdialytic weight gain. High UF rates are therefore required to achieve the desired fluid removal within the allotted time, even though this intervention increases the likelihood of an imbalance between UF rate and vascular refilling. To cope with this problem, UF-guided BVM may be useful as it allows the prescribed dry weight to be achieved more safely. Since high UF rates are independently associated with an increased mortality risk, longer or more frequent dialysis sessions should be considered in order to prevent the deleterious consequences of excessive UF rates [30]. The BVM algorithm used in our study may also be more beneficial than the constant UF protocol in terms of decreasing the risk of cardiovascular complications. However, the BVM alone cannot determine all of the variables that interfere with vascular refilling, such as intradialytic sodium balance. Hothi et al. [31] were able to achieve higher UF volumes without symptoms in pediatric patients when they prescribed HD using both dialysate sodium and UF profiles. The duration of HD was kept constant in our study because our aim was to evaluate the efficacy of BVM for assessing the effects of optimizing dry weight on BP control. However, prolonged or daily dialysis regimens can be useful to achieve dry weight, especially in diet-uncompliant hypertensive children [32].
Our study has several limitations that should be mentioned. First, the study was based on a relatively small number of children and adolescents. If a larger number of patients were to be analyzed, more definite results could be obtained. Second, the study period may have been too short for evaluation purposes, especially ABPM data and IDS at the end of the study. It is well-known that there may be a delay between the normalization of extracellular volume and the fall in BP readings (lag phenomenon) [33]. On the other hand, although not significantly different, the increase in IDS at the end of the study can be criticized. We believe that IDS can be decreased with a strict volume control regimen on the long-term course: even though we were unable to prove this on the basis of our data, Patel et al. have confirmed that this is indeed possible [18].
In conclusion, our study supports the routine use of online BVM to determine dry body weight and decreased chronic fluid overload and to decrease the need for antihypertensive medications to control hypertension in children maintained with chronic hemodialysis.
References
Mitsnefes M, Stablein D (2005) Hypertension in pediatric patients on long-term dialysis: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis 45:309–315
Parekh RS, Carroll CE, Wolfe RA, Port FK (2002) Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141:191–197
Van De Voorde RG, Barletta GM, Chand DH, Dresner IG, Lane J, Leiser J, Lin JJ, Pan CG, Patel H, Valentini RP, Mitsnefes MM (2007) Blood pressure control in pediatric hemodialysis: the Midwest Pediatric Nephrology Consortium Study. Pediatr Nephrol 22:547–553
Hörl MP, Hörl WH (2002) Hemodialysis-associated hypertension: pathophysiology and therapy. Am J Kidney Dis 39:227–244
Jaeger JQ, Mehta RL (1999) Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol 10:392–403
Ishibe S, Peixoto AJ (2004) Methods of assesment of volume status and intercompartmental fluid shifts in hemodialysis patients: implications in clinical practice. Semin Dial 17:37–43
Leypoldt JK, Cheung AK (1998) Evaluating volume status in hemodialysis patients. Adv Ren Replace Ther 5:64–74
Leypoldt JK, Lindsay RM (1999) Hemodynamic monitoring during hemodialysis. Adv Ren Replace Ther 6:233–242
De Vries JP, Kouw PM, Nardo JM, Van der Meer NJ, Olthof CG, Oe LP, Donker AJ, De Vries PM (1993) Non-invasive monitoring of blood volume during hemodialysis: its relation with post-dialytic dry weight. Kidney Int 44:851–854
Steuer RR, Leypoldt JK, Cheung AK, Senekjian HO, Conis JM (1996) Reducing symptoms during hemodialysis by continuously monitoring the hematocrit. Am J Kidney Dis 27:525–532
Steuer RR, Conis JM (1996) The incidence of hypovolemic morbidity in hemodialysis. Dial Transplant 25:272–274
Wilkie ME, Lindley EJ, Edwards L, Roebuck AF, Warwick G, Brown CB (1996) Improved ultrafiltration control using an on-line blood volume monitor (BVM). Nephrol Dial Transplant 11:A202
Steuer RR, Germain MJ, Leypoldt JK, Cheung AK (1998) Enhanced fluid removal guided by blood volume monitoring during hemodialysis. Artif Organs 22:627–632
Rodriguez HJ, Domenici R, Diroll A, Goykhman I (2005) Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int 68:854–861
Jain SR, Smith L, Brewer ED, Goldstein SL (2001) Non-invasive intravascular monitoring in the pediatric hemodialysis population. Pediatr Nephrol 16:15–18
Michael M, Brewer ED, Goldstein SL (2004) Blood volume monitoring to achieve target weight in pediatric hemodialysis patients. Pediatr Nephrol 19:432–437
Goldstein SL, Smith JM, Currier H (2003) Noninvasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol 14:2127–2131
Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, Flynn JT (2007) A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol 2:252–257
Leypoldt JK, Cheung AK, Steuer RR, Harris DH, Conis JM (1995) Determination of circulating blood volume by continuously monitoring hematocrit during hemodialysis. J Am Soc Nephrol 6:214–219
National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W (1997) Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr 130:178–184
Fischbach M, Edefonti A, Schröder C, Watson A (2005) Hemodialysis in children: general practical guidelines. Pediatr Nephrol 20:1054–1066
Cohen EP (2000) Hypertension in chronic hemodialysis: viewing a paradox, and some notes on therapy. Dial Transplant 29:535–539
Sankaranarayanan N, Sergio Santos FF, Peixoto AJ (2004) Blood pressure measurement in dialysis patients. Adv Chronic Kidney Dis 11:134–142
Mitra S, Chandna SM, Farrington K (1999) What is hypertension in chronic hemodialysis? The role of interdialytic blood pressure monitoring. Nephrol Dial Transplant 14:2915–2921
Mitsnefes MM, Kimball TR, Daniels SR (2003) Office and ambulatory blood pressure elevation in children with chronic renal failure. Pediatr Nephrol 18:145–149
Mailloux LU, Haley WE (1998) Hypertension in ESRD patient: pathophysiology, therapy, outcomes, and future directions. Am J Kidney Dis 32:705–719
Sorof JM, Brewer ED, Portman RJ (1999) Ambulatory blood pressure monitoring and interdialytic weight gain in children receiving chronic hemodialysis. Am J Kidney Dis 33:667–674
Özkahya M, Töz H, Ünsal A, Özerkan F, Asçı G, Gürgün C, Akçiçek F, Dorhout Mees EJ (1999) Treatment of hypertension in dialysis patients by ultrafiltration: role of cardiac dilatation and time factor. Am J Kidney Dis 34:218–221
Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G (2007) Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22:3547–3552
Hothi DK, Harvey E, Goia CM, Geary D (2008) Blood-volume monitoring in paediatric haemodialysis. Pediatr Nephrol 23:813–820
Fiscbach M, Terzic J, Laugel V, Dheu C, Menouer S, Helms P, Livolsi A (2004) Daily on-line hemodiafiltration: a pilot trial in children. Nephrol Dial Transplant 19:2360–2367
Charra B, Bergström J, Scribner BJ (1998) Blood pressure control in dialysis patients: importance of the lag phenomenon. Am J Kidney Dis 32:720–724
Acknowledgements
This work was supported by the Turkish Foundation of Pediatrics, and Turkish Society of Nephrology Istanbul Branch. It was partly presented at the 39th Annual Meeting of the European Society for Pediatric Nephrology, September 10–13, 2005, Istanbul, Turkey, and has been published in abstract form (abstract; Candan C et al, Pediatr Nephrol 20: C56, 2005). The authors thank the nurses of the Children’s Hospital Hemodialysis Unit of Cerrahpasa Medical Faculty, especially Gulseren Pehlivan and Refiye Basegmez for their assistance in performing this study. The authors thank to Mehmet Sukru Sever M.D. for his criticism and advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Candan, C., Sever, L., Civilibal, M. et al. Blood volume monitoring to adjust dry weight in hypertensive pediatric hemodialysis patients. Pediatr Nephrol 24, 581–587 (2009). https://doi.org/10.1007/s00467-008-0985-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0985-9