Abstract
Cyclodextrins are cyclic oligosaccharides obtained by enzymatic degradation of starch. They are remarkable macrocyclic molecules that have led major theoretical and practical advances in chemistry, biology, biochemistry, health science, and agriculture. Their molecular structure is composed of a hydrophobic cavity that can encapsulate other substances to form inclusion complexes through host-guest interactions. This unique feature is at the origin of many applications. Cyclodextrins and their derivatives have a wide variety of practical applications in almost all sectors of the industry, including pharmacy, medicine, foods, cosmetics, chromatography, catalysis, biotechnology, and the textile industry.
Villiers published the first reference to cyclodextrins in 1891. Since the beginning of the twentieth century, major researchers, such as Schardinger, Pringsheim, Karrer, Freudenberg, French, Cramer, Casu, Bender, Saenger, Nagai, Szejtli, and Pitha, have paved the history of the cyclodextrins. Several time periods have marked their history. After their discovery and characterization from 1891 to 1911, there has been a period of doubt and disagreement from 1911 to 1935. Then, the 1935–1950 exploration period was marked by structural results on the “Schardinger dextrins.” In 1949, Cramer introduced the cyclodextrin-based nomenclature. Research between 1950 and 1970, the period of maturation, focused on conformations and spectroscopic data of cyclodextrins and their inclusion complexes, with applications in catalysis and as enzyme models. Finally, the period of use has been ongoing since 1970 and has seen cyclodextrins find many industrial applications. Cyclodextrins have then found many industrial applications, initially in the pharmaceutical and food sectors. In 1984, the first chromatographic columns were commercialized. At that time, many cyclodextrin-based catalysts were developed for biomimetic chemistry and other applications such as artificial enzymes. Currently, more than 2000 publications on cyclodextrins are published each year.
In this chapter, we present a historical overview of the discovery, development, and applications of cyclodextrins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- History
- Schardinger dextrins
- Discovery
- Production
- Separation
- Native cyclodextrins
- Development
- Inclusion complexes
- Applications
1.1 Introduction
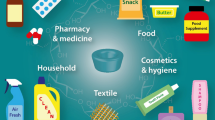
Figure 1.1 shows that cyclodextrins occur in many daily products such as an ibuprofen tablet, a nonsteroidal anti-inflammatory drug, a whooping cough vaccine, a curative antidote, a hair loss solution, a stop smoking aid, toothpastes, shampoo, colognes, a deodorant toilet, razors, a turmeric-based food supplement, a butter, a mayonnaise, fish sausages, modified steaks, a horseradish powder, mustard sauces, a sweetener, honey, a cinnamon extract, green tea without bitterness, vanilla coffee, clarified fruit juices, chewing gums, chromatographic columns, biopesticides, catalysts, tubular materials, a curtain, cosmetotextiles, an ink, a detergent, a bioflocculant for swimming pool, or a bioadsorbent for water treatment.
Cyclodextrins are cyclic oligomers obtained from the enzymatic degradation of starch. They are one of the most remarkable macrocyclic molecules with significant impacts in our daily lives. Cyclodextrins have a particular structure composed of a hydrophobic cavity that can encapsulate other substances to form inclusion complexes through host-guest interactions. This characteristic feature is at the origin of many applications. Today, all industrial sectors are concerned, e.g., pharmaceuticals, cosmetics, food, hygiene and toiletries, biotechnology, medical, radiology, agrochemistry, catalysis, packaging, textile industry, nanotechnology, and soil and water treatment.
The French pharmacist Villiers published the first reference to cyclodextrins in 1891 (Villiers 1891a, b, c, d). Since the beginning of the twentieth century, Schardinger, Pringsheim, Karrer, Freudenberg, French, Cramer, Casu, Bender, Saenger, Nagai, Szejtli, and Pitha have marked the history of cyclodextrins (Szejtli 1998; Loftsson and Duchêne 2007; Kurkov and Loftsson 2013; Crini 2014; Morin-Crini et al. 2015; Crini et al. 2018).
The first important period on the history of cyclodextrins, from 1891 to 1911, covers their discovery by Villiers, and their characterization and chemistry by Schardinger (Thoma and Stewart 1965; Caesar 1968; Clarke et al. 1988; Szejtli 1998). In 1891, Villiers discovered a crystalline dextrin from the Bacillus amylobacter digest of potato starch, which he named cellulosine. At the beginning of the last century, Schardinger also observed the formation of two crystallized products during his investigations of food spoilage, which he called crystallized dextrin-α and crystallized dextrin-β. Schardinger gave the first detailed description of the preparation and separation of these two dextrins. He was also the first to isolate the strain of bacteria responsible for dextrin formation, i.e., Bacillus macerans. However, from 1911 to 1935 came a period of doubt and disagreement, in particular between the groups of Pringsheim and Karrer, although they published numerous studies on the composition, properties, and chemistry of the crystallized dextrins (Crini 2014).
It was not until the mid-1930s that research on dextrins developed again. The exploration period from 1935 to 1950 was marked by the numerous results obtained by Freudenberg and French on the structure of the “Schardinger dextrin” molecules. In 1935, Freudenberg was the first to develop a relatively simple method for the obtention and purification of the two Schardinger dextrins. Freudenberg also suggested in 1936 a cyclic structure for α-dextrin and β-dextrin, which was confirmed in 1938. In the 1940s, French proposed that Schardinger dextrins be called cycloamyloses and described new protocols for the preparation of cycloamyloses with high purity. In 1942, Hudson discovered the enzyme in Bacillus macerans responsible for the conversion of starch into dextrins, and the same year, French published the exact molecular weights of the cyclohexaamylose and cycloheptaamylose, i.e., α-dextrin and β-dextrin, respectively. In 1948, Freudenberg discovered γ-dextrin or cyclooctaamylose, and 1 year later, Cramer, his PhD student, introduced the cyclodextrin-based nomenclature. From 1950 onward, this terminology was increasingly used although the nomenclature of cyclodextrins remained a subject of debate until the end of the 1990s.
The period between 1950 and 1970, known as the period of maturation of notions, focused on inclusion complexes with Cramer’s work in the foreground. At the beginning of the 1950s, French finally demonstrated the chemical cyclic structures of cycloamyloses. In 1953, Cramer gave the basis for supramolecular catalysis involving cyclodextrins, and the same year, with Freudenberg and Plieninger, he published the first patent concerning the applications of cyclodextrins in pharmaceutical formulations. In 1956, Cramer introduced and detailed the notion of an inclusion complex. From that time on, the interest in cyclodextrins increased. During the maturation period, the works of Casu on the conformation and spectroscopic characterization of cyclodextrins were acknowledged to have brought an important contribution. At the same time, much attention was also focused on their use for catalysis and as enzyme models, and one name stands out in particular in the enzymology and catalysis by cyclodextrins: Bender (Crini 2014). Nonetheless, until the mid-1970s, the three main native cyclodextrins, i.e., α-, β-, and γ-cyclodextrins, available only in small quantities, were long considered as just laboratory curiosities (Thoma and Stewart 1965; Caesar 1968; Kainuma 1984; Clarke et al. 1988). In the way of industrial development, the three main obstacles were their price, e.g., in 1975 1 kg of β-cyclodextrin had a price of about 1500 $ (Szejtli 1982a), their presumed toxicity (French 1957a), and the lack of sufficient knowledge of these substances (Szejtli 1982a). In addition, very few researchers were convinced of the industrial potential of cyclodextrins.
The 1970s were marked by two important events: firstly, several manufacturers started to produce and to commercialize cyclodextrins; at that time, due to improvements in the production of cyclodextrins, their prices have dropped significantly. Secondly, the first toxicological studies had established that β-cyclodextrin administrated orally was a harmless substance. As a result, this has led to spectacular progress. From then on, the period of use began and cyclodextrins found many industrial applications. During this period of utilization, four names stand out: Saenger, Szejtli, Nagai, and Pitha. In the mid-1970s, pharmaceutical and food applications started to appear and rapidly gained ground, especially in Japan (Hamada et al. 1975; Szejtli 1977; Pitha et al. 1983; Uekama and Otagiri 1987; Frömming and Szejtli 1994). In 1980, Saenger published the first comprehensive review about the potential industrial applications of cyclodextrins (Saenger 1980). The first International Cyclodextrin Symposium organized by Szejtli took place in Budapest in 1981, and 1 year later, he wrote the first comprehensive cyclodextrin book (Szejtli 1982a). At that time, many interesting catalysts based on cyclodextrins were also constructed for biomimetic chemistry and other processes of interest such as artificial enzymes (Breslow 1979; Breslow and Dong 1998). Both from an academic and industrial point of view, the number of communications then started to increase exponentially, as did the filing of patents.
In the mid-1980s, cyclodextrins were produced in large quantities and commercialized at a reasonable price, i.e., 10–15 $/kg (Szejtli 1982a). Other industrial applications have become possible. In 1984, the first chromatographic columns were marketed (Armstrong 1984; Ward and Armstrong 1986, 1988; Armstrong and Jin 1989). Since then, an increasing interest in cyclodextrins and their possible applications has existed (Duchêne 1987, 1991; Szejtli 1988). An abundant scientific literature has built up since the 1980s. Currently, every year, more than 2000 publications, including articles and book chapters, are devoted to cyclodextrins (Cyclodextrin News, CycloLab Ltd., Hungary). Nowadays, these molecules still fascinate researchers and industrials.
The objective of this chapter is to describe historical landmarks of the discovery, exploration, and utilization of cyclodextrins. We also present some highlights of their early industrial applications. To this end, an extensive list of data from about 500 original publications has been compiled. Although this historical chapter cannot hope to be exhaustive, it does highlight the work of those researchers who have contributed to the knowledge of cyclodextrins throughout the 129 years of its history.
1.2 Discovery and First Chemical Studies of Cyclodextrins
1.2.1 Discovery: 1891–1911
During experiments on the degradation and reduction of carbohydrates under the action of ferments, Antoine Villiers, a French pharmacist and chemist, was the first to observe in 1891 the formation of unwanted crystals with particular properties, i.e., the formation of cyclodextrins. Among various Villiers’ biographies, those by French (1957a), Thoma and Stewart (1965), Caesar (1968), Szejtli (1998), Loftsson and Duchêne (2007), Kurkov and Loftsson (2013), Crini (2014), and Morin-Crini et al. (2015) deserve particular mention.
Studying the degradation and reduction of carbohydrates, Villiers showed how easy it was to transform starch to yield “novel crystalline dextrins” with particular properties under the action of ferments. He first obtained a small amount of crystalline dextrins from digests of Bacillus amylobacter, i.e., Clostridium butyricum, on potato starch under certain conditions (Villiers 1891a, b): 50 g potato starch in 1 L of water at 100 °C subsequently seeded with Bacillus amylobacter and incubated for several days in an oven at 40 °C. Villiers presented his results to the French Académie des Sciences in February 1891 (Fig. 1.2). At that time, the dextrins, previously discovered in 1821, were the degradation products and/or the intermediate decomposition products of starch through heating. For Villiers, his dextrins were degradation products of starch. When purified by fractional precipitation, the crystals presented very different optical rotation properties and were difficult to hydrolyze any further. Iodine stains red those dextrins that had a high optical activity, and the intensity of the stain decreased with the optical activity. The butyric ferment caused the transformation of the starch directly into dextrin without the involvement of intermediates such as diastases secreted by the ferment. Later, Villiers considered his dextrins as the intermediate decomposition products of starch (Villiers 1891b). Villiers also obtained un curieux sous-produit, i.e., a curious by-product, in small quantities after several weeks of incubation: 3 g of this carbohydrate was obtained as crystals after bacterial digestion of 1000 g of starch. This new substance was found in the alcohol that was used for the precipitation of dextrins (Villiers 1891b).
In a second proceedings of the French Académie des Sciences of June 1891 (Fig. 1.3), Villiers described the chemical composition of the novel highly crystalline dextrin having a composition between that of starch and that of dextrin (Villiers 1891c). In air, the crystals, containing water and alcohol of crystallization (the proportion of the latter is rather small, about 4%), became opaque. They lose alcohol and absorbed water without any change in weight. After purification in large amount of hot water, Villiers obtained small brilliant crystals, most probably β-cyclodextrin, and determined the chemical composition of this crystalline carbohydrate. He gave the first empirical formula: [(C6H10O5)2 + 3H2O]. Its solubility in water at room temperature was low but raised with temperature. The white crystals with a very slight sweetness showed extremely high optical activity, much higher than those of certain dextrins formed under the action of the butyric ferment. Villiers then considered this novel substance as an isomer of starch (Villiers 1891c, d). By manipulating the experimental conditions, Villiers obtained two distinct crystalline dextrins, most probably α-cyclodextrin and β-cyclodextrin, having a composition represented by a multiple of the formula [(C6H10O5) + 3H2O]. Villiers noted again that the white crystals with a very slight sweetness showed extremely high optical activity. Pursuing his experiments, he observed that the two dextrins, always considered as isomers of starch, were almost insoluble in water, soluble in alcohol, non-fermentable, and acid resistant, and they could also be converted into ethers under the action of acid chlorides. Villiers finally concluded that the properties of these two particular dextrins were very clearly different from those of the various saccharides and polysaccharides known at the time and proposed the name of cellulosines due to the similarities with cellulose, e.g., with “regard to difficulty of acid hydrolysis” (Villiers 1891c, d).
At the beginning of the 1900s, Heinrich Robert Koch, a famous German physician and microbiologist, who received a Nobel Prize in 1905, remained unconvinced by Villiers’ conclusions (Crini 2014). In Koch’s opinion, Villiers used “primitive bacteriological techniques and probably impure cultures.” This was also pointed out by Schardinger (1904). Later, French (1957a) indicated that “Villiers used impure cultures but his digests contained sufficient Bacillus macerans to account for the small amount of crystalline dextrin obtained.”
The recognition to cyclodextrins is attributed to Franz Schardinger, an Austrian chemist and bacteriologist. Schardinger is the first Great Scientist who has left its mark on the history of these oligosaccharides. He is considered the “Founding Father” of cyclodextrin (Szejtli 1982a; Crini 2014).
At the beginning of the last century, Schardinger also observed the formation of dextrins during his investigations of resistant microorganisms that can lead to food poisoning (Fig. 1.4). Like other researchers at that time, Schardinger studied these dextrins with the expectation that they would shed some light on the synthesis and degradation of starch. In 1903, Schardinger discovered that a type of extremely heat-resistant microorganism was able to dissolve starch and form crystalline by-products (Schardinger 1903a), remarkably similar to cellulosines reported by Villiers. Using the iodine test, Schardinger distinguished two types of krystallisiertes dextrins which he called crystallized dextrin A and crystallized dextrin B. The B form resembled Villiers’ cellulosine. Indeed, the chemical behavior and the physical constants given by Schardinger for his substance agree very well with those of the dextrin previously described by Villiers. Schardinger found that it was possible to isolate pure fractions with a maximum yield of 30% crystallized dextrins from starch, the main form obtained being always dextrin B. Krystallisiertes dextrins were first considered as the degradation products of starch through heating (Schardinger 1903a). Schardinger also managed to isolate the strain of bacteria responsible for the degradation of starch – he called it strain II (Schardinger 1903b). He observed that this heat-resistant organism had considerable starch-fermenting power. When sub-cultured on starch, strain II broke down starch, giving an alcohol-insoluble “soluble starch” together with crystallized dextrin A (fine hexagonal plates) and crystallized dextrin B (stout prismatic crystals). Schardinger also observed that with time, the activity of the strain II microorganism decreased. Indeed, he was unsuccessful in maintaining a culture of strain II which had the characteristic starch-degrading activity.
In 1904, Schardinger isolated a new microorganism, considered as “an accidental contaminant,” which he first called Rottebacillus I owing to its action on potato starch, i.e., it produced acetone and ethyl alcohol by fermentation of carbohydrate media (Schardinger 1904). The name Rottebacillus I was used to express the fact the microorganism was able to form both acetone and ethyl alcohol. Several months later, Schardinger used the Latin term Bacillus macerans to name his microbe, i.e., macerare, to rot (Fig. 1.5). This bacillus was able to give the same crystalline dextrins as before, which he designated as krystallisiertes polysaccharides, i.e., crystallized polysaccharides, considered then as the intermediate decomposition products of starch (Schardinger 1904). Using the characteristic reaction that starch derivatives show with iodine, Schardinger proposed a distinction between a “crystallized amylose” and a “crystallized amylodextrin.” The yields obtained were tenfold those reported by Villiers. To explain this result, Schardinger suggested that, in the conditions of sterilization described by Villiers, the bacillus used was “probably not pure” (Schardinger 1904). One year later, Schardinger was also the first to observe that different starchy substrates differed in their behavior with Bacillus macerans, especially in the yields obtained (Schardinger 1905).
1.2.2 The Foundation of the Cyclodextrin Chemistry
Schardinger is acknowledged as being the first to lay down the basis of the cyclodextrin chemistry (French 1957a; Thoma and Stewart 1965; Szejtli 1998). Indeed, he was the first researcher to describe the fundamental properties of cellulosines, to introduce the terms crystallized α-dextrin and crystallized Β-dextrin, to isolate the microorganism able to synthesize the enzyme that catalyzes the degradation of starch into crystallized dextrins, to hypothesize that the crystallized substances were cyclic “polysaccharides,” and also to suggest their ability to form complexes.
Between 1905 and 1911, Schardinger made several important observations (Schardinger 1903a, b, 1904, 1905, 1909, 1911). He observed that cellulosines were often formed in starch-based media containing putrefying microorganisms. The formation of the two crystallized dextrins depended on the type of bacteria digesting starch. The distinction between the two forms was always made through their ability to form complexes of different colors with iodine. Schardinger also studied the chemistry of the two dextrins, pointing out their lack of reducing power and hydrolysis to reducing sugar. Dextrins were non-reducing to copper reagents and non-fermentable by yeast. Schardinger also reported their behavior in the presence of alcohols, chloroform, ether, and iodine solution. He used the complexes with these solvents as a means of precipitation of dextrins (Schardinger 1911). This was the first indication of the ability of dextrins to form “inclusion” complexes (Crini 2014). Finally, Schardinger proposed empirical formulae of dextrins. However, he did not propose a structure for his crystallized dextrins and also did not attempt their molecular-weight determinations. It will take another 20 years before the cyclic nature of Schardinger dextrins will be recognized. Professor Schardinger decided to stop his research into dextrins in 1911, and as a conclusion he wrote: “I realize that still very many questions remain unsolved; the answer to these I must leave to another, who, owing to more favorable external conditions, can deal with the subject more intensively.”
In the 24 years following Schardinger’s final paper (Schardinger 1911), the field of research on crystallized dextrins was dominated by the groups of Pringsheim and Karrer. Pringsheim is recognized as the first researcher to have published prolifically on dextrins. However, the works were repetitive, marred by frequently contradictory results and by even hot debate between the two groups (French 1957a; Szejtli 1998; Crini 2014; Morin-Crini et al. 2015).
1.3 Historical Landmarks in the Exploration of Cyclodextrins: From 1911 to 1970
1.3.1 Nomenclature
In 1891, cyclodextrin was initially called cellulosine by Villiers because he assumed that the novel crystalline substance, obtained from digests of Bacillus amylobacter, was une sorte de cellulose, i.e., a kind of cellulose (Crini 2014).
In 1903, Schardinger reported the formation of two krystallisiertes dextrins during his investigations of food spoilage, which he called crystallized dextrin A and crystallized dextrin B, because most of their properties were similar to the already known partial degradation products of starch, i.e., the dextrins (Schardinger 1903a, b). One year later, the krystallisiertes dextrins, considered as the intermediate crystallized decomposition products/by-products of starch, were designated by the term krystallisiertes polysaccharides, i.e., crystallized polysaccharides (Schardinger 1904). Pursuing his investigations on the structure of starch, Schardinger then introduced a distinction between a crystallized amylose for dextrin A and a crystallized amylodextrin for dextrin B, because, for him, there was an analogy between his dextrins and amylose and amylodextrin, especially with respect to their iodine color reactions (Schardinger 1905, 1907). Finally, Schardinger considered that these names were inappropriate and thus decided to rename it crystallized dextrin-α and crystallized dextrin-β (Schardinger 1911).
In the mid-1910s, the German chemist and biochemist Hans Pringsheim used the name of krystallisiertes polyamylosen, i.e., crystallized polyamyloses, distinguishing two series, the α-series of dextrins containing 2n D-glucose units per molecule and the β-series containing 3n D-glucose units per molecule. Four substances, i.e., α-diamylose, α-tetraamylose, α-hexaamylose, and α-octaamylose, were included in the α-series of dextrins, while the β-series only contained two substances, i.e., β-triamylose and β-hexaamylose. Indeed, for Pringsheim, the Schardinger dextrins arose through the bacterial depolymerization of starch to the fundamental units: the amylose fraction being broken down into the α-series of dextrins, i.e., polyamyloses, and the amylopectin fraction being degraded to the β-series (French 1957a; Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Morin-Crini et al. 2015). Pringsheim also used the terms of α-amylosan, α-allo-amylosan, and α-iso-amylosan and β-amylosan, β-allo-amylosan, and β-iso-amylosan for α-dextrin and β-dextrin, respectively (Crini 2014). At the same time, the Swiss chemist Paul Karrer also introduced the notion of series of crystallized dextrins. Like Pringsheim, Karrer was convinced that the α-series of dextrins was composed of at least four distinct substances differing in molecular size. However, he disagreed with the subdivision of the β-series into triamylose and hexaamylose. For Karrer, these two products were identical. In addition, Karrer regarded maltose as the fundamental unit of the whole of the starch molecule, while Pringsheim considered the polyamyloses as the basic units of the starch molecule (French 1957a; Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Morin-Crini et al. 2015). Like Pringsheim, Karrer also used the amylosan-based terminology (Crini 2014).
In the 1920s, as a tribute of the pioneering work of Schardinger, the German chemist Karl Johann Freudenberg called them “Schardinger dextrins” and referred to these compounds as α-dextrin and β-dextrin (French 1957a; Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Loftsson and Duchêne 2007; Kurkov and Loftsson 2013; Morin-Crini et al. 2015) and later as pentaosan and hexaosan, respectively (Crini 2014). For many years, cyclodextrins were called “Schardinger dextrins” in his honor, almost up to the 1970s, or also sometimes simply as dextrins (Szejtli 1998). Schardinger dextrins were subsequently named “cycloamyloses” by the American chemist Dexter French in 1942 (French and Rundle 1942), “cycloglucanes” by Freudenberg in 1943 (Freudenberg 1943), and finally “cyclodextrins” in 1949 by the German chemist Friedrich Cramer, a pupil of Freudenberg (Cramer 1949). The model of “cycloamyloses” was constructed from glucopyranose units in the boat conformation. For French, α-dextrin, β-dextrin, and γ-dextrin must be called cyclohexaamylose, cycloheptaamylose, and cyclooctaamylose, respectively, the Greek prefix to the “amylose” corresponding to the degree of polymerization, i.e., indicating the number of glucose units in the ring (French and Rundle 1942). However, at that time, Freudenberg claimed that “this new nomenclature was inappropriate and ambiguous” (Freudenberg 1943). Again, in 1947, Freudenberg wrote: “It appears to be premature to rename the α-dextrin cyclohexa-amylose and the β-dextrin cyclohepta-amylose” (Freudenberg et al. 1947a, b). In 1943, Freudenberg proposed the cycloglucane-based nomenclature, e.g., cyclohexaglucane α(1→4), cycloheptaglucane α(1→4), and cyclooctaglucane α(1→4) for α-dextrin, β-dextrin, and γ-dextrin, respectively (Freudenberg 1943). During the mid-1940s, there was another system in current use (Crini 2014). In the alternate system, the number of residues in the cyclic polymer was indicated by prefixing a Greek letter to the series name. Since the smallest known cycloamylose was a hexamer, it was assigned the prefix α. The cyclic heptatose, octaose, etc. were referred to, respectively, as β, γ, etc. The first system introduced by French was however preferred because it was more descriptive of the structures.
At the end of the 1940s, Cramer first proposed the cyclo-based nomenclature for the nomenclature of the Schardinger dextrins, e.g., (6-ose)-cyclo, (7-ose)-cyclo, and (8-ose)-cyclo for α-, β-, and γ-dextrins, respectively. For the first time in 1949, Cramer introduced the term cyclodextrin. This name was included in the title of his PhD dissertation entitled Die Cyclodextrine aus Stärke (Cramer 1949). For Cramer, the term of cyclodextrin must be used to refer to cyclic oligosaccharides made up of 6, 7, or 8 units of D-glucose joined by α-(1 → 4) linkages termed α-, β-, and γ-cyclodextrin, respectively. Because of its relative brevity, the term cyclodextrin was soon accepted, but the nomenclature of cyclodextrins remained a subject of debate until the end of the 1990s (Szejtli 1998; Loftsson and Duchêne 2007; Kurkov and Loftsson 2013; Crini 2014; Morin-Crini et al. 2015). Indeed, at that time, several laboratories proposed clarifications of the nomenclature of cyclodextrins because the term cyclodextrin only specified the nature of the sugars but did not give any information on the bonding between them. Thus, the name cyclomaltohexaose was suggested in 1997. This name is composed of first the term cyclo followed by a term indicating the type of linkage, i.e., malto for glucose unit bound by α-(1→4) linkages, and the number of sugar units with the ending ose, i.e., hexa for 6 or hepta for 7. This final term, present in cyclomaltohexaose, implies a free anomeric center, which is not present in cyclodextrins. Both the terms cyclodextrins and cyclomalto-oligosaccharides were used (Crini 2014).
Other nomenclatures have also been proposed. For instance, α-cyclodextrin was named cyclohexakis-(1→4)-α-D-glycosyl or cyclo-α-(1→4)-glucohexaoside. The term of the glycosyl residue is preceded by the type of linkage between brackets, which in turn is preceded by the term cyclo plus an indication of the number, i.e., cyclohexakis, etc. The literature uses all of these nomenclatures. Nevertheless, the cyclodextrin-based nomenclature is still the most widely used in literature today. The nomenclature for large-ring cyclodextrins, i.e., LR-CDs with a degree of polymerization between 9 and >100, is more simple: each molecule is designated by an abbreviation CDn where n indicates the number of glucose units in the macrocycle, e.g., CD14 (boat-like structure) composed of 14 glucose units (Morin-Crini et al. 2015; Assaf et al. 2016; Sonnendecker and Zimmermann 2019a, b; Sonnendecker et al. 2018, 2019).
1.3.2 Native Cyclodextrins
Schardinger recognized only dextrin-α and dextrin-β, while Freudenberg obtained γ-dextrin in 1948, although previously regarded by him as a cyclic heptasaccharide (Freudenberg and Cramer 1948). Two years later, Freudenberg elucidated the structure of γ-dextrin (Freudenberg and Cramer 1950). The same year, using partial acid hydrolysis and enzyme digestion followed by X-ray measurements and paper chromatography, French also elucidated the structure of γ-dextrin, first named cycloöctaamylose and later cyclooctaamylose (French et al. 1950b). This dextrin was composed of eight glucose residues symmetrically arranged in a ring and linked together by α-1,4-glucosidic bonds. In the late 1950s, French and co-workers had established the molecular weight, the exact chemical structure, the dimensions, and the types of bonding in the three cycloamyloses, cyclohexaamylose, cycloheptaamylose, and cyclooctaamylose, i.e., α-dextrin, β-dextrin, and γ-dextrin, respectively (French and McIntire 1950; Norberg and French 1950; French et al. 1950a, b).
In 1948, the first indications of the existence of higher homologues of dextrins were published by Freudenberg and his young student Cramer (Freudenberg and Cramer 1948). Two years later, French also suggested the possible existence of cycloamyloses containing more than 8 glycosyl units (Norberg and French 1950; French et al. 1950b). The same year, Akiya and co-workers claimed the “discovery of new series of cyclic oligosaccharides” similar to the Schardinger dextrins, containing more than 8 glucose units (Akiya and Watanabe 1950a, b, c; Akiya and Okui 1951). Later, Caesar (1968) reported that these “new” compounds were the α- and β-dextrins. In fact, the existence of larger homologues of cycloamyloses was clearly demonstrated a decade later by French. In 1957, French discovered delta-dextrin or δ-dextrin and epsilon-dextrin or ε-dextrin, containing 9 and 10 units of glucose, respectively (French 1957a, b). He proved their existence using radioautography and chromatography measurements. However, French elucidated their structures only in 1965 (French et al. 1965). At that time, French also wrote: “there is no obvious reason why the series should stop here” (French 1957a), suggesting the existence of cycloamyloses with 11 and 12 units of glucose, i.e., ξ-dextrin or zeta-dextrin and η-dextrin or eta-dextrin, respectively. In the beginning of the 1960s, French continued to study cycloamyloses with a larger ring. His objective was to develop a fractionation method for isolation of larger homologues of cycloamyloses after extensive β-amylase digestion to hydrolyze maltooligosaccharides. In 1961, the existence of cycloamyloses with 11 and 12 units of glucose is confirmed using radioautography (Pulley and French 1961), and 4 years later, he was the first to propose a fractionation method for their isolation (French et al. 1965). The structure and the dimensions of ξ-dextrin and η-dextrin are reported. French finally introduced the notion of Schardinger dextrin series, “a Schardinger dextrin family” (French et al. 1965). The same year, Thoma and Stewart (1965) also published similar results, and the discovery of ξ-dextrin and η-dextrin is attributed to them (Caesar 1968; Szejtli 1998; Loftsson and Duchêne 2007).
French’s results had for many years been regarded as dubious since they were not able to experimentally distinguish the large cyclodextrins from branched derivatives. As late as 1988, Szejtli expressed his doubts, in his monograph Cyclodextrin Technology, to whether cyclodextrins larger than γ-cyclodextrin exist (Szejtli 1988). In fact, higher cyclic cyclodextrins than the three native cyclodextrins, reported in the 1960s, were probably so-called branched derivatives such as branched diglucosyl-cyclodextrins. When a section of the amylopectin molecule containing a branching point was incorporated into a cyclic structure, one or two glucosyl or maltosyl side chains were attached by α-(1→6) linkages to the ring formed (Frömming and Szejtli 1994). During the production of native cyclodextrins, these branched cyclodextrins were also produced. It was only during the mid-1990s that the existence of the large cyclodextrins has been fully proven (Miyazawa et al. 1995; Endo et al. 1997, 1999; Larsen 2002; Qi et al. 2004; Taira et al. 2006; Crini 2014).
1.3.3 Cyclodextrin Chemistry
For over 20 years, Pringsheim and his various collaborators penned an abundant literature on dextrins. Indeed, Pringsheim is considered to be the first researcher to have published prolifically on their preparation and chemistry (Pringsheim and Langhans 1912; Pringsheim and Eissler 1913, 1914; Pringsheim 1915, 1919, 1922, 1924, 1925, 1926, 1927, 1928a, b, 1931a, b, 1932; Pringsheim and Lichtenstein 1916; Pringsheim and Persch 1921, 1922; Pringsheim and Dernikos 1922; Pringsheim and Goldstein 1922, 1923; Pringsheim and Beiser 1924, 1932; Irvine et al. 1924; Pringsheim and Leibowitz 1924, 1925a, b, 1926a, b; Pringsheim and Steingroever 1924, 1926; Pringsheim and Wolfsohn 1924; Pringsheim and Schapiro 1926; Pringsheim and Meyersohn 1927; Irvine et al. 1929; Pringsheim et al. 1930, 1931a, b). However, these studies suffered from numerous errors due to the use of dextrins that were not pure and to problems arising from separation of the fractions and from the use of unsuitable analytical methods, e.g., determination of the masses by cryoscopy (Freudenberg and Jacobi 1935; Samec and Blinc 1941; French 1957a; Thoma and Stewart 1965; Caesar 1968). In 1935, Freudenberg dismissed the work of Pringsheim as practically valueless, since “most of it was based upon work with dextrin mixtures and upon serious misconceptions relating to the structural principles of high polymers” (Freudenberg and Jacobi 1935). French (1957a) also wrote: “Pringsheim’s literature was voluminous but much of it was repetitive, controversial, or based on erroneous concepts.”
From 1910, Pringsheim repeated Schardinger’s experiments. He reported higher yields of β-dextrin from glycogen crude preparations of amylopectin, and this is the reason why he postulated that amylose was polymerized α-diamylose and amylopectin and glycogen were polymerized β-triamylose. Like Schardinger, Pringsheim observed that the relative proportions of α- and β-dextrins depended on the different substrates used (Pringsheim and Langhans 1912). Pringsheim described the chemical behavior of dextrins and their properties, in agreement with the previous results published by Schardinger. The dextrins were soluble in water but insoluble in alcohol, ether, and chloroform. They do not reduce Fehling’s solution. To precipitate the dextrins, different solvents including benzene, toluene, xylene, bromobenzene, nitrobenzene, and petroleum ether were proposed (Pringsheim and Eissler 1913, 1914; Pringsheim 1915; Pringsheim and Lichtenstein 1916). Pringsheim confirmed that the simplest means to distinguish between the α- and β-dextrins was the iodine reaction (Pringsheim 1922; Pringsheim and Dernikos 1922). Pringsheim was the first to study the halogen complexes of dextrins (Pringsheim and Wolfsohn 1924; Pringsheim and Schapiro 1926). The first methylated β-dextrin was also obtained by his group: 43.6% of degree of methylation as against 45.6% required by theory. The compound was crystallized from ether (Pringsheim and Goldstein 1923). Several data can also be found referring to the preparation of dextrin derivatives including acetates, nitrates, and ethers (Pringsheim 1927, 1928a, b, 1931b; Pringsheim and Meyersohn 1927; Irvine et al. 1929; Pringsheim et al. 1930, 1931a, b; Pringsheim and Beiser 1932). However, all Pringsheim’s data are essentially of historic interest (Samec and Blinc 1941; French 1957a; Thoma and Stewart 1965; Caesar 1968; Szejtli 1982a; Crini 2014). From 1920 to 1925, Karrer also contributed greatly to the knowledge of the chemistry of the Schardinger dextrins (Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Crini 2014). Karrer published several important works on dextrins (Karrer 1920, 1921, 1922, 1923, 1925; Karrer and Nägeli 1921a, b; Karrer et al. 1921, 1922; Karrer and Bürkin 1922). Like Schardinger and Pringsheim, Karrer studied the crystallized dextrins with the expectation that they would shed some light on the features of starch. In 1921, Karrer published the first conclusions on the acetolysis of α-dextrin and β-dextrins. He demonstrated that this reaction gave essentially the same excellent yield of maltose as starch or maltose itself gives, when treated similarly (Karrer 1921; Karrer and Nägeli 1921a, b; Karrer et al. 1921). Karrer also investigated the interactions between dextrins and ions such as barium, sodium, and potassium (Karrer 1922; Karrer and Bürkin 1922; Karrer et al. 1922). In 1925, Karrer summarized the whole of his works and conclusions on dextrins in a famous comprehensive book (Karrer 1925).
Between 1911 and 1935, epoch called by Crini (2014) the “period of doubt,” other researchers have also published interesting works on the chemistry of the Schardinger dextrins (Biltz 1913; Biltz and Truthe 1913; Freudenberg and Ivers 1922; Miekeley 1930, 1932; Ulmann 1932, Ulmann et al. 1932; Hess et al. 1933). Miekeley (1930, 1932) published experimental data on the chemical composition of dextrins, which complemented those of Pringsheim. In 1933, Ulmann’s group observed that the α-dextrin-ethanol complex had two different crystal modifications which could be interconverted. This was the first observation that a same guest may form different crystal structures with the same dextrin (Hess et al. 1933). However, this period did nothing to stimulate the development of Schardinger dextrins, considered as by-products of starch degradation. So, the work on cyclodextrins reported before 1935 was of little consequence (Samec and Blinc 1941; Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Crini 2014). This can be explained by the fact that researchers used incompletely separated fractions and based too much reliance on cryoscopic measurements of molecular weights, which led to many anomalous results.
From 1935 to 1950, epoch called by French (1957a) the “maturation period,” the works of Freudenberg on the chemistry of the Schardinger dextrins were acknowledged to have made an important contribution to the cyclodextrin science (Freudenberg and Jacobi 1935; Freudenberg and Rapp 1936; Freudenberg et al. 1936, 1938, 1939, 1947a, b; Freudenberg and Meyer-Delius 1938, 1939; Freudenberg 1939, 1943; Freudenberg and Cramer 1948, 1950). Indeed, Freudenberg is recognized as a pioneer in this domain (Thoma and Stewart 1965; Caesar 1968). As far back as 1922, Freudenberg was the first researcher to focus on the chemical modification of dextrins, in particular of tosylated residues (Freudenberg and Ivers 1922). Later, the Schardinger dextrins were oxidized by iodite, “probably by a glycol-cleavage reaction” (Freudenberg 1934). Enzymatic hydrolysis gave no trace of a sugar unit other than D-glucose (Freudenberg and Jacobi 1935). During the hydrolysis of dextrins, Freudenberg also observed an increase in rotation due to hydrolysis of the β-linkage. During acetolysis, the dextrins were shown to be more nearly similar to starch than to compounds of the levoglucosan type. Using a cryoscopic method for the determination of molecular weights, Freudenberg reported (erroneously) the number of glucose units that the Schardinger dextrins contained: five for Α-dextrin and six for β-dextrin (Freudenberg and Jacobi 1935). In 1936, Freudenberg confirmed that enzymatic hydrolysis gave no trace of a sugar unit other than D-glucose. He also reported that methylation studies failed to reveal the presence of any D-glucose units, concluding that glucose was the only product of acid hydrolysis of dextrins (Freudenberg and Rapp 1936). The following pieces of experimental evidence were also published: (i) the rate of hydrolysis of dextrins in 51% sulfuric acid was too low for there to be any labile β-linkages present; (ii) the Schardinger dextrins were non-reducing, that is, they did not have a reducing chain termination; and (iii) methylation studies on dextrins gave no products than 2,3,6-O-methyl-D-glucose (Freudenberg and Rapp 1936). The same year, Freudenberg prepared fully methylated α- and β-dextrins and finally demonstrated that 2,3,6-tri-methylglucose was the only product of methylation of dextrins followed by hydrolysis (Freudenberg et al. 1936). Later, acetate derivatives of the dextrins were proposed and characterized for the first time (Freudenberg et al. 1947a, b). In 1955, Freudenberg published a detailed description of the chemistry of the three main cyclodextrins (Freudenberg 1955), and in 1962, he summarized all his results (Freudenberg 1962).
Between 1942 and 1950, French published numerous important contributions on the chemistry of the Schardinger dextrins (French and Rundle 1942; Rundle and French 1943; Bates et al. 1943; French et al. 1948, 1949a, b, 1950a, b, 1954; French and McIntire 1950; Norberg and French 1950). Very quickly, like Freudenberg, French became a pioneer in the understanding of their chemistry (Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Crini 2014). French showed that the Schardinger dextrins, being cyclic, had no non-reducing end group and they were extremely resistant to alpha-type amylases. Using data from periodate oxidation and methylation reactions, he demonstrated that Schardinger dextrins could not be open-chain compounds. Periodate oxidation was slow with Schardinger dextrins in comparison with that of straight-chain amylodextrin. French published protocols for the methylation of Schardinger dextrins and showed that 2,3,6-tri-methylglucose was the only product of methylation of cycloamyloses followed by hydrolysis (French et al. 1950b), in agreement with the previous results published by Pringsheim (Pringsheim 1924, 1925, 1926; Pringsheim and Beiser 1924) and Freudenberg (Freudenberg and Meyer-Delius 1938; Freudenberg et al. 1938). French also published solubility data on cycloamyloses, especially in presence of organic liquids. Solubility data of dextrins in water at room temperature were as follows: α-dextrin 14.5 g/100 mL, β-dextrin 1.8 g/100 mL, and γ-dextrin 23.2 g/100 mL (French et al. 1949a). Using data from periodate oxidation and methylation reactions, French definitively demonstrated that Schardinger dextrins could not be open-chain compounds and they were regarded as conical cylinders (French and McIntire 1950; Norberg and French 1950; French et al. 1950a, b). At that time, Schardinger dextrins were also found to be rather anomalous structures with interesting complexing properties when compared with the linear oligosaccharides. French indeed suggested for the first time the fact that cycloamyloses were capable of forming particular complexes. The nature of the complexes between halogen and Schardinger dextrins, particularly the iodine complexes, depended much on the amount of the halides added. However, the cavity of dextrins was referred to as hydrocarbon in nature by French. This result has been definitively abandoned in 1965 with the advent of the modern conformational theory.
1.3.4 Molecular Structure of Schardinger Dextrins
Schardinger was the first to hypothesize that the crystalline substances were “cyclic polysaccharides” (Schardinger 1907, 1909, 1911). However, he never managed to elucidate their structure.
In 1920, Karrer was the first to suggest that the dextrins were made up of several components (Karrer 1920), and 1 year later, he proved it using detailed acetolysis data (Karrer 1921; Karrer and Nägeli 1921a, b; Karrer et al. 1921). In 1923, Karrer was also the first to propose that dextrins are composed of maltose units only joined by α-(1→4) glucosidic linkages (Karrer 1923, 1925), although Pringsheim (1922, 1924) remained unconvinced by Karrer’s conclusions. Figure 1.6 is a schematic illustration of two glucopyranose units of a dextrin molecule showing details of the α-(1→4) glucosidic/glycosidic linkage and the numbering systems employed to describe the glucopyranose rings. Later, Miekeley (1930, 1932) also came to the same conclusions as Karrer. In 1926, Pringsheim is finally convinced by Karrer’s conclusions (Pringsheim 1926) although he continued to regard the polyamyloses as the basic units of the starch “molecule” (Pringsheim 1928a, 1931a). However, just like Schardinger, Karrer, and Miekeley, Pringsheim failed to elucidate the cyclic structure of the dextrins.
From 1934 for a period of approximately 25 years, the main contributions toward the molecular structure and size of the Schardinger dextrins were developed by Freudenberg (Freudenberg 1934, 1939, 1943, 1955, 1957a, b; Freudenberg and Jacobi 1935; Freudenberg and Rapp 1936; Freudenberg et al. 1936, 1938, 1939, 1947a, b, 1953; Freudenberg and Meyer-Delius 1938, 1939; Freudenberg and Cramer 1948, 1950). From 1922, Freudenberg was attracted by Schardinger dextrins since he wanted to obtain information on the degradation products of starch to be able to elucidate its structure (Freudenberg and Ivers 1922). For Freudenberg, Schardinger dextrins were first laboratory curiosities and/or unwanted by-products of starch degradation (Freudenberg 1934), and their chain molecules were intermediate between maltose and starch with non-reducing end groups. Indeed, it is only at the end of the 1930s that Freudenberg concluded that the dextrin-α and dextrin-β molecules were cyclic. In 1935, α-dextrin was considered as a mixture of chain molecules containing 4–5 D-glucose units (Freudenberg and Jacobi 1935). Using results of constructing molecular models with the monomer units in a boat rather than a chain conformation, the dextrins were lined with a hydrocarbon interior. One year later, studying the nature of the glycosidic bonds, Freudenberg showed that the dextrins gave rotation-time curves closely parallel to those given by starch and the rigid models such as Kekulé model did not allow free rotation about the individual bonds (Freudenberg and Rapp 1936). The presence of a Konstellation, i.e., a ring conformation, is suggested, and in 1936, Freudenberg hypothesized that Α-dextrin and β-dextrin have a cyclic structure (Freudenberg et al. 1936). During 2 years, he tried to prove it. On the basis of results obtained from methylation reactions and enzymatic hydrolysis of the dextrins, Freudenberg came, in 1938, to the “same conclusion” as Schardinger, Karrer, Pringsheim, and Miekeley, concerning the cyclic chemical structure of Α-dextrin and β-dextrin (Freudenberg and Meyer-Delius 1938; Freudenberg et al. 1938). Ten years later, Freudenberg and his doctoral student Cramer finally demonstrated his conclusion using optical activity data (Freudenberg and Cramer 1948). Schardinger dextrins had a cyclic structure composed of maltose units bound together by α-(1→4) glycosidic linkages. At that time, both French and Borchert also confirmed the cyclic structure of dextrins by X-ray crystallography (French et al. 1948; Borchert 1948). However, although Freudenberg had determined for the first time the correct chemical structure for the Schardinger dextrins, the number of D-glucosyl residues that he gave for the α- and β-dextrin rings, i.e., five and six, respectively, using a cryoscopic method were incorrect. The correct values were determined by French using both X-ray diffraction and crystal density measurements.
Between 1942 and 1965, French also contributed greatly to the molecular structural knowledge of the Schardinger dextrins, or, as he preferred to call them, cycloamyloses (Caesar 1968; Szejtli 1998; Crini 2014). Very quickly, French became a pioneer in the understanding of their structure, publishing an impressive number of results on cycloamyloses which are still used as references today (French and Rundle 1942; Rundle and French 1943; Bates et al. 1943; French et al. 1948, 1949a, b, 1950a, b, 1954; French and McIntire 1950; Norberg and French 1950; French 1957a, b, 1960, 1962; Bailey and French 1957; Thoma and French 1958, 1959, 1960, 1961; James et al. 1959; Thoma et al. 1959; Whelan et al. 1960; Pulley and French 1961; Robyt and French 1964; French and Abdullah 1965; French et al. 1963, 1965). French’s first work concerned the molecular weights of the Schardinger dextrins, considered as cyclic molecules in agreement with the previous results published by Freudenberg (Freudenberg and Meyer-Delius 1938; Freudenberg et al. 1938). French and Rundle (1942), using the X-ray diffraction technique and crystal density measurements, determined the molecular weights of α-dextrin and β-dextrins and discovered the exact number of glucose units per dextrin, i.e., six and seven, respectively, in disagreement with the results published by Freudenberg and Jacobi (1935). French and Rundle demonstrated that molecular weights were integral multiples of the value 162.1 for a glucose residue. They concluded that the X-ray diffraction technique was better suited to the determination of the molecular weights of high molecular weight crystalline substances since impurities, such as solvent of crystallization and inorganic ash, were of minor importance (French and Rundle 1942). In this paper, French also suggested that Schardinger dextrins were cyclic “macromolecules,” formed from starch polysaccharide (French and Rundle 1942). They were non-reducing “D-glucopyranosyl polymers” containing 6, 7, or 8 units linked by α-D-(1→4) bonds, in agreement with the results published by Karrer (1923) and Miekeley (1932). In each cycloamylose “macromolecule,” the D-glucose units were in the C1 conformation. Schardinger dextrins were then regarded as cylinders (French and Rundle 1942). However, Freudenberg did not agree with this point of view (Freudenberg 1943).
French pointed out three interesting features: (1) as a consequence of the C-1 conformation of the glucopyranose units, all the secondary hydroxyl groups were located on one side of the cylinder, whereas all the primary hydroxyl groups were located on the opposite side of the cylinder; (2) the interior of the cylinder consisted only of a ring of C-H groups, a ring of glucosidic oxygens, and another ring of C-H groups; and (3) the interior of the cavity was relatively apolar compared to water (French and Rundle 1942; Rundle and French 1943; Bates et al. 1943; French et al. 1948, 1949a, b). Freudenberg claimed again that all the structural and conformational conclusions of French were ambiguous due to “the use of products that were not pure” (Freudenberg et al. 1947a, b). One year later, Freudenberg and Cramer concurred with French’s results, after studying the X-ray measurements of Borchert (1948) and also his optical rotation data, publishing similar interpretations (Freudenberg and Cramer 1948; Cramer 1949). In 1950, French, studying the periodate oxidation of the three cycloamyloses, finally concluded that all three molecules had a cyclic structure in which each D-glucose unit was linked to the next by an α-D-(1→4)-glucosidic bond, and the interior of the cavity was apolar (French and McIntire 1950; Norberg and French 1950; French et al. 1950b). Schardinger dextrins were then regarded rather as conical cylinders than cylinders, in agreement with Cramer’s suggestion. Another interesting feature is made by French: γ-dextrin was “a noncoplanar, more flexible structure,” and therefore, it was the “most soluble of the three dextrins.” Later, cycloamyloses were finally regarded as truncated cones or “capsules” by French (French 1957a), in agreement with the results published by Cramer (Cramer 1952, 1953, 1956; Dietrich and Cramer 1954).
Cramer also contributed greatly to the molecular structural knowledge of the Schardinger dextrins. In 1948, the young student Cramer published his first result on Schardinger dextrins (Freudenberg and Cramer 1948). Using optical activity, Cramer demonstrated the cyclic nature of α- and β-dextrins. The same year, he discovered γ-dextrin and suggested that the three dextrins possessed an apolar cavity. One year later, Cramer received his PhD at Heidelberg University, under the supervision of Freudenberg (Cramer 1949). He introduced the cyclodextrin-based nomenclature, demonstrated the cyclic nature of cyclodextrins using optical activity data, and showed that the three cyclodextrins had different internal diameters and each cavity was filled with water molecules (Cramer 1949). His doctoral work was then published between 1951 and 1952 (Cramer 1951a, b, c, 1952), adding to the previous results of Freudenberg but mostly “confirming those of French” on the physical (cavity size) and chemical (reactivity) properties, the structure, and chemistry of cyclodextrins. For instance, investigating the configuration at the anomeric centers by hydrolytic methods, Cramer came to the same conclusions as Karrer (1923), Miekeley (1932), and French (French and Rundle 1942) as to the existence of α-(1→4) glucosidic/glycosidic linkages. Cramer also published for the first time a variety of other interesting features. Studying the molecular size of the three dextrins, he showed that a same dextrin could exist in different crystal forms. Cramer then discovered the toroidal form of the cyclodextrin molecules, considering cyclodextrins as truncated cones or “capsules” rather than cylinders, like previously reported by French (French et al. 1948, 1949a, b). The numbering system employed to describe the glucopyranose rings, reported in Fig. 1.5, was then accepted, and Cramer schematized his conclusions on the chemical structure of α-, β-, and γ-cyclodextrins by the two schemes reported in Fig. 1.7. Cramer finally concluded that cyclodextrins were non-reducing oligosaccharides containing 6, 7, or 8 units linked by α-D-(1→4) bonds, having both hydrophobic and hydrophilic regions. On the side where the secondary hydroxyl groups were situated, the diameter of the cavity was larger than on the side with the primary hydroxyls, since free rotation of the latter reduced the effective diameter of the cavity. Figure 1.8 illustrates the hydrophobic and hydrophilic regions of an α-dextrin “capsule” (Cramer 1953, 1956; Dietrich and Cramer 1954).
In 1965, both Casu et al. (1965) and Hybl et al. (1965) confirmed the conclusions published by French and Cramer on the cyclic structure of cyclodextrin and its features, using NMR spectra in dimethylsulfoxide solution and using X-ray crystallography of the α-cyclodextrin-potassium acetate complex, respectively. Their results clearly demonstrated that (i) all the glucose residues of cyclodextrins were in the 4C1 chair conformation; (ii) the cavity was lined by the hydrogen atoms and the glycosidic oxygen bridges, respectively; and (iii) the nonbonding electron pairs of the glycosidic oxygen bridges were directed toward the inside of the cavity, producing a high electron density. The schematic diagram of two glucopyranose units of a cyclodextrin molecule showing details of the α-(1→4) glycosidic linkage reported in Fig. 1.6 and the schematic representations of the chemical tridimensional structure and dimensions (Fig. 1.9) for α-, β-, and γ-cyclodextrin are finally accepted at the mid-1960s. Later, a more precise study of the conformation of α-cyclodextrin in solution was made by Saenger’s group using NMR spectroscopy (Wood et al. 1977). All six glucose units had identical conformations and the molecule had hexagonal symmetry. The secondary hydroxyl groups, which were located in one side of the torus of cyclodextrins, formed hydrogen bond with the secondary hydroxyl groups of contiguous glucose units, in agreement with the previous conclusions published by Casu et al. (1965) and by Hybl et al. (1965). In the cyclodextrin molecule, a complete secondary belt was formed by hydrogen bonds, making it a rigid structure. This was proposed to explain the fact that, among the three native cyclodextrins, β-cyclodextrin had the lowest solubility (Wood et al. 1977). The hydrogen belt was incomplete in the α-cyclodextrin molecule, and γ-cyclodextrin was a noncoplanar, more flexible structure, confirming the results published by French and McIntire (1950). At the beginning of the 1960s, French indicated the possible existence of “a Schardinger dextrin family,” describing the structure of δ-dextrin, ε-dextrin, ξ-dextrin, and η-dextrin containing 9, 10, 11, and 12 glucose units, called larger homologues of cycloamyloses (Pulley and French 1961; French et al. 1965). These larger dextrins were not regular cylinder-shaped structures. Indeed, they were collapsed and their real cavity was even smaller than the γ-dextrin (Fig. 1.10).
1.3.5 Preparation and Separation of Schardinger Dextrins
Between 1905 and 1911, Schardinger studied the first preparation, fractionation/separation, and purification of the two cellulosines (Schardinger 1905, 1907, 1909, 1911). In 1911, he published the first fractionation and purification scheme of the dextrins. Later, both Freudenberg, French, and Cramer published other important schemes: see the references French (1957a) and Thoma and Stewart (1965).
The dextrins were synthesized from several sources of starch, e.g., potatoes, rice, and wheat, and bacteria, e.g., the formation of dextrins depended on the type of bacteria digesting starch. About 25–30% of the starch was converted to crystalline dextrins depending on these parameters. The yield was tenfold those reported by Villiers (Schardinger 1907). Schardinger also based his method of separation on the ease of crystallization of the β-dextrin from water and its low solubility, about 1.5% at room temperature, followed by precipitation of the α-dextrin from the mother liquor by the addition of alcohol. Schardinger’ protocol was modified by Lange in 1925 who introduced trichloroethylene as a precipitating agent for the crystalline dextrins (Lange 1925). This protocol is described in detail in Pringsheim’s book (Pringsheim 1932).
In 1935, Freudenberg and his student Jacobi described a method for the synthesis of Schardinger dextrins with high purity (Freudenberg and Jacobi 1935) (Fig. 1.11). Freudenberg is indeed recognized as the first to prepare almost pure dextrins with high yields (Thoma and Stewart 1965; Caesar 1968; Clarke et al. 1988; Szejtli 1998; Crini 2014). Freudenberg improved the separation of dextrins and produced a scheme based not only on solubility differences of the dextrins themselves, as initially proposed by Schardinger, but also on the differences in solubilities and rates of crystallization of their acetates (Freudenberg and Jacobi 1935). However, the protocol was difficult since it involved many acetylation and saponification reactions. During more than 10 years, this protocol was studied and modified, and in 1947, Freudenberg’s group described the first scheme for the isolation of pure fractions of dextrins using bromobenzene as precipitant: α-dextrin did not precipitate, while β-dextrin and γ-dextrin were readily precipitated (Freudenberg et al. 1947a, b). This scheme was comprehensively discussed by French (1957a). In 1950, Freudenberg and Cramer also confirmed the possible existence of dextrins with 9 or 10 glucose units, identified during the preparation of α-, β-, and γ-dextrins (Freudenberg and Cramer 1950). However, these findings were only substantiated a decade later by French (Pulley and French 1961).
French was also among the early researchers, along with Freudenberg, to focus on improving the production of dextrins. French became a pioneer in the preparation of the compounds in a very pure state. Knowing the works of the group of Hudson on the enzymolysis conditions which affected the yield and proportion of the dextrins (Tilden and Hudson 1939, 1942; Tilden et al. 1942; McClenahan et al. 1942; Wilson et al. 1943) and using his own results on the solubilities of Schardinger dextrins (French et al. 1949a), French proposed in 1949 a new protocol for the separation and purification of dextrins (French et al. 1949b), which did not require the acetylation and saponification steps used by Freudenberg. Treatment of starch with the amylase of Bacillus macerans gave crude starch digests containing the three cycloamyloses, i.e., ~60% α-dextrin, ~20% β-dextrin, and ~ 20% γ-dextrin, together with small amounts of higher cycloamyloses. Moreover, the protocol permitted the facile separation of pure dextrins by differential precipitation using specific precipitants such as bromobenzene and propan-1-ol (French et al. 1949b). Later, French showed that high temperature cellulose column chromatography was one of the most effective methods for the quantitative analysis of mixtures of cycloamyloses (Pulley and French 1961; French et al. 1965). This method was required in connection with the production of cycloamyloses since these products were simultaneously produced from starch together with the higher series of cycloamyloses. In 1961, French also reported the preparation, isolation, and partial characterization of large cyclodextrins with 9, 10, 11, and 12 glycosyl units in the macrocycle (Pulley and French 1961), identified during the preparation of α-, β-, and γ-dextrins like Freudenberg.
In the mid-1950s, Cramer also investigated the enzymatic production of cyclodextrins, their separation and purification, and characterization (Cramer 1955, 1956; Cramer and Steinle 1955; Cramer and Henglein 1957a, b). Cramer described an easy protocol to separate α-, β-, and γ-cyclodextrins from the digest by selective precipitation using appropriate organic compounds and optimize parameters, e.g., pH = 6 and temperature = 40 °C (Cramer 1956). The three cyclodextrins are precipitated by addition of a tetrachloroethylene-tetrachloroethane mixture, followed by the addition of p-cumene. α-Cyclodextrin was isolated by selective precipitation with cyclohexane, β-cyclodextrin with fluorobenzene, and γ-cyclodextrin with anthracene. Cramer explained his results by the difference in the sizes of cavities of the three cyclodextrins and concluded that the superiority of his method over previous procedures, particularly those of French, resided in the technical ease and the completeness of precipitation.
To summarize, during the periods of reaching maturity from 1935 to 1950 and of exploration from 1950 to 1970, the separation and the purification of the mixture were difficult (Crini 2014; Crini et al. 2018). The period of reaching maturity was also marked by several contradictory results, due, at least in part, to differences in the protocols used for the preparation of Schardinger dextrins and dubious purity of the samples (Thoma and Stewart 1965; Caesar 1968; Szejtli 1998). The work during these periods was even marred by hot debate between the different laboratories, especially those of Freudenberg and Cramer and of French. In addition, in the early 1950s, researchers had not fully realized the potential of cycloamyloses and had little faith in their complexation properties (Szejtli 1998). The three main cyclodextrins were considered just laboratory curiosities difficult to produce. In 1963, French was the first to propose the preparation of cycloamyloses on a larger-than-laboratory scale (French et al. 1963). However, at the end of the 1960s, French concluded that “cycloamyloses were very promising molecules although they remained very expensive products, available only in small amounts as fine chemicals, and also toxic.”
1.3.6 The Action of Amylases on Cycloamyloses
Up to 1939, the Schardinger dextrins were known only as products of the bacterial breakdown of starch. For Freudenberg’s opinion, Bacillus macerans was able to transform starch structure into cyclic and linear breakdown products, and starch was based upon a cyclic Schardinger nucleus with side branches which would be broken in the bacterial breakdown (Fig. 1.11). During the same period, Tilden and Hudson (1942), studying the bacteria that produced the dextrins, also concluded that the resulting Schardinger dextrins were derived from some basic configuration pre-existing in the starch “molecule.” Similar conclusions were published by Kerr (1942, 1943), Myrbäck (1942), and Samec (1942).
Using this concept, Freudenberg was the first scientist to investigate the enzymatic production of dextrins and to propose a first mechanism of action for Bacillus macerans (Freudenberg et al. 1938, 1939; Freudenberg and Meyer-Delius 1938, 1939; Freudenberg 1939). This mechanism indicated that the dextrins were preformed within the starch macromolecules (Freudenberg 1939), in agreement with the results of Hudson’ group (Tilden and Hudson 1939). However, Freudenberg did not agree with the generally accepted point of view at that time concerning the nature of the bonding of the D-glucose units, and he rapidly abandoned this mechanism. Using the helical model of the structure of starch proposed by Hanes (1937) and the α-D nature of the glucose units, Freudenberg proposed a second mechanism based on a transglucosylation reaction (Fig. 1.12). He suggested that the enzyme involved was able to degrade the helical structure of the starch, i.e., the amylose fraction, and that there ensued a rearrangement of the glucose units which were then able to form a ring structure (Freudenberg et al. 1939; Freudenberg and Meyer-Delius 1939). Because of the helical arrangement, the first and fifth or sixth D-glucosyl residues were situated close to one another and were able to unite to form rings of five or six D-glucose units. The reactions proposed by Freudenberg to explain the formation of dextrins are given in Scheme 1.1 (Freudenberg 1939). Freudenberg concluded that the cyclodextrins were not preformed in starch “molecule” but that formation was made possible by the helicity of the starch chain. However, he was unable to prove this mechanism. It would be confirmed a few years later by French using chromatography (French et al. 1954) and later by Takeo and Kuge (1969) using X-ray crystallography.
Freudenberg’s initial (above) and final (below) model of formation of Schardinger dextrins/cyclodextrin; adapted from Freudenberg (1939)
Reactions proposed by Freudenberg (1939) to explain the formation of dextrins (Glc = a D-glucose or a D-glucosyl residue)
From the 1940s, numerous research groups worked on the bacteria that produced the dextrins (Myrbäck 1938, 1942, 1949a, b; Samec and Blinc 1939, 1941; Myrbäck and Ahlborg 1940; Blinc 1941, 1942; Samec 1942; Tilden and Hudson 1939, 1942; Tilden et al. 1942; Kerr 1942, 1943, 1949; Kerr and Severson 1943; Wilson et al. 1943; Myrbäck and Gjorling 1945; Cori and Cori 1946; French et al. 1948; Kerr and Cleveland 1949; Hale and Rawlins 1951). However, the discovery of the enzyme in Bacillus macerans, responsible for the conversion of starch into dextrin, is attributed to Tilden and Hudson. In 1939, Tilden and Hudson isolated a cell-free enzyme preparation from Bacillus macerans, i.e., Acrobacillus macerans, that had the ability to convert starch into crystalline dextrins with interesting yields, ~55%, (Tilden and Hudson 1939). They introduced the name of cycloamylose glucanotransferase, i.e., CGTase or cyclodextrin glucanotransferase. Prior to this discovery, dextrins were made using live cultures of Bacillus macerans. In 1942, the authors proposed the following protocol (Tilden and Hudson 1942; Tilden et al. 1942): they cultivated Bacillus macerans on sterilized potato slices or on a medium containing 5% oatmeal, in presence of 2% calcium carbonate; after 2–3 weeks of cultivation at 37–40 °C, the cell mass was recovered by filtering or centrifuging; the filtrate contained the enzyme in an activity of 0.7 units/mL, which was separated either by freeze-drying or, after concentration, by precipitation with acetone. Their results mainly showed that it was essential to determine the optimal culturing conditions for the production of the enzyme and the optimal pH, temperature, and fermentation time for enzyme activity for effective use of the enzyme. Later, Hale and Rawlins (1951) also attained similar yields of the enzyme on a scale of 20 L in 10–12 days in an aerated culture. Tilden and Hudson were also the first to propose a simple protocol for purifying the amylase of Bacillus macerans using both precipitation by acetone, adsorption, and dialysis steps (Tilden and Hudson 1942; Tilden et al. 1942). The enzyme purified had an activity 140 times that of the initial enzyme solution and was able to convert 1000 times its weight of starch in 30 min at 40 °C.
Since Tilden and Hudson’s discovery of Bacillus macerans cycloamylose glucanotransferase, effort was devoted to working out methods for cyclodextrin production and the details of the mechanism. These two researchers also laid down the basis of the enzymology of dextrins, and their findings were validated and used for over 30 years. Myrbäck and Gjorling (1945) and Cori and Cori (1946) also confirmed that the enzyme which catalyzed the degradation of starch into dextrins was mainly produced by bacillus strains. These two groups were the first to describe the action mechanism involved in the enzyme synthesis of Schardinger dextrins and to point out that the rate of hydrolysis of dextrins was much slower in its initial phase than later on. In addition, the authors noted that, after a few days, the Schardinger dextrins formed under the action of Bacillus macerans gradually disappeared. This was also observed by Kneen and Beckord (1946), by French (French et al. 1948), and later by Hale and Rawlins (1951). The explanation was given by French which introduced the reversibility of the action of the enzyme (French et al. 1948). In 1948, French’s group proposed a mechanism of action based on a transglucosylation reaction to interpret the formation of dextrins by Bacillus macerans (French et al. 1948). Their results demonstrated that Bacillus macerans was capable of producing a glycosidic exchange reaction between maltose and cyclohexaamylose which resulted in the formation of higher weight saccharides, confirming the concept of glycosidic exchange introduced by Cori and Cori (1946). French pointed out that the enzyme performed three transglucosylation reactions involving cyclization, coupling, and disproportionation, as well as a hydrolysis reaction. This mechanism proposed by French and detailed in his comprehensive review (French 1957a) was only demonstrated in the 2000s (Lee and Robyt 2001; Qi et al. 2004). In 1950, Akiya and co-workers also proposed a mechanism of action and claimed the “discovery” of a new strain of Bacillus macerans (Akiya and Watanabe 1950a, b, c), although Hudson’s group have previously shown that all examined strains of Bacillus macerans were capable of forming Schardinger dextrins (Tilden and Hudson 1939, 1942; Tilden et al. 1942).
1.3.7 The First Schardinger Dextrin-Related Patents
The first patent on Schardinger dextrins was registered in 1925 by the German Fritz Lange for IG Farbenindustrie (Fig. 1.13). This patent entitled Verfahren zur gewinnung von polyamylosen focused on the isolation of polyamyloses (Lange 1925).
Freudenberg, Cramer, and Plieninger filed the first industrial cyclodextrin-related patent in 1953, called “Method for preparation of inclusion compounds of physiologically active organic compound” (Freudenberg et al. 1953). The patent described the most important aspects of the applications of cyclodextrins in drug formulations (Fig. 1.14). The authors detailed specific effects that could be achieved by complexation of drugs with cyclodextrin complexation such as enhancement of solubility of poorly soluble drugs, protection of easily oxidizable substances against atmospheric oxidation, reduction of the loss of highly volatile substances, etc. In 1987, Cramer wrote: “At that time, I saw the first possibilities for a technology transfer and I took a patent. This, unfortunately, never found any industrial application” (Cramer 1987). The potential use of cyclodextrins in pharmaceuticals had been launched. It was not till the 1970s and 1980s though that the first industrial-scale applications in pharmacy appeared (Szejtli 1998; Loftsson and Duchêne 2007; Morin-Crini et al. 2015).
1.3.8 Cyclodextrins as Models
Cramer knew everything about cyclodextrins, publishing an impressive number of results over a period of 25 years on their preparation, separation, characterization, structure, properties, chemistry, biochemistry, and their potential application in pharmacy, catalysis, and enzymology (Freudenberg and Cramer 1948, 1950; Cramer 1949, 1951a, b, c, 1952, 1953, 1954, 1956, 1961; Freudenberg et al. 1953; Cramer and Steinle 1955; Cramer and Henglein 1956, 1957a, b; Cramer and Dietsche 1958, 1959a, b; Lüttringhaus et al. 1958; Cramer and Kampe 1962, 1965; Cramer and Hettler 1967; Cramer et al. 1967, 1969).
In 1953, Cramer was the first to show the catalytic role that cyclodextrins could play in chemical reactions through a key-lock interaction similar to that of an enzyme-substrate complex (Cramer 1953). Later, Cramer gave the basis for supramolecular catalysis involving cyclodextrins (Cramer and Dietsche 1958, 1959a; Cramer 1961; Cramer and Kampe 1962, 1965). For instance, he reported a cyclodextrin-accelerated reaction studying the hydrolysis of ethyl p-chloromandelate in the presence of α-cyclodextrin (Cramer and Dietsche 1958). Cyclodextrins can also be used as an asymmetric agent (Cramer 1961; Cramer and Kampe 1962, 1965). The potential use of cyclodextrins in catalysis and supramolecular chemistry had been launched. The first reports of their potential use as enzyme models were also attributed to Cramer. Enzyme-substrate interactions can be established either by non-covalent bond such as hydrogen bonding or van der Waals forces or by a covalent bond. When a covalent bond is formed, the cyclodextrin established a covalent bond with some entering or leaving component, e.g., in the case of ester hydrolysis. This process was called covalent catalysis. The model of non-covalent catalysis was inclusion complex catalysis, introduced by Cramer (1953) and later studied in detail by Bender (van Etten et al. 1967a, b; Griffiths and Bender 1973). An interesting example is given in Fig. 1.15. The hydrolysis of 3,3-disubstituted phenyl-glutarate proceeded via intramolecular catalysis. This was suppressed by cyclodextrin inclusion complex formation. The stabilizing effect depended on the cyclodextrin concentration according to the hyperbolic curve of the Michaelis-Menten enzyme kinetics. The reaction rate reached a minimum value when all the ester molecules were complexed. The hydrolysis was also independent of the pH; consequently cyclodextrin was only a binding site and was not involved directly in the reaction mechanism.
At the end of the 1950s, the numerous fundamental studies of French also led to growth in the interest in cycloamyloses not only as model enzymes but also as aroma-stabilizing agents for the food industry (Thoma and French 1958; Thoma et al. 1959; French et al. 1963), even though at the time, industrial application of cycloamyloses was still not considered feasible. This last point was only clarified in the 1970s. In the 1960s, Bender reported that cycloamyloses had a great potential for acid-base catalysis similar to that of naturally occurring enzymes. His numerous works made the creation of artificial enzymes possible. Bender, studying cycloamyloses-catalyzed reactions, showed that cycloamyloses can accelerate or decelerate various kinds of reactions including oxidation, hydrolysis, decarboxylation, nitrosation, and isomerization. The reaction rates depended on the cycloamylose used and the kind and stability of the inclusion compound formed. The first review on the phenomenon of cycloamylose catalysis has been published in 1973 by Bender (Griffiths and Bender 1973). This comprehensive review also summarized the developments in the chemistry of cycloamyloses and its derivatives used as enzyme models. It was updated by Bender in 1978 (Bender and Komiyama 1978). Cycloamylose-catalyzed reactions were classified in two categories: (i) covalent catalysis in which cycloamyloses catalyze reactions via the formation of covalent intermediates and (ii) non-covalent catalysis in which cycloamyloses provide their cavities as apolar or sterically restricted reaction fields without the formation of any covalent intermediates. Bender pointed out that non-covalent catalysis by cycloamyloses might be the result of either a solvent effect or of a conformational effect, e.g., in decarboxylation and stereoselective reactions. Bender is recognized as the initiator of the era of biomimetic chemistry, including artificial enzymes, molecular recognition, and bio-inspired reactivity (see his famous book: Bender and Komiyama (1978)).
1.3.9 Complex Formation and Inclusion Compounds
In 1938, Freudenberg suggested, for the first time, the hydrophobicity of the inner surface of the dextrin and noted how dextrins had the ability to form complexes due to their cyclic structure (Freudenberg and Meyer-Delius 1938; Freudenberg et al. 1938). The dextrins exhibited a complexing capacity which was discussed in connection with the blue starch/iodine complex. For the first time, the ability of Schardinger dextrins to form complexes was suggested. To explain these complexes, Freudenberg was the first to show the involvement of hydrophobic forces in the formation of the complexes (Freudenberg 1939; Freudenberg and Meyer-Delius 1939; Freudenberg et al. 1939). Originally, however, Freudenberg was convinced that the dextrins and the amylose helix were lined with a hydrocarbon interior, and thus the cavity of the dextrins has been referred to as hydrocarbon in nature (Thoma and Stewart 1965; Caesar 1968; Clarke et al. 1988; Szejtli 1998; Crini 2014; Morin-Crini et al. 2015; Crini et al. 2018).
Cramer was recognized not only for having introduced the cyclodextrin-based nomenclature but mostly for his important work on inclusion complexes, although they were only fully acknowledged at the end of the 1970s (Crini 2014). In 1949, in his PhD dissertation entitled Die cyclodextrine aus Stärke, Cramer evoked for the first time the fact that the three native cyclodextrins, considered as cylinders with different internal diameters, were able to accommodate molecules of different sizes: this was the first indication on their ability to form “inclusion” complexes (Cramer 1949). Two years later, Cramer published his first results on the complexes (Cramer 1951a, b, c, 1952). For instance, he observed that a number of dyes showed characteristic changes in their absorption spectra in aqueous solutions of the cyclodextrins (Cramer 1952). Between 1952 and 1954, Cramer discovered that the toroidal form of the cyclodextrin molecules, regarded as truncated cones, enabled them to accept various molecules inside their cavity (Cramer 1952, 1953; Dietrich and Cramer 1954). He was the first to demonstrate the hypotheses Schardinger put forward at the beginning of the nineteenth century on their ability to form complexes. In 1952, Cramer adopted the term einschlussverbindungen, i.e., inclusion compound, to characterize a complex (Cramer 1952), and later he also used the terms “occlusion compound” and “molecular encapsulation” (Cramer and Dietsche 1959a). In 1953, he registered his first patent where he highlighted the fact that “the formation of an inclusion complex could modify the physical, chemical and biological characteristics of a guest molecule such as a drug” (Freudenberg et al. 1953). The inclusion phenomena and the term einschlussverbindungen were however used by Schlenk in 1950 for the first time (Schlenk et al. 1955; French 1957a; Szejtli 1982a; Crini 2014). The term “inclusion compound” has been coined to describe the positional relationship of two components which form certain types of crystals. These components were designated as host and guest, again in reference to the solid state (Schlenk et al. 1955; Schlenk and Sand 1961).
In 1956, Cramer introduced the notion of “inclusion complex” (Cramer 1956). He considered the interior of the cavity as a lipophilic microenvironment into which a non-polar hydrophobic molecule can “slide” (Fig. 1.16). The guest was maintained within the cavity by non-covalent forces, which were thus weak and enable the whole system to be reversible. Cramer showed that formation of an inclusion complex was the result of an association/dissociation equilibrium between the free guest and the free host and the complex. This was governed by a constant, denoted formation constant, Kf. The higher its value, the more stable the inclusion is, and the less dissociation that occurred. Cramer then conducted important research between 1955 and 1965 on the inclusion phenomena (Cramer 1956, 1961; Cramer and Henglein 1956; Cramer and Dietsche 1959a, b; Cramer and Kampe 1962, 1965). He studied in detail the molecular dispositions of numerous guest organic compounds in the cyclodextrin cavity in solution by the use of UV-visible and circular dichroism experiments, in order to demonstrate the formation of inclusion complexes. These experiments also permitted to calculate the formation constant or dissociation constant of the different complexes.
Figure 1.17 shows the two possible penetration pathways for benzoic acid, phenol, and methylated benzoic acids. α-Cyclodextrin complexes with phenol and benzoic acid guests in the head first position were more stable than in the tail first position, while β-cyclodextrin complexes with the same guests preferred the tail first position. A substituted benzene ring with a van der Waals radii of about 6.8 Å could only penetrate into the ring of a β-cyclodextrin molecule (diameter cavity: 7.5 Å) either “head first” or “bottom first” but never crosswise. The stability of the complex was proportional to the hydrophobic character of the substituents (Cramer and Henglein 1956; Cramer and Dietsche 1959a, b; Cramer 1961). In the 1970s, this was also demonstrated by van Hooidonk and Breebaart-Hansen (1970, 1972), Harata and Uedaira (1975), and later Szejtli (1982a) using the same methods, i.e., UV-visible spectroscopy and circular dichroism. Demarco and Thakkar (1970) were the first in a pioneering work to demonstrate the formation of inclusion complexes between several organic substances studied by Cramer and β-cyclodextrin using NMR spectroscopy.
Another interesting example was the complex between β-cyclodextrin and naphthalene substituted in position 1 or 2 (Fig. 1.18). The modification of the circular dichroism that occurred depended on the geometry of both the host and the guest molecules. A positive circular dichroism band implied axial inclusion, i.e., along the Cn symmetry axis, whereas a negative band signified equatorial inclusion, i.e., perpendicular to the Cn axis (Cramer 1961; Cramer and Kampe 1962, 1965). Later, Szejtli (1982a) also indicated that the sign and intensity of the induced Cotton effects were quite sensitive to the orientation of the guest chromophore in the cyclodextrin cavity. If the electric dipole moment coincided with the axis of the cyclodextrin, a positive Cotton effect was observed. When they were perpendicular to each other, a negative Cotton effect was observed. The circular dichroism spectra of 1- and 2-naphthols were therefore quite different: the naphthalene ring in one case was accommodated crosswise and in the other case lengthwise in the cyclodextrin cavity. In the complexes of 2-naphthalenes, the inclusion was axial (Harata and Uedaira 1975; Szejtli 1982a) (Fig. 1.18).
(a) Equatorial inclusion of a 2-substituted naphthalene and (b) axial of a 1-substituted naphthalene. (Adapted from Harata and Uedaira 1975)
Cramer concluded that the preferred position for the guest compound inside the cavity depended on steric interactions, due to the chemical structure and geometry of each guest compound. The complex was strong when there was size complementarity between the guest and the cyclodextrin cavity. In solution, two main components of the driving forces of the process are suggested: the first was the presence of repulsive forces between the included water molecules and the apolar cavity, and the second was the presence of repulsive forces between the bulk water and the apolar organic guest. Cramer also indicated that hydrogen bonding between the guest and the cyclodextrin was of only minor importance as a driving force for complex formation (Cramer 1956). The stability of cyclodextrin inclusion complexes was due to a favorable change in enthalpy during the inclusion process. However, later, Cramer also considered that a complex was stabilized not only by van der Waals forces but also by hydrogen bonds (Cramer and Kampe 1965; Cramer et al. 1967).
In 1958, Cramer was the first to propose a scheme for the preparation of so-called catenanes, compounds consisting of two rings connected to each other without chemical bond, by using cyclodextrin complexes (Lüttringhaus et al. 1958). Figure 1.19 shows the structural scheme of catenanes and the principle of their attempted preparation. Incorporating a dithiol with sufficiently long chain into cyclodextrin, and oxidizing the two protruding terminal SH groups to give an -S-S- bridge, a catenane was formed. These molecules were studied in the 1990s and proposed for the synthesis of supramolecular materials, scaffolds and templates, and in biomimetism (Nepogodiev and Stoddart 1998). In 1959, Cramer observed that, in the case of guest molecules that cannot be totally included by a single molecule, a second cyclodextrin molecule may bind: this was the first observation of 2:1 complexes in solution (Cramer and Dietsche 1959a, b). Figure 1.20 illustrates the association of free cyclodextrin and substrate to form various substrate-cyclodextrin complexes. Depending on the respective size of the guest and host molecules, one guest molecule can interact with one or two (or more) cyclodextrins, i.e., host-guest complexes 1:1 and 2:1, or two (or more) guest molecules can interact with one cyclodextrin or two (or more), i.e., host-guest complexes 1:2 and 2:2.
(a) Structural scheme of catenanes and (b) principle of their attempted preparation. (Adapted from Lüttringhaus et al. 1958)
In 1967, Cramer comprehensively discussed all the possible penetration pathways and the effect of substituents on the stability of the inclusion complexes (Cramer and Hettler 1967). The same year, he gave the first scientific explanation to explain the mechanism of formation of an inclusion complex between a substrate and a cyclodextrin molecule both in solution and solid state (Cramer et al. 1967). To explain the formation of an inclusion complex, Cramer introduced five elementary steps: (1) the substrate approaches the cyclodextrin molecule; water molecules escape from the cyclodextrin cavity and acquire a new energy level, corresponding to that of the gaseous state; the van der Waals interactions and the number of hydrogen bonds decrease, whereas the degrees of freedom of translation and rotation of the freed water molecules increase; (2) the guest molecule becomes released from the layer of water that envelops it and also acquires a different state; the layer of water becomes dispersed and rearranges; (3) the guest molecule, considered to be in a perfect gas state, enters the cavity, and the complex formed is stabilized by van der Waals forces and/or hydrogen bonds; (4) the expelled water molecules are rearranged and form hydrogen bonds between each other; and (5) the structure of the water is restored around the part of the substrate that remains in contact with the solvent and that is integrated into the hydration shell around the CD. Cramer finally concluded that the most important property of cyclodextrins was the ability to establish specific interactions, i.e., molecular encapsulation, with various types of molecules through the formation of non-covalently bonded entities such as hydrophobic interactions, van der Waals forces, and hydrogen bonding (Cramer and Hettler 1967; Cramer et al. 1967). Cramer’s work on inclusion complexes established much of our modern understanding of the behavior of cyclodextrins during complexation and remains a commonly cited source to this day.
At the same time as Cramer, French also studied the formation of inclusion complexes and showed that evidence for a guest inclusion into the cycloamylose cavity may be proved by analytical techniques such as UV-visible absorption spectrophotometry, optical rotatory dispersion, circular dichroism, and X-ray measurements (Thoma and French 1958, 1959, 1960, 1961; James et al. 1959; Thoma et al. 1959). The guests studied were the same as that of Cramer such as phenol, benzoic acids, or iodine. For instance, in 1958, French showed that absorption spectroscopy was an interesting method to determine the dissociation constant of the inclusion complex between cycloamylose and iodine (Thoma and French 1958). The values of dissociation constant could be easily obtained from the observed change in absorbance and the concentration of cycloamylose added according the Benesi-Hildebrand method. For French, the driving forces for complex formation included solvent effects, van der Waals forces, and hydrogen bonds. One year later, French demonstrated that X-ray diffraction was also a powerful method to study the inclusion complexes between iodine and cycloamyloses (James et al. 1959). French also observed that, because of their structure, some guests can be included in one or two cycloamylose molecules, and depending on the cavity size, it was a different part of the guest molecule that can be included. This was clearly demonstrated in the 1970s by NMR spectroscopy (Szejtli 1982a).
Later, Lichtenthaler and Immel (1996) demonstrated, using molecular modelling, the need for compliance between hydrophobic host surfaces and hydrophobic domains in the cyclodextrin cavity. This striking tendency to optimize the reciprocal concurrence of lipophilic as well as hydrophilic domains at the guest-host interface may accordingly be concluded to be an important if not the decisive element in orienting the guest into the cavity and in determining the stability of the complex, particularly in cases where the guest is devoid of polar groups. This was the first study showing the importance of the reciprocal interplay of such interactions.
1.3.10 The First Reviews on Schardinger Dextrins
Relatively few reviews of cyclodextrins were published during the period from 1911 to 1970 as recently reported by Crini et al. (2018). The first review of Schardinger dextrins was published in German by Pringsheim in 1931 in a chapter entitled Dextrine: Charakteristik, Gewinnung und Eigenschaften and published in his book Die polysaccharide (Pringsheim 1931a). Pringsheim also published an English version in 1932 in another book The Chemistry of the Monosaccharides and of the Polysaccharides (Fig. 1.21). This mini-revue, entitled “The dextrins: Characteristics, Sources, and Properties,” summarized the works of Schardinger on dextrins but mostly his own works (Pringsheim 1932).
Later, the dextrins were described in German by Samec and Blinc in a review dated 1941 and entitled “Die Neuere Entwicklung der Kolloidchemie der Stärke,” in which they concluded that, “because of the use of impure dextrins, the work performed prior to about 1935 was judged to be of little consequence” (Samec and Blinc 1941). In 1954, an interesting brief overview on cyclodextrins was published by Cramer in his famous book on inclusion complexes entitled Einschlussverbindungen (Cramer 1954). However, the very first state of the art on the subject was only published by French in 1957 in a special volume of the journal Advances in Carbohydrate Chemistry. French wrote up an excellent fundamental review of over 70 pages with 159 references, entitled “The Schardinger Dextrins,” where he described the history of Schardinger dextrins, their characteristics, chemistry and derivatives, and biochemical properties (French 1957a). French divided their history into two general periods, their discovery between 1891 and 1935 and their maturity from 1935 to 1950. Transfer reactions due to the Bacillus macerans action were also critically reviewed by French. In 1965, Thoma and Stewart also published a comprehensive review on the characteristics of Bacillus macerans amylase (Thoma and Stewart 1965). Three years later, Caesar summarized the use of cycloamyloses as models for enzymes (Caesar 1968).
1.3.11 Toxicological Considerations
Pringsheim was the first to conduct several biological tests to determine whether the dextrins were physiologically available either to plants or animals (Pringsheim and Müller 1922; Hoesslin and Pringsheim 1923; Pringsheim 1928b, 1931a). Pringsheim studied the absorption and metabolism of dextrins in rats, pigs, and humans. Polyamylose, probably α-cyclodextrin, administered in a dose of 50 g to diabetic persons, did not cause any change in the urinary glucose level, and no polyamylose was found in the feces (Pringsheim and Müller 1922). Hoesslin and Pringsheim (1923) fed polyamylose to starved rabbits and guinea pigs. No glycogen synthesis was detected in the liver 3 h after administration. Pringsheim suggested that polyamylose was metabolized in the rat. His experiments also showed that the Schardinger dextrins were not fermentable and hence not utilized by yeast (Pringsheim 1928b). In 1931, for the treatment of diabetes, Pringsheim concluded: “α-dextrin directly utilized would be a suitable source of energy for diabetics since it only occasionally cause nausea and there is no noticeable increase in urine sugar” (Pringsheim 1931a).
However, in 1957, French wrote: “It would appear that the Schardinger dextrins exhibit a toxic effect, possibly by virtue of their remarkable complexing ability, and in any case, the suggestion of Professor Pringsheim that they be used as an energy source by diabetics looks risky” (French 1957a). Indeed, the first studies carried out by him in the rat led to the conclusion that Schardinger dextrins presented a certain toxicity (French 1957a). In the same paper, it is noted that “In unpublished attempts to investigate the ability of animals to utilize Schardinger dextrins, B.H. Thomas and D. French fed rats a diet in which a part of the carbohydrate was supplied by higher purified β-dextrin. The animals refused to eat the test diet except in very small quantities and within a week all animals on the ration were dead. Postmortem examination did not reveal the cause of death” (French 1957a). Their experiments indeed showed that the rats refused to eat food containing highly refined dextrins except in very small quantities. In spite of the small doses, rat mortality was 100% within a week of introducing “highly purified” β-dextrin into the diet (French 1957a). However, in these studies, experimental conditions, such as the purity of dextrins, the number of rats treated, or the existence of a control group, were not mentioned. Later, Szejtli suggested that one of the hypotheses was traces of solvent remaining in the dextrins which the rats could have smelt (Szejtli 1982a, 1988; Frömming and Szejtli 1994). This was the only result of French’s that posed a problem and which led to extensive debate of the toxicity of the cycloamyloses (French 1957a), and most importantly this result deterred many scientists from developing cycloamylose-containing products for human use. The observations and conclusions drawn by French were only refuted much later following studies with the same animal model (Andersen et al. 1963; Lach and Cohen 1963; Lach and Chin 1964; Szejtli et al. 1980a; Chow and Karara 1986).
1.3.12 Other Works During the Period from 1911 to 1970
Other historical landmarks on the discovery of cyclodextrins published by other researchers during the period from 1911 to 1970 are reported in Table 1.1 (Thoma and Stewart 1965; Caesar 1968; Szejtli 1998; Loftsson and Duchêne 2007; Kurkov and Loftsson 2013; Crini 2014; Morin-Crini et al. 2015; Crini et al. 2018).
1.4 Historical Landmarks in the Development of Cyclodextrins: From 1970 Until Now
1.4.1 Production of Cyclodextrins
At the mid-1970s, industrial-scale production really started (Horikoshi 1979). Indeed, several manufacturers started to produce and to market cyclodextrins, e.g., Nihon Shokuhin Kako, Japan; Sanraku Ocean Co. Ltd., Japan; Toyo Joso, Japan; and Chinoin, Hungary. The first pilot plant at Nihon Shokuhin Kako firm, Japan, started up in 1978 with a production capacity of about 20 tons per year (Sicard and Saniez 1987). However, cyclodextrins were expensive.
The first to believe in the possibilities of industrial production and the multiple applications of cyclodextrins was probably Szejtli, a Hungarian carbohydrate chemist who organized the cyclodextrin technology in Hungary in the 1970s (Szejtli 1988, 1998). Among the list of prestigious researchers who have contributed to the development of cyclodextrins, Szejtli played a fundamental role as eminent scientist, visionary, and entrepreneur, creating, in 1989, a private company, CycloLab Ltd., totally devoted to cyclodextrins. Without Szejtli, the feasible production of cyclodextrins on an industrial scale probably would not be as advanced as it is today.
In the mid-1980s, advancements in biotechnology led to drastic improvements in the production and purification of cyclodextrins. Cyclodextrins were then produced in large quantities with high purity and marketed at a reasonable price, and as a result, more industrial applications have become possible. In addition, this period from 1970 to 1980 was also marked by another important event: the first toxicological studies had established that β-cyclodextrin administrated orally was a harmless substance. Pharmaceutical, food, chromatographic, and cosmetic applications started to appear and rapidly gained ground. Since then, an increasing interest in cyclodextrins as raw materials and their possible applications has existed (Szejtli 1982a, 1988; Duchêne 1987, 1991).
1.4.2 Mechanism of Inclusion Complexes
In the mid-1970s, several researchers such as Saenger, a pupil of Cramer, Bender, and Szejtli pursued and reformulated the interpretations made by Cramer on the mechanisms of formation of inclusion complexes.
On the basis of the mechanism proposed in 1967 by Cramer, Saenger gave in 1976 three important explanations for the formation of an inclusion complex with α-cyclodextrin in aqueous solution (Saenger et al. 1976): (1) the guest molecule directly replaces the water molecules in the cavity; (2) the cyclodextrin molecules absorb the energy of the water molecules retained in the cavity and take on a relaxed conformation; in this state, the water molecules can be easily substituted by another guest; and (3) the guest becomes associated with the outer surface of the cyclodextrin and only enters the cavity once it has absorbed the activation energy, i.e., transfer of the conformation from a state of high energy of the cyclodextrin-water complex to a state of lower energy of the cyclodextrin-guest molecule complex. Saenger introduced the notion of the release of the tension energy within the α-cyclodextrin molecule upon formation of the complex. This relief of strain in the cyclodextrin ring contributed to the enthalpy of association and to the stabilization of the inclusion complex. In water, the α-cyclodextrin was in a strained, high-energy conformation, and that, when another guest molecules displaced the water, thus forming an inclusion complex, a conformational change of the cyclodextrin molecule occurred, transforming the α-cyclodextrin structures into an unstrained, relaxed state. This was demonstrated using detailed X-ray diffraction studies. A scheme of inclusion-complex formation involving relief of strain energy and release of high-energy water is proposed (Fig. 1.22). This scheme shows the possible pathways of α-cyclodextrin inclusion complex formation in aqueous solution. Owing to the inclusion of water, the ring of cyclodextrin is distorted, only four of the six possible hydrogen bonds are formed, and introducing a suitable guest molecule, the ring is relaxed. Later, Saenger showed that, in the cases of β- and γ-cyclodextrins, the strain energy relief mechanism did not seem to be operative and the cyclodextrin-water adducts were not strained (Lindner and Saenger 1978). In 1980, Saenger published a thorough state of the art of inclusion complexes, including his own interpretations (1980).
Saenger’s theory of formation of α-cyclodextrin complexes in aqueous solution: G, guest; H2O*, activated water. Hydrogen bonds are marked by dashed lines. (Adapted from Saenger et al. 1976)
In 1978, Bender elucidated the mechanism of formation of the complexes developed by Saenger. He pointed out that the complexation involved hydrophobic interactions, like that of Saenger 2 years before. The driving force of inclusion is “an example of an atypical hydrophobic interaction” (Bender and Komiyama 1978). Bender proposed the fact that the complexation reaction involved a gain in enthalpy and a loss of entropy. The further the guest molecule penetrated into the cyclodextrin cavity, the greater was the change in enthalpy, and the higher was the stability of the complex. Moreover, the greater was the apolarity of the guest, the more this phenomenon was marked. The previous explanation that Bender offered to explain the favorable enthalpy change was that the empty cyclodextrin contained water molecules that were unable to form their full complement of hydrogen bonds to adjacent water molecules, and thus might be considered to enthalpy rich (Griffiths and Bender 1973). The inclusion of a guest “would then displace this high energy water from the cyclodextrin cavity, leading to a net increase in solvent-solvent hydrogen bonds and a favorable enthalpy of association.”
In 1982, in his first book on cyclodextrins, Szejtli pursued and reformulated all the interpretations made by Cramer, Saenger, and Bender on the mechanism of formation of inclusion complexes (Szejtli 1982a). The gain in enthalpy is then explained by the spontaneous arrival of the guest, displacing active water molecules retained in the non-polar cavity of the cyclodextrin in aqueous solution. Szejtli illustrated his conclusions using a schematic representation of cyclodextrin inclusion complex formation (Fig. 1.23). In this famous figure, p-xylene is the guest molecule, and the small circles represent the water molecules which are repulsed both by the hydrophobic potential guest and the hydrophobic cavity of the truncated cyclodextrin cylinder (Szejtli 1978; Szejtli et al. 1979). The three main conclusions were as follows: (1) the guest molecule, less polar than water, directly replaced the water molecules in the cavity; these water molecules were in an unfavorable energy state owing to the polar-apolar interactions and were thus easily displaced by more suitable molecules; (2) the cyclodextrin molecules absorbed the energy of the water molecules retained in the cavity; and (3) the organic guest dissolved in water entered in the cavity because it had a preference for hydrophobic environment.
Szejtli supported the idea that, although van der Waals interactions and hydrogen bonding played an important role, the main force behind the formation of the complexes was the stabilizing reduction of the whole system’s energy on the replacement of the high enthalpy water molecules in the cavity, by hydrophobic molecules leading to apolar-apolar bonding. He proposed that this bonding was too weak to be alone responsible for the higher stability of the complex and showed the parallel occurrence of steric interactions. Indeed, Szejtli demonstrated in various publications that the preferred position for the guest compound inside the cavity also depended on steric interactions. He concluded that the complexation phenomenon results from a multitude of interactions between the three components of the system cyclodextrin-substrate-solvent leading to a state that is more thermodynamically stable overall (Szejtli 1995). In the 1990s, there was a general agreement in the literature that during the formation of an inclusion complex, a whole set of intermolecular interactions come into play and that each one has its own role in the overall process.
1.4.3 Inclusion Phenomena and Its Effects
Since Cramer’s discovery of inclusion phenomena, effort was devoted to physical and chemical properties of inclusion complexes and their consequences. Indeed, his results were not only of fundamental interest but also of industrial interest, and in the 1970s, this interest has grown considerably. Numerous works showed that inclusion of a guest active ingredient molecule in a host cyclodextrin molecule was a real molecular encapsulation, and the resulting inclusion complex superstructure had new physicochemical properties, stability, solubility, and also better therapeutic efficacy. So, several technological characteristics used in pharmacy could also be advantageously modified.
Rapidly, the pharmaceutical industry understood the advantages of using cyclodextrins. Szejtli (1982a, 1988) summarized them in six points:
-
1.
The modification of the physicochemical properties of the guest molecule: e.g., liquid compounds can be transformed into crystalline, compressible forms; substances with low solubility in water become more soluble after complexation; the rate of dissolution of poorly soluble substances can also be increased; certain unpleasant tastes can be eliminated; smell can also be covered by complex forming; the color of certain substances can be altered since inclusion can change the spectral properties of the guest; etc.
-
2.
The modification of the chemical activity of the guest: e.g., reactive substances can be protected by inclusion reducing the risks when they are mixed with other substances; chemical reactions can be carried out selectively, the cyclodextrins playing the role of catalysts; etc.
-
3.
The stabilization of substances sensitive to light or to oxygen, etc., e.g., protection of active ingredients against oxidation, heat-promoted decomposition, or light-induced reactions.
-
4.
The uptake of volatile substances: e.g., volatile drug can be stabilized without losses through evaporation; storage and handling of certain toxic substances such as pesticides can be improved; savings can be made on the quantity of substance required owing to reduced evaporation; etc.
-
5.
The complexation, extraction, and transport of pollutants.
-
6.
Several technological advantages, e.g., stable, standardized compositions, simple dosage and handling of dry powders, reduced packing and storage costs, and also saving of energy and manpower.
1.4.4 Large Cyclodextrins
Although the existence of cyclodextrins with over 8 glucose units is described and studied for the first time in the 1950s by Freudenberg, French, and Cramer, it was only in the middle of the 1990s that the cyclodextrins containing 9, 10, 11, and 12 units of glucose, called large-ring cyclodextrins, were studied in any depth (Miyazawa et al. 1995; Endo et al. 1997, 1999; Larsen 2002; Taira et al. 2006). The difficulties to purify them and the low yields prevented their study until then. Using specific enzymes and/or particular experimental conditions, some works also reported the existence of cyclodextrins of over 100 glucose units (Terada et al. 1997; Koizumi et al. 1999; Qi et al. 2004). Larsen (2002) published the first interesting review on large cyclodextrins.
1.4.5 First Comprehensive Reviews on Cyclodextrins
From a fundamental point of view, an abundant scientific literature has built up since the 1970s. Indeed, a large number of generalist reviews, book chapters and books, and articles and patents have been published on practically all the aspects of cyclodextrins. Crini et al. (2018) recently reported that in the periods 1961–1985 (~24 years), 1986–2000 (~14 years), and 2001–2015 (~14 years), 2654, 18,856, and 42,284 cyclodextrin-related publications have been published, respectively, so many that it would not be feasible to cite them all. For this, readers should refer to the library database “Cyclodextrin News” from CycloLab Ltd. (Hungary) which is a periodical collecting all the cyclodextrin works, i.e., articles, reviews, proceedings, patents, conferences, and lectures, through different disciplines, i.e., chemistry, biology, health science, agriculture, nanotechnology, etc. This database is searchable until 2018, when it was turned into a blog and stopped collecting literature. Table 9 shows the first comprehensive reviews and book chapters on cyclodextrins during the period 1957–1998.
As already stated, the very first comprehensive review on cyclodextrins was published by French in 1957 (French 1957a). French’s state of the art was continued a few years later by Kainuma (1984) and Clarke et al. (1988). Research on the action of Bacillus macerans on starch before 1980 was comprehensively reviewed by Kainuma. Clarke et al. discussed topics on detection, thermodynamics, and kinetics of complex formation from a literature survey of 182 previous papers. In 1980, Saenger published the very first review of industrial applications of cyclodextrins (Saenger 1980). In a very well-cited review published in the journal Chemical Reviews, Szejtli wrote: “Cyclodextrins can be consumed by humans as ingredients of drugs, foods, or cosmetics” (Szejtli 1998). In this review, Szejtli, pursuing the history of cyclodextrins written by French, divided the chemical and industrial developments of cyclodextrins into three stages, the discovery period from 1891 to 1935, the exploratory period in 1935–1970, and the utilization period from 1970 to the present day. The history was then updated later by Loftsson and Duchêne (2007) and by Crini (2014). Other fundamental reviews published in the period 1970–1990 are reported in Table 1.2.
1.4.6 First Books on Cyclodextrins
The first books devoted entirely to the chemistry of cyclodextrins and their applications were only edited in 1978 and in 1982 by Bender and Komiyama and Szejtli, respectively (Table 10). Bender and Komiyama (1978) detailed the formation of an inclusion complex and covered all the aspects of catalysis by cyclodextrins. In his first book entitled Cyclodextrins and Their Inclusion Complexes (296 pages with more than 650 references), Szejtli reviewed the first industrial applications of cyclodextrins in pharmacy, food industry, chromatography, and chemical industry (Szejtli 1982a). In this book, Szejtli also summarized his interpretations on the concept on inclusion complex. Other important books published in the period from 1970 to 1990, which are still considered as reference works in the cyclodextrin community, are reported in Table 1.3.
1.4.7 The Dissemination of Knowledge About Cyclodextrins
Szejtli is distinguished not only for his contribution to chemistry, biology, and technology of cyclodextrins but also for his important contribution to the dissemination of knowledge about cyclodextrins. From 1975 to 2004, Szejtli published more than 500 publications on cyclodextrins (Cyclodextrin News Database, CycloLab Ltd., Hungary), including 106 patents and more than 100 general reviews and book chapters, and has given more than 200 invited lectures throughout his career. Szejtli’s comprehensive reviews are still considered as reference works in the cyclodextrin community (Szejtli 1977, 1982a, b, 1983, 1984, 1985, 1986, 1987, 1988, 1989, 1990, 1994, 1996, 1997, 1998, 2002, 2003, 2004). His name is very often cited in the bibliographic references of articles dealing with cyclodextrins.
From the point of view of the dissemination of knowledge about cyclodextrins, we should also cite the publications of Duchêne (Duchêne 1987, 1991; Duchêne and Wouessidjewe 1990a, b, 1992; Duchêne et al. 1992, 2009), Loftsson (Loftsson and Brewster 1996, 2010, 2012; Loftsson and Stefansson 1997; Brewster and Loftsson 2002, 2007; Loftsson et al. 2004, 2005; Loftsson and Duchêne 2007; Kurkov and Loftsson 2013; Jansook et al. 2018), and Szente (Szente and Strattan 1991; Szente and Szejtli 1996; Szente and Szemán 2013; Fenyvesi and Szente 2016; Fenyvesi et al. 2016; Szente and Fenyvesi 2018).
1.4.8 The First International Cyclodextrin Symposium
Realizing the exponentially increasing number of articles, chapters, and patents, Szejtli organized the first International Cyclodextrin Symposium in Budapest in 1981 (Szejtli 1982a). This first symposium was a great success, with participants coming from all over the world – more than 180 participants from 17 countries – while Szejtli “expected 25-30 participants outside Hungary.” The 63 submitted manuscripts filled a 544-page volume of proceedings published by Reidel Publishing (Szejtli 1982c). The second cyclodextrin symposium was organized in 1984 in Tokyo, Japan.
Since 1984 and Szejtli’s initiative, a broad community of researchers has met every 2 years to exchange and share their works on cyclodextrins. Each symposium provides opportunities for scientists who work in several aspects of cyclodextrin research to discuss their advances in all cyclodextrin fields. The 19th International Cyclodextrin Symposium was held in 2018 in Tokyo, Japan, and the next will be organized in Sicily, Italy, in 2020. In Asia and in Europe, the cyclodextrin scientific community is also very active with the organization of meetings where academic researchers and industrials come together to present the latest achievements in the field of cyclodextrin science and technology, e.g., Asian Cyclodextrin Conferences, European Conferences, and French Cyclodextrin Days.
1.4.9 Cyclodextrin Derivatives
The underivatized or native β-cyclodextrin’s low water solubility as showed by French in the 1940s restricted its advantage. For this reason, a number of derivatives such as hydrophilic methylated derivatives have been synthesized. Whereas the first methylated derivatives of β-cyclodextrins were studied as early as 1924 by Pringsheim and collaborators (Irvine et al. 1924), it was however not until 1969 that the alkylated cyclodextrins were comprehensively described by Gramera (1969). Indeed, in the older literature, particularly in the works of Pringsheim and Freudenberg, several methods were proposed referring to the preparation of such derivatives, but these are essentially of historic interest.
Literature on the preparation and investigation of cyclodextrin derivatives only proliferated in the 1960s. Since, numerous cyclodextrin derivatives and polymers were described, including alkylated and acylated derivatives, nitrogen- and sulfur-containing derivatives, halogenated products, and 6-deoxy derivatives. The derivatives of practical importance were the methylated and hydroxypropyl cyclodextrin derivatives in the 1980s and 10 years later the sulfobutyl ether derivatives. Casu was the first to prepare cyclodextrin derivatives such as methylated (Casu et al. 1968a, c) and acetylated (Casu et al. 1970) products. The experimental protocols were repeated in the 1980s by Szejtli (Szejtli 1982a, 1983, 1984). His work, particularly on methylated cyclodextrins, showed great promise for both human and animal use (Szejtli 1982a). Methylation could be either partial, i.e., esterification in positions 2 and 6, giving dimethyl-cyclodextrins or complete giving trimethyl-cyclodextrins. The randomized derivative called RAMEB was often used. These derivatives were much more soluble than the parent cyclodextrins, but their solubility was temperature-dependent. For this reason, hydroxypropyl cyclodextrins with high water solubility were more advantageous. However, because hydroxypropylation occurred randomly, the resulting products were not pure chemical entities but amorphous complex mixtures.
Croft and Bartsch (1983) were the first to publish a review on all the different chemically modified cyclodextrins which had been synthetized up to late 1982. In 1987, Sébille showed that cyclodextrin chemistry offered various possibilities to synthetize derivatives with different functions for various industrial uses, e.g., sulfur- and nitrogen- and imidazole- or histamine-containing derivatives, alkylated and acyl derivatives, halogenated products, polymers from cyclodextrins, etc. (Sébille 1987). By working in carefully controlled conditions, mono-, di-, and poly-substitution were possible and opened the way to several functional derivatives with catalytic or biological activity, for instance. One of the most popular derivative was mono-substituted 6-O-p-toluenesulfonyl-cyclodextrin, used as starting material to prepare modified cyclodextrins (Saenger 1980; Szejtli 1982a; Sébille 1987). Jicsinszky et al. (1996) published a comprehensive chapter (137 pages, 865 references) on cyclodextrin derivatives. In 1998, Khan et al. (1998) proposed a global schema for the modification of cyclodextrins. These two last publications are still reference today.
Thousands of derivatives containing cyclodextrin have been proposed in the literature, particularly for pharmaceutical uses. Szejtli (2004) estimated that over 15,000 derivatives had been studied. In reality, most of these derivatives will never find applications, especially for reasons of production costs and essentially lengthy and difficult synthesis involving complicated steps. Among industrially produced, standardized, and available derivatives, the most important ones are the methylated β-cyclodextrins such as RAMEB (randomly methylated-β-cyclodextrin; considered as a mixture) and DIMEB (a particular methylated cyclodextrin: heptakis(2,6-di-O-methyl-β-cyclodextrin), considered a single isomer; its solubility decreases with an increase in temperature), the 2-hydroxypropylated β-cyclodextrins or HPBCD (the real advantage of this derivative over the methylated derivatives is the lower affinity for cholesterol binding), and the sulfobutylether-β-cyclodextrins or SBEBCD (Fig. 1.24).
Brauns and Müller (1983) and Pitha (1984) registered the first patents on 2-hydroxypropyl-β-cyclodextrin. This compound called hydroxypropylbetadex was the first commonly applied cyclodextrin derivative, used as pharmaceutical excipient in drug formulations in the 1990s (Szente and Strattan 1991). Rapidly, a monograph for this substance has been published in both the US Pharmacopeia and European Pharmacopeia (Brewster and Loftsson 2002, 2007; Brewster et al. 2004). Nowadays, 2-hydroxypropyl-β-cyclodextrin is the most versatile excipient among the cyclic oligosaccharides (Malanga et al. 2016). It can be used in oral, rectal, dermal, ocular, and parenteral formulations, and several pharmaceutical products are marketed, e.g., Indocid® (eye drop), Vorzu® (tablet for fungal infection), Strepfen® (oromucosal spray with flurbiprofen), Vibativ® (i.v. infusion with telavancin), and Lubion® (injection with progesterone as active ingredient). This substance is used as excipient and/or as active component, e.g., at the end of the 2000s, it was discovered that it had beneficial effects for patients in Niemann-Pick type C disease (Liu et al. 2009). Stella and Rajewski (1992) patented the sulfobutylether-β-cyclodextrin product as a potential alternate solubilizing excipient to 2-hydroxypropyl-β-cyclodextrin. This derivative, developed by CyDex under the brand name Captisol®, was found a more efficient complex-forming host than parent cyclodextrins with no apparent toxicity and very high water solubility. Captisol® became generic worldwide in 2011, e.g., Dexolve® developed by CycloLab Ltd. (Hungary). It is used not only as a solubilizing agent but also as an osmotic agent (Puskás et al. 2015).
In the last four decades, numerous cyclodextrin-based materials have also been synthetized. The list includes modified polymers obtained using grafting reactions (Crini and Morcellet 2002; Kozlowski and Sliwa 2010); cross-linked materials using reticulation or polymerization reaction, e.g., soluble or insoluble polymers (gels/hydrogels, beads, sponges) and fibers (Fenyvesi 1988; Armspach et al. 1999; Mocanu et al. 2001; Crini and Morcellet 2002; Crini 2005; Li 2009; Yang and Yang 2013; Aytac and Uyar 2017); functionalized materials prepared through coating or grafting such as silica beads, e.g., Cyclobond® columns, or resins (Crini and Morcellet 2002; Landy et al. 2012); and nanoporous frameworks containing cyclodextrins (Mahmud and Wilson 2016; Morin-Crini et al. 2018). These materials have been proposed for potential applications in pharmacy (Fenyvesi 1988; Van de Manakker et al. 2009; Muankaew and Loftsson 2018), chromatography (Ward and Armstrong 1988; Schneiderman and Stalcup 2000; Szejtli 2002; Vetter and Bester 2006; Xiao et al. 2012), electrophoresis (Fanali 1993; Chankvetadze 2004; Escuder-Gilabert et al. 2014), textile (Buschmann et al. 1998; Szejtli 2003; Buschmann and Schollmeyer 2004; Ammayappan and Moses 2009; Andreaus et al. 2010; Voncina 2011), supramolecular chemistry (Wenz 1994; Nepogodiev and Stoddart 1998; Schneider and Yatsimirsky 2000; Wenz et al. 2006; Schneider 2012; Zhang and Ma 2013), enzymology (Komiyama 1996; Villalonga et al. 2007; Sonnendecker and Zimmermann 2019a), food industry (Han 2005; Fenyvesi et al. 2016), and cosmetics (Buschmann and Schollmeyer 2002, 2004; Bilensoy 2011) and for environmental purposes (Gruiz et al. 2011; Morin-Crini et al. 2018; Crini et al. 2018).
1.4.10 The First Applications of Cyclodextrins
Table 1.4 shows selected historical landmarks in cyclodextrin fundamental science and applications during the period of their development, from 1970 to 1990. Since the 1990s, a large number of generalist reviews and book chapters have been published on practically all the aspects of cyclodextrins, so many that it would not be feasible to cite them all. In this section, we chose to highlight selected works on the first applications of cyclodextrins. A recent review on this topic can be found in the review by Crini et al. (2018) who summarized the literature published in the last four decades. Readers interested in cyclodextrin applications should also refer to the library database “Cyclodextrin News” from CycloLab Ltd., Hungary.
Cyclodextrins have been used in pharmaceutical industry since the 1970s (Szejtli 1977; Uekama and Hirayama 1978; Uekama and Otagiri 1987; Frömming and Szejtli 1994). With a more accurate picture of their toxicity and better understanding of molecular encapsulation, several inclusion complexes appeared on the market (Davis and Brewster 2004; Brewster and Loftsson 2007).
In 1976, the first product, i.e., prostaglandin E2/β-cyclodextrin Prostarmon E™ sublingual tablet, was marketed in Japan (Ono Co.) (Uekama and Hirayama 1978; Hirayama et al. 1980). Prostaglandin E2, a substance with potent oxytocin-like effects, was of interest as a possible agent for the induction of labor in childbirth (Davis and Brewster 2004). However, this substance was highly unstable, and this feature complicated its formulation and development. The solution was to encapsulate it using β-cyclodextrin, resulting in a significant increase in its solid-state stability. This led to spectacular progress in pharmaceutical domain. The second prostaglandin marketed was Prostavasin®, a complex between prostaglandin E1 and α-cyclodextrin. In 1979, this product was approved for the treatment of peripheral vascular complications. Uekama’s group studied the molecular motions of prostaglandin F2α-cyclodextrin inclusion compounds (Uekama and Hirayama 1978; Hirayama et al. 1980; Uekama et al. 1984). As a consequence of the inclusion, the internal motion of the ϖ-side alkyl chain of the prostaglandin is selectively reduced by α-cyclodextrin, while the internal motion around the five-membered ring, as well as the overall motion, is decreased by β-cyclodextrin. In the case of the γ-cyclodextrin inclusion compound, total motion of the prostaglandin is reduced. The inclusion of prostaglandin E1 in γ-cyclodextrin increased its heat stability and slowed down its conversion to prostaglandin A1 (Uekama et al. 1984). The structures proposed by Uekama’s group and illustrated in Fig. 1.25 were demonstrated using NMR data (Uekama and Hirayama 1978; Hirayama et al. 1980).
Another interesting formulation was the complex β-cyclodextrin/piroxicam, used, e.g., in the treatment of acute pain of rheumatic disease. In 1988, Chiesi Farmaceutici, Italy, has put on the market this complex Brexin® or Cycladol® (Fig. 1.26). Nine years later, the first US-approved product, 2-hydroxypropyl-β-cyclodextrin/itraconazole oral solution (Sporanox® Janssen), was introduced (Fig. 1.26). Itraconazole is an orally active triazole antifungal agent to inhibit most human fungal pathogens but is practically insoluble in water at physiological pH. In 2002, two formulations containing sulfobutylether β-cyclodextrin were introduced by Pfizer in the USA and Europe: an intravenous formulation of the antifungal agent voriconazole (Vfend®) and an intramuscular dosage form for the antipsychotic agent ziprasidone (Geodon®).
All these formulations are preformed prior to be administrated. There are also some cases when the complexes are formed within the body. The best-known example is the one containing the active compound sugammadex (Bridion®): it is a modified γ-CD used as an antidote to certain curare-like muscle relaxants in anesthesia since 2008. After intravenous administration, it neutralizes steroid curare-like agents such as rocuronium and vecuronium by forming an inactive complex (Fig. 1.26) in the plasma which is then eliminated in the urine.
Actually, more than 80 pharmaceutical products can be found (CycloLab Ltd., Hungary). In these formulations, native cyclodextrins and their derivatives, such as the hydroxypropyl derivatives of β- and γ-cyclodextrins, sulfobutylether β-cyclodextrin, and maltosyl-β-cyclodextrin (this derivative might be a component of several drugs marketed in Japan, but information are scarce), are mainly used as complexing agents to increase the aqueous solubility of poorly water-soluble drugs, to increase their stability and bioavailability. Cyclodextrins are also used to replace organic solvents in parenteral and topical formulations, to reduce gastrointestinal irritation, and to increase dermal availability of drugs. Initially, cyclodextrins were mainly used in solid dosage forms for oral administration, but rapidly, other administration routes were proposed such as ocular, nasal, parenteral, rectal, or dermal route. For instance, RAMEB is included in Clorocil® eye drop (Oftalder, Portugal) and Aerodiol® nasal spray (Servier, France). Both are non-parenteral, and the latter was withdrawn from the market because of economic reasons.
Food applications also started to appear in the mid-1970s. Japan authorized the use of cyclodextrin as a food additive in 1976 (Szejtli 1982a, 1998; Vaution et al. 1987; Hedges et al. 1995; Hashimoto 1996; Hedges 1998). Powdered flavors, e.g., apple and citrus fruits; spices, e.g., horseradish wasabi and mustard; and herbs such as peppermint were marketed. Other marketed food products containing cyclodextrins or made by cyclodextrin-aided technology included chocolate (Choco Bar™), chewing gum (Flavono™), powdered green tea (Stick Lemon™), and dietary fibers (Hedges 1998; Hashimoto 2002). In 1992, low-cholesterol butter, prepared by mixing cyclodextrin with the melted butter, under the trade name of Balade™ was marketed in Belgium (Comini and Mentink 1991). Other low-cholesterol milk products such as cheese (Natual™, France), cream, and egg (Simply Eggs™, USA) were produced. Other examples include bubbling coffee (Nescafé®, Nestlé), beer (FlavorAktiv™, Great Britain), and supplements. In the 2000s, the three native cyclodextrins were introduced into the generally regarded safe list of the US Food and Drug Administration for use as a food additive (Hashimoto 2002). Now the food industry, along with the pharmaceutical domain, is one of the sectors that consumes the most cyclodextrins, at least in Japan.
Hinze (1981) was the first to describe the application of cyclodextrins in analytical chemistry, focusing on their use in chromatography and purification methods. At that time, the first studies had established that cyclodextrins were interesting complexing agents, chiral selectors, and/or additives in chromatography (Smolková-Keulemansová and Krysl 1980; Hinze 1981; Smolková-Keulemansová 1982; Sybilska and Smolková-Keulemansová 1984; Krysl and Smolková-Keulemansová 1985; Li and Purdy 1992). Cyclodextrins were first proposed for thin-layer chromatography, gel electrophoresis, gas chromatography, and liquid chromatography and later for capillary electrophoresis, electrokinetic chromatography, and dialysis (Armstrong 1980, 1984; Smolková-Keulemansová and Krysl 1980; Smolková-Keulemansová 1982; Hinze 1981; Cserhati et al. 1983; Ward and Armstrong 1986; Li and Purdy 1992; Fanali 1993; Fanali et al. 1994; Schneiderman and Stalcup 2000). The first cyclodextrin-based chiral gas chromatography was published by Smolková-Keulemansová (1982).
At the beginning of the 1980s, Armstrong laid down the fundamentals of cyclodextrin-assisted separation science. Between 1980 and 1988, Armstrong and his collaborators pioneered the development and optimization of analytical methods suitable for cyclodextrin-based isomer separation. In 1984, the first chromatographic columns were marketed (Advanced Separation Technologies Inc., Whippany, NJ), and this led to spectacular progress in chromatography (Armstrong 1980, 1984; Hinze 1981; Ward and Armstrong 1986, 1988; Armstrong and Jin 1989; Han and Armstrong 1989; Menges and Armstrong 1991). These chromatographic packings consisted of cyclodextrin molecules linked to silica gel via a 6–10-atom spacer. Both the linkage and the cyclodextrin were hydrolytically stable under high-performance liquid chromatography. Easily the most popular cyclodextrin-based stationary phases were based on β-cyclodextrin, e.g., they have been shown to be very effective at resolving the enantiomers of many compounds. Subsequently, other stationary phases were developed, based on other native cyclodextrins, e.g., α- and γ-cyclodextrin, or derivatized cyclodextrins such as naphthyl-ethyl-carbamate derivative. The α-cyclodextrin and γ-cyclodextrin columns, while less broadly applicable in the reversed-phase mode than the β-cyclodextrin columns, were useful for specific applications such as the separation of enantiomers of underivatized aromatic amino acids and substituted analogues or of polycyclic aromatic compounds and steroid stereoisomers. The aromatic-derivatized cyclodextrin phases were used to separate the enantiomers of many classes of compounds including pesticides, biological compounds, drugs, and amino acids (Menges and Armstrong 1991; Mitchell and Armstrong (2004). The chiral recognition mechanisms in analytical separation sciences were reviewed by Scriba (2012). Li and Purdy (1992) and later Szente and Szemán (2013) comprehensively reviewed the application of cyclodextrins in diverse fields of analytical chemistry and covered the structural aspects of cyclodextrins that enabled the improvement of different chromatographic separations.
The first comprehensive review on potential applications in cosmetology, toiletry, and hygiene has been published by Szejtli (1982b). This topic was later updated by Vaution et al. (1987), Hashimoto (1996, 2006), Citernesi and Sciacchitano (1995), Buschmann and Schollmeyer (2002), and Duchêne et al. (2009). In the mid-1980s, some products appear in the market although the cyclodextrins were often used without any indication of their precise role: Epicutin® TT, Chemishes Laboratorium Dr Kurt Richter, a complex between cyclodextrin and Melaleuca alternifolia leaf oil, used in skin care applications; Vivace®, Shiseifo Co., a powder cologne; Klorane®, Klorane Laboratories, a dry shampoo; Novoflex®, Revlon, a vitamin shampoo; Eucerin® Vital Active, Beiersdorf, a vitamin A cream; etc. In the cosmetic industry, cyclodextrin complexes were useful for modifying solubility, improving stabilization, transforming liquids into solids, preserving color, decreasing an unpleasant smell, or diminishing the odor of mercaptan used in permanent hair preparations. Cyclodextrins were used as empty capsules in dry shampoos to eliminate fatty substances such as sebum, or in toothpaste and mouthwash to remove undesirable odors (Duchêne et al. 2009).
In the 1980s, applications were found in textiles including textile finishing and functional textiles (Szejtli 1985; Hashimoto 1996; Buschmann and Schollmeyer 2002). The permanent fixation of cyclodextrin molecules offered textiles with interesting properties, e.g., the formation of body odor was reduced by the complexation, the release of perfumes from cyclodextrins was possible, etc. The first cellulose-cyclodextrin copolymer was described in 1980 by Szejtli’s group. Alkali-swollen cellulose fibers were reacted with epichlorohydrin. The chemically bound cyclodextrin retained its complex-forming ability and could be loaded with chemically and/or biologically active guests such as perfumes to prepare perfumed textiles or drugs to prepare medical bandages (Szejtli et al. 1980b). For instance, Fig. 1.27 illustrates a guest exchange by a cellulose fiber containing cyclodextrin. The process consists to load the fixed cyclodextrin molecules with perfume molecules. During wearing and by sweat humidity, excreted short-chain fatty acids displace and release the perfume molecules and will be in turn entrapped (Szejtli 2003). Procter & Gamble recognized that similar effects can be achieved without chemical fixation by simple spraying of hydroxypropyl-β-cyclodextrin solution on the fabric. This odor-eliminating spray (Febreze®) has become a great success in the USA marketed since 1998 ensuring a continuous demand for cyclodextrin production. Actually, cyclodextrins play an important role in the textile industry as a tool to remove odors, e.g., encapsulation of sweat or cigarette smoke components permits to reduce the intensity of odors of clothing or furniture textiles, as showed in Fig. 1.28. The mechanism of drug release is similar from medical textiles such as antimicrobial textiles for wound dressing (Aubert-Viard et al. 2019), intraperitoneal meshes (Chai et al. 2019), and other implants (Vermet et al. 2017). CycloMesh™, a polyester visceral implant soaked in ropivacaine hydrochloride developed for slow anesthetic release and in situ activity after inguinal hernia surgery, will be soon subjected to clinical trials in France.
Guest exchange by a cellulose fiber containing cyclodextrin: the process consists to load the fixed cyclodextrin molecules with perfume molecules; then during wearing and by sweat humidity, excreted short-chain fatty acids displace and release the perfume molecules and will be in turn entrapped. (Adapted from Szejtli 2003)
During the period from 1980 to 1990, manufactured products using cyclodextrins have emerged such as pesticide formulations, reproduction processes, e.g., photography and inks, tobaccos, corrosion inhibitors, industrial detergents, sterilizing agents, anti-foam agents, glues, wood treatment, etc. (Szejtli 1982a, 1984, 1988; Vaution et al. 1987). In the agricultural sector, the use of complexation by cyclodextrins has facilitated the formulation of pesticides in many ways, e.g., by enhancing water solubility and biological activity, through stabilization of labile substances, by enabling easier handling of hazardous substance, and also through the formation of crystalline substances from volatile liquids. An important feature of the cyclodextrin-formulated pesticides is the water-triggered release of the active compounds. A large number of patents concerning the use of cyclodextrins in agrochemistry were filled (Szejtli 1982a, 1984; Szente and Szejtli 1996; Morillo 2006).
1.4.11 Cyclodextrins: Trends and Outlook
Although an important number of works, patents, and products have been realized, the sector of cyclodextrin-based pharmacy continues to interest the scientific community. Cyclodextrins are still regarded as “novel” excipients, active ingredients, drug delivery vehicles, and anti-aggregation agents (Conceição et al. 2018). New formulations continue to be reported, generating new interests in medicine and biomedicine (Higashi et al. 2018; Higashi 2019; Menezes et al. 2019; Pawar and Shende 2019). The 18 ongoing clinical trials in 2019 give the promise of new marketed formulations (Cyclodextrin News, CycloLab Ltd., Hungary).
The recent research has clearly revealed that cyclodextrins cannot be considered inactive excipients any more (Arima et al. 2017). Hydroxypropyl-β-cyclodextrin was found by serendipity to be useful for slowing down the progression of Niemann-Pick type C disease, the fatal genetic disorder. Based on the promising results of the clinical trials, this cyclodextrin received orphan drug status both from Food and Drug Administration and European Drug Agency. The mechanism of action which was thought first to be based on cholesterol complexation is still under debate.
Yokoo et al. (2015) demonstrated that hydroxypropyl-β-cyclodextrin was a potential anticancer agent in leukemia. This derivative was found effective in inhibition of leukemic cell proliferation at various leukemic cell lines. It was proved to influence autophagy, a catabolic process with an essential function in the maintenance of cellular and tissue homeostasis. As solubilizing agent to increase cholesterol solubility, hydroxypropyl-β-cyclodextrin is used for prevention and treatment of atherosclerosis, a chronic inflammatory disease driven primarily by a continuous retention of cholesterol within the subendothelial space to hinder its precipitation in the form of cholesterol crystals (Zimmer et al. 2016).
Several research groups are developing special cyclodextrin derivatives with various functions, for instance, for targeted drug delivery. Folate-appended cyclodextrins are recognized by folate-receptor-expressing tumor cells; therefore they can be utilized as potent anticancer agents (Motoyama et al. 2015). Lactosyl-β-cyclodextrin was found effective for hepatomegaly in Niemann-Pick type C disease (Maeda et al. 2019). Cyclodextrins decorated with a photosensitizer group can be used for drug delivery in phototherapy where the release of active ingredients is controlled by light (Benkovics et al. 2017). Multifunctional cyclodextrin derivatives having nitric oxide-releasing moiety in addition to the photosensitizer are efficient antimicrobial and antitumor agents in photodynamic therapy (Malanga et al. 2019). Many bacteria display mannose-binding lectins on their surfaces, and so mannosylated cyclodextrins are target-specific antimicrobial delivery systems to be used in fighting against antimicrobial resistance (Cutrone et al. 2018).
Another direction of recent cyclodextrin research is the design and synthesis of specific cyclodextrins tailored to the guest molecules to be entrapped. Encouraged by the extreme success of sugammadex tailored for encapturing rocuronium muscle relaxant, further cyclodextrin-based detoxicants were prepared: e.g., a cyclodextrin dimer as antidote for cyanide poisoning (Yamagiwa et al. 2014), another dimer designed for binding and removal of bisretinoid lipofuscins from the eye to prevent aging-related blindness (Nociari et al. 2014), and specially substituted cyclodextrins to catalyze the decomposition of organophosphorus chemical weapons getting importance in view of increasing terrorist threat (Müller et al. 2013). Even the social media shared the news on methyl- and hydroxypropyl-β-cyclodextrins as possible antidotes to box jellyfish venom (Lau et al. 2019). Similarly, quaternary amino β-cyclodextrin was found to bind ochratoxin A, a widely spread nephrotoxic contaminant mycotoxin, with an association constant more than 200-fold higher than that of β-cyclodextrin, making this derivative useful for decontamination of ochratoxin A-contaminated drinks (Poór et al. 2015).
Actually, fundamental research is also focusing on cyclodextrin-based nanoparticles/nanomaterials for pharmaceutical and biomedical applications and nanomedicine, e.g., molecular diagnosis, medical imaging, antifungal treatment, antimicrobial therapy, gene therapy, or tissue engineering, and on self-association of cyclodextrins for applications not only for formulation and drug delivery, and medicine, but also for materials science, supramolecular chemistry, and asymmetric catalysis (Hirakawa and Tomita 2013; Morohoshi et al. 2013; Zhang and Ma 2013; Chilajwar et al. 2014; Melotti et al. 2014; Simoes et al. 2014; Dong et al. 2015; Macaev and Boldescu 2015; Mavridis and Yannakopoulou 2015; Miller et al. 2015; Perez-Anes et al. 2015; Wu et al. 2015; Brackman et al. 2016; Junthip et al. 2016; Okano et al. 2016; Oliveri and Vecchio 2016; Ryzhakov et al. 2016; Sharma and Baldi 2016; Silva et al. 2016; Yuan and Zhang 2016; Saokham and Loftsson 2017; Egele et al. 2019; Fenyvesi et al. 2019; Hammoud et al. 2019; Kumar and Rao 2019; Neva et al. 2019; Pawar and Shende 2019; Topuz and Uyar 2019; Zhang et al. 2019a).
Nanoparticles of various compositions have been engineered in ever smaller sizes to function in both diagnostic and therapeutic capacities. They are available on a scale similar to many biological molecules and infectious agents, thereby opening the possibility of biological intervention on the molecular level (Gilmore and Colson 2011). Nanoparticle-based systems can improve bioavailability, reduce immunogenicity, modify drug metabolism, reduce toxicity, and increase the biological half-life of drugs after systemic administration. The use of cyclodextrin-based nanoaggregates both in oral and ophthalmic drug delivery could be a promising strategy to improve the bioavailability of poorly soluble drugs (Loftsson and Stefansson 2017; Kumar and Rao 2019). Nanomaterials are also interesting because they can be formulated as oral, parenteral, topical, or inhalation dosage forms (Chilajwar et al. 2014). They can be targeted by using specific components and/or moieties, e.g., antibody-targeted cyclodextrin-based nanoparticles were developed for siRNA delivery in the treatment of myeloid leukemia (Guo et al. 2017). Various innovative ideas for the purpose-oriented design of such systems have been published, e.g., “ship-in-a-bottle” (Xu et al. 2019), modern Trojan horse (Gilmore and Colson 2011), and molecular Lego approach for the diversity-oriented synthesis of cyclodextrin analogues as scaffolds for multivalent systems (Lepage et al. 2015). Promising developments for nanoparticles are under way in emerging domains such as nutraceuticals and cosmeceuticals (Fenyvesi et al. 2016; Adeoye et al. 2017). Further contributions are also expected in the near future in bacterial resistance and chemotherapy (Carneiro et al. 2019; Zhang et al. 2019b).
Recently, Higashi et al. (2018) introduced a new concept in pharmaceutical sciences termed “supramolecular pharmaceutical sciences” which combines pharmacy domain and supramolecular chemistry. This concept is focused on the development of cyclodextrin-based supermolecules, such as polyspeudorotaxanes, polyrotaxanes, polycatenanes, and daisy chains, as active pharmaceutical ingredients used, for instance, against Niemann-Pick type C disease, leukemia, Alzheimer’s disease, chronic renal failure, or sterility. These biodegradable polyrotaxanes ensure longer residence time and slow release of the cyclodextrin – mostly hydroxypropyl-β-cyclodextrin – as active ingredient. The low local concentrations result in reduced toxicity even in the case of the methylated derivatives.
The number of publications on the use of nanofibers containing cyclodextrins, e.g., prepared by electrospinning, is also growing (Celebioglu and Uyar 2012, 2013; Aytac et al. 2015, 2016; Topuz and Uyar 2019). These nanofibers are proposed as innovative products for medicine, biomedicine, and nanomedicine applications, e.g., for medical devices, tissue engineering scaffolds, stents, prosthesis, and bone implants. Most of these studies are in the proof-of-concept stage, and only a few therapeutic nanosystems/nanomaterials have been comprehensively investigated. Nanofibers are also proposed for textile and environmental applications (Celebioglu et al. 2016), e.g., innovative clothing, filtration media, and membranes.
The use of cyclodextrins for veterinary purposes seems to be a promising domain (Chiu et al. 2016). New formulations continue to be reported, e.g., Itrafungol™, Voriconazole Dexolve™, Nexterone™, Cereni™, Vetmedin™, Suvaxyn™, etc. Itrafungol™ is an antifungal containing 2-hydroxypropyl-β-cyclodextrin used as antimycotic drug in oral form in cats. Another example is Voriconazole Dexolve™, a commercial formulation containing sulfobutylether-β-cyclodextrin as an excipient, used as an antimycotic drug for veterinary and human use. Suvaxyn™ containing a sulfolipo-cyclodextrin as adjuvant is used as vaccine for the active immunization of pigs.
The global market of cyclodextrins used in food industries is continuously increasing (Fenyvesi and Szente 2016; Fenyvesi et al. 2016). The main application is the stabilization of flavors and aromas. α-Cyclodextrin, being non-digestible, is recognized a dietary fiber with beneficial effects on digestion of fat and carbohydrates (Artiss et al. 2006). It has been marketed for body weight control in several countries. Further studies and industrial developments are expected in the near future in the following domains: functional food, nutritional supplements, nutraceuticals, wrapping materials, and packaging. One of the most promising functional food groups is those enriched in antioxidant compounds of a lipophilic nature (Fenyvesi and Szente 2016; Kfoury et al. 2016; Zarzycki et al. 2016).
Other applications have been reported in sectors such as supramolecular catalysis (Hapiot et al. 2014; Chen et al. 2019; Fernandez et al. 2019), asymmetric and stereospecific synthesis (Macaev and Boldescu 2015), click chemistry (Celebioglu et al. 2016; Hou et al. 2016), metal-organic frameworks (Rajkumar et al. 2019), agrochemistry (Campos et al. 2015; Yusoff et al. 2016), supercritical fluid chromatography (Xiao et al. 2012; West 2014), imprinting techniques (Lay et al. 2016), nanofibers (Topuz and Uyar 2019), and environment (Taka et al. 2017; Crini et al. 2018).
Research on cyclodextrins is also very active in fields such as the formulation of detergents and sugar-based surfactants (Valente and Söderman 2014), glues and adhesives (Osaki 2019), silicon industry (Grachev et al. 2019), flame-retardant formulation (Luda and Zanetti 2019), the sector of plastics for packaging or for automotive (Szente and Fenyvesi 2018), the industry of fibers and paper, soil remediation (Atteia et al. 2013; Lau et al. 2014; Madrid et al. 2019; Gruiz et al. 2019), materials for wastewater treatment (Cova et al. 2018; Morin-Crini et al. 2018; Barbosa et al. 2019), biodiesel production (Zhang et al. 2018), and hydrogen storage (Han et al. 2018).
The slide ring gels (polyrotaxane gels) found their application in the car industry and telecommunication (Ito 2017; Kashiwagi et al. 2018; Jiang et al. 2018). There are also many possibilities for the development of new textiles and cosmetic products, called cosmetotextiles (Singh et al. 2011), with advanced properties (Shende and Trotta 2019; Yao et al. 2019). Their applications seem promising.
Cosmeceuticals containing cyclodextrins also seem to be a promising domain for medicine, dermatology, and aromatherapy (Adeoye et al. 2017; Kaur et al. 2018). These products are cosmetics with pharmaceutical and therapeutic benefits.
1.5 Conclusion
In this chapter, we described historical landmarks of the discovery, exploration, and utilization of cyclodextrins, cyclic oligosaccharides obtained from the enzymatic degradation of starch and discovered serendipitously in 1891 by Villiers. Of course, this historical chapter cannot hope to be exhaustive, but it highlighted the work of those researchers who have contributed to the knowledge of cyclodextrins throughout the 129 years of its history.
Expensive to produce, the three main native cyclodextrins, i.e., α-, β-, and γ-cyclodextrins, were long considered just laboratory curiosities. Until the mid-1970s, the main obstacles were the lack of sufficient knowledge of these molecules, their high price, and also their presumed toxicity. In addition, very few researchers were convinced of the industrial potential of cyclodextrins.
As reported in this comprehensive chapter, since Villiers’ discovery, several great scientists, including Schardinger, Freudenberg, Cramer, French, and Szejtli, have left their mark on the history, characterization, properties, and potential applications of these molecules over a period of 86 years, from 1903, i.e., the first paper on cyclodextrin chemistry published by Schardinger, to 1989, i.e., the creation by Szejtli of the first company totally devoted to cyclodextrins.
Since the 1980s, cyclodextrins have considerably attracted the interest of scientists and industries in different disciplines including health science, agriculture, chemistry, biochemistry, and environment. The main reason for this growing interest was their ability to form inclusion complexes with various molecules through host-guest interactions.
Today, cyclodextrins continue to offer new horizons to scientists and industrials with a wide range of possible modifications and forms for multiple classical, e.g., pharmacy, food industry, chromatography, cosmetology, and biotechnology, and emerging, e.g., biomedicine, agrochemistry, and nanomaterials, applications.
References
Adeoye O, Figueiredo A, Cabral Marques H (2017) Chapter 13: Cyclodextrins and skin disorders: therapeutic and cosmetic applications. In: Ascenso A, Ribeiro H, Simões S (eds) Carrier-mediated dermal delivery. Jenny Stanford Publishing, New York, 39 p. https://doi.org/10.4324/9781315364476
Akiya S, Okui S (1951) Studies of the degeneration process of sugars. 3. Reactions of periodic acid on 2 hexasaccharides. J Pharm Soc Japan 71:865–869
Akiya S, Watanabe T (1950a) A crystalline decomposition product of starch by a bacillus. 3. A new strain of Bacillus macerans. J Pharm Soc Japan 70:572–576
Akiya S, Watanabe T (1950b) A crystalline decomposition product of starch by a bacillus. 4. A new hexasaccharide. J Pharm Soc Japan 70:576–578
Akiya S, Watanabe T (1950c) A crystalline decomposition product of starch by a bacillus. 5. Structure of the new hexasaccharide. J Pharm Soc Japan 70:579–582
Albers E, Muller BW (1995) Cyclodextrin derivatives in pharmaceutics. Crit Rev Ther Drug Carrier Syst 12:311–337
Ammayappan L, Moses JJ (2009) An overview on application of cyclodextrins in textile product enhancement. J Text Assoc 70:9–18
Andersen GH, Robbins FM, Domingues FJ, Moores RG, Long CL (1963) The utilization of Schardinger dextrins by the rat. Toxicol Appl Pharmacol 5:257–266
Andreaus J, Dalmolin MC, De Oliveira IB, Barcellos IO (2010) Application of cyclodextrins in textile processes. Quim Nova 33:929–937. https://doi.org/10.1590/S0100-40422010000400031
Antenucci RN, Palmer JK (1984) Enzymic degradation of α- and β-cyclodextrins by bacteroides of the human colon. J Agric Food Chem 32:1316–1321
Arima H, Motoyama K, Higashi T (2017) Potential use of cyclodextrins as drug carriers and active pharmaceutical ingredients. Chem Pharm Bull 65:341–348. https://doi.org/10.1248/cpb.c16-00779
Armbruster FC (1970) Method of preparing pure alpha-cyclodextrin. US Patent 3541077
Armspach D, Gattuso G, Koeniger R, Stoddart JF (1999) Cyclodextrins. In: Hecht SM (ed) Bioorganic chemistry: carbohydrates. Oxford University Press, New York, pp 458–488
Armstrong DW (1980) Pseudophase liquid chromatography: applications to TLC. J Liq Chromatogr 3:895–900
Armstrong DW (1984) Chiral stationary phases for high performance liquid chromatographic separation of enantiomers: a mini-review. J Liq Chromatogr 2:353–376
Armstrong DW, Jin HL (1989) Liquid-chromatographic separation of anomeric forms of saccharides with cyclodextrin bonded phases. Chirality 1:27–37. https://doi.org/10.1002/chir.530010108
Artiss JD, Brogan K, Brucal M, Moghaddam M, Jen KL (2006) The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism 55:195–202
Assaf KI, Gabela D, Zimmermann W, Nau WM (2016) High-affinity host-guest chemistry of large-ring cyclodextrins. Org Biomol Chem 14:7702–7706. https://doi.org/10.1039/c6ob01161f
Atteia O, Estrada ED, Bertin H (2013) Soil flushing: a review of the origin of efficiency variability. Rev Environ Sci Biotechnol 12:379–389. https://doi.org/10.1007/s11157-013-9316-0
Atwood JL, Davies JED, MacNicol DD (1984) Inclusion compounds. Oxford University Press, London
Aubert-Viard F, Mogrovejo-Valdivia A, Tabary N, Maton M, Chai F, Neut C, Martel B, Blanchemain N (2019) Evaluation of antibacterial textile covered by layer-by-layer coating and loaded with chlorhexidine for wound dressing application. Mater Sci Eng C 100:554–563. https://doi.org/10.1016/j.msec.2019.03.044
Aytac Z, Uyar T (2017) Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: enhanced water solubility and slow release of curcumin. Int J Pharm 518:177–184. https://doi.org/10.1016/j.ijpharm.2016.12.061
Aytac Z, Sen HS, Durgun E, Uyar T (2015) Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B: Biointerfaces 128:331–338. https://doi.org/10.1016/j.colsurfb.2015.02.019
Aytac Z, Yildiz ZI, Kayaci F, San NO, Kusku SI, Durgun E, Tekinay T, Uyar T (2016) Fast-dissolving, prolonged release and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J Agric Food Chem 64:7325–7334. https://doi.org/10.1021/acs.jafc.6b02632
Bailey JM, French D (1957) The significance of multiple reactions in enzyme-polymer systems. J Biol Chem 226:1–14
Barbosa PFP, Cumba LR, Andrade RDA, do Carmo DR (2019) Chemical modifications of cyclodextrin and chitosan for biological and environmental applications: metals and organic pollutants adsorption and removal. J Polym Environ 27:1352–1366. https://doi.org/10.1007/s10924-019-01434-x
Bates FL, French D, Rundle RE (1943) Amylose and amylopectin content of starches determined by their iodine complex formation. J Am Chem Soc 65:142–148. https://doi.org/10.1021/ja01242a003
Beadle JB (1969) Analyses of cyclodextrin mixtures by gas chromatography of their dimethylsilyl ethers. J Chromatogr 42:201–206
Bekers O, Uijtendaal EV, Beijnen JH, Bult JH, Underberg WJM (1991) Cyclodextrins in the pharmaceutical field. Drug Dev Ind Pharm 17:1503–1549
Bender H (1986) Chapter 6: Production, characterization and applications of cyclodextrins. In: Liss AR (ed) Advances in biotechnological processes. Wiley, New York, pp 31–71
Bender ML, Komiyama M (1978) Cyclodextrin chemistry. Reactivity and structure: concepts in organic chemistry, vol 6. Springer-Verlag, Berlin
Benkovics G, Afonso D, Darcsi A, Béni S, Conoci S, Fenyvesi É, Szente L, Malanga M, Sortino S (2017) Novel β-cyclodextrin-eosin conjugates. Beilstein J Org Chem 13:543–551. https://doi.org/10.3762/bjoc.13.52
Bergeron RJ (1984) Chapter 12: Cycloamylose-substrate binding. In: Atwood JL, JED D, DD MN (eds) Inclusion compounds, vol 3. Academic, London, pp 391–443
Bergeron RJ, McPhie P (1979) Circular dichroism studies on the structure of p-nitro-phenolate-cycloamylose complexes. Bioorg Chem 6:465–436
Bilensoy E (ed) (2011) Cyclodextrins in pharmaceutics, cosmetics and biomedicine. Current and future industrial applications. John Wiley & Sons, Inc., Hoboken, 395 p. https://doi.org/10.1002/9780470926819
Biltz W (1913) Über den osmotischen druck der kolloide. Fünfte mitteilung: zur kolloidchemie der dextrine (On the osmotic pressure of colloids. Fitfh announcement: on the colloid chemistry of dextrins). Z Phys Chem 83:683–707. https://doi.org/10.1515/zpch-1913-8348
Biltz W, Truthe W (1913) Über die molekulargrösse von dextrin β (The molecular size of β-dextrin). Ber Dtsch Chem Ges 46:1377–1380. https://doi.org/10.1002/cber.19130460217
Blinc M (1941) Gewinnung des enzyms von Bacillus macerans und seine wirkungsweise. Arch Mikrobiol 12:183–191
Blinc M (1942) Experiments to concentrate the macerans enzyme. Kolloid Z 101:126–128
Borchert W (1948) Röntgenographische untersuchungen an Shardinger-dextrinen. Z Naturforsch B 3:464–465
Brackman G, Garcia-Fernandez MJ, Lenoir J, De Meyer L, Remon JP, De Beer T, Concheiro A, Alvarez-Lorenzo C, Coenye T (2016) Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosci 16:859–869. https://doi.org/10.1002/mabi.201500437
Brauns U, Müller BW (1983) Pharmaceutical compositions drugs which are unstable or sparingly soluble in water, and methods for their preparation. European Patent No. 149197, 21 March 1990, with priority from German Patent DE No. 3346123, 21 December 1983
Breslow R (1979) Biomimetic chemistry in oriented systems. Isr J Chem 18:187–191
Breslow R, Dong SD (1998) Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem Rev 98:1997–2012. https://doi.org/10.1021/cr970011j
Breslow R, Czarniecki MF, Emert J, Hamaguchi H (1980) Improved acylation rates within cyclodextrin complexes from flexible capping of the cyclodextrin and from adjustment of the substrate geometry. J Am Chem Soc 102:762–770
Brewster ME, Loftsson T (2002) The use of chemically modified cyclodextrins in the development of formulations for chemical delivery systems. Pharmazie 57:94–101
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 59:645–666. https://doi.org/10.1016/j.addr.2007.05.012
Brewster ME, Loftsson T, Bodor N (2004) Applications of chemically-modified cyclodextrins: use of hydroxypropyl-beta-cyclodextrin as an enabling excipient for brain targeting, redox-based derivatives of estradiol a review of preclinical and clinical findings. J Drug Del Sci Technol 14:32–34
Buschmann HJ, Schollmeyer EJ (2002) Applications of cyclodextrins in cosmetic products: a review. J Cosmet Sci 53:185–191
Buschmann HJ, Schollmeyer E (2004) Cosmetic textiles: a new functionality of clothes. Cosmet Toiletries 11:105–112
Buschmann HJ, Denter U, Knittel D, Schollmeyer E (1998) The use of cyclodextrins in textile processes – an overview. J Text Inst 89:554–561. https://doi.org/10.1080/00405009808658641
Cael JJ, Koenig JL, Blackwell J (1973) Infrared and Raman spectroscopy of carbohydrates. III. Raman spectra of the polymorphic forms of amylose. Carbohydr Res 29:123–134
Caesar GV (1968) Chapter 5: The Schardinger dextrins. In: Radley JA (ed) Starch and its derivatives, 4th edn. Chapman and Hall Ltd., EC4, London, pp 290–305
Campos EVR, de Oliveira JL, Fraceto LF, Singh B (2015) Polysaccharides as safer release systems for agrochemicals. Agron Sustain Dev 35:47–66. https://doi.org/10.1007/s13593-014-0263-0
Carneiro SB, Duarte FIC, Heimfarth L, Quintans JDS, Quintans LJ, da Veiga VF, de Lima AAN (2019) Cyclodextrin-drug inclusion complexes: in vivo and in vitro approaches. Int J Mol Sci 20:642. https://doi.org/10.3390/ijms20030642
Casu B (1964) Spectrometric identification of organic compounds. Chim Ind 46:1425–1429
Casu B (1966) Recent contributions to knowledge of amylose and cyclodextrins structure. Chim Ind 48:921–923
Casu B, Reggiani M (1964) Infrared spectra of amylose and its oligomers. J Polym Sci Part C 7:171–185
Casu B, Reggiani M (1966) Conformation of amylose and its derived products. I. Infrared spectra of amylose and its oligomers in the amorphous solid phase and in solution. Starch/Stärke 18:218–229. https://doi.org/10.1002/star.19660180704
Casu B, Reggiani M, Gallo GG, Vigevani A (1964) Hydroxyl proton resonances of sugars in dimethylsulphoxide solution. Tetrahedron Lett 39-4:2839–2284
Casu B, Reggiani M, Gallo GG, Vigevani A (1965) NMR spectra and conformation of glucose and some related carbohydrates in dimethylsulphoxide solution. Tetrahedron Lett 27:2253–2259
Casu B, Gallo GG, Reggiani M, Vigevani A (1968a) Applications of magnetic resonance spectroscopy of hydroxyl protons to analysis of starch-derived products. Starch/Stärke 20:387–391. https://doi.org/10.1002/star.19680201202
Casu B, Reggiani M, Gallo GG, Vigevani A (1968b) Applications of magnetic resonance spectroscopy of the hydroxyl protons to the analysis of starch-derived products. X Congress of the Italian Chemical Society, Padova, Italy, June 1968
Casu B, Reggiani M, Gallo GG, Vigevani A (1968c) Conformation of O-methylated amylose and cyclodextrins. Tetrahedron 24:803–821. https://doi.org/10.1016/0040-4020(68)88030-5
Casu B, Reggiani M, Gallo GG, Vigevani A (1970) Conformation of acetylated cyclodextrins and Amylose. Carbohydr Res 12(2):157–170
Celebioglu A, Uyar T (2012) Electrospinning of nanofibers from non-polymeric systems: polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 4:621–631. https://doi.org/10.1039/c1nr11364j
Celebioglu A, Uyar T (2013) Electrospinning of nanofibers from non-polymeric systems: electrospun nanofibers from native cyclodextrins. J Colloid Interface Sci 404:1–7. https://doi.org/10.1016/j.jcis.2013.04.034
Celebioglu A, Sen HS, Durgun E, Uyar T (2016) Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemosphere 144:736–744. https://doi.org/10.1016/j.chemosphere.2015.09.029
Chacko KK, Saenger W (1981) Topography of cyclodextrin inclusion complexes. Crystal and molecular structure of the cyclohexaamylose 7.57 water complex. J Am Chem Soc 103:1708–1715
Chai F, Maton M, Degoutin S, Vermet G, Simon N, Rousseaux C, Martel B, Blanchemain N (2019) In vivo evaluation of post-operative pain reduction on rat model after implantation of intraperitoneal PET meshes functionalized with cyclodextrins and loaded with ropivacaine. Biomaterials 192:260–270. https://doi.org/10.1016/j.biomaterials.2018.07.032
Chankvetadze B (2004) Combined approach using capillary electrophoresis and NMR spectroscopy for an understanding of enantioselective recognition mechanisms by cyclodextrins. Chem Soc Rev 33(6):337–347. https://doi.org/10.1039/b111412n
Chen YQ, Gui X, Duan ZB, Zhu LJ, Xiang YZ, Xia DH (2019) Transition metal catalyzed organic reaction involving cyclodextrin. Chin J Org Chem 39:1284–1292. https://doi.org/10.6023/cjoc201809012
Chilajwar SV, Pednekar PP, Jadhav KR, Gupta GJC, Kadam VJ (2014) Cyclodextrin-based nanosponges: a propitious platform for enhancing drug delivery. Expert Opin Drug Deliv 11:111–120. https://doi.org/10.1517/17425247.2014.865013
Chiu KW, Robson S, Devi JL, Woodward A, Whittem T (2016) The cardiopulmonary effects and quality of anesthesia after induction with alfaxalone in 2-hydroxypropyl-beta-cyclodextrin in dogs and cats: a systematic review. J Veterinary Pharmacol Ther 39:525–538. https://doi.org/10.1111/jvp.12312
Chow DD, Karara AH (1986) Characterization, dissolution and bioavailability in rats of ibuprofen-β-cyclodextrin complex. Int J Pharm 28:95–101
Citernesi U, Sciacchitano M (1995) Cyclodextrins in functional dermocosmetics. Cosmetics Toiletries 110:53–61
Clarke RJ, Coates JH, Lincoln SF (1988) Inclusion complexes of cyclomalto-oligosaccharides (cyclodextrins). Adv Carbohydr Chem Biochem 46:205–249
Comini S, Mentink L (1991) Refining mixtures containing complexes of cyclodextrins with lipophilic compounds such as fatty acids. European patent 440539
Conceição J, Adeoye O, Cabral-Marques HM, Sousa Lobo JM (2018) Cyclodextrins as excipients in tablet formulations. Drug Discov Today 23:1274–1284. https://doi.org/10.1016/j.drudis.2018.04.009
Connors KA (1997) The stability of cyclodextrin complexes in solution. Chem Rev 97:1325–1357
Connors KA, Lin SF, Wong AB (1982) Potentiometric study of molecular complexes of weak acids and bases applied to complexes of α-cyclodextrin with para-substituted benzoic acids. J Pharm Sci 71:217–222
Cori CF, Cori GT (1946) Carbohydrate metabolism. Annu Rev Biochem 15:193–218
Cova TFGG, Murtinho D, Pais AACC, Valente AJM (2018) Cyclodextrin-based materials for removing micropollutants from wastewater. Curr Org Chem 22:2150–2181. https://doi.org/10.2174/1385272822666181019125315
Cramer F (1949) Die cyclodextrine aus Stärke. Dissertation, Heidelberg
Cramer F (1951a) Einschlussverbindungen von cyclodextrinen und die jod-reaktion der starke. Angew Chem 63:487–487
Cramer F (1951b) Über Einschlussverbindungen, I. Mitteilung, additionsversbindungen der cycloamylosen. Chem Ber 84:851–854
Cramer F (1951c) Über Einschlussverbindungen, II. Mitteilung, die blauen jodadditionsverbindungen organischer moleküle. Chem Ber 84:855–859
Cramer F (1952) Einschluβverbindungen. Angew Chem 64:437–447
Cramer F (1953) Über einschlussverbindungen. 5. Basenkatalyse durch innermolekulare hohlraume. Chem Ber Recl 86:1576–1581
Cramer F (1954) Einschlussverbindungen. Springer-Verlag, Berlin. ISBN: 978-3-642-49192-4
Cramer F (1955) Umglucosidierung mit einer amylase aus Bacillus macerans. Angew Chem Int Ed 67:714–714
Cramer F (1956) Einschluβverbindungen. Angew Chem 68(1956):115–120
Cramer F (1961) Probleme der Chemischen polynucleotide synthese. Angew Chem Int Ed 73:49–49
Cramer (1987) Introduction. In: Duchêne D (ed) Cyclodextrins and their industrial uses. éditions de santé, Paris, pp 11–18. ISBN: 2-86411-019-9
Cramer F, Dietsche W (1958) Asymmetric catalysis by inclusion compounds. Chem Ind 28:892–893
Cramer F, Dietsche W (1959a) Occlusion compounds. 15. Resolution of racemates with cyclodextrins. Chem Ber 92:378–384
Cramer F, Dietsche W (1959b) Uber einschlussverbindungen. 16. Sterospezifische reaktionen mit einschlussverbindungen. Chem Ber 92:1739–1755
Cramer F, Henglein FM (1956) Einschlussverbindungen der cyclodextrine mit gasen. Angew Chem Int Ed 68:649–649
Cramer F, Henglein FM (1957a) Über einschlussverbindungen. 11. Gesetzmassigkeiten bei der bildung von addukten der cyclodextrine. Chem Ber Recl 90:2561–2571
Cramer F, Henglein FM (1957b) Über einschlussverbindungen. 12. Verbindungen von alpha-cyclodextrin mit gasen. Chem Ber Recl 90:2572–2575
Cramer F, Hettler H (1967) Inclusion compounds of cyclodextrins. Naturwissenschaften 54:625–632
Cramer F, Kampe W (1962) Katalyse der decarboxylierung durch cyclodextrine. Eine modellreaktion fur die wirkungsweise der enzyme. Tetrahedron Lett 8:353–356
Cramer F, Kampe W (1965) Inclusion compounds. 17. Catalysis of decarboxylation by cyclodextrins. A model reaction for mechanism of enzymes. J Am Chem Soc 87:1115–1118
Cramer F, Steinle D (1955) Die wirkungsweise der amylase aus Bacillus macerans. Ann Chem Justus Liebig 595:81–100
Cramer F, Saenger W, Spatz HC (1967) Inclusion compounds. XIX. The formation of inclusion compounds of α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics. J Am Chem Soc 89:14–20
Cramer F, MacKensen G, Sensse K (1969) Über einschlussverbindungen, XX ORD-spektren und konformation der glucose-einheiten in cyclodextrin. Chem Ber 102:494–508
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70. https://doi.org/10.1016/j.progpolymsci.2004.11.002
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. https://doi.org/10.1021/cr500081p
Crini G, Morcellet M (2002) Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci 25:789–813. https://doi.org/10.1002/1615-9314(20020901)25:13%3c789:AID-JSSC789%3e3.0.CO;2-J
Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N (2018) Cyclodextrins, from molecules to applications. Environ Chem Lett 16:1361–1375. https://doi.org/0.1007/s10311-018-0763-2
Croft AP, Bartsch RA (1983) Synthesis of chemically modified cyclodextrins. Tetrahedron 9:1417–1474
Cserhati T, Dobrovolszky A, Fenyvesi E, Szejtli J (1983) Beta-cyclodextrin polymer beads as GC packings. J High Resoult Chromatogr 6:442–443
Cutrone G, Benkovics G, Malanga M, Casas-Solvas JM, Fenyvesi É, Sortino S, García-Fuentes L, Vargas-Berenguel A (2018) Mannoside and 1,2-mannobioside β-cyclodextrin-scaffolded NO-photodonors for targeting antibiotic resistant bacteria. Carbohydr Polym 199:649–660. https://doi.org/10.1016/j.carbpol.2018.07.018
Davis ME, Brewster ME (2004) Cyclodextrin-based pharmaceutics: past, present and future. Natl Rev 3:1023–1035. https://doi.org/10.1038/nrd1576
Demarco PV, Thakkar AL (1970) Cyclohepta-amylose inclusion complexes. A proton magnetic resonance study. J Chem Soc Chem Commun 1:2–4. https://doi.org/10.1039/C29700000002
Dietrich HV, Cramer F (1954) Über Einschlussverbindungen, VII. Mitteilung, zür struktur der jodketten in kanal-einschlussverbindungen. Chem Ber 87:806–817
Dong RJ, Zhou YF, Huang XH, Zhu XY, Lu YF, Shen J (2015) Functional supramolecular polymers for biomedical applications. Adv Mater 27:498–526. https://doi.org/10.1002/adma.201402975
Duchêne D (1987) Cyclodextrins and their industrial uses. Éditions de Santé, Paris
Duchêne D (1991) New trends in cyclodextrins and derivatives. Éditions de Santé, Paris
Duchêne D, Wouessidjewe D (1990a) The current state of beta-cyclodextrin in pharmaceutics. Int J Drug Formul Biopharm 36:1–6
Duchêne D, Wouessidjewe D (1990b) Pharmaceutical uses of cyclodextrins and derivatives. Drug Dev Ind Pharm 16:2487–2499. https://doi.org/10.3109/03639049009058543
Duchêne D, Wouessidjewe D (1992) Industrial uses of cyclodextrins and their derivatives. J Coord Chem 27:223–236. https://doi.org/10.1080/00958979209407955
Duchêne D, Ponchel G, Wouessidjewe D (1992) Cyclodextrins in targeting – application to nanoparticles. Adv Drug Deliv Rev 36:29–40. https://doi.org/10.1016/S0169-409X(98)00053-2
Duchêne D, Bochot A, Loftsson T (2009) Cyclodextrins and their use in pharmacy and cosmetology. STP Pharm Pratiques 19:15–27
Egele K, Samaddar S, Schneider N, Thompson D, Wenz G (2019) Synthesis of the anionic hydroxypropyl-β-cyclodextrin:poly(decamethylenephosphate) polyrotaxane and evaluation of its cholesterol efflux potential in Niemann-Pick C1 cells. J Mater Chem B 7:528–537. https://doi.org/10.1039/C8TB02950D
Endo T, Nagase H, Ueda H, Kobayashi S, Nagai T (1997) Isolation, purification, and characterization of cyclomaltodecaose (epsilon-cyclodextrin), cyclomaltoundecaose (zeta-cyclodextrin) and cyclomaltroridecaose (theta-cyclodextrin). Chem Pharm Bull 45:532–536
Endo T, Nagase H, Ueda H, Kobayashi S, Shiro M (1999) Crystal structure of cyclomaltodecaose (epsilon-cyclodextrine) at 203K. Anal Sci 15:613–614
Escuder-Gilabert L, Martin-Biosca Y, Medina-Hernandez MJ, Sagrado S (2014) Cyclodextrins in capillary electrophoresis: recent developments and new trends. J Chromatogr A 1357:2–23. https://doi.org/10.1016/j.chroma.2014.05.074
Fanali S (1993) Use of cyclodextrins in capillary electrophoresis. In: Guzman NA (ed) Capillary electrophoresis technology, Chromatographic science series, vol 64, part V. Marcel Dekker Inc, New York, pp 731–752. ISBN: 0-8247-9042-1
Fanali S, Cristalli M, Vespalec R, Bocek P (1994) Chapter 7: Chiral separations in capillary electrophoresis. In: Chrambach A, Dunn MJ, Radola BJ (eds) Advances in electrophoresis. VCH Verlagsgesellschaft mbH, Weinheim, pp 1–88
Fenyvesi É (1988) Cyclodextrin polymers in the pharmaceutical industry. J Incl Phenom 6:537–545
Fenyvesi É, Szente L (2016) Chapter 18: Nanoencapsulation of flavors and aromas by cyclodextrins. In: Grumezescu A (ed) Encapsulations. Nanotechnology in the agri-food industry, vol 2, 1st edn, pp 769–792. ISBN: 978-0-12-804378-3
Fenyvesi É, Vikmon M, Szente L (2016) Cyclodextrins in food technology and human nutrition: benefits and limitations. Crit Rev Food Sci Nutr 56:1981–2004. https://doi.org/10.1080/10408398.2013.809513
Fenyvesi É, Puskas I, Szente L (2019) Applications of steroid drugs entrapped in cyclodextrins. Environ Chem Lett 17:375–391. https://doi.org/10.1007/s10311-018-0807-7
Fernandez MA, Fernando OF, Vico RV, de Rossi RH (2019) Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr Res 480:12–34. https://doi.org/10.1016/j.carres.2019.05.006
French D (1957a) The Schardinger dextrins. In: Wolfrom ML (ed) Advances in carbohydrate chemistry, vol 12. Academic, New York, pp 189–260
French D (1957b) Preparation of Schardinger dextrins. Methods Enzymol 3:17–20. https://doi.org/10.1016/S0076-6879(57)03341-8
French D (1960) Determination of starch structure by enzymes. Bull Soc Chim Biol 42:1677–1689
French D (1962) Cyclodextrin transglycosylase (Bacillus macerans amylase). In: Collowick SP, Kaplan NO (eds) Methods in enzymology, vol 5. Academic, New York, pp 148–155. https://doi.org/10.1016/S0076-6879(62)05197-6
French D, Abdullah M (1965) Branched Schardinger dextrins. Fed Proc 24:221–223
French D, McIntire RL (1950) Studies on the Schardinger dextrins. V. Periodate oxidation. J Am Chem Soc 72:5148–5150. https://doi.org/10.1021/ja01167a095
French D, Rundle RE (1942) The molecular weights of the Schardinger alpha and beta dextrins. J Am Chem Soc 64:1651–1653. https://doi.org/10.1021/ja01259a050
French D, Pazur JH, Levine ML, Norberg E (1948) Reversible action of macerans amylase. J Am Chem Soc 70:3145–3145. https://doi.org/10.1021/ja01189a512
French D, Levine ML, Pazur JH, Norberg E (1949a) Studies on the Schardinger dextrins. The preparation and solubility characteristics of alpha-dextrins, beta-dextrins, and gamma-dextrins. J Am Chem Soc 71:353–356. https://doi.org/10.1021/ja01169a100
French D, Levine ML, Pazur JH (1949b) Studies on the Schardinger dextrins. II. Preparation and properties of amyloheptaose. J Am Chem Soc 71:356–358. https://doi.org/10.1021/ja01169a101
French D, Levine ML, Pazur JH, Norberg E (1950a) Studies on the Schardinger dextrins. IV. The action of soy bean beta amylase on amyloheptaose. J Am Chem Soc 72:1746–1748. https://doi.org/10.1021/ja01160a093
French D, Knapp D, Pazur JH (1950b) Studies on the Schardinger dextrins. VI. The molecular size and structure of the γ-dextrin. J Am Chem Soc 72:5150–5152. https://doi.org/10.1021/ja01167a096
French D, Levine ML, Norberg E, Nordin P, Pazur JH, Wild GM (1954) Studies on the Schardinger dextrins. VII. Co-substrate specificity in coupling reactions of Macerans amylase. J Am Chem Soc 76:2387–2390
French D, Pulley AO, Whelan WJ (1963) Preparation of Schardinger dextrins on a larger-than-laboratory scale. Die Stärke 8:280–284
French D, Pulley AO, Effenberger JA, Rougvie MA, Abdullah M (1965) Studies on the Schardinger dextrins. XII. The molecular size and structure of the delta-, epsilon-, zeta-, and eta-dextrins. Arch Biochem Biophys 111:153–160. https://doi.org/10.1016/0003-9861(65)90334-6
Freudenberg K (1934) Beiträge zur chemie der stärke und anderer polysaccharide. Angew Chem 39:675–677
Freudenberg K (1939) Polysaccharides and lignin. Annu Rev Biochem 8:81–112
Freudenberg K (1943) Beiträge zur chemie der kohlenhydrate. Ber Dtsch Chem Ges 76:A71–A96
Freudenberg K (1955) Contributions to the chemistry of high molecular natural substances. J Polym Sci 16:155–162
Freudenberg K (1957a) Hydrolysis and optical rotation of cellulose, starch, and cycloglucans. J Polym Sci 23:791–799
Freudenberg K (1957b) Beiträge zur chemie der stärke und der cycloglucane (Schardinger-dextrine). Angew Chem 69:419–422
Freudenberg K (1962) Beiträge zur chemie der cellulose und der stärke. Starch-Stärke 15:199–208
Freudenberg K, Cramer F (1948) Die constitution der Schardinger-dextrine dextrine-alpha, dextrin-beta and dextrin-gamma. Z Naturforsch B 3:464–464
Freudenberg K, Cramer F (1950) Über die Schardinger-dextrine aus stärke. Chem Ber Recl 83:296–304
Freudenberg K, Ivers O (1922) Synthesen gemischt-acylierter halogen zucker. Ber Dtsch Chem Ges 55:929–941. https://doi.org/10.1002/cber.19220550416
Freudenberg K, Jacobi R (1935) Über Schardingers dextrin aus stärke. Justus Liebigs Ann Chem 518:102–108
Freudenberg K, Meyer-Delius M (1938) Über die Schardinger-dextrine aus stärke. Ber Dtsch Chem Ges 71:1596–1600
Freudenberg K, Meyer-Delius M (1939) Neue ansichten über die stärke. Naturwissenschaften 27:850–853. https://doi.org/10.1007/BF01489430
Freudenberg K, Rapp W (1936) Zur kenntnis der stärke und der Schardinger-dextrine. Ber Dtsch Chem Ges 69:2041–2045
Freudenberg K, Blomqvist G, Ewald L, Soff K (1936) Hydrolyse und acetolyse der stärke und der Schardinger-dextrine. Ber Dtsch Chem Ges 69:1258–1266
Freudenberg K, Boppel H, Meyer-Delius M (1938) Observations on starch. Naturwissenschaften 26:123–124
Freudenberg K, Schaaf E, Dumpert G, Ploetz T (1939) New aspects of starch. Naturwissenschaften 27:850–853
Freudenberg K, Plankenhorn E, Knauber H (1947a) Uber Schardinger dextrine aus stärke. Ann Chem Justus Liebig 558:1–10
Freudenberg K, Plankenhorn E, Knauber H (1947b) Schardinger’s dextrins – derived from starch. Chem Ind 48:731–735
Freudenberg K, Cramer F, Plieningen H (1953) Verfahren zur Herstellung von Einschlussverbindungen Physiologisch Wirksamer Organischer Verbindungen. Knoll AG, Chemische Fabriken, German Patent DBP 895.769, November 1953
Frömming KH, Szejtli J (1994) Cyclodextrins in pharmacy. Topics in inclusion science, vol 5. Kluwer Academic Publishers, Dordrecht
Gilmore D, Colson YL (2011) Tumor targeted nanoparticles: a modern day Trojan horse. Semin Thorac Cardiovasc Surg 23:10–11. https://doi.org/10.1053/j.semtcvs.2011.03.004
Grachev MK, Kurochkina GI, Popkov AV (2019) The features of synthesis and chemical behavior of some silicon-containing cyclodextrin derivatives. Russ Chem Bull 68:708–716. https://doi.org/10.1007/s11172-019-2477-4
Gramera R (1969) Cyclodextrin polyethers and their production. US Patent No. 3459731, October 1969
Griffiths DW, Bender ML (1973) Cycloamyloses as catalysts. Adv Catal 23:209–261. https://doi.org/10.1016/S0360-0564(08)60302-8
Gruenhut NS, Cushing ML, Caesar GV (1948) Alpha and beta-Schardinger dextrin nitrates. J Am Chem Soc 70:424–425
Gruiz K, Molnar M, Fenyvesi É, Cs H, Atkari A, Barkacs K (2011) Cyclodextrins in innovative engineering tools for risk-based environmental management. J Incl Phenom Macrocycl Chem 70:299–306. https://doi.org/10.1007/s10847-010-9909-y
Gruiz K, Meggyes T, Fenyvesi É (eds) (2019) Engineering tools for environmental risk management. Volume 4. Risk reduction technologies and case studies. CRC Press, Boca Raton. https://doi.org/10.1201/b20405
Guo J, Russell EG, Darcy R, Cotter TG, McKenna SL, Cahill MR, O’Driscoll CM (2017) Antibody-targeted cyclodextrin-based nanoparticles for siRNA Delivery in the treatment of acute myeloid leukemia: physicochemical characteristics, in vitro mechanistic studies, and ex vivo patient derived therapeutic efficacy. Mol Pharm 14:940–952. https://doi.org/10.1021/acs.molpharmaceut.6b01150
Hale WS, Rawlins LC (1951) Amylase of Bacillus macerans. Cereal Chem 28:49–58
Hamada Y, Nambu N, Nagai T (1975) Pharmaceutical interactions in dosage forms and processing. III. Interactions of α- and β-cyclodextrin with several nonsteroidal antiinflammatory drugs in aqueous solution. Chem Pharm Bull 23:1205–1211
Hammoud Z, Khreich N, Auezova L, Fourmentin S, Elaissari A, Greige-Gerges H (2019) Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int J Pharm 564:59–76. https://doi.org/10.1016/j.ijpharm.2019.03.063
Han JH (2005) Innovations in food packaging. Food science and technology, international series. Academic, Amsterdam, 503 p. ISBN: 0-12-311632-5
Han SM, Armstrong DW (1989) Chapter 10: HPLC separation of enantiomers and other isomers with cyclodextrin-bonded phases: rules for chiral recognition. In: Krstulovic AM (ed) Chiral separations by HPLC. Ellis Horwood Limited/Wiley, New York, pp 208–284
Han YY, Liu WCJ, Huang JW, Qiu SR, Zhong H, Liu D, Liu JQ (2018) Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics 10:271. https://doi.org/10.3390/pharmaceutics10040271
Hanes CS (1937) The action of amylases in relation to the structure of starch and its metabolism in the plant. Parts IV-VII. New Phytol 36:189–239
Hapiot F, Bricout H, Menuel S, Tilloy S, Monflier E (2014) Recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis. Cat Sci Technol 4:1899–1908. https://doi.org/10.1039/C4CY00005F
Harata K, Uedaira H (1975) Structure of α-cyclodextrin-p-iodoaniline complex. Nature 253:190–191
Harvey AE, Manning DL (1950) Spectrophotometric methods of establishing empirical formulas of colored complexes in solution. J Am Chem Soc 72:4488–4493
Hashimoto HJ (1996) Cyclodextrins in foods, cosmetics, and toiletries. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon, London/Oxford, pp 483–502
Hashimoto HJ (2002) Present status of industrial applications of cyclodextrins in Japan. J Incl Phenom Macrocycl Chem 44:57–62
Hashimoto H (2006) Chapter 16: Cyclodextrin applications in food, cosmetic, toiletry, textile and wrapping materiel fields. In: Dodziuk H (ed) Cyclodextrins and their complexes. Chemistry, analytical methods, applications. Wiley-VCH/Verlag GmbH & Co. KGaA, Weinheim, pp 452–459. https://doi.org/10.1002/3527608982.ch16
Hedges AR (1998) Industrial applications of cyclodextrins. Chem Rev 98:2035–2044
Hedges AR, Shieh WJ, Sikorski CT (1995) Use of cyclodextrins for encapsulation in the use and treatment of food products. In: Risch SJ, Reineccius GA (eds) Encapsulation and controlled release of food ingredients, ACS symposium series 590. American Chemical Society, Washington, DC, pp 60–71
Hess K, Trogus M, Ulmann M (1933) Information on the modifications of alpha-dextrin by F Schardinger. Z Phys Chem Abt B 21:1–6
Higashi T (2019) Cyclodextrin-based molecular accessories for drug discovery and drug delivery. Chem Pharm Bull 67:289–298. https://doi.org/10.1248/cpb.c18-00735
Higashi T, Iohara D, Motoyama K, Arima H (2018) Supramolecular pharmaceutical sciences: a novel concept combining pharmaceutical sciences and supramolecular chemistry with a focus on cyclodextrin-based supermolecules. Chem Pharm Bull 66:207–216
Higuchi T, Connors KA (1965) Phase solubility techniques. Adv Anal Chem Instrum 4:117–212
Higuchi S, Tanaka K, Tanaka S (1982) Raman circular intensity differential spectra of methyl orange induced by inclusion in cyclodextrin. Chem Lett 11:635–638. https://doi.org/10.1246/cl.1982.635
Hinze WL (1981) Applications of cyclodextrins in chromatographic separations and purification methods. Sep Purif Methods 10:159–237
Hirakawa H, Tomita H (2013) Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front Microbiol 4:114. https://doi.org/10.3389/fmicb.2013.00114
Hirayama F, Uekama K, Koinuma H (1980) Molecular dynamics of prostaglandin F2α-cyclodextrin complexes in aqueous solution. Chem Pharm Bull 28:1975–1980
Hiroshi K (1981) Edible film. Japan Patent JPS 5618555
Hoesslin H, Pringsheim H (1923) Physiology of the polyamyloses. II. Glycogen formation and animal combustion. Hoppe-Seiler’s Z Physiol Chem 131:168–176
Horikoshi K (1979) Production and industrial applications of beta-cyclodextrin. Process Biochem 14:26–30
Hou XS, Ke CF, Stoddart JF (2016) Cooperative capture synthesis: yet another playground for copper-free click chemistry. Chem Soc Rev 45:3766–3780. https://doi.org/10.1039/c6cs00055j
Hybl A, Rundle RE, Williams DE (1965) The crystal and molecular structure of the cyclohexaamylose-potassium acetate complex. J Am Chem Soc 87:2779–2788. https://doi.org/10.1021/ja01091a001
Irvine JC, Pringsheim H, MacDonald J (1924) CXIV.– The constitution of polysaccharides. Part VIII. The molecular structure of β-hexa-amylose. J Chem Soc Trans 125:942–947
Irvine JC, Pringsheim H, Skinner AF (1929) Die methylierung der α-tetra-amylose. Ber Dtsch Chem Ges 62:2372–2378. https://doi.org/10.1002/cber.19290620873
Ito K (2017) Slide-ring materials using cyclodextrin. Chem Pharm Bull 65:326–329. https://doi.org/10.1248/cpb.c16-00874
James WJ, French D, Rundle RE (1959) Studies on the Schardinger dextrins. 9. Structure of the cyclohexaamylose-iodine complex. Acta Crystallogr 12:385–389. https://doi.org/10.1107/S0365110X59001141
Jansook P, Ogawa N, Loftsson T (2018) Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int J Pharm 535:272–284. https://doi.org/10.1016/j.ijpharm.2017.11.018
Jiang L, Liu C, Mayumi K, Kato K, Yokoyama H, Ito K (2018) Highly stretchable and instantly recoverable slide-ring gels consisting of enzymatically synthesized polyrotaxane with low host coverage. Chem Mater 30:5013–5019. https://doi.org/0.1021/acs.chemmater.8b01208
Jicsinszky L, Fenyvesi É, Hashimoto H, Ueno A (1996) Chapter 4: Cyclodextrin derivatives. In: Atwood JL, Davies JE, DD MN, Vögtle F, Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Elsevier Science Ltd, London, pp 57–188. ISBN: 978-008-0912-844
Junthip J, Tabary N, Chai F, Leclercq L, Maton M, Cazaux F, Neut C, Paccou L, Guinet Y, Staelens JN, Bria M, Landy D, Hedoux A, Blanchemain N, Martel B (2016) Layer-by-layer coating of textile with two oppositely charged cyclodextrin polyelectrolytes for extended drug delivery. J Biomed Mater Res Part A 104:408–1424. https://doi.org/10.1002/jbm.a.35674
Kainuma K (1984) Chapter V: Starch oligosaccharides: linear, branched, and cyclic. In: Whistler RL, JN BM, Paschall EF (eds) Starch – chemistry and technology, 2nd edn. Academic, London, pp 125–152. https://doi.org/10.1016/B978-0-12-746270-7.50011-2
Karrer P (1920) The understanding of polysaccharides I. methylation of starch. Helv Chim Acta 3:620–625
Karrer P (1921) Polysaccharides XI. The compounds of anhydrosugar with caustic alkali. A method for determining the basic elements of polymeric anhydrosugar. Helv Chim Acta 4:811–816
Karrer P (1922) Untersuchungen über polymere kohlenhydrate. Angew Chem 35:85–90
Karrer P (1923) Polysaccharides XX. Zur kenntniss polymerer kohlenhydrate. Helv Chim Acta 6:402–409
Karrer P (1925) Polymere kohlenhydrate. Akademische Verlagsgesellschaft, Leipzig
Karrer P, Bürkin E (1922) Polysaccharides XIV. On the understanding of amylose. Helv Chim Acta 5:181–187
Karrer P, Nägeli C (1921a) Polysaccharides II. On the constitution of diarrylose. Helv Chim Acta 4:169–172
Karrer P, Nägeli C (1921b) Structure of potato starch. Helv Chim Acta 4:185–202
Karrer P, Nägeli C, Hurwitz O, Wälti A (1921) Polysaccharides VIII. Zur kenntnis der stärke und der amylosen. Helv Chim Acta 4:678–699
Karrer P, Staub M, Wälti A (1922) Polysaccharides XIII. On the understanding of inulin and the alkali hydroxide bonding of anhydrous sugar. Helv Chim Acta 5:129–139
Kashiwagi Y, Katashima T, Nakahata M, Takashima Y, Harada A, Inoue T (2018) Linear viscoelastic studies on a transient network formed by host-guest interaction. J Polym Sci B Polym Phys 56:1109–1117. https://doi.org/10.1002/polb.24630
Kaufmann HP, Wessels H (1964) Die dünnschicht-chromatographie auf dem fettgebiet XIV: die trennung der triglyceride durch kombination der adsorptions- und der umkehrphasen-chromatographie. Fette Seifen Anstrichmittel 66:81–86. https://doi.org/10.1002/lipi.19640660201
Kaur R, Kukkar D, Bhardwaj SK, Kim KH, Deep A (2018) Potential use of polymers and their complexes as media for storage and delivery of fragrances. J Control Release 285:8–95. https://doi.org/10.1016/j.jconrel.2018.07.008
Kerr RW (1942) Action of macerans enzyme on a component of corn starch. J Am Chem Soc 64:3044–3045
Kerr RW (1943) On the significance of the degradation of starch by macerans amylase. J Am Chem Soc 65:188–193
Kerr RW (1949) Action of beta amylase on amylase. Nature 164:757–759
Kerr RW, Cleveland FC (1949) The molecular weight of the beta-amylase limit dextrin from corn starch. J Am Chem Soc 71:3455–3457
Kerr RW, Severson GM (1943) On the multiple amylase concept on starch. III. The isolation of an amylase crystalline form. J Am Chem Soc 65:193–198
Kfoury M, Hădărugă NG, Hădărugă DI, Fourmentin S (2016) Chapter 4: Cyclodextrins as encapsulation material for flavors and aroma. In: Encapsulations. Nanotechnology in the agri-food industry, vol 2, 1st edn, pp 127–192. ISBN: 978-0-12-804378-3
Khan AR, Forgo P, Stine KJ, D’Souza VT (1998) Methods for selective modifications of cyclodextrins. Chem Rev 98:1977–1996. https://doi.org/10.1021/cr970012b
Kneen E, Beckord LD (1946) Quantity and quality of amylase produced by various bacterial isolates. Arch Biochem 10:41–54
Koizumi K, Sanbe H, Kubota Y, Terada Y, Takaha T (1999) Isolation and characterization of cyclic alpha-(1→4)-glucans having degrees of polymerization 9-31 and their quantitative analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. J Chromatogr A 852:407–416
Komiyama M (1996) Cyclodextrins as enzyme models. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 401–422
Komiyama M, Hirai H (1981) Carbon 13 NMR study on the cyclodextrin inclusion complexes in solution. Bull Chem Soc Jpn 54:828–831
Kozlowski CA, Sliwa W (2010) Use of cyclodextrin polymers in separation of organic species, Polymer science and technology series. Nova Science Publishers, Inc, New York
Krysl S, Smolková-Keulemansová E (1985) Cyclodextrins and their utilization in chromatographic methods. Chem List 79:919–942
Kumar S, Rao R (2019) Analytical tools for cyclodextrin nanosponges in pharmaceutical field: a review. J Incl Phenom Macrocycl Chem 94:11–30. https://doi.org/10.1007/s10847-019-00903-z
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453:167–180. https://doi.org/10.1016/j.ijpharm.2012.06.055
Kurozumi M, Nambu N, Nagai T (1975) Inclusion compounds of non-steroidal anti-inflammatory and other slightly water soluble drugs with α- and β-cyclodextrins in powdered form. Chem Pharm Bull 23:3062–3068
Lach JL, Chin TF (1964) Interaction of pharmaceuticals with Schardinger dextrins. III. J Pharm Sci 53:69–73
Lach JL, Cohen J (1963) Interaction of pharmaceuticals with Schardinger dextrins. II. J Pharm Sci 52:137–142
Lammers JNJJ (1967) A new purity criterion for α- and β-cyclodextrin using partition chromatography on cellulose columns. Die Stärke 19:70–73. https://doi.org/10.1002/star.19670190304
Landy D, Mallard I, Ponchel A, Monflier E, Fourmentin S (2012) Remediation technologies using cyclodextrins: an overview. Environ Chem Lett 10:225–237. https://doi.org/10.1007/s10311-011-0351-1
Lange F (1925) Verfahren zur gewinnung von polyamylosen. German Patent, Patentschrift n° 442963, I.G. Farbenindustrie Akt.-Ges. In Frankfurt
Larsen KL (2002) Large cyclodextrins. J Incl Phenom 43:1–13. https://doi.org/10.1023/A:1020494503684
Lau EV, Gan SY, Ng HK, Poh PE (2014) Extraction agents for the removal of polycyclic aromatic hydrocarbons (PAHs) from soil in soil washing technologies. Environ Pollut 184:640–649. https://doi.org/10.1016/j.envpol.2013.09.010
Lau MT, Manion J, Littleboy JB, Oyston L, Khuong TM, Wang QP, Nguyen DT, Hesselson D, Seymour J, Neely GG (2019) Molecular dissection of box jellyfish venom cytotoxicity highlights an effective venom antidote. Nat Commun 10:1655. https://doi.org/10.1038/s41467-019-09681-1
Lautsch W, von Wiechert R, Lehman H (1954) Tosyl- and mesyl derivative der cyclodextrine. Kollloid Z 135:134–136
Lay S, Ni XF, Yu HN, Shen SR (2016) State-of-the-art applications of cyclodextrins as functional monomers in molecular imprinting techniques: a review. J Sep Sci 39:2321–2331. https://doi.org/10.1002/jssc.201600003
Lee SB, Robyt JF (2001) Trapping of a covalent enzyme intermediate in the reaction of Bacillus macerans cyclomaltodextrin glucanyltransferase with cyclomaltohexaose. Carbohydr Res 336:47–53. https://doi.org/10.1016/S0008-6215(01)00247-6
Lepage ML, Schneider JP, Bodlenner A, Compain P (2015) Toward a molecular Lego approach for the diversity-oriented synthesis of cyclodextrin analogues designed as scaffolds for multivalent systems. J Organomet Chem 80:10719–10733. https://doi.org/10.1021/acs.joc.5b01938
Lewis EA, Hansen LD (1973) Thermodynamics of binding of guest molecules to α- and β-cyclodextrins. J Chem Soc Perkin Trans II:2081–2085
Li J (2009) Cyclodextrin inclusion polymers forming hydrogels. In: Wenz G (ed) Inclusion polymers, Advances in polymer science, vol 222, pp 79–112. https://doi.org/10.1007/12_2008_9
Li S, Purdy WC (1992) Cyclodextrins and their applications in analytical chemistry. Chem Rev 92:1457–1470
Lichtenthaler FW, Immel S (1996) Towards understanding formation and stability of cyclodextrin inclusion complexes: computation and visualization of their molecular lipophilicity patterns. Starch/Stärke 48:145–154
Lincoln SF, Easton CJ (1998) Chapter 14: inclusion complexes of the cyclodextrins. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. Marcel Dekker, Inc, New York, pp 473–521
Lindner K, Saenger W (1978) β-cyclodextrine dodecahydrate: crowing of water molecules within a hydrophobic cavity. Angew Chem Int Ed 17:694–695
Lipkowitz KB (1998) Applications of computational chemistry to the study of cyclodextrins. Chem Rev 98:1829–1873
Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM (2009) Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci U S A 106:2377–2382. https://doi.org/10.1073/pnas.0810895106
Loftsson T, Brewster ME (1996) Pharmaceutical applications of cyclodextrins.1. Drug solubilization and stabilization. J Pharm Sci 85:1017–1025. https://doi.org/10.1021/js950534b
Loftsson T, Brewster ME (2010) Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol 62:1607–1621. https://doi.org/10.1111/j.2042-7158.2010.01030.x
Loftsson T, Brewster ME (2012) Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci 101:3019–3032. https://doi.org/10.1002/jps.23077
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications: historical perspectives. Int J Pharm 329:1–11. https://doi.org/10.1016/j.ijpharm.2006.10.044
Loftsson T, Stefánsson E (1997) Effect of cyclodextrins on topical drug delivery to the eye. Drug Dev Ind Pharm 23:473–481. https://doi.org/10.3109/03639049709148496
Loftsson T, Stefánsson E (2017) Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int J Pharm 531:413–423. https://doi.org/10.1016/j.ijpharm.2017.04.010
Loftsson T, Másson M, Brewster ME (2004) Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93:1091–1099. https://doi.org/10.1002/jps.20047
Loftsson T, Jarho P, Másson M, Järvinen T (2005) Cyclodextrins in drug delivery. Expert Opin Drug Deliv 2:335–351. https://doi.org/10.1517/17425247.2.1.335
Luda MP, Zanetti M (2019) Cyclodextrins and cyclodextrin derivatives as green char promoters in flame retardants formulations for polymeric materials. A review. Polymers 11:664. https://doi.org/10.3390/polym11040664
Lüttringhaus A, Cramer F, Prinzbach H, Henglein FM (1958) Cyclisationen von langkettigen dithiolen. Versuche zur darstellung sich umfassender ringe mit hilfe von einschlußverbindungen. Liebigs Ann Chem 613:185–198. https://doi.org/10.1002/jlac.19586130120
Macaev F, Boldescu V (2015) Cyclodextrins in asymmetric and stereospecific synthesis. Symmetry Basel 7:1699–1720. https://doi.org/10.3390/sym7041699
Madrid F, Ballesteros R, Lacorte S, Villaverde J, Morillo E (2019) Extraction of PAHS from an aged creosote-polluted soil by cyclodextrins and rhamnolipids. Side effects on removal and availability of potentially toxic elements. Sci Total Environ 635:384–392. https://doi.org/10.1016/j.scitotenv.2018.10.316
Maeda Y, Motoyama K, Nishiyama R, Higashi T, Onodera R, Hakamura H, Takeo T, Nakagata H, Yamada Y, Ishitsuka Y, Kondo Y, Irie T, Era T, Arima H (2019) In vivo efficacy and safety evaluation of lactosyl-β-cyclodextrin as a therapeutic agent for hepatomegaly in Niemann-pick type C disease. Nano 9:802. https://doi.org/10.3390/nano9050802
Mahmud ST, Wilson LD (2016) Synthesis and characterization of surface-modified mesoporous silica materials with beta-cyclodextrin. Cogent Chem 2:1132984. https://doi.org/10.1080/23312009.2015.1132984
Malanga M, Szemán J, Fenyvesi E, Puskas I, Csabai K, Gyemant G, Fenyvesi F, Szente L (2016) “Back to the future”: a new look at hydroxypropyl beta-cyclodextrins. J Phamr Sci 105:2921–2931. https://doi.org/10.1016/j.xphs.2016.04.034
Malanga M, Seggio M, Kirejev V, Fraix A, Di Bari I, Fenyvesi É, Ericson MB, Sortino S (2019) A phototherapeutic fluorescent β-cyclodextrin branched polymer delivering nitric oxide. Biomater Sci 7:2272–2276. https://doi.org/10.1039/C9BM00395A
Matzuzawa M, Kawano M, Nakamura N, Horikoshi K (1975) An improved method for the preparation of Schardinger β-dextrin on an industrial scale by cyclodextrin glycosyl transferase of an alkalophilic Bacillus sp. Starch/Die Stärke 27:410–413. https://doi.org/10.1002/star.19750271204
Mavridis IM, Yannakopoulou K (2015) Anionic cyclodextrins as versatile hosts for pharmaceutical nanotechnology: synthesis, drug delivery, enantioselectivity, contrast agents for MRI. Int J Pharm 492:275–290. https://doi.org/10.1016/j.ijpharm.2015.06.004
McClenahan WS, Tilden EB, Hudson CS (1942) A study of the products obtained from starch by the action of the amylase of Bacillus macerans. J Am Chem Soc 64:2139–2144
McMullan RK, Saenger W, Fayos J, Mootz D (1973a) Topography of cyclodextrin inclusion complexes. 1. Classification of crystallographic data of alpha-cyclodextrin inclusion complexes. Carbohydr Res 31:37–46
McMullan RK, Saenger W, Fayos J, Mootz D (1973b) Topography of cyclodextrin inclusion complexes. 2. Iodine-cyclohexaamylose tetrahydrate complex. Its molecular geometry and cage-type crystal-structure. Carbohydr Res 31:211–227
Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Ruiz i Altaba A (2014) The river blindness drug ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med 6:1263–1278. https://doi.org/10.15252/emmm.201404084
Menezes PD, Andrade TD, Frank LA, de Souza EPBSS, Trindade GDG, Trindade IAS, Serafini MR, Guterres SS, Araujo AAD (2019) Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int J Pharm 559:312–328. https://doi.org/10.1016/j.ijpharm.2019.01.041
Menges RA, Armstrong DW (1991) Chiral separations using native and functionalized cyclodextrin-bonded stationary phases in high-pressure liquid-chromatography. ACS Symp Ser 471:67–100
Miekeley A (1930) Über die fragliche existenz der sog. α-diamylose. Ber Dtsch Chem Ges 63:1957–1961
Miekeley A (1932) Bemerkung zür existenz des α-diamylose. Ber Dtsch Chem Ges 65:69–69
Miller KP, Wang L, Chen YP, Pellechia PJ, Benicewicz BC, Decho AW (2015) Engineering nanoparticles to silence bacterial communication. Front Microbiol 6:189. https://doi.org/10.3389/fmicb.2015.00189
Mitchell CR, Armstrong DW (2004) Cyclodextrin-based chiral stationary phases for liquid chromatography: a twenty-year overview. In: Gübitz G, Schmid MG (eds) Chiral separations – methods and protocols, Methods in molecular biology, vol 243. Humana Press Inc, Totowa, pp 61–112. ISBN: 1-58829-150-2
Miyazawa I, Ueda H, Nagase H, Endo T, Kobayashi S, Nagai T (1995) Physicochemical properties and inclusion complex formation of δ-cyclodextrin. Eur J Pharm Sci 3:153–162
Mocanu G, Vizitiu D, Carpov A (2001) Cyclodextrin polymers. J Bioact Compat Polym 16:315–342
Morillo E (2006) Chapter 16: Application of cyclodextrins in agrochemistry. In: Dodziuk H (ed) Cyclodextrins and their complexes. Chemistry, analytical methods, applications. Wiley-VCH, Verlag GmbH & Co. KGaA, Weinheim, pp 459–467
Morin-Crini N, Fourmentin S, Crini G (eds) (2015) Cyclodextrines. PUFC, Besançon, 370 p. ISBN: 978-2-84867-520-6
Morin-Crini N, Fourmentin M, Fourmentin S, Torri G, Crini G (2018) Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ Chem Lett 16:1361–1375. https://doi.org/10.1007/s10311-018-00818-0
Morohoshi T, Tokita K, Ito S, Saito Y, Maeda S, Kato K, Ikeda T (2013) Inhibition of quorum sensing in gram-negative bacteria by alkylamine-modified cyclodextrins. J Biosci Bioeng 116:175–179. https://doi.org/10.1016/j.jbiosc.2013.01.022
Motoyama K, Onodera R, Tanaka N, Kameyama K, Higashi T, Kariya R, Okada S, Arima H (2015) Evaluation of antitumor effects of folate-conjugated methyl-β-cyclodextrin in melanoma. Biol Pharm Bull 38:374–379. https://doi.org/10.1248/bpb.b14-00531
Muankaew C, Loftsson T (2018) Cyclodextrin-based formulations: a non-invasive platform for targeted drug delivery. Basic Clin Pharmacol Toxicol 122:46–55. https://doi.org/10.1111/bcpt.12917
Müller S, Estour F, Kalakuntla RK, Le P, Romain LO, Worek F, Thiermann H, Reiter G (2013) New modified beta-cyclodextrin derivatives as detoxifying agents of chemicalwarfare agents (II). In vitro detoxification of cyclosarin (GF): general screening and toxicokineticaspects of OP scavengers. Toxicol Lett 216:206–212
Myrbäck K (1938) Beta-dextrin and starch. I. Announcement. Biochem Z 297:160–171
Myrbäck K (1942) Article on border dextrin and starch. XVI. On the nature of alpha-dextrin. Biochem Z 311:242–246
Myrbäck K (1949a) On the action of Bacillus macerans amylase. Acta Chem Scand 3:91–93
Myrbäck K (1949b) Nonoxidative, nonproteolytic enzymes. Annu Rev Biochem 18:59–86
Myrbäck K, Ahlborg K (1940) About limit dextrin and starch. VIII. Announcement: constitution of starch limit dextrin. Evidence of alpha-glycoside 1,6-bindings in dextrin and starch. Biochem Z 307:69–78
Myrbäck K, Gjörling LG (1945) Über die starkespaltung durch Bacillus macerans. Ark Kemi Min Och Geol 20:1–13
Nepogodiev S, Stoddart FJ (1998) Cyclodextrin-based catenanes and rotaxanes. Chem Rev 98:1959–1576. https://doi.org/10.1021/cr970049w
Neva T, Mellet CO, Fernandez JMG, Benito JM (2019) Multiply-linked cyclodextrin-aromatic hybrids: caps, hinges and clips. J Carbohydr Chem. https://doi.org/10.1080/07328303.2019.1609020
Nociari MM, Lehmann GL, Perez Bay AE, Radu RA, Jiang Z, Goicochea S, Schreiner R, Warren JD, Shan J, de Beaumais SA, Ménand M, Sollogoub M, Maxfield FR, Rodriguez-Boulan E (2014) Beta cyclodextrins bind, stabilize, and remove lipofuscin bisretinoids from retinal pigment epithelium. Proc Natl Acad Sci U S A 111:E1402–E1408. https://doi.org/10.1073/pnas.1400530111
Norberg E, French D (1950) Studies on the Schardinger dextrins. III. Redistribution reactions of Macerans amylase. J Am Chem Soc 72:1202–1205. https://doi.org/10.1021/ja01159a036
Okada S (1979) Application of cyclodextrin glycosyltransferase – review. Hakkokogaku Kaishi J Soc Ferment Technol 57:396–406
Okano C, Nasuno E, Iimura K, Kato N (2016) Cyclodextrin-immobilized microspheres for uptake of the quorum-sensing signaling molecule N-acylhomoserine lactone. J Appl Polym Sci 133:43198. https://doi.org/10.1002/app.43198
Oliveri V, Vecchio G (2016) Cyclodextrins as protective agents of protein aggregation: an overview. Chem Asian J 11:1648–1657. https://doi.org/10.1002/asia.201600259
Osa T, Matsue T, Fujihira M (1977) Cyclodextrin-nitrophenol systems studied by polarography. Heterocycles 6:1833–1839
Osaki M (2019) Functionalization of cyclodextrin derivatives to create supramolecular pharmaceutical materials. J Pharm Soc Japan 139:165–173
Pawar S, Shende P (2019) A comprehensive patent review on beta-cyclodextrin cross-linked nanosponges for multiple applications. Recent Pat Nanotechnol 14(1):75–89. https://doi.org/10.2174/1872210513666190603083930
Perez-Anes A, Gargouri M, Laure W, Van Den Berghe H, Courcot E, Sobocinski J, Tabary N, Chai F, Blach JF, Addad A, Woisel P, Douroumis D, Martel B, Blanchemain N, Lyskawa J (2015) Bioinspired titanium drug eluting platforms based on a poly-beta-cyclodextrin-chitosan layer-by-layer self-assembly targeting infections. ACS Appl Mater Interfaces 7:12882–12893. https://doi.org/10.1021/acsami.5b02402
Pictet A, Jahn R (1922) Sur un nouveau produit de dépolymérisation de l’amidon. Helv Chim Acta 5:640–644. https://doi.org/10.1002/hlca.19220050503
Pitha J (1984) Pharmaceutical preparations containing cyclodextrin derivatives. US Patent No. 4277064, 23 February 1988, with priority from US Patent No. 4596795, 25 April 1984
Pitha J, Szente L, Szejtli J (1983) Molecular encapsulation of drugs by cyclodextrins and congeners. In: Bruck SD (ed) Controlled drug delivery, vol I. CRC Press, Boca Raton, pp 125–148
Poór M, Kunsági-Máté S, Szente L, Matisz G, Secenji G, Czibuya Z, Kőszegi T (2015) Interaction of ochratoxin A with quaternary ammonium beta-cyclodextrin. Food Chem 172:143–149. https://doi.org/10.1016/j.foodchem.2014.09.034
Pringsheim H (1915) Neue ergebnisse der stärkechemie. Naturwissenschaften 3:95–99. https://doi.org/10.1007/BF01546143
Pringsheim H (1919) Die chemische anpassung der mikroorganismen. Naturwissenschaften 7:319–327. https://doi.org/10.1007/BF01546143
Pringsheim H (1922) Problematisches aus der polysaccharide chemie. Angew Chem 35:345–349
Pringsheim H (1924) Über die konstitution der stärke, des glykogens und der flechtenstärke (Beiträge zur Chemie der Stärke, XII). Ber Dtsch Chem Ges 57:1581–1598. https://doi.org/10.1002/cber.19240570870
Pringsheim H (1925) Über die chemie komplexer naturstoffe. Naturwissenschaften 13:1084–1090. https://doi.org/10.1007/BF01585591
Pringsheim H (1926) Abbau und aufbau der polysaccharide. Ber Dtsch Chem Ges 59:3008–3018. https://doi.org/10.1002/cber.19260591205
Pringsheim H (1927) Über die zusammensetzung des holzgeistöls und acetonöls. Angew Chem 40:1387–1393
Pringsheim H (1928a) In: Walton RP (ed) A comprehensive survey of starch chemistry. Chemical Catalog Co. Inc, New York, p 35
Pringsheim H (1928b) Twenty-five years of biochemistry. Science 68:603–608
Pringsheim H (1931a) Chapter VII: Dextrine: charakteristik, gewinnung und eigenschaften. In: Die polysaccharide. Verlag von Julius Springer, Berlin, pp 248–274
Pringsheim H (1931b) Ein umriβ der heutigen zuckerchemie. Angew Chem 44:677–682
Pringsheim H (1932) Chapter XV: The dextrins: characteristics, sources, and properties. In: The chemistry of the monosaccharides and of the polysaccharides, vol 6. McGraw-Hill Book Company, Inc./Cornell University, New York, pp 271–295
Pringsheim H, Beiser A (1924) A complement of amylase and the marginal dextrin. Third memorandum. Biochem Z 148:336–343
Pringsheim H, Beiser A (1932) Diamylose und tetraamylose (Beiträge zur chemie der stärke, XXVII). Ber Dtsch Chem Ges 65:1870–1873. https://doi.org/10.1002/cber.19320651124
Pringsheim H, Dernikos D (1922) Weiteres über die polyamylosen (Beiträge zur Chemie der Stärke, VI). Ber Dtsch Chem Ges 55:1433–1445. https://doi.org/10.1002/cber.19220550534
Pringsheim H, Eissler F (1913) Über Schardingers krystallisierte dextrin (Beiträge zur chemie der stärke, II.). Ber Dtsch Chem Ges 46:2959–2974. https://doi.org/10.1002/cber.19130460370
Pringsheim H, Eissler F (1914) Über Schardingers krystallisierte dextrin (Beiträge zur chemie der stärke, III.). Ber Dtsch Chem Ges 47:2565–2572. https://doi.org/10.1002/cber.19140470331
Pringsheim H, Goldstein K (1922) Die Beziehung der α- und β-polyamylosen zur inhalts- und hüllsubstanz des stärkekorns (Beiträge zur Chemie der Stärke, VII). Ber Dtsch Chem Ges 55:1446–1449. https://doi.org/10.1002/cber.19220550535
Pringsheim H, Goldstein K (1923) Zür charakterisierung der polyamylosen (Beiträge zur Chemie der Stärke, VIII). Ber Dtsch Chem Ges 56:1520–1526. https://doi.org/10.1002/cber.19230560704
Pringsheim H, Langhans A (1912) Über krystallisierte polysaccharides aus stärke. Ber Dtsch Chem Ges 45:2533–2546. https://doi.org/10.1002/cber.191204502156
Pringsheim H, Leibowitz J (1924) Über die constitution der polyamylosen (Beiträge zur Chemie der Stärke, IX). Ber Dtsch Chem Ges 57:884–887. https://doi.org/10.1002/cber.19240570536
Pringsheim H, Leibowitz J (1925a) Über α- und β-amylasen (Beiträge zur Chemie der Stärke, XIII). Ber Dtsch Chem Ges 57:1262–1265. https://doi.org/10.1002/cber.19250580710
Pringsheim H, Leibowitz J (1925b) Über die beziehung zwischen optischem drehungsvermögen und struktur in der polysaccharide-chemie. Ber Dtsch Chem Ges 58:2808–2814. https://doi.org/10.1002/cber.19250581217
Pringsheim H, Leibowitz J (1926a) Über die spezifität der amylasen (Beiträge zur Chemie der Stärke, XV). Ber Dtsch Chem Ges 57:991–995. https://doi.org/10.1002/cber.19260590523
Pringsheim H, Leibowitz J (1926b) Molekulargröβe und assoziation der polyamylosen (Beiträge zur Chemie der Stärke, XVIII). Ber Dtsch Chem Ges 59:2058–2064. https://doi.org/10.1002/cber.19260590863
Pringsheim H, Lichtenstein S (1916) On crystallized polysaccharides from glycogen. Ber Dtsch Chem Ges 49:364–369. https://doi.org/10.1002/cber.19160490141
Pringsheim H, Meyersohn P (1927) Über die dispergierung der polyamylosen (Beiträge zur Chemie der Stärke, XX). Ber Dtsch Chem Ges 60:1709–1716. https://doi.org/10.1002/cber.19270600743
Pringsheim H, Müller KO (1922) Physiology of the polyamyloses. Hoppe-Seiler’s Z Physiol Chem 118:236–240
Pringsheim H, Persch W (1921) Über die methylierung der poly-amylosen (Beiträge zur Chemie der Stärke, IV). Ber Dtsch Chem Ges 54:3162–3168. https://doi.org/10.1002/cber.19210541128
Pringsheim H, Persch W (1922) Über methyl- und acetylprodukte der ‘polyamylosen’ (Beiträge zur Chemie der Stärke, V). Ber Dtsch Chem Ges 55:1425–1433. https://doi.org/10.1002/cber.19220550533
Pringsheim H, Schapiro E (1926) Über den fermentativen abbau der stärke durch biolase. (Beiträge zur chemie der stärke, XVI). Ber Dtsch Chem Ges 59:996–1000. https://doi.org/10.1002/cber.19260590524
Pringsheim H, Steingroever F (1924) Über die halogenuerbindungen der polyamylosen (Beiträge zur Chemie der Stärke, XI). Ber Dtsch Chem Ges 57:1579–1581. https://doi.org/10.1002/cber.19240570869
Pringsheim H, Steingroever F (1926) Zur kenntnis der amylobiose (Beiträge zur Chemie der Stärke, XVII). Ber Dtsch Chem Ges 59:1001–1006. https://doi.org/10.1002/cber.19260590525
Pringsheim H, Wolfsohn K (1924) Über den verschiedenen aufbau der beiden stärke-bestandteile (Beiträge zur Chemie der Stärke, X). Ber Dtsch Chem Ges 57:887–891. https://doi.org/10.1002/cber.19240570537
Pringsheim H, Wiener A, Weidinger A (1930) Über neue polyamylosen. I. (Beiträge zur chemie der stärke, XXIV). Ber Dtsch Chem Ges 63:2628–2636. https://doi.org/10.1002/cber.19300630943
Pringsheim H, Weidinger A, Sallentien H (1931a) Diamylose und tetramylose; triamylose und hexaamylose (Beiträge zur Chemie der Stärke, XXV). Ber Dtsch Chem Ges 64:2117–2125. https://doi.org/10.1002/cber.19310640841
Pringsheim H, Weidinger A, Sallentien H (1931b) Über neue polyamylosen, II. (Beiträge zur Chemie der Stärke, XXVI). Ber Dtsch Chem Ges 64:2125–2130. https://doi.org/10.1002/cber.19310640842
Pulley AO, French D (1961) Studies on the Schardinger dextrins. 11. Isolation of new Schardinger dextrins. Biochem Biophys Res Commun 5:11–15. https://doi.org/10.1016/0006-291X(61)90071-7
Puskás I, Varga E, Tuza K, Szemán J, Fenyvesi É, Sohajda T, Szente L (2015) Chapter 10: Sulfobutylether-cyclodextrins: structure, degree of substitution and functional performance. In: Ramirez FG (ed) Cyclodextrins. Nova Science Publishers, Inc, Hauppauge, pp 293–320. ISBN: 978-1-63482-788-1
Qi QS, She XY, Endo T, Zimmermann W (2004) Effect of the reaction temperature on the transglycosylation reactions catalyzed by the cyclodextrin glucanotransférase from Bacillus macerans for the synthesis of large-ring cyclodextrins. Tetrahedron 60:799–806
Rajkumar T, Kukkar D, Kim KH, Sohn JR, Deep A (2019) Cyclodextrin-metal-organic framework (CD-MOF): from synthesis to for applications. J Ind Eng Chem 72:50–66. https://doi.org/10.1016/j.jiec.2018.12.048
Rao VSR, Foster JF (1963) On the conformation of the D-glucopyranose ring in maltose and in higher polymers of D-glucose. J Phys Chem 67:951–952. https://doi.org/10.1021/j100798a524
Rekharsky MV, Inoue Y (1998) Complexation thermodynamics of cyclodextrins. Chem Rev 98:1875–1917
Robyt JF (1998) Chapter 8: Cyclodextrins. In: Essentials of carbohydrate of chemistry. Springer-Verlag Inc, New York, pp 245–261. https://doi.org/10.1007/978-1-4612-1622-3
Robyt J, French D (1964) Purification and action pattern of an amylase from Bacillus polymyxa. Arch Biochem Biophys 104:338–345. https://doi.org/10.1016/S0003-9861(64)80024-2
Rundle RE, French D (1943) The configuration of starch and the starch-iodine complex. II. Optical properties of crystalline starch fractions. J Am Chem Soc 65:558–561. https://doi.org/10.1021/ja01244a018
Ryzhakov A, Thi TD, Stappaerts J, Bertoletti L, Kimpe K, Couto ARS, Saokham P, Van den Mooter G, Augustijns P, Somsen GW, Kurkov S, Inghelbrecht S, Arien A, Jimidar MI, Schrijnemakers K, Loftsson T (2016) Self-assembly of cyclodextrins and their complexes in aqueous solutions. J Pharm Sci 105:2556–2569. https://doi.org/10.1016/j.xphs.2016.01.019
Saenger W (1976) α-Cyclodextrin inclusion complexes: mechanism of adduct formation and intermolecular interactions. In: Pullman B (ed) Environmental effects on molecular structure and properties. D. Reidel Publishing Company, Dordrecht, pp 265–305
Saenger W (1980) Cyclodextrin inclusion-compounds in research and industry. Angew Chem Int Ed 19:344–362. https://doi.org/10.1002/anie.198003441
Saenger W (1984) Structural aspects of cyclodextrins and their inclusion complexes. In: Atwood JL, JED D, DD MN (eds) Inclusion compounds – structural aspects of inclusion compounds formed by organic host lattices, vol 2. Academic, London, pp 231–259. ISBN-13: 978-0120671021
Saenger W, Noltemeyer M, Manor PC, Hingerty B, Klar B (1976) “Induced fit” type complex formation of the model enzyme α-cyclodextrin. Bioorg Chem 5:187–195. https://doi.org/10.1016/0045-2068(76)90007-9
Samec M (1942) Über die hydrolyse von abbauprodukten der stärke durch den Bacillus macerans und sein enzym. Ber Dtsch Chem Ges 75:1758–1760
Samec M, Blinc M (1939) Die neueren ergebnisse der stärkeforschung, II. Kolloidchem Beih 49:211–223
Samec M, Blinc M (1941) Die neuere entwicklung der kolloidchemie der stärke. T. Steinkopff, Dresden/Leipzig, p 543
Sand DM, Schlenk H (1961) Acylated cyclodextrins as polar phases for gas liquid chromatography. Anal Chem 33:1624–1625
Saokham P, Loftsson T (2017) Gamma-cyclodextrin. Int J Pharm 516:278–292. https://doi.org/10.1016/j.ijpharm.2016.10.062
Schardinger F (1903a) Über thermophile bakterien aus verschiedenen speisen und milch, sowie über einige umsetzungsprodukte derselben in kohlenhydrathaltigen nährlösungen, darunter krystallisierte polysaccharide (dextrine) aus stärke. Zeitschrrift für Untersuchuing von Nahrungs- und Genussmittel 6:865–880
Schardinger F (1903b) Ueber die Zulässigkeit des warmhaltens von zum genuβ bestimmten nahrungsmitteln mittelst wärme speichernder apparate, sog. thermophore. Wien Klin Wochenschr 16:468–474
Schardinger F (1904) Mitteilung aus der staatlichen untersuchungsanstalt für lebensmittel in Wien Azetorgärung. Wien Klin Wochenschr 17:207–209
Schardinger F (1905) Bacillus macerans, ein aceton bildender rottebacillus. Centralblatt für Bakteriologie Parasitenkunde und Infektionskrankheiten 14:772–781
Schardinger F (1907) Zur biochemie des Bacillus macerans. Centralblatt für Bakteriologie Parasitenkunde und Infektionskrankheiten 19:161–163
Schardinger F (1909) Ueber die bildung kristallisierter fehlingsche lösung nicht reduzierender körper (polysaccharide) aus stärke durch mikrobielle tätigkeit. Centralblatt für Bakteriologie und Parasitenkunde 22:98–103
Schardinger F (1911) Bildung kristallisierter polysaccharide (dextrine) aus stärkekleister durch mikrobien. Centrablatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 29:188–197
Schlenk H, Sand DM (1961) The association of α- and β-cyclodextrins with organic acids. J Am Chem Soc 83:2312–2320. https://doi.org/10.1021/ja01471a022
Schlenk H, Sand DM, Tillotson JA (1955) Stabilization of autoxidizable materials by means of inclusion. J Am Chem Soc 77:3587–3590. https://doi.org/10.1021/ja01618a049
Schlenk H, Gellerman JL, Sand DM (1962) Acylated cyclodextrins as stationary phases for comparative gas liquid chromatography. Anal Chem 34:1529–1532. https://doi.org/10.1021/ac60192a006
Schneider HJ (2012) Applications of supramolecular chemistry. CRC Press/Taylor & Francis Group, Boca Raton
Schneider HJ, Yatsimirsky AK (2000) Principles and methods in supramolecular chemistry. Wiley, Chichester
Schneiderman E, Stalcup AM (2000) Cyclodextrins: a versatile tool in separation science. J Chromatogr B 745:83–102. https://doi.org/10.1016/S0378-4347(00)00057-8
Schwimmer S (1953) Evidence for the purity of Schardinger dextrinogenase. Arch Biochem Biophys 43:108–117. https://doi.org/10.1016/0003-9861(53)90089-7
Scriba GKE (2012) Chiral recognition mechanisms in analytical separation sciences. Chromatographia 75:815–838. https://doi.org/10.1007/s10337-012-2261-1
Sébille B (1987) Cyclodextrin derivatives. In: Duchêne D (ed) Cyclodextrins and their industrial uses. Edition de Santé, Paris, pp 351–393
Sharma N, Baldi A (2016) Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 23:739–757. https://doi.org/10.3109/10717544.2014.938839
Shende P, Trotta F (2019) Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int J Pharm 565:333–350. https://doi.org/10.1016/j.ijpharm.2019.05.015
Sicard PJ, Saniez MH (1987) Chapter 2: Biosynthesis of cycloglycosyltransferase and obtention of its enzymatic reaction products. In: Duchêne D (ed) Cyclodextrins and their industrial uses. Éditions de Santé, Paris, pp 77–103
Silva A, Duarte A, Sousa S, Ramos A, Domingues FC (2016) Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT Food Sci Technol 73:481–489. https://doi.org/10.1016/j.lwt.2016.06.043
Simoes SMN, Veiga F, Torres-Labandeira JJ, Ribeiro ACF, Concheiro A, Alvarez-Lorenzo C (2014) Syringeable self-assembled cyclodextrin gels for drug delivery. Curr Top Med Chem 14:494–509. https://doi.org/10.2174/1568026613666131219124308
Singh MK, Varun VK, Behera BK (2011) Cosmetotextiles: state of art. Fibres Text East Eur 19:27–33
Sirlin C (1984) Supramolecular catalysis by cyclodextrin and macrocyclic polyether (a review). Bull Soc Chim France Partie II Chim Moléculaire Organique et Biologique 1-2:5–40
Smolková-Keulemansová E (1982) Cyclodextrins as stationary phases in chromatography. J Chromatogr 251:17–34
Smolková-Keulemansová E, Krysl S (1980) Inclusion compounds in chromatography. J Chromatogr 184:347–361. https://doi.org/10.1016/S0021-9673(00)89005-6
Solms J, Egli RH (1965) Harz emit einschlusshohlräumen von cyclodextrin-strukture. Helv Chim Acta 48:1225–1228
Sonnendecker C, Zimmermann W (2019a) Domain shuffling of cyclodextrin glucanotransferases for tailored product specificity and thermal stability. FEBS Open Bio 9:384–395. https://doi.org/10.1002/2211-5463.12588
Sonnendecker C, Zimmermann W (2019b) Change of the product specificity of a cyclodextrin glucanotransferase by semi-rational mutagenesis to synthesize large-ring cyclodextrins. Catalyts 9:242. https://doi.org/10.3390/catal9030242
Sonnendecker C, Melzer S, Zimmermann W (2018) Engineered cyclodextrin glucanotransferases from Bacillus sp. G-825-6 produce large-ring cyclodextrins with high specificity. MicrobiologyOpen 8(6):e00757. https://doi.org/10.1002/mbo3.757
Sonnendecker C, Thürmann S, Przybylski C, Zitmann FD, Heinke N, Krauke Y, Monks K, Robitzki BD, Zimmermann W (2019) Large-ring cyclodextrins as chiral selectors for enantiomeric pharmaceuticals. Angew Chem Int Ed 58:6411–6414. https://doi.org/10.1002/anie.201900911
Stella VJ, Rajewski RA (1992) Derivatives of cyclodextrin exhibiting enhanced aqueous solubility and pharmaceutical uses thereof US Patent No. 5,134,127
Sundararajan PR, Rao VCR (1970) Conformational studies on cycloamyloses. Carbohydr Res 13:351–358. https://doi.org/10.1016/S0008-6215(00)80592-3
Sybilska D, Smolková-Keulemansová E (1984) Application of inclusion compounds in chromatography. In: Inclusion compounds, vol 3. Academic, London, pp 173–243
Sybilska D, Zukowski J (1989) Chapter 7: Cyclodextrin additives. In: Krstulovic AM (ed) Chiral separations by HPLC. Ellis Horwood Limited/John Wiley & Sons, New York, pp 147–172
Szejtli J (1977) Some application possibilities of cyclodextrins in pharmaceutical industries. Starch/Stärke 29:26–33. https://doi.org/10.1002/star.19770290107
Szejtli J (1978) Neue untersuchungsmethoden in der cyclodextrinchemie (new analytical methods in chemistry of cyclodextrins). Starch/Stärke 30:427–431. https://doi.org/10.1002/star.19780301207
Szejtli J (1982a) Cyclodextrins and their inclusion complexes. Akadémiai Kiadó, Budapest. ISBN: 963-05-2850-9
Szejtli J (1982b) Cyclodextrins in food, cosmetics and toiletries. Starch/Stärke 34:379–385. https://doi.org/10.1002/star.19820341106
Szejtli J (1982c) Proceedings of the first international symposium on cyclodextrins. Akadémiai Kiadó, Budapest. ISBN 963 05 3056 2
Szejtli J (1983) Dimethyl-β-cyclodextrin as parenteral drug carrier. J Incl Phenom 1:135–150
Szejtli J (1984) Industrial applications of cyclodextrins. In: Atwood JL, JED D, DD MN (eds) Inclusion compounds, vol 3. Academic, London, pp 331–390
Szejtli J (1985) Cyclodextrins: a new group of industrial basic materials. Nahrung/Food 29:911–924
Szejtli J (1986) Cyclodextrins in biotechnology. Starch/Stärke 38:388–390. https://doi.org/10.1002/star.19860381107
Szejtli J (1987) Application of cyclodextrins in the chromatography. Starch-Stärke 39:357–362. https://doi.org/10.1002/star.19870391006
Szejtli J (1988) Cyclodextrin technology. Kluwer Academic Publishers, Dordrecht, p 450. ISBN: 90-277-2314-1
Szejtli J (1989) Downstream processing using cyclodextrins. Trends Biotechnol 7:171–174. https://doi.org/10.1016/0167-7799(89)90094-
Szejtli J (1990) The cyclodextrins and their applications in biotechnology. Carbohydr Polym 12:375–392. https://doi.org/10.1016/0144-8617(90)90088-A
Szejtli J (1994) Medicinal applications of cyclodextrins. Med Res Rev 14:353–386. https://doi.org/10.1002/med.2610140304
Szejtli J (1995) Selectivity/structure correlation in cyclodextrin chemistry. Supramol Chem 6:217–223. https://doi.org/10.1080/10610279508032537
Szejtli J (1996) Chapter 2: Chemistry, physical and biological properties of cyclodextrins. In: Atwood JL, Davies ED, MacNicol DD and Vögtle F, executive editors; Szejtli J and Osa T (eds), Cyclodextrins, comprehensive supramolecular chemistry, vol 3. Pergamon, London/Oxford, pp 5–40. ISBN: 9780080912844
Szejtli J (1997) Utilization of cyclodextrins in industrial products and processes. J Mater Chem 7:575–587. https://doi.org/10.1039/A605235E
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1753
Szejtli J (2002) The role of cyclodextrins in chiral selective chromatography. Trends Anal Chem 21:379–388
Szejtli J (2003) Cyclodextrins in the textile industry. Starch/Stärke 55:191–196
Szejtli J (2004) Past, present and future of cyclodextrin research. Pure Appl Chem 76:1825–1845. https://doi.org/10.1351/pac200476101825
Szejtli J, Szente L, Bánky-Előd E (1979) Molecular encapsulation of volatile, easily oxidizable labile flavour substances by cyclodextrins. Acta Chim Acad Sci Hung 101:27–46
Szejtli J, Gerloczy A, Fonagy A (1980a) Intestinal absorption of 14C-labelled β-cyclodextrin in rats. Arzeim Forsch 30:808–810
Szejtli J, Zsadon B, Fenyvesi É, Otta K, Tudos F (1980b) Hungarian Patent 181733, U.S. Patent 4,357,468 (1982)
Szente L, Fenyvesi É (2018) Cyclodextrin-enabled polymer composites for packaging. Molecules 23:1556. https://doi.org/10.3390/molecules23071556
Szente L, Strattan CE (1991) Chapter 2: Hydroxypropyl-β-cyclodextrins, preparation and physicochemical properties. In: Duchêne D (ed) New trends in cyclodextrins and derivatives. Editions de Santé, Paris, pp 55–96
Szente L, Szejtli J (1996) Cyclodextrins in pesticides. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 503–514
Szente L, Szemán J (2013) Cyclodextrins in analytical chemistry: host-guest type molecular recognition. Anal Chem 85:8024–8030. https://doi.org/10.1021/ac400639y
Taira H, Nagase H, Endo T, Ueda H (2006) Isolation, purification and characterization of large-ring cyclodextrins (CD36~CD39). J Incl Phenom Macrocycl Chem 56:23–28
Taka AL, Pillay K, Mbianda XY (2017) Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: a review. Carbohydr Polym 159:94–107. https://doi.org/10.1016/j.carbpol.2016.12.027
Takahashi K (1998) Organic reactions mediated by cyclodextrins. Chem Rev 98:2013–2033
Takeo K (1971) Studies on complexes of amylose and cyclodextrins with organic compounds. Complexation of amylose and cyclodextrins and X-ray diffraction of the crystalline complexes. Kyoto Furitsu Daigaku Houkoku 23:168–208
Takeo K, Kuge T (1969) Complexes of starchy materials with organic compounds. 3. X-ray studies on amylase and cyclodextrin complexes. Agric Biol Chem 33:1174–1180
Takeo K, Kuge T (1970) Conformation of peracetylated cyclodextrins. Agric Biol Chem 34:1416–1419
Terada Y, Yanase M, Takata H, Takaha T, Okada S (1997) Cyclodextrins are not the major cyclic alpha-(1→4)-glucans produced by the initial action of cyclodextrin glucanotransferase on amylase. J Biol Chem 272:15729–15733
Thoma JA, French D (1958) Studies on the Schardinger dextrins. X. The interaction of cyclohexaamylose, iodine and iodide. Part I. Spectrophotometric studies. J Am Chem Soc 80:6142–6146. https://doi.org/10.1021/ja01555a060
Thoma JA, French D (1959) The dissociation constant for the cyclohexaamylose-iodine complex. J Phys Chem 62:1603–1603. https://doi.org/10.1021/j150570a041
Thoma JA, French D (1960) The starch iodine iodide interaction. 1. Spectrophotometric investigations. J Am Chem Soc 82:4144–4147. https://doi.org/10.1021/ja01501a004
Thoma JA, French D (1961) The starch iodine iodide interaction. 2. Potentiometric investigations. J Phys Chem 65:1825–1828. https://doi.org/10.1021/j100827a032
Thoma JA, Stewart L (1965) Chapter IX: Cycloamyloses. In: Whistler RL, Paschall EF (eds) Starch, chemistry and technology, Volume 1: Fundamental aspects. Academic, New York, pp 209–249
Thoma JA, Wright HB, French D (1959) Partition chromatography of homologous saccharides on cellulose columns. Arch Biochem Biophys 85:452–460. https://doi.org/10.1016/0003-9861(59)90510-7
Tilden EB, Hudson CS (1939) Conversion of starch to crystalline dextrins by the action of a new type of amylase separated from cultures of Aerobacillus macerans. J Am Chem Soc 61:2900–2902
Tilden EB, Hudson CS (1942) Preparation and properties of the amylases produced by Bacillus macerans and Bacillus polymyxa. J Bacteriol 43:527–544
Tilden EB, Adams M, Hudson CS (1942) Purification of the amylase of Bacillus macerans. J Am Chem Soc 64:1432–1433
Topuz F, Uyar T (2019) Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 11:1–35. https://doi.org/10.3390/pharmaceutics11010006
Uekama K, Hirayama F (1978) Inclusion complexation of prostaglandin F2α with α- and β-cyclodextrins in aqueous solution. Chem Pharm Bull 26:1195–1200
Uekama K, Otagiri M (1987) Cyclodextrins in drug carrier systems. Crit Rev Ther Drug Carrier Syst 3:1–40
Uekama K, Fujise A, Hirayama F, Otagiri M, Inaba K (1984) Improvement of dissolution characteristics and chemical stability of prostaglandins E1 by γ-cyclodextrin complexation. Chem Pharm Bull 32:275–279
Uekama K, Hirayama F, Irie T (1998) Cyclodextrin drug carrier systems. Chem Rev 98:2045–2076
Ueno A, Takahashi K, Osa T (1980) One host/two guest complexation between γ-cyclodextrin and sodium α-naphthylacetate as shown by excimer fluorescence. J Chem Soc Chem Commun 19:921–922. https://doi.org/10.1039/C39800000921
Ulmann M (1932) The molecular size of the alpha-dextrin of F Schardinger. Biochem Z 251:458–477
Ulmann M, Trogus M, Hess K (1932) Zur kenntnis des α-dextrins von F. Schardinger (information on the α-dextrins by F. Schardinger). Ber Dtsch Chem Ges 65:682–686
Valente AJM, Söderman O (2014) The formation of host-guest complexes between surfactants and cyclodextrins. Adv Colloid Interface 205:156–176. https://doi.org/10.1016/j.cis.2013.08.001
Van de Manakker F, Vermonden T, Van Nostrum CF, Hennink WE (2009) Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 10:3157–3175. https://doi.org/10.1021/bm901065f
van Etten RL, Sebastian JF, Clowes GA, Bender ML (1967a) Acceleration of phenyl ester cleavage by cycloamyloses: a model for enzymatic specificity. J Am Chem Soc 89:3242–3253
van Etten RL, Clowes GA, Sebastian JF, Bender ML (1967b) The mechanism of the cycloamylose-accelerated cleavage of phenyl esters. J Am Chem Soc 89:3253–3262
van Hooidonk C, Breebaart-Hansen CAE (1970) Stereospecific reaction of isopropyl methylphosphonofluoridated (sarin) with α-cyclodextrin: a model for enzyme inhibition. Rec Trav Chim 89:289–299. https://doi.org/10.1002/recl.19700890309
van Hooidonk C, Breebaart-Hansen CAE (1972) Model studies for enzyme inhibition. Part IV: the association of some alkyl methylphosphonates with α-cyclodextrin in an aqueous medium. Rec Trav Chim 91:958–964. https://doi.org/10.1002/recl.19720910811
Vaution C, Hutin M, Glomot F, Duchêne D (1987) Chapter 8: The use of cyclodextrins in various industries. In: Duchêne D (ed) Cyclodextrins and their industrial uses. Éditions de Santé, Paris, pp 299–350
Vermet G, Degoutin S, Chai F, Maton M, Danjou PE, Martel B, Blanchemain N (2017) Cyclodextrin modified PLLA parietal reinforcement implant with prolonged antibacterial activity. Acta Biomater 53:222–232. https://doi.org/10.1016/j.actbio.2017.02.017
Vetter W, Bester K (2006) Chapter 6: Gas chromatographic enantioseparation of chiral pollutants – techniques and results. In: Busch KW, Busch MA (eds) Chiral separation. Elsevier, Amsterdam, pp 131–228
Villalonga R, Cao R, Fragoso A (2007) Supramolecular chemistry of cyclodextrin in enzyme technology. Chem Rev 107:3088–3116. https://doi.org/10.1021/cr050253g
Villiers A (1891a) Sur la transformation de la fécule en dextrine par le ferment butyrique. Chimie Organique – Compte Rendus des Séances de l’Académie des Sciences (France) Février, CXII, pp 435–437
Villiers A (1891b) Sur la transformation de la fécule en dextrine par le ferment butyrique. Bulletin de la Société Chimique de Paris. 1er semestre, 3ème série, tome V, vol 45, pp 468–470
Villiers A (1891c) Sur la fermentation de la fécule par l’action du ferment butyrique. Chimie Organique – Compte Rendus des Séances de l’Académie des Sciences (France) Juin, CXII, pp 536–538
Villiers A (1891d) Sur la fermentation de la fécule par l’action du ferment butyrique. Bulletin de la Société Chimique de Paris. 1er semestre, 3ème série, tome V, vol 46, pp 470–472
Voncina B (2011) Chapter 17: application of cyclodextrins in textile dyeing. In: Hauser P (ed) Textile dyeing. InTech, Tijeka, pp 373–392. ISBN: 978-953-307-565-5
Ward TJ, Armstrong DW (1986) Improved cyclodextrin chiral phases – a comparison and review. J Liq Chromatogr 9:407–423. https://doi.org/10.1080/01483918608076644
Ward TJ, Armstrong DW (1988) Chapter 5: Cyclodextrin-stationary phases. In: Zief M, Crane LJ (eds) Chromatographic chiral separations. Marcel Dekker Inc, New York, pp 131–163
Wenz G (1994) Cyclodextrins as building blocks for supramolecular structures and functional units. Angew Chem Int Ed 33:803–822
Wenz G, Han BH, Müller A (2006) Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev 106:782–817. https://doi.org/10.1021/cr970027+
West C (2014) Enantioselective separations with supercritical fluids – review. Curr Anal Chem 10:99–120. https://doi.org/10.2174/1573411011410010009
Whelan WJ, Manners DJ, Rosenfeld E, Gottschalk A, French D, Bell DJ, Courtois JE, Hasside WZ (1960) Determination of starch structure by enzymes – discussion. Bull Soc Chim Biol 42:1690–1700
Wiedenhoff N, Lammers JNJJ (1968) Properties of cyclodextrins: part II. Preparation of a stable β-cyclodextrin hydrate and determination of its water content and enthalpy of solution in water from 15-30°. Carbohydr Res 7:1–6. https://doi.org/10.1016/S0008-6215(00)81426-3
Wilson EJ, Schoch TJ, Hudson CS (1943) The action of macerans amylase on the fractions from starch. J Am Chem Soc 65:1380–1383
Wood DJ, Hrsuka FE, Saenger W (1977) 1H NMR study of the inclusion of aromatic molecules in α-cyclodextrin. J Am Chem Soc 99:1735–1740
Wu ZL, Song N, Menz R, Pingali B, Yang YW, Zheng YB (2015) Nanoparticles functionalized with supramolecular host-guest systems for nanomedicine and healthcare. Nanomedicine 10:1493–1514. https://doi.org/10.2217/NNM.15.1
Xiao Y, Ng SC, Tan TTY, Wang Y (2012) Recent development of cyclodextrin chiral stationary phases and their applications in chromatography. J Chromatogr A 1269:52–68. https://doi.org/10.1016/j.chroma.2012.08.049
Xu J, Wu L, Guo T, Zhang G, Wang C, Li H, Li X, Singh V, Chen W, Gref R, Zhang J (2019) A “ship-in-a-bottle” strategy to create folic acid nanoclusters inside the nanocages of γ-cyclodextrin metal-organic frameworks. Int J Pharm 556:89–96. https://doi.org/10.1016/j.ijpharm.2018.11.074
Yamagiwa T, Kawaguchi AT, Saito T, Inoue S, Morita S, Watanabe K, Kitagishi H, Koji K, Inokuchi S (2014) Supramolecular ferric porphyrins and a cyclodextrin dimer as antidotes for cyanide poisoning. Hum Exp Toxicol 33:360–368. https://doi.org/10.1177/0960327113499041
Yamamoto Y, Inoue Y (1989) NMR-studies of cyclodextrin inclusion complex. J Carbohydr Chem 8:29–46. https://doi.org/10.1080/07328308908047990
Yang JS, Yang L (2013) Preparation and application of cyclodextrin immobilized polysaccharides. J Mater Chem B 1:909–918. https://doi.org/10.1039/c2tb00107a
Yao XK, Huang P, Nie ZH (2019) Cyclodextrin-based polymer materials: from controlled synthesis to applications. Prog Polym Sci 93:1–35. https://doi.org/10.1016/j.progpolymsci.2019.03.004
Yokoo M, Kubota Y, Motoyama K, Higashi T, Taniyoshi M, Tokommaru H, Nishiyama R, Tabe Y, Mochinaga S, Sato A, Sueoka-Aragane N, Sueoka E, Arima H, Irie T, Kimura S (2015) 2-Hydroxypropyl-β-cyclodextrin acts as a novel anticancer agent. PlosOne 10:e0141946. https://doi.org/10.1371/journal.pone.0141946
Yuan Z, Zhang L (2016) Photoinduced controlled-release drug delivery systems for applications in nanomedicine. Curr Org Chem 20:1768–1785. https://doi.org/10.2174/1385272820666160112001944
Yusoff SNM, Kamari A, Aljafree NFA (2016) A review of materials used as carrier agents in pesticide formulations. Int J Environ Sci Technol 13:2977–2994. https://doi.org/10.1007/s13762-016-1096-y
Zacherov JP (1930) Some morphological and physiological characteristics of Bacillus macerans. Zentrabl Bakteriol Parasitenkd Infektionskrankh Hyg Abt 80:205–218
Zarzycki PK, Fenert BE, Głód BK (2016) Chapter 17: Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. In: Grumezescu A (ed) Encapsulations, Nanotechnology in the agri-food industry. Academic, London, pp 717–767. ISBN: 978-0-12-804378-3
Zhang J, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233. https://doi.org/10.1016/j.addr.2013.05.001
Zhang GQ, He LQ, Yuan MX, Li H, Chang T, Qin SJ (2018) Clean and green procedure for the synthesis of biodiesel from the esterification of free fatty acids and alcohol catalyzed by 6-O-(sulfobutyl)-cyclodextrin. Russ J Appl Chem 91:1123–1128. https://doi.org/10.1134/S1070427218070091
Zhang DJ, Lv P, Zhou C, Zhao YL, Liao XL, Yang B (2019a) Cyclodextrin-based delivery systems for cancer treatment. Mater Sci Eng C Mater Biol Appl 96:872–886. https://doi.org/10.1016/j.msec.2018.11.031
Zhang YM, Xu QY, Liu Y (2019b) Molecular recognition and biological application of modified cyclodextrins. Sci China Chem 62:549–560. https://doi.org/10.1007/s11426-018-9405-3
Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Bjorkhem I, Gustafsson JA, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lutjohann D, Latz E (2016) Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med 8:333ra50. https://doi.org/10.1126/scitranslmed.aad6100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morin-Crini, N. et al. (2020). History of Cyclodextrins. In: Crini, G., Fourmentin, S., Lichtfouse, E. (eds) The History of Cyclodextrins. Environmental Chemistry for a Sustainable World, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-030-49308-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-49308-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49307-3
Online ISBN: 978-3-030-49308-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)