Abstract
Experimental results, which may serve as basis for innovative applications of cyclodextrins (CDs) in environmental technologies, are presented here. Some newly developed CD-aided tools are used in sampling, measuring the concentration or testing the effect of contaminants in water and soil. The innovative methods such as the bacterial bioassays with CD-increased sensitivity or the CD-filled absorptive samplers for air and water sampling are utilised in environmental exposure, hazard and risk assessment. Technological developments aim the reduction of the risk of chemical substances in waters and soils. CD-aided environmental remediation is introduced through examples for the elimination of organic contaminants from water by CD-filters, and for the enhancement of the mobility and availability of soil contaminants in this way increasing the efficiency of soil remediation by water extraction, chemical oxidation, biodegradation, etc.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental management of chemical substances and polluted land requires a complex approach integrating (1) the conservation of the environment and the protection of human health as well as (2) maintaining sustainable development and rising living standards all over the world. To reconcile these two, seemingly contradictory requirements, an economically and ecologically efficient management of human activities and contaminated land is necessary [1].
A comprehensive environmental management and decision-making as well as legislation of hazardous chemical substances and contaminated land have two main tasks: risk assessment and if necessary, risk reduction as it is shown on the scheme in Fig. 1. Two further, supporting elements of the environmental risk management are legislation and monitoring. All of these elements and the strategies toward their use and integration into the whole process need innovative engineering tools, such as assessment and monitoring methods as well as risk reduction, on the first line, sustainable technologies for the remediation of the environment [2, 3].
A co-operation between Budapest University of Technology & Economics and Cyclolab Cyclodextrin R&D Laboratory Ltd. made possible the utilisation of cyclodextrins (CDs) in innovative environmental technologies in the following cases:
-
to concentrate the pollutant when its concentration is low compared to the sensitivity of the chemical or biological analytical method
-
to create a “moderate pessimistic” simulation system: it means that instead of a worse case in contaminant mobility, availability and adverse effects a more realistic scenario is established in analyses and testing for the safe prevention of ecosystem and human, without extreme overestimates and consequent costs, such as e.g. decisions based on the total contaminant content of soils or sediments instead of the mobile ones,
-
to mimic bioavailability and biodegradation,
-
to prevent ecosystems or its members from toxic effects of contaminants in waters and soils,
-
to sample air, water, pore-water in soil or sediment and soil moisture,
-
to decontaminate air, water and soil by direct CD application in physico-chemical technologies based on filtration, partitioning, extraction, or leaching
-
to increase contaminant mobility by CD instead of hazardous chemical additives, such as surfactants or co-solvents to intensify physico-chemical soil treatment technologies
-
to increase biological availability in bioremedial technologies such as biodegradation-based soil remediation to increase the technological efficiency.
The ability of CD to modify the risk-components, such as mobility, water solubility, and biological availability of the contaminants makes possible its use in the environmental risk assessment and risk reduction technologies. It is extremely useful in the management of contaminated soil, which is a three phase environmental compartment, with a complex texture and structure, and many folded interaction between soil phases, organic and inorganic soil constituents, the microflora and the often multi-component contaminant, resulting in a hardly predictable situation. The suitable testing methods in such cases are bioassays and microcosms, where all important factors, such as matrix, biota and contaminants are represented and a moderately pessimistic scenario can be created by CD-application. Based on the results of these moderately pessimistic test-methods direct risk-based decision making is possible.
Some successful and thought-provoking applications of CDs from our previously published research results are enlisted in the followings:
-
We measured the mutagenicity-increasing effect of random methylated β-cyclodextrin (RAMEB) in Ames mutagenicity test applied for soil contaminated with pentachlorophenol (PCP), a questionable substance from the point of view of direct mutagenesis [4, 5].
-
We proved the bioavailability-mimicking effect of hydroxypropyl β-cyclodextrin for multicomponent pollutants of petroleum origin, such as diesel- and transformer-oil [6].
-
We evidenced the water solubilising and biodegradation intensifying effect in soil remediation, resulting in 1 year time requirement instead of 2.5 years [7, 8].
-
We explored the protection of the ecosystem thanks to CDs toxicity buffering effect [9].
-
We published the very first application of RAMEB in contaminated soil bioremediation, which goes back to 1996 [10] and lead to successful field demonstrations [11–13].
In this paper we give further examples on CD-aided new environmental risk assessment and risk reduction technologies.
Materials and methods
In this chapter we define the experimental conditions of the CD-applications in environmental testings for risk assessment and in technologies for risk reduction.
Efficient testing and assessment of the contaminated environment
Dehydrogenase-enzyme activity
The dehydrogenase-enzyme activity bioassay is carried out in a soil suspension, where the partition and solubility of the contaminant may limit the sensitivity of the test. It can be used as a simple method to investigate soil microbial activities and possible inhibitory effect of the contaminants on the soil microorganisms. Nearly all microorganisms reduce 2,3,5-triphenyl-tetrazolium-chloride (TTC) (Fluka Analytical, Sigma-Aldrich Schweiz) as an artificial electron acceptor to triphenyl formazan (TPF), which is colorimetrically measured at 546 nm by SANYO SP55 UV/Vis Spectrophotometer. The testorganism is Azomonas agilis in the direct contact toxicity test [14], carried out in a soil suspension, with 20 % solid content. The soil was contaminated with transformer oil, a high Kow (log Kow = 5.1, according to our own results) petroleum hydrocarbon mixture. In the bioassay 1% RAMEB (Wacker Chemie, Munich, Germany) was added to the soil, 24 h before testing.
CD-aided air-sampling
This is useful for concentrating relative diluted air-pollutants, before air analyses. Beta-cyclodextrin (BCD) immobilized on cellulose fabric (approx. 30% BCD content) was used in the model-experiment. The immobilization was carried out by epichlorohydrin (Epi). Blank sample for comparison was prepared with Epi without CD. An artificial and standardized vapour-mixture of trichloroethylene (TCE), toluene and ethylalcohol (EtOH) with a concentration of 0.40, 0.12 and 0.11 g/L, respectively was prepared by equilibrating the free solvents in an exsiccator at 25 °C.
Air sampling was implemented by 1 day passive contact of the sampler (1 cm2 cellulose-fabric with or without CD-content) with contaminated air, following by 1, 3 and 24 h storage in contaminant-free atmosphere at 25 °C. Gas-chromatographic analyses of the immobilised air-polluting vapours—bound to the CD-impregnated fabric—was carried out by head space chromatography on BP20 (30 m × 0.32 mm × 1 μm) column with an oven program starting at 60 °C, heated by 10 °C/min to 100 °C. The headspace vials containing the sampler (1 cm2) and 0.1 mL dimethyl formamide and 5 mL water were equilibrated at 90 °C for 20 min, then 1 mL of the head space was injected into the gas chromatograph. The temperature of the injector and of the FID detector was 220 °C.
CD-aided water sampling
This process makes possible to collect and analyse diluted and specific contaminants from water. The adequacy was demonstrated for biologically active, hazardous organic water-contaminants. The contaminant in the model-experiment was bisphenol A (BPA). BPA was collected (concentrated) from the water by using a 20 mL volume column (a hypodermic syringe), filled with 1 g of BCD polymer beads (BCDP) (CY-2011, CycloLab, Hungary) with ~60% BCD content (swelling in water: 4–5 mL/g). For comparison 1 g granulated activated charcoal (Reanal 29903, Hungary) was tested. 400 mL of 5 and 50 ppm BPA test-solution was filtered through the fillings. The contaminated and filtered water samples were analysed by gas chromatography–mass spectrometry (GC–MS). BPA as its trimethylsilyl derivative was measured by Varian 4000 GC–MS/MS system (Varian, Walnut Creek, CA, USA), equipped with a Varian CP-8400 AutoSampler, and with septum-equipped programmable injector (SPI) according to Sebők et al. [15]. In addition to the chemical analysis, toxicity testing was used for the monitoring of the concentrating effect of the sampler: in the test system the toxicity of water before and after sampling was measured by using Vibrio fischeri bioluminescence-inhibition test, Tetrahymena pyriformis reproduction-inhibition test, Daphnia magna and Folsomia candida mortality tests according to Gruiz et al. [14]. Evaluation of the acute toxicity test is based on the EC50 values read from the concentration response curve, using StatSoft 8.0 software.
Environmental risk reducing technologies
Decontamination of water by CD-filter
This technic is based on the same principle as sampling low concentration contaminants from water (point 3), the aim here, however, is not the concentration, but the removal of the contaminant from water. The contaminant in the model-experiment was nicotine. Its elimination from the water was done by a 20 mL volume column (a hypodermic syringe) filled with 1 g of BCD polymer beads with ~60% BCD content. For comparison 1 g granulated activated charcoal and 1 g of BCDP + 1 g of AC was used instead of BCDP. An aqueous solution (400 mL) of 10 and 50 ppm nicotine was filtered through the columns. Only toxicity testing was used in this experiment for checking the efficiency of the CD-filter: toxicity of water before and after treatment by CD-filtration.
A testorganism with high nicotine-sensitivity, Tetrahymena pyriformis was applied according to Leitgib et al. [16]. The reproduction-inhibition of the testorganism—calculated from microscopic living cell counts—was plotted against nicotine concentration, and from that concentration response curve EC50 values were read, using StatSoft 8.0 software.
Intensification of anoxic groundwater bioremediation
This technic with RAMEB was investigated in 15-day long model experiments, using closed batch-reactors filled with 500–500 mL contaminated groundwater–soil suspension from a contaminated fuel- and service-station. Groundwater on the site was highly contaminated with BTEX (benzene, toluene, ethyl benzene and xylene) and gasoline. Before starting the experiment 22,700 μg/L total aliphatic hydrocarbons and 46,040 μg/L BTEX were measured in the groundwater.
0.1 and 0.5% RAMEB was applied for the intensification of the anoxic biodegradation based remediation. The hydrocarbon content of the water was extracted by hexane before analyses. The concentration of Extractable Petroleum Hydrocarbons (EPH C10–C40) and Volatile Petroleum Hydrocarbons (VPH C5–C12) were determined by gas chromatography. The results of EPH and VPH were summarized to get Total Petroleum Hydrocarbon (TPH) concentration. For the determination of EPH Agilent 6890N gas chromatograph equipped with HP-1 (15 m × 0.25 mm × 1.0 μm) column and FID detector was used with hydrogen as carrier gas and with oven temperature of 40 °C for 2 min, rising by 15 °C/min till 310 °C (for 4 min). The temperature of injector/detector was 280 °C/300 °C. Pulsed splitless injection (3 μL) was applied. In the case of VPH-determination Agilent 6890N gas chromatograph equipped with Rtx-VMS (20 m × 0.18 mm × 1.0 μm) column and FID detector was used with helium as carrier gas and with oven temperature of 40 °C for 2 min, rising by 50 °C/min till 250 °C (for 1.7 min). The temperature of injector was 250 °C. The split ratio was 10:1.
Intensification of in situ chemical oxidation
ISCO of TCE-contaminated groundwater using sodium persulfate was modelled in batch reactors. 250 mL of 1 g/L TCE spiked groundwater was placed into each glass reactors of 300 mL volume, shielded from light. Sodium persulfate was added to all soils in reaction bottles (18.1 g/L), and supplied with FeSO4 (10.6 g/L) or 5% (w/w) RAMEB or both. We compared the results to control without iron and RAMEB. The reactors were shaken by 100 rpm in the dark. Samples were withdrawn after 4 h moreover 24 h and chloride, conductivity as well as TCE concentration were measured. Chloride (Cl−) was determined by capillary electrophoresis, using a HP 3D CE instrument, equipped with silica capillary of 33 cm at 25 °C: injection at 50 mbar for 4 s, background electrolyte diamino-propane: tris:benzene-dicarboxylic acid buffer (pH 8); conductivity was determined by using WTW Multi 340i/SET. TCE concentration was measured by gas chromatography before and after soil treatment. Shimadzu 17A gas chromatograph equipped with Rtx-624 (30 m × 0.32 mm × 1.8 mm) column and FID detector was used with helium as carrier gas and with oven temperature of 60 °C for 13 min, rising by 40 °C/min till 220 °C. The temperature of injector/detector was 200 °C/220 °C. The split ratio was 10:1.
Results and discussion
In this chapter we first discuss newly developed CD-aided tools, which are used in sampling and testing the concentration or effect of contaminants, applying their results in the environmental exposure, hazard and risk assessment. In the second part of the chapter we introduce our developments on the field of reduction of risk of chemical substances in waters and soils.
CD-aided environmental risk assessment
The examples introduced here prove the suitability of CD in water- and soil-testing to modify partition of the contaminants between solid and liquid phases.
Moderate pessimistic soil toxicity tests
Goal: enhancing sensitivity of toxicity bioassays to be used in early warning and moderately pessimistic risk estimation.
Results (Fig. 2) clearly demonstrate that the testorganism Azomonas agilis shows higher sensitivity when RAMEB is added to the test-suspension: lower transformer oil concentration with RAMEB and the higher without RAMEB caused identical rate of inhibition.
The addition of 1% RAMEB shifted the partition of the transformer oil (log Kow = 5.1) toward the water phase, increasing its solubilised and bioavailable ratio as well as toxicity in soil. Contaminants of an aged pollution are strongly bound to the organic matrix of the soil. In the risk assessment it results in seemingly low risk. In reality such contaminants represent high latent risk due to mobilising potential of temperature, to other contaminants functioning as co-solvents or to increasing microbial activity in the environment. It is important, not to overestimate exaggeratedly, but also not to underestimate the risk based on test results. The moderately pessimistic models intend to fulfil the requirement of being pessimistic, but within a realistic frame.
CD-increased effect of the contaminant in the bioassay makes the risk assessment safer, it means that lower safety factors can be applied in the risk assessment methodology, and negative cases can be excluded sooner.
CD-aided atmospheric air analyses: sampling solvent-vapours from air
Goal: analyses and testing of low-concentration air and water contaminants.
Figure 3 shows the differences between the filter-bound concentrations of the air pollutants, comparing the filters of CD bound to cellulose (Cell-Epi-CD), untreated cellulose (Cell) and blank filter, treated similarly but without CD (Cell-Epi). The vapour components bound on the material of the samplers were measured in 1, 3 and 24 h after saturating them in polluted air containing 0.40 g/L TCE, 0.12 g/L toluene and 0.11 g/L ethanol vapours.
The cellulose fabric with covalently bound CD was able to concentrate the air contaminants to 3–5 fold compared to the untreated cellulose and to the “blank” sample (cellulose treated similarly with the coupling agent Epi, without CD) and 25–100 fold compared to the original air concentration. The three organic vapours had different concentrations in the air and have different affinity toward complex formation with cyclodextrin. The formation constants with BCD are as follows: 60 M−1 [17], 170 M−1 [18] and 1.2 M−1 [19] for trichloroethylene, toluene and ethanol, respectively. We can see that the higher the affinity (binding constant) the less decrease in concentration can be observed during storing the sampler. While the toluene content has not changed in the sampler during 24 h, a slight and a dramatic decrease were observed in case of TCE and ethanol, respectively.
These kinds of CD-samplers are not only integrative, but they may have special affinity to the air-pollutant to be analysed, so we can create efficient early warning and air-monitors of high-sensitivity.
CD-aided water analyses: sampling bisphenol A from water
Goal: To concentrate, possibly selectively concentrate low concentration water contaminants in the CD-containing absorptive sampler (BCDP beads).
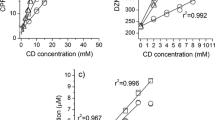
Bisphenol A (BPA) from the applied artificially contaminated water could be collected with the sampler very efficiently thanks to the high affinity of BPA to BCDP (association constant of BPA/BCD, Ka = 5800 M−1 [20]. Under the experimental conditions the CD-containing sampler was able to absorb 100% of the BPA from the water, even in 50 ppm concentration, as it is shown in Table 1. BPA concentration on the BCDP-filled sampler was 400 fold compared to water. The effect of the waters before and after the sampling on the luminescence of Vibrio fisheri is shown in Fig. 4; the 67% inhibition of 50 ppm bisphenol A decreased to 4% on the effect of filtering by the BCDP sampler.
CD-aided risk reduction by environmental remediation
The examples introduced here prove the suitability of CD in water and soil treatment, for the immobilisation from water or for the mobilisation in soil and for protecting of the ecosystem of waters and soils.
Immobilisation of contaminants on CD-filters
Nicotine contaminated model-water was decontaminated by using column-reactor filled with BCDP. This CD-filter was compared to an activated carbon filter and the mixture of them. The efficiency of the elimination of the contaminant from water was checked by a toxicity bioassay, as a fast and cheap method for screening the CD-filters.
The inhibition of the nicotine-contaminated model-water decreases on the effect of filtration with BCDP from 80 to 4% as Fig. 5 shows. The activated carbon used for comparison was not able to eliminate nicotine the only toxic component in this experiment. The mixture of BCDP and activated carbon may cause an integrated filtering effect for organic contaminants in real water, which are not specifically bound to cyclodextrins used in the filters.
Nicotine and beta-cyclodextrin are efficiently complexing with each other (Ka = 252 M−1) [21], the result is the decreased toxicity of the outflow compared to the inflow. The specific CD-filters are able to remove low concentration contaminants from drinking waters, industrial waters or waste-waters.
BTEX-removal from saturated soil by groundwater extraction and biodegradation
The technology of “pump-and-treat” for extraction and surface treatment of groundwater is one of the most popular, but not always efficient remedial technologies. Contaminant removal with the groundwater works efficiently only for those soil contaminants, which are easy to mobilize. Soil contaminants depending on their partition coefficients are distributed between soil and pore-water of the water-saturated soil or sediment. The aim of using cyclodextrins in case of high Kow contaminants is to shift partition toward water phase prior groundwater extraction from soil, in other words: to enhance the availability of the contaminants for water.
Surfactants and co-solvents may also be efficient, but not environmental-friendly, so that CD is a good alternative, in spite of its higher price.
We can see in Fig. 6 that BTEX compounds sorbed by solid phase get dissolved in the water phase (pore water, groundwater) on the effect of RAMEB. The result of this change in the partition of the soil contaminants is not only the higher concentration of the contaminant in the water-phase, but also an enhanced microbial decomposition in our case, when biodegradative activity is present. It means that in the case of biodegradable contaminants both the water-removal and the biodegradation based remediation can be intensified by RAMEB.
CD-enhanced soil remediation applying ISCO
ISCO means In Situ Chemical Oxidation of contaminants in soil or/and groundwater, without removal of the soil. Chemical degradation is an alternative of biodegradation, applicable mainly for those contaminants, which are not or very slowly biodegradable. The efficiency of chemical degradation depends on the concentration of the reactants in aqueous phase.
The results of the laboratory experiments are summarised in Fig. 7. We observed the catalytic effect of RAMEB on the chemical oxidation carried out in groundwater. Behind we suspect a complex mechanism, not only solubilisation of the contaminants, but also a kind of catalytic effect, which, together with Fe2+ accelerates in situ chemical oxidation.
Summary
The real environment is much more complex than any industrial conditions: interactions, partitions, competitions are not easily predictable. CDs may play an important role in modelling environment.
The effect of CD depends on the physical phase of the contaminant and its distribution between the physical phases of the soil. In the case of soil minimum three main phases are present, plus the biofilm and the biota itself.
Based on the results introduced here and published earlier we can summarise the conditions, which influence the effect of CD in soil:
-
the properties (Kow and Henry constant, association constant with CDs, etc.) of the contaminant
-
other substances present and the composition of mixtures
-
water content, water “activity”, dilution, and consistency (solid, dense or diluted suspension, liquid) of the sample to test
-
CD concentration.
The introduced and documented beneficial applications of CD are the following:
-
1.
CDs are promising additives for the creation of a pessimistic environmental scenario in toxicity tests ensuring safe but not exaggerated overestimates.
-
2.
CD-enhanced sampling is a promising tool for environmental monitoring by
-
selectively concentrating the pollutants,
-
increasing their mobility and bioavailability
-
increasing sensitivity of test-methods.
-
-
3.
Mobility- and availability-enhancing effect of CDs can be utilised for the intensification of remediation both in the course of physico-chemical and biological soil treatments.
-
4.
CD as a complexing agent may concentrate contaminants from water and air. CD-containing filters and sieves can industrially be applied for water treatment for the elimination of special organic contaminants from process waters, drinking waters or from surface-waters preventing by this the sensitive aquatic ecosystem.
References
Gruiz, K.: Scientific and engineering ‘improvement’ of environmental risk management by MOKKA. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 343–346. EPP Publ. Limited, Southampton (2009)
Gruiz, K.: Integrated and efficient assessment of contaminated sites. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 373–386. EPP Publ. Limited, Southampton (2009)
Gruiz, K.: Early warning and monitoring in efficient environmental management, In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 387–406. EPP Publ. Limited, Southampton (2009)
Hajdu, Cs., Gruiz, K., Fenyvesi, É.: Bioavailability- and bioaccessibility-dependent mutagenicity of pentachlorophenol. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 375–384. EPP Publ. Limited, Southampton (2009)
Hajdu, Cs., Nagy, Zs. M., Fenyvesi, É., Gruiz, K.: Cyclodextrins in environmental bioassays. J. Incl. Phenom. Macrocycl Chem. (2010). Special issue on 15th International cyclodextrin symposium (meeting date and venue: May 9–12, 2010, Vienna, Austria)
Molnár, M., Fenyvesi, É., Gruiz, K., Illés, G., Nagy, Z., Hajdu, Cs., Kánnai, P.: Laboratory testing of biodegradation in soil: a comparison of chemical and biological methods. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 497–510. EPP Publ. Limited, Southampton (2009)
Molnár, M., Leitgib, L., Gruiz, K., Fenyvesi, É., Szaniszló, N., Szejtli, J., Fava, F.: Enhanced biodegradation of transformer oil in soils with cyclodextrin—from the laboratory to the field. Biodegradation 16, 159–168 (2005)
Gruiz, K., Molnár, M., Fenyvesi, É.: Evaluation and verification of soil remediation. In: Kurladze, G.V. (ed.) Environmental Microbiology Research Trends, pp. 1–57. Nova Science Publishers Inc., NY (2008)
Molnár, M., Gruiz, K.: Interactive soil toxicity test. In: Book of Abstracts of MOKKA Conference and EURODEMO Workshop (14–15 June 2007), Budapest, BME & MOKKA, p. 40 (2007)
Gruiz, K., Fenyvesi, É., Kriston, É., Molnár, M., Horváth, B.: Potential use of cyclodextrins in soil bioremediation. J. Incl. Phenom. Macrocycl. Chem. 25, 233–236 (1996)
Molnár, M., Fenyvesi, É., Gruiz, K., Leitgib, L., Balogh, G., Murányi, A., Szejtli, J.: Effects of RAMEB on bioremediation of different soils contaminated with hydrocarbon. J. Incl. Phenom. Macrocycl. Chem. 44, 447–452 (2002)
Fenyvesi, É., Leitgib, L., Gruiz, K., Balogh, G., Murányi, A.: Demonstration of soil bioremediation technology enhanced by cyclodextrin. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, pp. 612–620. EPP Publ. Limited, Southampton (2009)
Molnár, M., Leitgib, L., Fenyvesi, É., Gruiz, K.: Development of cyclodextrin-enhanced soil remediation: from the laboratory to the field. In: Gruiz, K., Meggyes, T. (eds.) Land Contamination & Reclamation, vol. 17, pp. 601–612. EPP Publ. Limited, Southampton (2009)
Gruiz, K., Horváth, B., Molnár, M.: Environmental Toxicology. Műegyetemi Kiadó, Budapest (2001). (in Hungarian)
Sebők, Á., Vasanits-Zsigrai, A., Helenkár, A., Záray, Gy., Molnár-Perl, I.: Multiresidue analysis of pollutants as their trimethylsilyl derivatives, by gas chromatography–mass spectrometry. J. Chromatogr. A 1216(12), 2288–2301 (2009)
Leitgib, L., Kálmán, J., Gruiz, K.: Comparison of bioassays by testing whole soil and their water extract from contaminated sites. Chemosphere 66(3), 428–434 (2007)
Shirin, S., Buncel, E., vanLoon, G.W.: Enhanced solubilization of organic pollutants (TCE and PCE) through complexation by cyclodextrins. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (eds.) Environmental Chemistry, pp. 569–583. Springer-Verlag, Berlin (2005)
Szaniszló, N., Fenyvesi, É., Balla, J.: Structure-stability study of cyclodextrin complexes with selected volatile hydrocarbon contaminants of soils. J. Incl. Phenom. Macrocycl. Chem. 53(3–4), 241–248 (2005)
Buvari, A., Szejtli, J., Barcza, L.: Complexes of short-chain alcohols with beta-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 1(2), 151–157 (1983)
Wang, G., Wu, F., Zhang, X., Luo, M., Deng, N.: Enhanced TiO2 photocatalytic degradation of bisphenol A by ß-cyclodextrin in suspended solutions. J. Photochem. Photobiol. A 179, 49–56 (2005)
Berglund, J., Cedergren, L., Andersson, S.B.: Determination of the stability constant for the inclusion complex between beta-cyclodextrin and nicotine using capillary electrophoresis. Int. J. Pharm. 156(2), 195–200 (1997)
Acknowledgment
The financial support of the Hungarian R&D programs (MOKKA NKFP-3-020/2005 and CDFILTER TECH-08-4A-161) as well as the technical assistance of Norbert Lantos and Eszter Oláh are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cyclodextrin Symposium in Vienna.
Rights and permissions
About this article
Cite this article
Gruiz, K., Molnár, M., Fenyvesi, E. et al. Cyclodextrins in innovative engineering tools for risk-based environmental management. J Incl Phenom Macrocycl Chem 70, 299–306 (2011). https://doi.org/10.1007/s10847-010-9909-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9909-y