Abstract
Cyclodextrins are among the most remarkable macrocyclic molecules with significant theoretical and practical impacts in chemistry and biology. Cyclodextrins belong to the family of cage molecules due to their structure, which is composed of a hydrophobic cavity that can encapsulate other molecules. Indeed, the most characteristic feature of these compounds is their ability to form inclusion complexes with various molecules through host–guest interactions. This is at the origin of many applications. It is well known and widely reported in the literature that cyclodextrins and their derivatives have a wide variety of practical applications including pharmacy, medicine, foods, cosmetics, toiletries, catalysis, chromatography, biotechnology, nanotechnology, and textile industry. Cyclodextrins are also the object of numerous fundamental studies. In this review, we chose to highlight selected works on cyclodextrins published over the last 5 years by different research groups. The main objective is to summarize some of the recent developments related to the applications of cyclodextrins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cyclodextrins: an introduction
This article is an abridged version of the chapter published by Crini et al. (2018) in the series Environmental Chemistry for a Sustainable World.
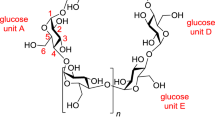
Cyclodextrins are cyclic oligomers obtained from the enzymatic degradation of starch, one of the most essential polysaccharide in the nature. Cyclodextrins make up a family of cyclic oligosaccharides. They are composed of six or more d-glucopyranoside units linked in α(1–4), like in amylose, a component of starch. Typical native cyclodextrins contain six, seven, or eight glucose units and are denoted α-, β-, and γ-cyclodextrins, respectively (Fig. 1). They are produced in a highly pure form at an industrial scale. Cyclodextrins are hollow, truncated-cone-shaped molecules made up of several glucose units linked together covalently by oxygen atoms and held in shape via hydrogen bonding between the secondary hydroxyl groups on adjacent units at the wider rim of the cavity. Indeed, cyclodextrins are ring molecules and they are toroidal or cone-shaped (Fig. 1).
β-Cyclodextrin (β-CD) is the most studied and most frequently used, based on its cheapness, availability, and complex-forming capacities toward a large range of substances. Indeed, as others cyclodextrins, the most characteristic feature of the β-CD molecule is its ability to form inclusion compounds with various substances through host–guest interactions (Fig. 2). Its central cavity which is composed of seven glucose units is hydrophobic when the external part is hydrophilic because the presence of 21 hydroxyl groups. The core of its structure can trap or encapsulate other substances. These remarkable encapsulation properties can modify and/or improve the physical, chemical, and/or biological characteristics of the guest molecule (Szejtli 1988; Morin-Crini et al. 2015).
Although they have been known for 127 years, cyclodextrins only really took off in the 1980s with the first applications in the chromatography, pharmaceutical and food industries (Crini 2014). Actually, applications are found in practically all sectors of industry (Morin-Crini et al. 2015). The literature on cyclodextrins is vast and spread across different disciplines: Chemistry, Biochemistry, Health Science, Agriculture, etc. Interesting books and book chapters on the different aspects of cyclodextrins such as the preparation, description, characterization, properties, chemistry, and derivatives for these disciplines can be consulted (Table 1). Figure 3 demonstrates how the number of publications increased using cumulative numbers of 5-year periods. In 2015, with an average of 4.5 papers and 2.5 patent applications daily, the literature data show that cyclodextrin research and development is still in the focus (Source: Cyclodextrin News, Cyclolab).
The main objective of this review is to summarize some of the recent developments related to the applications of cyclodextrins. To do this, we chose to highlight selected works on cyclodextrins published over the last 5 years by different research groups in order to have an update overview. Of course, this review is not exhaustive. Readers interested in cyclodextrin topics should refer to the library database “Cyclodextrin News” from Cyclolab, Ltd. (Hungary), which is a periodical collecting all of the cyclodextrin papers, proceedings, patents, conferences and lectures.
Cyclodextrins: an update 2012–2016—present situation, trends and outlook
Self-association of cyclodextrins
It is now well known that cyclodextrin molecules can adopt various types of assembly modes in aqueous solution as well as crystal structures (Gonzalez-Gaitano et al. 2003). New micro- and nanostructures including aggregates, formed by the self-assembly of cyclodextrins, have been useful, particularly in the fields of supramolecular chemistry, materials science, pharmacy including formulation and drug delivery, and medicine as reported by Kurkov and Loftsson (2013), Simoes et al. (2014), Ryzhakov et al. (2016), and Oliveri and Vecchio (2016).
The most important property of the cyclodextrins is the ability to establish specific interactions (molecular encapsulation) with various types of molecules through the formation of non-covalently bonded entities, either in the solid phase or in aqueous solution. However, cyclodextrins are not only able to form these host/guest inclusion complexes but also non-inclusion complexes (Gonzalez-Gaitano et al. 2003; Loftsson et al. 2004; Messner et al. 2010; Moya-Ortega et al. 2012; Kurkov and Loftsson 2013). The hydroxyl groups present on the outer surface can form hydrogen bonds with other molecules which make them able, just like dextrins, non-cyclic oligosaccharides, and polysaccharides, to form complexes (molecular structures) with lipophilic substances insoluble in water. In pure aqueous cyclodextrin solutions, CD molecules self-assemble to form nanoparticles with diameter from about 20 to 200 nm (Loftsson et al. 2004; He et al. 2008). At low concentrations (below 1% w/v), the fraction of CD molecules forming such aggregates is insignificant but the aggregation increases with increasing cyclodextrin concentration. Another possibility that is mentioned is the formation of aggregates able to dissolve water-insoluble lipophilic molecules (structures similar to micelles). The CD/drug complex aggregates are frequently from 100 to 4000 nm in diameter or in the nanoparticle and small microparticle size range. The complexes are kept together by weak hydrogen bonds and hydrophobic forces and dissociate readily upon media dilution. Such systems are called cyclodextrin nanostructures, nanoassemblies, or self-aggregates.
It has been observed, in pharmaceutical applications, that other types of cyclodextrin complexes such as non-inclusion complexes are also participating in CD solubilization of poorly soluble drugs (Bilensoy 2011; Kurkov and Loftsson 2013; Morin-Crini et al. 2015). There are some indications that formation of CD/drug complex aggregates might play an important role in CD enhancement of drug bioavailability. The cyclodextrin aggregates present the ability to form complexes, and nanosized aggregates and nanotube-type host/guest architectures can be envisaged. This is a generally unexplored domain and often causes controversies since the results obtained are closely dependent on the technique used, as pointed out by Ryzhakov et al. (2016). Another problem discussed in the literature is the stability of the cyclodextrin aggregates, in particular when these systems are used as drug delivery systems. The publications of Loftsson’s group summarized the most important features and the main conclusions of these cyclodextrin-based aggregates (Fülöp et al. 2012; Kurkov and Loftsson 2013; Ryzhakov et al. 2016; Saokham and Loftsson 2017). The use of CD-based nanoaggregates in oral drug delivery could be a promising strategy to improve the bioavailability of poorly soluble drugs. Much work is necessary to study the behavior of these nanoaggregates under conditions that are representative for the gastrointestinal tract and the effects which may cause disaggregation (Ryzhakov et al. 2016).
Cyclodextrins as drug delivery vehicles
Although cyclodextrins can be found in 56 pharmaceutical products (source: Cyclolab), they are still regarded as novel pharmaceutical excipients, drug delivery vehicles, and anti-aggregation agents (Loftsson and Brewster 2010, 2012; Bilensoy 2011; Ahuja et al. 2011; Kurkov and Loftsson 2013; Chilajwar et al. 2014; Morin-Crini et al. 2015; Jansook et al. 2018). In the last decade, as already mentioned, it has been observed that cyclodextrins and cyclodextrin complexes in particular self-assemble to form nanoparticles and that, under certain conditions, these nanoparticles can self-assemble to form microparticles, a tendency that increases upon formation of inclusion complexes with lipophilic drugs. These properties have changed the way we perform cyclodextrin pharmaceutical research and have given rise to new cyclodextrin formulation opportunities as summarized by Bilensoy (2011), and Kurkov and Loftsson (2013). The design of functional cyclodextrin nanoparticles formed by self-assembly is also a developing area in the field of nanomedicine (cancer therapy) as reported by Fülöp et al. (2012). Nanoparticle-based systems can improve bioavailability, reduce immunogenicity, modify drug metabolism, reduce toxicity, and increase the biological half-life of drugs after systemic administration (Bilensoy 2011; Fülöp et al. 2012; Kurkov and Loftsson 2013; Tejashri et al. 2013; Concheiro and Alvarez-Lorenzo 2013; Simoes et al. 2014; Morin-Crini et al. 2015; Sharma and Baldi 2016).
Oliveri and Vecchio (2016) reviewed the use of cyclodextrins and their derivatives as anti-aggregation agents in a number of proteins such as insulin, prion protein, and amyloid-beta, and some multimeric enzymes. There are many diseases that are correlated to protein misfolding and amyloid formation processes affecting numerous organs and tissues. There are over 30 different amyloid proteins and a number of corresponding diseases including Alzheimer’s diseases the most common neurodegenerative disease. Treatment of these diseases is still a goal to reach, and many molecules including cyclodextrins were studied in this perspective.
Actually, cyclodextrins are also perceived as dream molecules for the development of applications in biomedicine and nanomedicine including nanovectorization, such as, for instance, nanoparticles for drug delivery, innovative biosensors for detection of biological targets, biorecognition events, molecular diagnosis and medical imaging, gene therapy or tissue engineering (Concheiro and Alvarez-Lorenzo 2013; Zhang and Ma 2013; Tan et al. 2014; Simoes et al. 2014; Dong et al. 2015; Wu et al. 2015b; Yuan and Zhang 2016).
Medical devices including catheters, prosthesis, vascular grafts, and bone implants can also benefit from surface grafting or thermofixation of cyclodextrins. This explains the recent increase in the number of research papers dealing with these topics (Concheiro and Alvarez-Lorenzo 2013). However, most of these studies are in the proof-of-concept stage, and only a few therapeutic nanosystems have been comprehensively investigated. The successful translation of these laboratory innovations to clinical reality remains challenging.
A new generation of drug eluting stents based on the strong anchorage of a biocompatible and bioresorbable cyclodextrin-based polymer onto metallic devices has been elaborated (Perez-Anes et al. 2015). Polydopamine, a strong adhesive polymer, was applied as a first coated layer. Cyclodextrin was fixed by in situ polycondensation with citric acid (polycyclodextrins formation). As a third layer an amine-rich polymer, polyethyleneimine, was used to stabilize the anionic cyclodextrin layer. As an alternative polycyclodextrins and chitosan layers were applied using layer-by-layer deposition technique.
Functionalized cyclodextrins are interesting scaffolds for contrast agents used in magnetic resonance imaging as reported by Gouhier’s group (Idriss et al. 2013; Zgani et al. 2017). For the further improvement of the sensitivity of this medical diagnostic tool, it is necessary to fully understand the role of the cyclodextrins in the efficiency of the contrast agents. The review by Mavridis and Yannakopoulou (2015) can be also consulted on the same topic.
Cyclodextrins as active ingredients
Currently, the field of medicine is closely concerned with cyclodextrin inclusion complexes. These complexes are preformed prior to be administrated. There are, however, some cases when the complexes are formed within the body. The best-known example is the one containing the active compound sugammadex (Bridion®): it is a modified γ-CD used as an antidote to certain curare-like muscle relaxants in anesthesia since 2008 (Booij 2009; Yang and Keam 2009). After intravenous administration, it neutralizes steroid curare-like agents (rocuronium, vecuronium) by forming an inactive complex in the plasma which is then eliminated in the urine. Sugammadex has improved effectiveness compared with currently available methods of accelerating reversal of neuromuscular blockade. Its mechanism of action also differs from that of other commonly used reversal agents, e.g., neostigmine and edrophonium. Sugammadex is biologically inactive, does not bind to plasma proteins, and appears to be safe and well tolerated although it has few side effects. Its cost is markedly higher than that of any of the other drugs used in anesthesia (Donati 2011). The literature data are actually abundant with a matter of considerable debate. Sugammadex shows an unexpected rise from 17 papers in 2000–2005 to 780 in 2010–2015 (Source: Cyclodextrin News, Cyclolab).
Application of hydroxypropyl-β-cyclodextrin (HP-β-CD) against Niemann–Pick C disease (NPC) started with a surprising observation in 2007 that this cyclodextrin aimed as excipient was more effective than allopregnanolone, the active (Walkley et al. 2016). As no alternative treatment existed at that time the NPC1 disease families pushed FDA for approval for individual access for their children and as a result of a large collaborative work in all areas of drug development, including chemistry and manufacturing, formulation, pharmacology, pharmacokinetics, toxicology, and regulatory affairs, the preclinical clinical Phase 1 and Phase 2 studies have been concluded to start Phase 3 studies in 2015. In the meantime, the research focuses on the still not completely understood mechanism how HP-β-CD can help to NPC patients (Tanaka et al. 2015; Davidson et al. 2016).
Cyclodextrins are used in autophagy, a catabolic process with an essential function in the maintenance of cellular and tissue homeostasis. It is primarily recognized for its role in the degradation of dysfunctional proteins and unwanted organelles. Actually, the range of substrates also includes lipids. Autophagy is a self-degradative process that is important for balancing sources of energy at critical times in development and in response to nutrient stress. It also plays a housekeeping role in removing misfolded or aggregated proteins, clearing damaged organelles, such as mitochondria, endoplasmic reticulum and peroxisomes, as well as eliminating intracellular pathogens. Thus, autophagy is generally thought of as a survival mechanism, although its deregulation has been linked to non-apoptotic cell death (Glick et al. 2010). As cellular membranes play important role in autophagy, their modulation by cyclodextrins modifies this important housekeeping process of the cells. However, the results of various studies seem to be controversial partly because different cell types, different cyclodextrins were used at different concentrations. The accumulation of autophagosomes, the intermediary products of autophagy, was interpreted both as a sign of activated and impaired autophagy. Hydroxypropyl-β-cyclodextrin treatment at high concentration leads to cholesterol depletion in an extent which leads to hindered fusion of the cellular membranes and this way to the diminished fusion of autophagosomes with lysosomes or to reduced expulsion of the autophagolysosomes. At low HP-β-CD concentrations, however, the autophagy is not perturbed or might be even improved. A combination of cholesterol removal by HP-β-CD and autophagy stimulation by rapamycin or carbamazepine seems to be a promising strategy in the treatment of impaired autophagy in lysosomal storage disorders, such as Niemann–Pick type C disease (Maetzel et al. 2014; Ward et al. 2016). Other interesting works have been published on cyclodextrin role (Yokoo et al. 2015; Tamura and Yui 2015; Motoyama et al. 2015, 2016; Manchon et al. 2016). Hydroxypropyl-β-cyclodextrin was found effective in inhibition of leukemic cell proliferation at various leukemic cell lines as recently reported by Arima’s group (Yokoo et al. 2015). Their results demonstrated that hydroxypropyl-β-cyclodextrin was a potential anticancer agent in leukemia. Further studies are needed to understand the effect of cyclodextrins on autophagy and specially to learn how these effects can be utilized in the therapy of various illnesses including neurodegenerative diseases and cancers.
Cyclodextrins are also used for prevention and treatment of atherosclerosis as recently reported by Zimmer et al. (2016). These authors published a comprehensively study on the anti-atherosclerotic and anti-inflammatory effects of hydroxypropyl-β-cyclodextrin used as solubilizing agent to increase cholesterol solubility.
A recent review on the effect of cyclodextrins on blood brain barrier summarizes the findings of several research groups on various animal models of the central nervous system diseases (Vecsernyés et al. 2014). The CD-mediated cholesterol modulations change the action of various proteins in the membrane of epithelial cells in blood brain barrier. These proteins play significant role in pathogenesis of stroke, cerebral hypoxia and ischemia, Alzheimer, Parkinson and Huntington disease, epilepsia, central nervous system infections, and brain tumor. Extensive research is going on to translate these effects into therapy.
The alarming spread of bacterial resistance to traditional antibiotics has warranted the study of alternative antimicrobial agents. Quorum sensing is a chemical cell-to-cell communication mechanism utilized by bacteria to coordinate group behaviors and establish infections (Hirakawa and Tomita 2013; Morohoshi et al. 2013; Miller et al. 2015; Okano et al. 2016). It is known that cyclodextrins can interact with N-acyl-L-homoserine lactones (AHLs), the main signaling molecules for the bacterial cell-to-cell communication quorum sensing system. Many bacteria regulate their cooperative activities through releasing, sensing, and responding to small signaling molecules. This mechanism called quorum sensing makes possible for a population of bacteria to behave as a multi-cellular organism in host colonization, formation of biofilms, defense against competitors, and adaptation to changing environment. Quorum sensing is a new target for the development of antibiotic agents.
The concept of complexation of signal molecules (AHLs or peptides) by cyclodextrins does not aim to kill the bacteria but to control their growth and to decrease their virulence (Hirakawa and Tomita 2013). The development of novel strategies in the prevention and treatment of biofilm infections are expected in the next years. This will be useful not only in medicine but also in cosmetic, textile, and packaging fields (Brackman et al. 2016; Silva et al. 2016).
Cyclodextrins and nanotechnology
Cyclodextrins and their derivatives have been successfully employed to create novel nanomaterials, often called nanosponges (Bilensoy and Hincal 2009; Goyal et al. 2011; Davis and Higson 2011, Tejashri et al. 2013, Chilajwar et al. 2014; Trotta et al. 2014, 2015; Mavridis and Yannakopoulou 2015). A broad spectrum of cyclodextrin-containing materials with versatile supramolecular architectures such as nanoparticles, nanosponges, nanomicelles, and nanovesicles has been synthesized to assemble functional platforms. For pharmaceutical and biomedical applications (Bilensoy and Hincal 2009; Goyal et al. 2011), nanomaterials can be formulated as oral, parenteral, topical or inhalation dosage forms. These materials have also found applications in nanomedicine. Cyclodextrin-based nanosponges have been also developed as tool for the delivery of anticancer drugs, e.g., paclitaxel, doxorubicin, 5-fluorourcil, and tamoxifen, as reported by Trotta et al. (2014, 2015). These innovative materials can be considered as a challenging technology for the development of innovative formulations, suitable for various administration routes for anticancer drugs.
Cyclodextrins and foods
In recent years, the growth of the functional foods industry has increased research into new compounds with high added value for use in the fortification of traditional products (Martina et al. 2013; Lopez-Nicolas et al. 2014; Calo et al. 2015; Sharma and Baldi 2016; Kfoury et al. 2016; Fenyvesi and Szente 2016; Zarzycki et al. 2016). One of the most promising functional food groups is those enriched in antioxidant compounds of a lipophilic nature. In spite of the numerous advantages reported for such antioxidant molecules, they may also have disadvantages that impede their use in functional foods, although these problems may be avoided by the use of encapsulant agents such as cyclodextrins (Fenyvesi et al. 2016).
An excellent review of the most recent studies on the complexes formed between several important types of antioxidant compounds and cyclodextrins was published by López-Nicolás et al. (2014). This comprehensive review focuses on the contradictory data reported in the literature concerning the antioxidant activity of the host/guest molecule complexes, the different complexation constants reported for identical complexes, the bioavailability of the antioxidant compound in the presence of cyclodextrins, and the recommendations concerning the use of natural or modified cyclodextrins. The authors also concluded that cyclodextrins will act as secondary antioxidants, enhancing the ability of traditional antioxidants to prevent enzymatic browning in different foods. Another interesting review on the encapsulation of antioxidants such as flavonoids and phenolic acids and their applications in food products, food supplements, and also packaging has been published by Zarzycki et al. (2016).
A general overview on the nanoencapsulation of flavors and aromas was also given in a chapter published by Fenyvesi and Szente (2016). Starting with the history dating back to the sixties of the last century, the advantages were summarized, the approval status of cyclodextrins in food was evaluated, and the methods of preparation and analysis were shortly outlined. Flavor complexes in food processing to reduce the loss in color, odor, and taste were revealed. Several examples were given to illustrate the advantages of nanoencapsulation of flavors in aroma preserving food packaging.
Cyclodextrins and cosmetics
Encapsulation techniques using cyclodextrins as (nano)-encapsulating agents are increasingly used not only by food, aroma, and pharmaceutical industries but also by cosmetic, fragrance and flavor industries, toiletry and personal care sectors for improving the efficiency of odorant and aroma substances, odor control in perfumes, masking unpleasant smells and tastes of some compounds, improving the physical and/or stability of essential oils and volatile compounds, stabilizing volatiles by reducing or eliminating any losses through evaporation, modifying the physicochemical and/or biological properties of the guest to afford a protective effect, or transforming of liquid compounds into crystalline form (Bilensoy 2011; Hougier and Kircik 2012; Kfoury et al. 2016; Zarzycki et al. 2016; Fenyvesi and Szente, 2016). The fragrance and flavor industry is a large and innovate sector of the chemical industry. Fragrance chemicals are added to consumer products such as personal care products, perfumes, deodorants, laundry detergents. Encapsulation techniques using cyclodextrins are increasingly used by this industry for protecting fragile molecules (eye-drop solutions) and improving the efficiency of odorant and aroma substances, but also to avoid the degradation of flavors by processing or, on storage, allowing the use of minor amounts of flavors. The use of these host compounds is also promising for various emerging fields such as aromatherapy and cosmeto-textiles—these new products also called ‘cosmeceuticals’ (Bilensoy 2011).
Cyclodextrins and textiles
Cyclodextrin molecules can be an ecofriendly alternative and used as finishing agents in textile applications. In this sector, their main characteristics are: ecofriendly nature, cost-effectiveness and ease of production at large-scale, physicochemical (e.g., inclusion complex-forming ability, solubilizing activity, chelating activity, slow release of fragrances) and biological (e.g., biocompatibility, biodegradability, drug carrier ability, insecticidal delivery) properties. These properties can be applied to different areas of applications such as deodorant (odor absorption, stabilization of active ingredients), fragrance/aroma, UV protection, water resistance, antimicrobial resistance, flame retardancy, and also insect repellent. In general, cyclodextrins are grafted in materials using binding and cross-linking agents. The comprehensive reviews of Islam et al. (2013) and Voncina and Vivod (2013), and the previous works by Hebeish and El-Hilw (2001), Martel et al. (2002), Romi et al. (2005), and Abdel-Halim et al. (2010) can be consulted. Cyclodextrin is considered as a promising reagent in textile finishing. Cosmeto-textiles also meet an increasing demand on the market and the cosmetic and textile industries are on the forefront of the research on this topic. These innovative materials are increasingly used not only in these sectors but also in pharmaceutical and medical industries, and in food packaging. Development of textiles with an antimicrobial activity can be also useful for water and air treatment.
Cyclodextrins and separation techniques
Although an important number of works have been published since the 1980s, the sector of cyclodextrin-based chromatography and electrophoresis continues to interest the scientific community (Hongdeng et al. 2011; Xiao et al. 2012; West 2014; Scriba 2016). The number of publications continues to grow not only in high performance liquid chromatography, ultra-high performance liquid chromatography, capillary chromatography, and gas chromatography, but also in supercritical fluid chromatography. Actually, liquid chromatography is considered as a green separation technique, as it avoids the use of organic mobile phase and is an ideal alternative technique with fast and efficient separation for the preparation and separation of pure substances, e.g., enantioselective separation, chiral extraction).
Scriba (2016) published an interesting update review on the contributions to the understanding of the binding mechanism between chiral selectors and cyclodextrins in analytical enantioseparations dating between 2012 and early 2016. The author showed that many tools are available nowadays to study the mechanism of enantiorecognition including spectroscopic techniques (NMR) as well as molecular modeling for the visualization and analysis of the dynamics of the process. Selectors such as cyclodextrins appeared advantageous due to the much broader range of applications for structurally diverse analytes. Either random substituted cyclodextrins, which are mixtures of isomers with similar structure, or single isomer cyclodextrins are used. This high versatility makes cyclodextrins the first-choice selectors. The single isomers have the advantage of uniform structure, while the random substituted cyclodextrins often suffer from the batch-to-batch reproducibility (Li and Vigh 2004; Benkovics et al. 2016).
Cyclodextrins and catalysis
Cyclodextrins have long been known to be good contributors to the development of catalytic processes. They have been used for mass-transfer catalytic reactions and for the production of new catalysts mimicking enzymatic activity (Macaev and Boldescu 2015; Hong et al. 2015). The following classification for catalytic applications of cyclodextrins was proposed by Monflier’s group (Hapiot et al. 2006, 2011, 2014): (1) to significantly increase the rate and selectivity of reactions catalyzed by water-soluble organometallic complexes; (2) to design new water-soluble ligands for aqueous organometallic catalysis; (3) to stabilize catalytically active noble metal nanoparticles in water; and (4) to facilitate reactions catalyzed by supported metals or metallic powder in water. Cyclodextrins were used in organic synthesis as promoters or catalysts of different reactions, as components of artificial enzymatic systems, as stabilizers for the nanodimensional metallic catalysts. A comprehensive collection of recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis can be found in the review by Hapiot et al. (2014).
Macaev and Boldescu (2015) published a state of the art of cyclodextrins in asymmetric and stereospecific synthesis. Three topics were summarized: (1) cyclodextrins’ complexes with transition metals as asymmetric and stereospecific catalysts; (2) cyclodextrins’ non-metallic derivatives as asymmetric and stereospecific catalysts; and (3) cyclodextrins promoting asymmetric and stereospecific catalysis in water. The authors concluded that cyclodextrins and their derivatives can be a feasible alternative to traditional catalysts in a variety of reactions.
Cyclodextrin-based supramolecular architectures
The state-of-the-art reviews of the design of complex macromolecular architectures based on cyclodextrin were presented by Schmidt et al. (2014) and Dong et al. (2015) and comprehensively discussed. In particular, by an elegant combination of dynamic/reversible structures with exceptional functions, functional supramolecular polymers are attracting increasing attention in various fields such as biomedical, e.g., gene transfection, protein delivery, bioimaging and diagnosis, tissue engineering, and biomimetic chemistry, and material science (polymer science, nanotechnology). Although extensive work has been done on cyclodextrin-based supramolecular architectures, future research needs to take into account their precise physicochemical characterization (Dong et al. 2011, 2015; Schmidt et al. 2014; Agostoni et al. 2015; Karim and Loh 2016; Gontero et al. 2017; Valetti et al. 2017). Indeed, the understanding and the design of supramolecular systems require a detailed characterization with respect to stoichiometry, affinity, structure, heterogeneity, and supramolecular dynamics.
Cyclodextrins and sugar-based surfactants
Carbohydrate-based surfactants are today an important class of amphiphilic compounds which play an important role with both fundamental and practical applications. The growing interest in such compounds is due to, inter alia, their preparation from renewable raw materials, their ready biodegradability and biocompatibility, as well as other more basic reasons of practical, economic, and environmental order. When complexed with cyclodextrins, carbohydrate-based surfactants considerably increase their performance and potential application range (Dodziuk 2006; Villalonga et al. 2007; Li et al. 2011). The formation of CD/surfactant host–guest compounds leads to an increase in the critical micelle concentration and in the solubility of surfactants. The use of these new systems is promising as reported by Valente and Söderman (2014).
Cyclodextrins and click chemistry
Click chemistry describes a family of modular, efficient, versatile, and reliable reactions which have acquired a pivotal role as one of the most useful synthetic tools for functionalization of cyclodextrins with a potentially broad range of applications (Faugeras et al. 2012; Dondoni and Marra 2012; Schmidt et al. 2014; Hou et al. 2016). Cyclodextrins modified by the click reaction are building blocks of superstructures used for drug delivery systems (Zhou and Ritter 2010; Nielsen et al. 2010), for the modification of macromolecular surfaces (Celebioglu et al. 2014a), for the preparation of cyclodextrin dimers and trimers (Tungala et al. 2013), and for the generation of various glycoconjugates (glycopeptides, glycodendrimers, etc.) (Kushwaha et al. 2013). A tutorial review with the development in thiol-ene coupling for peptide glycosylation was published by Dondoni and Marra (2012). Cyclodextrins will play a very important role in all these new developments.
Cyclodextrins and agrochemistry
An interesting sector for cyclodextrins is agrochemistry (Ho et al. 2014; Garrido et al. 2014; Fernandes et al. 2014; Campos et al. 2015; Yusoff et al. 2016). Indeed, the efficacy of cyclodextrins for pesticide formulations has been evaluated. Cyclodextrins can effectively encapsulate or bind the pesticide’s active ingredients in the material’s matrix with a sustained release profile and slow mobility in soil (Yusoff et al. 2016). Cyclodextrin molecules form complexes with a wide variety of agricultural substances such as insecticides, fungicides, herbicides, repellents, pheromones, and growth regulators (Luca and Grigoriu 2007; Venturini et al. 2008). This is at the origin of many benefits: modification of the physicochemical properties of the included guest, i.e., physical state, stability, solubility, and bioavailability, stabilization against the effects of light or biochemical degradation, and reduction of volatility. All these benefits are interesting during the preparation of the commercial formulations. It is important to note that most of the pesticides-CD inclusion complexes studied in the literature have used β-CD because of its lower price, and most of the published papers related to pesticides are not directly practice-oriented (Garrido et al. 2014; Fernandes et al. 2014).
Electrospinning of functional nanofibers with cyclodextrins
Electrospinning is one of the most useful techniques for nanofibers production due to its versatility and cost-effectiveness. Electrospinning of functional nanofibers with cyclodextrin molecules was proposed, and an important contribution was made by Uyar’s group on this topic (Celebioglu and Uyar 2012, 2013a, b; Kayaci and Uyar 2012, 2014; Kayaci et al. 2013a, b, c, 2014, 2015; Celebioglu et al. 2014a, b, 2016; Keskin et al. 2015a, b; Aytac et al. 2015, 2016a, b; Aytac and Uyar 2016, 2017; Senthamizhan et al. 2016). Functional nanofibers incorporating cyclodextrin were developed via electrospinning using different polymers and reactions (cross-linking, grafting, click chemistry). Cyclodextrin molecules were also proposed to produce nanofibers without using any polymer carrier because cyclodextrin can form aggregates via intermolecular hydrogen bonding in their concentrated solutions or polymeric structures through cross-linked reactions. Various applications were target of including functional materials for textile and medical textile, drug delivery, control release of antibacterials, packaging and food applications, complexation and microencapsulation (essential oils, antioxidants), and environmental purposes (e.g., filtration, purification and separation processes).
Cyclodextrins and remediation
In the last two decades, cyclodextrins as complexing agents have attracted considerable attention in environmental science in terms of removal of pollutants present in all environmental compartments, soils, air, waters and wastewaters, and sediments.
Soil flushing using cyclodextrin-based aqueous solutions was employed to solubilize pollutants. Cyclodextrin molecules are used as additives to enhance efficiencies and reduce the treatment time compared to the use of water alone or conventional surfactants. The review by Atteia et al. (2013) can be consulted on this topic.
Cyclodextrins found their role in the environmental risk management. Both risk assessment and risk reduction technologies may benefit from the CD’s ability of forming inclusion complexes with the typical organic contaminants in soils as reported by Gruiz et al. (2011). The solubility enhancement of these compounds of usually poor water solubility can be utilized. Extraction of soils with aqueous solutions of well soluble cyclodextrin derivatives (HP-β-CD or RAMEB, randomly methylated-β-CD) gives information on the contaminant fraction easy to mobilize. As this fraction is the most available for the soil microbes, extracting the soil with HP-β-CD solution is considered as a measure of the bioavailable, biodegradable fraction. Nowadays, this method has become a part of the everyday protocol to predict the microbial bioavailability of organic pollutants of soils.
Cyclodextrin-based materials for water and wastewater treatment include cross-linked polymers and nanosponges, membranes, nanofibers, and functionalized systems such as polymers, silica, and organic resins. Morin-Crini and Crini (2013) reviewed the developments in the use of cross-linked cyclodextrin-based polymers as complexing polymeric matrices for pollutant removal by oriented-adsorption processes. The authors summarized the features of these polymers and how they were used in decontamination applications. Numerous interesting studies on the treatment of real effluents can be found in the literature (Nagy et al. 2014; Khaoulani et al. 2015; Morin-Crini et al. 2015; Charles et al. 2016; Euvrard et al. 2016; Alsbaiee et al. 2016).
Imprinting technology makes possible to develop sorbents with extremely high specificity (Lay et al. 2016). Cyclodextrin-containing molecular imprinting polymers and cyclodextrins grafted to the surface of silica or other support via imprinting technology offer multiple binding sites with tunable surface properties for sorption of selected chemicals, e.g., perfluorinated compounds (Karoyo and Wilson 2015).
Nanofibers containing cyclodextrin molecules are another interesting material for pollutant removal. All the results published by Uyar’s group demonstrated that these new materials may have high potential to be used as air filters for the removal of organic vapor wastes, adsorbents/filters for pollutant removal from aqueous solutions, and also drug delivery system.
There are several other interesting works for synthesizing membranes and nanofibers containing cyclodextrins (Meng et al. 2014; Xiao et al. 2014; Ghemati and Aliouche 2014; Zhao et al. 2015; Zhang et al. 2015; Wu et al. 2015a; Norena-Caro and Alvarez-Lainez 2016; Wei et al. 2016; Costoya et al. 2017). For example, Norena-Caro and Alvarez-Lainez (2016) proposed two different methods for the production of electrospun polyacrylonitrile (PAN) nanofibers containing cyclodextrin capable of capturing formaldehyde, a common indoor pollutant. The former comprised the addition of cyclodextrins to PAN/dimethyl sulfoxide solutions and the subsequent electrospinning of the mixture. The latter involved the cross-linking of cyclodextrins on electrospun PAN fibers by alkaline hydrolysis and esterification with citric acid. The results showed that both functionalized nanofibers might be used for indoor air purification. However, functionalized fibers obtained by addition of cyclodextrins were more effective for capturing formaldehyde than fibers obtained by cross-linking of cyclodextrins.
Cyclodextrin-functionalized silica networks used as adsorbents/filters for environmental applications has recently received a lot of attention as reported by Gibson (2014), Samiey et al. (2014), Lee and Park (2015), Dinker and Kulkarni (2015), Vunain et al. (2016), Mahmud and Wilson (2016), and Yamamoto and Kuroda (2016). Two main classes of materials have been proposed: cyclodextrin-functionalized silicas prepared through grafting reactions and cyclodextrin-silica hybrid systems prepared through sol–gel or self-assembly process. These materials could be applied in the elimination, enrichment and detection of environmental pollutants in air and water samples.
Actually, fundamental research is also focusing on cyclodextrin-based nanoparticles for environmental applications (Taka et al. 2017). Indeed, nanosponges have not only been explored for their pharmaceutical applications but for water purification and wastewater treatment (Landy et al. 2012; Tong and Chen 2013; Taka et al. 2017). This emerging technology of cyclodextrin-based nanosponges is expected to provide technical solutions to water treatment.
Conclusion
This review gives an overview of recent selected works on cyclodextrins used in various fields. The increasing number of publications on cyclodextrins shows that there is always an increasing interest in these molecules and their applications from academic and practical point of views. The emergence of cyclodextrins as active and smart molecules rather than complexing molecules in numerous cosmetic, textile, therapeutic, and biomedical products seems to be the next step in the development of cyclodextrin technology. Further studies, contributions, and industrial developments are expected in the near future in the following domains: catalysis, bacterial resistance, anticancer drugs (chemotherapy), magnetic resonance imaging, biotechnology, e.g., biotransformation, fermentation processing, enzyme models, peptide and protein delivery, material science, e.g., wrapping materials and packaging, cosmeto-textiles, agrochemistry, and sensor applications.
References
Abdel-Halim ES, Fouda MMG, Hamdy I, Abdel-Mohdy FA, El-Sawy SM (2010) Incorporation of chlorohexidin diacetate into cotton fabrics grafted with glycidyl methacrylate and cyclodextrin. Carbohydr Polym 79:47–53. https://doi.org/10.1016/j.carbpol.2009.07.050
Agostoni V, Horcajada P, Noiray M, Malanga M, Aykaç A, Jicsinszky L, Vargas-Berenguel A, Semiramoth N, Daoud-Mahammed S, Nicolas V, Martineau C, Taulelle F, Vigneron J, Etcheberry F, Serre C, Gref R (2015) A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci Rep 5:7925. https://doi.org/10.1038/srep07925
Ahuja A, Baboota S, Ali J, Mustafa G (2011) Cyclodextrins as potential excipients in pharmaceutical formulations: Solubilizing and stabilizing effects. In: Bilensoy E (ed) Cyclodextrins in pharmaceutics, cosmetics and biomedicine. Current and future industrial. Wiley, Hoboken, pp 19–43. https://doi.org/10.1002/9780470926819
Alsbaiee A, Smith BJ, Xiao L, Ling YH, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 529:190. https://doi.org/10.1038/nature16185
Atteia O, Estrada ED, Bertin H (2013) Soil flushing: a review of the origin of efficiency variability. Rev Environ Sci Bio Technol 12:379–389. https://doi.org/10.1007/s11157-013-9316-0
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry. Marcel Dekker Inc, New York
Atwood JL, Davies EE, MacNicol DD (1984) Inclusion compounds. Academic, Michigan
Aytac Z, Uyar T (2016) Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur Polym J 79:140–149. https://doi.org/10.1016/j.eurpolymj.2016.04.029
Aytac Z, Uyar T (2017) Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: enhanced water solubility and slow release of curcumin. Int J Pharm 518:177–184. https://doi.org/10.1016/j.ijpharm.2016.12.061
Aytac Z, Sen HS, Durgun E, Uyar T (2015) Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B Biointerf 128:331–338. https://doi.org/10.1016/j.colsurfb.2015.02.019
Aytac Z, Yildiz ZI, Kayaci F, San NO, Kusku SI, Durgun E, Tekinay T, Uyar T (2016a) Fast-dissolving, prolonged release and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J Agric Food Chem 64:7325–7334. https://doi.org/10.1021/acs.jafc.6b02632
Aytac Z, Yildiz ZI, Kayaci F, San NO, Tekinay T, Uyar T (2016b) Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv 6:46089–46099. https://doi.org/10.1039/C6RA07088D
Bender ML, Komiyama M (1978) Cyclodextrins chemistry. Springer, Berlin
Benkovics G, Fejős I, Darcsi A, Varga E, Malanga M, Fenyvesi É, Sohajda T, Szente L, Sz Béni, Szemán J (2016) Single-isomer carboxymethyl-γ-cyclodextrin as chiral resolving agent for capillary electrophoresis. J Chromatog A 1467:445–453. https://doi.org/10.1016/j.chroma.2016.06.083
Bilensoy E (2011) Cyclodextrins in pharmaceutics, cosmetics and biomedicine. Current and future industrial applications. Wiley, Hoboken. https://doi.org/10.1002/9780470926819
Bilensoy E, Hincal AA (2009) Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opinion Drug Delivery 6:1161–1173. https://doi.org/10.1517/17425240903222218
Booij LHDJ (2009) Cyclodextrins and the emergence of sugammadex. Anaesthesia 64:31–37. https://doi.org/10.1111/j.1365-2044.2008.05868.x
Brackman G, Garcia-Fernandez MJ, Lenoir J, De Meyer L, Remon JP, De Beer T, Concheiro A, Alvarez-Lorenzo C, Coenye T (2016) Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosc 16:859–869. https://doi.org/10.1002/mabi.201500437
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems - A review. Food Control 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Campos EVR, de Oliveira JL, Fraceto LF, Singh B (2015) Polysaccharides as safer release systems for agrochemicals. Agron Sustain Dev 35:47–66. https://doi.org/10.1007/s13593-014-0263-0
Celebioglu A, Uyar T (2012) Electrospinning of nanofibers from non-polymeric systems: polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 4:621–631. https://doi.org/10.1039/c1nr11364j
Celebioglu A, Uyar T (2013a) Electrospinning of nanofibers from non-polymeric systems: electrospun nanofibers from native cyclodextrins. J Colloid Int Sci 404:1–7. https://doi.org/10.1016/j.jcis.2013.04.034
Celebioglu A, Uyar T (2013b) Electrospun gamma-cyclodextrin (gamma-CD) nanofibers for the entrapment of volatile organic compounds. RSC Adv 3(45):22891–22895. https://doi.org/10.1039/C3RA44870C
Celebioglu A, Demirci S, Uyar T (2014a) Cyclodextrin-grafted electrospun cellulose acetate nanofibers via click reaction for removal of phenanthrene. Appl Surf Sci 305:581–588. https://doi.org/10.1016/j.apsusc.2014.03.138
Celebioglu A, Umu OCO, Tekinay T, Uyar T (2014b) Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloids Surf B Biointerf 116:612–619. https://doi.org/10.1016/j.colsurfb.2013.10.029
Celebioglu A, Sen HS, Durgun E, Uyar T (2016) Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemosphere 144:736–744. https://doi.org/10.1016/j.chemosphere.2015.09.029
Charles J, Bradu C, Morin-Crini N, Sancey B, Winterton P, Torri G, Badot PM, Crini G (2016) Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: chemical abatement. J Saudi Chem Soc 20:185–194. https://doi.org/10.1016/j.jscs.2013.03.007
Chilajwar SV, Pednekar PP, Jadhav KR, Gupta GJC, Kadam VJ (2014) Cyclodextrin-based nanosponges: a propitious platform for enhancing drug delivery. Expert Opin Drug Deliv 11:111–120. https://doi.org/10.1517/17425247.2014.865013
Concheiro A, Alvarez-Lorenzo C (2013) Chemically cross-linked and grafted cyclodextrin hydrogels: from nanostructures to drug-eluting medical devices. Adv Drug Deliv Rev 65:1188–1203. https://doi.org/10.1016/j.addr.2013.04.015
Costoya A, Concheiro A, Alvarez-Lorenzo C (2017) Electrospun fibers of cyclodextrins and poly(cyclodextrins). Molecules 22:230. https://doi.org/10.3390/molecules22020230
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. https://doi.org/10.1021/cr500081p
Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N (2018) Fundamentals and applications of cyclodextrins. In: Fourmentin S, Crini G, Lichtfouse E (eds) Environmental chemistry for a sustainable world, vol 1. Springer, Berlin, pp 1–56. https://doi.org/10.1007/978-3-319-76159-6_1
Davidson CD, Fishman YI, Puskás I, Szemán J, Sohajda T, McCauliff LA, Sikora J, Storch J, Vanier MT, Szente L, Walkley SU, Dobrenis K (2016) Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann Clin Transl Neuro 3:366–380. https://doi.org/10.1002/acn3.306
Davis F, Higson S (2011) Cyclodextrins. In: Davis F, Higson S (eds) Macrocycles: construction, chemistry and nanotechnology applications. Wiley, New York
Dinker MK, Kulkarni PS (2015) Recent advances in silica-based materials for the removal of hexavalent chromium: a review. J Chem Eng Data 60:2521–2540. https://doi.org/10.1021/acs.jced.5b00292
Dodziuk H (2002) Introduction to supramolecular chemistry. Dordrecht: Kluwer Academic Publishers. ISBN: 0-306-47587-1
Dodziuk H (2006) Cyclodextrins and their complexes. Chemistry, analytical methods, applications. Wiley, Weinheim. https://doi.org/10.1002/3527608982
Donati F (2011) Sugammadex: a cyclodextrin-based novel formulation and marketing story. In: Bilensoy E (ed) Cyclodextrins in pharmaceutics, cosmetics and biomedicine: current and future industrial applications. Wiley, Hoboken, pp 363–370. https://doi.org/10.1002/9780470926819
Dondoni A, Marra A (2012) Recent applications of thiol-ene coupling as a click process for glycoconjugation. Chem Soc Rev 41:573–586. https://doi.org/10.1039/c1cs15157f
Dong HQ, Li YY, Li L, Shi DL (2011) Cyclodextrins/polymer based (pseudo)polyrotaxanes for biomedical applications. Prog Chem 23:914–922
Dong RJ, Zhou YF, Huang XH, Zhu XY, Lu YF, Shen J (2015) Functional supramolecular polymers for biomedical applications. Adv Mat 27:498–526. https://doi.org/10.1002/adma.201402975
Duchêne D (ed) (1987) Cyclodextrins and their industrial uses. Éditions de Santé, Paris
Duchêne D (ed) (1991) New trends in cyclodextrins and derivatives. Éditions de Santé, Paris
Euvrard E, Morin-Crini N, Druart C, Bugnet J, Martel B, Cosentino C, Moutarlier V, Crini G (2016) Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein J Org Chem 12:1826–1838. https://doi.org/10.3762/bjoc.12.172
Faugeras PA, Boens B, Elchinger PH, Brouillette F, Montplaisir D, Zerrouki R, Lucas R (2012) When cyclodextrins meet click chemistry. Eur J Org Chem 22:4087–4105. https://doi.org/10.1002/ejoc.201200013
Fenyvesi É, Szente (2016) Nanoencapsulation of flavors and aromas by cyclodextrins. In: Grumezescu A (ed) Encapsulations: Nanotechnology in the agri-food industry, 1st edn, vol 2, pp. 769–792. ISBN: 978-0-12-804378-3
Fenyvesi É, Vikmon MA, Szente L (2016) Cyclodextrins in food technology and human nutrition: benefits and limitations. Crit Rev Food Sci Nutr 56:1981–2004. https://doi.org/10.1080/10408398.2013.809513
Fernandes C, Encarnação I, Gaspar A, Garrido J, Borges F, Garrido EM (2014) Influence of hydroxypropyl-cyclodextrin on the photostability of fungicide pyrimethanil. Int J Photoenergy 2014:1–8. https://doi.org/10.1155/2014/489873
Fourmentin S, Crini G, Lichtfouse É (2018) Cyclodextrins fundamentals, reactivity and analysis. In: Fourmentin S, Crini G, Lichtfouse É (eds) Environmental chemistry for a sustainable world. Springer, Berlin. https://doi.org/10.1007/978-3-319-76159-6
Frömming KH, Szejtli J (1994) Cyclodextrins in pharmacy. Kluwer Academic Publishers, Dordrecht
Fülöp Z, Kurkov SV, Nielsen TT, Larsen KL, Loftsson T (2012) Self-assembly of cyclodextrins: formation of cyclodextrin polymer based nanoparticles. J Drug Deliv Sci Technol 22:215–221. https://doi.org/10.1016/S1773-2247(12)50032-8
Garrido J, Cagide F, Melle-Franco M, Borges F, Garrido EM (2014) Microencapsulation of herbicide MCPA with native β-cyclodextrin and its methyl and hydroxypropyl derivatives: an experimental and theoretical investigation. J Mol Struct 1061:76–81. https://doi.org/10.1016/j.molstruc.2013.12.067
Ghemati D, Aliouche D (2014) Dye adsorption behavior of polyvinyl alcohol/glutaraldehyde/beta-cyclodextrin polymer membranes. J Appl Spectroscopy 81(2):257–263. https://doi.org/10.1007/s10812-014-9919-4
Gibson LT (2014) Mesosilica materials and organic pollutant adsorption: part B—removal from aqueous solution. Chem Soc Rev 43(15):5173–5182. https://doi.org/10.1039/C3CS60095E
Glick D, Barth S, MacLeod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Gontero D, Lessard-Viger M, Brouard D, Bracamonte AG, Boudreau D, Veglia AV (2017) Smart multifunctional nanoparticles design as sensors and drug delivery systems based on supramolecular chemistry. Microchem J 130:316–328. https://doi.org/10.1016/j.microc.2016.10.007
Gonzalez-Gaitano G, Rodriguez P, Isasi JR, Fuentes M, Tardajos G, Sanchez M (2003) The aggregation of cyclodextrins as studied by photon correlation spectroscopy. J Incl Phenom Macrocycl Chem 44:101–105. https://doi.org/10.1023/A:1023065823358
Goyal AK, Johal ES, Rath G (2011) Nanotechnology for water treatment. Curr Nanosci 7:640–654. https://doi.org/10.2174/157341311796196772
Gruiz K, Molnár M, Fenyvesi É, Cs Hajdu, Atkári Á, Barkács K (2011) Cyclodextrins in innovative engineering tools for risk-based environmental management. J Incl Phenom Macro 70:299–306. https://doi.org/10.1007/s10847-010-9909-y
Grumezescu AM (2016) Encapsulations. In: nanotechnology in the agri-food industry, 1st edn, vol 2. Academic Press, Elsevier. ISBN: 978-0-12-804378-3
Hapiot F, Tilloy S, Monflier E (2006) Cyclodextrins as supramolecular hosts for organometallic complexes. Chem Rev 106:767–781. https://doi.org/10.1021/cr050576c
Hapiot F, Ponchel A, Tilloy S, Monflier E (2011) Cyclodextrins and their applications in aqueous-phase metal-catalyzed reactions. C R Chim 14:149–166. https://doi.org/10.1016/j.crci.2010.04.003
Hapiot F, Bricout H, Menuel S, Tilloy S, Monflier E (2014) Recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis. Catal Sci Technol 4:1899–1908. https://doi.org/10.1039/C4CY00005F
He Y, Fu P, Shen X, Gao H (2008) Cyclodextrin-based aggregates and characterization by microscopy. Micron 39:495–516. https://doi.org/10.1016/j.micron.2007.06.017
Hebeish A, El-Hilw ZH (2001) Chemical finishing of cotton using reactive cyclodextrin. Color Technol 117:104–110. https://doi.org/10.1111/j.1478-4408.2001.tb00343.x
Hirakawa H, Tomita H (2013) Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00114
Ho TM, Howes T, Bhandari BR (2014) Encapsulation of gases in powder solid matrices and their applications: a review. Powder Technol 259:87–108. https://doi.org/10.1016/j.powtec.2014.03.054
Hong SB, Liu MY, Zhang W, Deng W (2015) Organic reactions catalyzed by cyclodextrin and its derivatives. Chin J Org Chem 35:325–336. https://doi.org/10.6023/cjoc201409001
Hongdeng Q, Xiaojing L, Min S, Shengxiang J (2011) Development of silica-based stationary phases for high-performance liquid chromatography. Anal Bioanal Chem 399:3307–3322. https://doi.org/10.1007/s00216-010-4611-x
Hou XS, Ke CF, Stoddart JF (2016) Cooperative capture synthesis: yet another playground for copper-free click chemistry. Chem Soc Rev 45:3766–3780. https://doi.org/10.1039/c6cs00055j
Hougier EG, Kircik L (2012) A review of delivery systems in cosmetics. Dermatol Therm 25:234–237. https://doi.org/10.1111/j.1529-8019.2012.01501.x
Idriss H, Estour F, Zgani I, Barbot C, Biscotti A, Petit S, Galaup C, Hubert-Roux M, Nicol L, Mulder P, Gouhier G (2013) Effect of the second coordination sphere on new contrast agents based on cyclodextrin scaffolds for MRI signals. RSC Adv 3:4531. https://doi.org/10.1039/C3RA40314A
Islam SU, Shahid M, Mohammad F (2013) Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers: a review. Ind Eng Chem Res 52:5245–5260. https://doi.org/10.1021/ie303627x
Jansook P, Ogawa N, Loftsson T (2018) Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int J Pharm 535:272–284. https://doi.org/10.1016/j.ijpharm.2017.11.018
Karim AA, Loh XJ (2016) Towards cyclodextrin-based supramolecular materials. In: Polymers for personnal care products and cosmetics, pp 154–177. https://doi.org/10.1039/9781782623984-00154
Karoyo AH, Wilson LD (2015) Nano-sized cyclodextrin-based molecularly imprinted polymer adsorbents for perfluorinated compounds: a mini-review. Nanomater 5:981–1003. https://doi.org/10.3390/nano5020981
Kayaci F, Uyar T (2012) Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: prolonged shelf-life and high temperature stability of vanillin. Food Chem 133:641–649. https://doi.org/10.1016/j.foodchem.2012.01.040
Kayaci F, Uyar T (2014) Electrospun polyester/cyclodextrin nanofibers for entrapment of volatile organic compounds. Polym Eng Sci 54(12):2970–2978. https://doi.org/10.1002/pen.23858
Kayaci F, Aytac Z, Uyar T (2013a) Surface modification of electrospun polyester nanofibers with cyclodextrin polymer for the removal of phenanthrene from aqueous solution. J Hazard Mat 261:286–294. https://doi.org/10.1016/j.jhazmat.2013.07.041
Kayaci F, Umu OCO, Tekinay T, Uyar T (2013b) Antibacterial electrospun polylactic acid (PLA) nanofibrous webs incorporating triclosan/cyclodextrin inclusion complexes. J Agric Food Chem 61:3901–3908. https://doi.org/10.1021/jf400440b
Kayaci F, Ertas Y, Uyar T (2013c) Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J Agric Food Chem 61:8156–8165. https://doi.org/10.1021/jf402923c
Kayaci F, Sen HS, Durgun E, Uyar T (2014) Functional electrospun polymeric nanofibers ıncorporating geraniol-cyclodextrin ınclusion complexes: high thermal stability and enhanced durability of geraniol. Food Res Int 62:424–431. https://doi.org/10.1016/j.foodres.2014.03.033
Kayaci F, Sen HS, Durgun E, Uyar T (2015) Electrospun nylon 6,6 nanofibers functionalized with cyclodextrins for removal of toluene vapor. J Appl Polym Sci 132:41941. https://doi.org/10.1002/app.41941
Keskin NOS, Celebioglu A, Uyar T, Tekinay T (2015a) Microalgae immobilized by nanofibrous web for removal of reactive dyes from wastewater. Ind Eng Chem Res 54:5802–5809. https://doi.org/10.1021/acs.iecr.5b01033
Keskin NOS, Celebioglu A, Sarioglu OF, Ozkan AD, Uyar T, Tekinay T (2015b) Removal of a reactive dye and hexavalent chromium by a reusable bacteria attached electrospun nanofibrous web. RSC Adv 5:86867–86874. https://doi.org/10.1039/C5RA15601G
Kfoury M, Hădărugă NG, Hădărugă DI, Fourmentin S (2016) Cyclodextrins as encapsulation material for flavors and aroma. In: Encapsulations: nanotechnology in the agri-food industry, 1st edn, vol 2, chapter 4, pp. 127-192. ISBN: 978-0-12-804378-3
Khaoulani S, Chaker H, Cadet C, Bychkov E, Cherif L, Bengueddach A, Fourmentin S (2015) Wastewater treatment by cyclodextrin polymers and noble metal/mesoporous TiO2 photocatalysts. C R Chim 18:23–31. https://doi.org/10.1016/j.crci.2014.07.004
Kozlowski CA, Sliwa W (2010) Use of cyclodextrin polymers in separation of organic species. In: Polymer science and technology series. Nova Science Publishers, Inc, New York
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453:167–180. https://doi.org/10.1016/j.ijpharm.2012.06.055
Kushwaha D, Dwivedi P, Kuanar SK, Tiwari VK (2013) Click reaction in carbohydrate chemistry: recent developments and future perspective. Curr Org Synth 10:90–135. https://doi.org/10.2174/1570179411310010005
Landy D, Mallard I, Ponchel A, Monflier E, Fourmentin S (2012) Cyclodextrins for remediation technologies. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry for a sustainable world: nanotechnology and health risk, 1. Springer, Berlin, pp 47–81
Lay S, Ni XF, Yu HN, Shen SR (2016) State-of-the-art applications of cyclodextrins as functional monomers in molecular imprinting techniques: a review. J Sep Sci 39:2321–2331. https://doi.org/10.1002/jssc.201600003
Lee SY, Park SJ (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11. https://doi.org/10.1016/j.jiec.2014.09.001
Li S, Vigh G (2004) Single-isomer sulfated alpha-cyclodextrins for capillary electrophoresis: hexakis(2,3-di-O-methyl-6-O-sulfo)-alpha-cyclodextrin, synthesis, analytical characterization, and initial screening tests. Electrophoresis 25:2657–2670. https://doi.org/10.1002/elps.200405839
Li JJ, Zhao F, Li J (2011) Polyrotaxanes for applications in life science and biotechnology. Appl Microbiol Biotechnol 90:427–443. https://doi.org/10.1007/s00253-010-3037-x
Loftsson T, Brewster ME (2010) Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol 62:1607–1621. https://doi.org/10.1111/j.2042-7158.2010.01030.x
Loftsson T, Brewster ME (2012) Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Pharm 101:3019–3032. https://doi.org/10.1002/jps.23077
Loftsson T, Masson M, Brewster ME (2004) Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93:1091–1099. https://doi.org/10.1002/jps.20047
López-Nicolás JM, Rodríguez-Bonilla P, García-Carmona F (2014) Cyclodextrins and antioxidants. Crit Rev Food Sci Nutr 54:251–276. https://doi.org/10.1080/10408398.2011.582544
Luca C, Grigoriu AM (2007) Cyclodextrins inclusion compounds in macromolecular chemistry. Cell Chem Technol 41:1–12
Macaev F, Boldescu V (2015) Cyclodextrins in asymmetric and stereospecific synthesis. Symmetry Basel 7:1699–1720. https://doi.org/10.3390/sym7041699
Maetzel D, Sarkar S, Wang H, Abi-Mosleh L, Xu P, Cheng AW, Gao Q, Mitalipova M, Jaenisch R (2014) Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Rep 2:866–880. https://doi.org/10.1016/j.stemcr.2014.03.014
Mahmud ST, Wilson LD (2016) Synthesis and characterization of surface-modified mesoporous silica materials with beta-cyclodextrin. Cogent Chem 2:1132984. https://doi.org/10.1080/23312009.2015.1132984
Manchon JFM, Uzor NE, Kesler SR, Wefel JS, Townley DM, Nagaraja AS, Pradeep S, Mangala LS, Sood AK, Tsvetkov AS (2016) TFEB ameliorates the impairment of the autophagy-lysosome pathway in neurons induced by doxorubicin. Aging (US) 8:3507–3519. https://doi.org/10.18632/aging.101144
Martel B, Morcellet M, Ruffin D, Vinet F, Weltrowski L (2002) Capture and controlled release of fragrances by CD finished textiles. J Incl Phenom Macrocycl Chem 44:439–442. https://doi.org/10.1023/A:1023028105012
Martina K, Binello A, Lawson D, Jicsinszky L, Cravotto G (2013) Recent applications of cyclodextrins as food additives and in food processing. Curr Nutr Food Sci 9:167–179. https://doi.org/10.2174/1573401311309030001
Mavridis IM, Yannakopoulou K (2015) Anionic cyclodextrins as versatile hosts for pharmaceutical nanotechnology: synthesis, drug delivery, enantioselectivity, contrast agents for MRI. Int J Pharma 492:275–290. https://doi.org/10.1016/j.ijpharm.2015.06.004
Meng QR, Bai J, Li CP, Huang YR, Liu H, Li HQ (2014) Electrospun functional cyclodextrins/polystyrene (PS) composite nanofibers and their applications for sorption of Cu (II) ions under aqueous solution. Nanosci Nanotechnol Lett 6:289–294. https://doi.org/10.1166/nnl.2014.1768
Messner M, Kurkov SV, Jansook T, Loftsson T (2010) Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm 387:199–208. https://doi.org/10.1016/j.ijpharm.2009.11.035
Miller KP, Wang L, Chen YP, Pellechia PJ, Benicewicz BC, Decho AW (2015) Engineering nanoparticles to silence bacterial communication. Front Microbiol 6:189. https://doi.org/10.3389/fmicb.2015.00189
Morin-Crini N, Crini G (2013) Environmental applications of water-insoluble & β-cyclodextrin-epichlorohydrin polymers. Prog Polym Sci 38:344–368. https://doi.org/10.1016/j.progpolymsci.2012.06.005
Morin-Crini N, Fourmentin S, Crini G (eds) (2015) Cyclodextrines. Besançon: PUFC. ISBN: 978-2-84867-520-6
Morohoshi T, Tokita K, Ito S, Saito Y, Maeda S, Kato K, Ikeda T (2013) Inhibition of quorum sensing in gram-negative bacteria by alkylamine-modified cyclodextrins. J Biosci Bioeng 116:175–179. https://doi.org/10.1016/j.jbiosc.2013.01.022
Motoyama K, Hirai Y, Nishiyama R, Maeda Y, Higashi T, Ishitsuka Y, Kondo Y, Irie T, Era T, Arima H (2015) Cholesterol lowering effects of mono-lactose-appended beta-cyclodextrin in Niemann-Pick type C disease-like HepG2 cells. Beilstein J Org Chem 11:2079–2086. https://doi.org/10.3762/bjoc.11.224
Motoyama K, Nishiyama R, Maeda Y, Higashi T, Kawaguchi Y, Futaki S, Ishitsuka Y, Kondo Y, Irie T, Era T, Arima H (2016) Cholesterol-lowering effect of octaarginine-appended beta-cyclodextrin in Npc1-Trap-CHO cells. Biol Pharm Bull 39:1823–1829. https://doi.org/10.1248/bpb.b16-00369
Moya-Ortega M, Alvarez-Lorenzo C, Concheiro A, Loftsson T (2012) Cyclodextrin-based nanogels for pharmaceuticals and biomedical applications. Int J Pharm 428:152–163. https://doi.org/10.1016/j.ijpharm.2012.02.038
Nagy ZM, Molnár M, Fekete-Kertész I, Molnár-Perl I, Fenyvesi É, Gruiz K (2014) Removal of emerging micropollutants from water using cyclodextrins. Sci Total Environ 485–486:711–719. https://doi.org/10.1016/j.scitotenv.2014.04.003
Nielsen TT, Wintgens V, Amiel C, Wimmer R, Larsen KL (2010) Facile synthesis of β-cyclodextrin-dextran polymers by “click” chemistry. Biomacromol 11:1710–1715. https://doi.org/10.1021/bm9013233
Norena-Caro D, Alvarez-Lainez M (2016) Functionalization of polyacrylonitrile nanofibers with beta-cyclodextrin for the capture of formaldehyde. Mater Des 95:632–640. https://doi.org/10.1016/j.matdes.2016.01.106
Okano C, Nasuno E, Iimura K, Kato N (2016) Cyclodextrin-immobilized microspheres for uptake of the quorum-sensing signaling molecule N-acylhomoserine lactone. J Appl Polym Sci. https://doi.org/10.1002/app.43198
Oliveri V, Vecchio G (2016) Cyclodextrins as protective agents of protein aggregation: an overview. Chem Asian J 11:1648–1657. https://doi.org/10.1002/asia.201600259
Perez-Anes A, Gargouri M, Laure W, Van Den Berghe H, Courcot E, Sobocinski J, Tabary N, Chai F, Blach JF, Addad A, Woisel P, Douroumis D, Martel B, Blanchemain N, Lyskawa J (2015) Bioinspired titanium drug eluting platforms based on a poly-beta-cyclodextrin-chitosan layer-by-layer self-assembly targeting infections. ACS Appl Mat Int 7:12882–12893. https://doi.org/10.1021/acsami.5b02402
Romi R, Nostro PL, Bocci E, Ridi F, Baglioni P (2005) Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnol Prog 21:1724–1730. https://doi.org/10.1021/bp050276g
Ryzhakov A, Thi TD, Stappaerts J, Bertoletti L, Kimpe K, Couto ARS, Saokham P, Van den Mooter G, Augustijns P, Somsen GW, Kurkov S, Inghelbrecht S, Arien A, Jimidar MI, Schrijnemakers K, Loftsson T (2016) Self-assembly of cyclodextrins and their complexes in aqueous solutions. J Pharm Sci 105:2556–2569. https://doi.org/10.1016/j.xphs.2016.01.019
Samiey B, Cheng CH, Wu JG (2014) Organic-inorganic hybrid polymers as adsorbent for removal of heavy metal ions from solutions: a review. Materials 7:673–726. https://doi.org/10.3390/ma7020673
Saokham P, Loftsson T (2017) γ-Cyclodextrin. Int J Pharm 516:278–292. https://doi.org/10.1016/j.ijpharm.2016.10.062
Schmidt BVKJ, Hetzer M, Ritter H, Barner-Kowollik C (2014) Complex macromolecular architecture design via cyclodextrin host/guest complexes. Prog Polym Sci 39:235–249. https://doi.org/10.1016/j.progpolymsci.2013.09.006
Schneider HJ (2012) Applications of supramolecular chemistry. CRC Press, Taylor & Francis Group, Boca Raton
Schneider HJ, Yatsimirsky AK (2000) Principles and methods in supramolecular chemistry. Wiley, Chichester
Scriba GKE (2016) Chiral recognition in separation science: an update. J Chromatogr A 1467:56–78. https://doi.org/10.1016/j.chroma.2016.05.061
Senthamizhan A, Balusamy B, Celebioglu A, Uyar T (2016) Nanotraps in porous electrospun fibers for effective removal of lead(II) in water. J Mater Chem A 4:2484–2493. https://doi.org/10.1039/C5TA09166G
Sharma N, Baldi A (2016) Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 23:739–757. https://doi.org/10.3109/10717544.2014.938839
Silva A, Duarte A, Sousa S, Ramos A, Domingues FC (2016) Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. WT-Food Sci Technol 73:481–489. https://doi.org/10.1016/j.lwt.2016.06.043
Simoes SMN, Veiga F, Torres-Labandeira JJ, Ribeiro ACF, Concheiro A, Alvarez-Lorenzo C (2014) Syringeable self-assembled cyclodextrin gels for drug delivery. Curr Topics Med Chem 14:494–509. https://doi.org/10.2174/1568026613666131219124308
Szejtli J (1982) Cyclodextrins and their inclusion complexes. Akademiai Kiado, Budapest
Szejtli J (1988) Cyclodextrin technology. Kluwer Academic Publishers, Dordrecht
Szejtli J, Osa T (1996) Comprehensive supramolecular chemistry. In: Szejtli J, Osa T (eds) Cyclodextrins, vol 3. Pergamon, Oxford
Taka AL, Pillay K, Mbianda XY (2017) Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: a review. Carbohydr Polym 159:94–107. https://doi.org/10.1016/j.carbpol.2016.12.027
Tamura A, Yui N (2015) beta-Cyclodextrin-threaded biocleavable polyrotaxanes ameliorate impaired autophagic flux in Niemann-Pick type C disease. J Biol Chem 290:9442–9454. https://doi.org/10.1074/jbc.M114.636803
Tan S, Ladewig K, Fu Q, Blencowe A, Qiao GG (2014) Cyclodextrin-based supramolecular assemblies and hydrogels: recent advances and future perspectives. Macromol Rapid Comm 35:1166–1184. https://doi.org/10.1002/marc.201400080
Tanaka Y, Yamada Y, Ishitsuka Y, Matsuo M, Shiraishi K, Wada K, Uchio Y, Kondo Y, Takeo T, Nakagata N, Higashi T, Motoyama K, Arima H, Mochinaga S, Higaki K, Ohno K, Irie T (2015) Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann-pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol Pharm Bull 38:844–851. https://doi.org/10.1248/bpb.b14-00726
Tejashri G, Amrita B, Darshana J (2013) Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm 63:335–358. https://doi.org/10.2478/acph-2013-0021
Tong J, Chen LG (2013) Review: preparation and application of magnetic chitosan derivatives in separation processes. Anal Lett 46:2635–2656. https://doi.org/10.1080/00032719.2013.807815
Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R (2014) The application of nanosponges to cancer drug delivery. Expert Opin Drug Deliv 11–6:931–941. https://doi.org/10.1517/17425247.2014.911729
Trotta F, Caldera F, Dianzani C, Argenziano M, Barrera G, Cavalli R (2015) New glutathione bio-responsive cyclodextrin nanosponges. ChemPlusChem 81:439–443. https://doi.org/10.1002/cplu.201500531
Tungala K, Adhikary P, Krishnamoorthi S (2013) Trimerization of β-cyclodextrin through the click reaction. Carbohydr Polym 95:295–298. https://doi.org/10.1016/j.carbpol.2013.02.074
Valente AJM, Söderman O (2014) The formation of host-guest complexes between surfactants and cyclodextrins. Adv Colloid Int 205:156–176. https://doi.org/10.1016/j.cis.2013.08.001
Valetti S, Xia X, Costa-Gouveia J, Brodin P, Bernet-Camard MF, Andersson M, Feiler A (2017) Clofazimine encapsulation in nanoporous silica particles for the oral treatment of antibiotic-resistant mycobacterium tuberculosis infections. Nanomedicine. https://doi.org/10.2217/nnm-2016-0364
Vecsernyés M, Fenyvesi F, Bácskay I, Deli MA, Szente L, Fenyvesi É (2014) Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch Med Res 45:711–729. https://doi.org/10.1016/j.arcmed.2014.11.020
Venturini CDG, Nicolini J, Machado C, Machado VG (2008) Properties and recent applications of cyclodextrins. Quim Nova 31:360–368. https://doi.org/10.1590/S0100-40422008000200032
Villalonga R, Cao R, Fragoso A (2007) Supramolecular chemistry of cyclodextrin in enzyme technology. Chem Rev 107:3088–3116. https://doi.org/10.1021/cr050253g
Voncina B, Vivod V (2013) Cyclodextrins in textile finishing. In: Günay M (ed) Textile dyeing. InTech, Rijeka, pp 53–75. https://doi.org/10.5772/53777
Vunain E, Mishra AK, Mamba BB (2016) Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: a review. Int J Biol Macromol 86:570–586. https://doi.org/10.1016/j.ijbiomac.2016.02.005
Walkley SU, Davidson CD, Jacoby J, Marella PD, Ottinger EA, Austin CP, Porter FD, Vite CH, Ory DS (2016) Fostering collaborative research for rare genetic disease: the example of niemann-pick type C disease. Orphanet J Rare Dis 11:161. https://doi.org/10.1186/s13023-016-0540-x
Ward C, Martinez-Lopez N, Otten EG, Carroll B, Maetzel D, Singh R, Sarkar S, Korolchuk VI (2016) Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim Biophys Acta Mol Cell Biol Lipids 1861:269–284. https://doi.org/10.1016/j.bbalip.2016.01.006
Wei ZZ, Liu YL, Hu HM, Yu JY, Li FX (2016) Biodegradable poly(butylene succinate-co-terephthalate) nanofibrous membranes functionalized with cyclodextrin polymer for effective methylene blue adsorption. RSC Adv 6:108240–108246. https://doi.org/10.1039/C6RA22941G
West C (2014) Enantioselective separations with supercritical fluids: review. Curr Anal Chem 10:99–120. https://doi.org/10.2174/1573411011410010009
Wu HQ, Kong JH, Yao XY, Zhao CY, Dong YL, Lu XH (2015a) Polydopamine-assisted attachment of beta-cyclodextrin on porous electrospun fibers for water purification under highly basic condition. Chem Eng J 270:101–109. https://doi.org/10.1016/j.cej.2015.02.019
Wu ZL, Song N, Menz R, Pingali B, Yang YW, Zheng YB (2015b) Nanoparticles functionalized with supramolecular host-guest systems for nanomedicine and healthcare. Nanomedicine 10:1493–1514. https://doi.org/10.2217/NNM.15.1
Xiao Y, Ng SC, Tan TTY, Wang Y (2012) Recent development of cyclodextrin chiral stationary phases and their applications in chromatography. J Chromatogr A 1269:52–68. https://doi.org/10.1016/j.chroma.2012.08.049
Xiao N, Wen Q, Liu QW, Yang QB, Li YX (2014) Electrospinning preparation of beta-cyclodextrin/glutaraldehyde crosslinked PVP nanofibrous membranes to adsorb dye in aqueous solution. Chem Res Chin Univ 30:1057–1062. https://doi.org/10.1007/s40242-014-4203-y
Yamamoto E, Kuroda K (2016) Colloidal mesoporous silica nanoparticles. Bull Chem Soc Jpn 89:501–539. https://doi.org/10.1246/bcsj.20150420
Yang LPH, Keam SJ (2009) Sugammadex: a review of its use in anaesthetic practice. Drugs 69:919–942. https://doi.org/10.2165/00003495-200969070-00008
Yokoo M, Kubota Y, Motoyama K, Higashi T, Taniyoshi M, Tokommaru H, Nishiyama R, Tabe Y, Mochinaga S, Sato A, Sueoka-Aragane N, Sueoka E, Arima H, Irie T, Kimura S (2015) 2-Hydroxypropyl-β-cyclodextrin acts as a novel anticancer agent. PlosOne 10:e0141946. https://doi.org/10.1371/journal.pone.0141946
Yuan Z, Zhang L (2016) Photoinduced controlled-release drug delivery systems for applications in nanomedicine. Curr Org Chem 20:1768–1785. https://doi.org/10.2174/1385272820666160112001944
Yusoff SNM, Kamari A, Aljafree NFA (2016) A review of materials used as carrier agents in pesticide formulations. Int J Environ Sci Technol 13:2977–2994. https://doi.org/10.1007/s13762-016-1096-y
Zarzycki PK, Fenert BE, Głód BK (2016) Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. In: Grumezescu A (ed) Encapsulations: nanotechnology in the agri-food industry, pp. 717–767. ISBN: 978-0-12-804378-3
Zgani I, Idriss H, Barbot C, Djedaïni-Pilard F, Petit S, Hubert-Roux M, Estour F, Gouhier G (2017) Positive variation of the MRI signal via intramolecular inclusion complexation of a C-2 functionalized & β-cyclodextrin. Org Biomol Chem 15:564–569. https://doi.org/10.1039/c6ob02583h
Zhang J, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233. https://doi.org/10.1016/j.addr.2013.05.001
Zhang XM, Li HZ, Cao ML, Shi L, Chen CY (2015) Adsorption of basic dyes on beta-cyclodextrin functionalized poly (styrene-alt-maleic anhydride). Sep Sci Technol 50:947–957. https://doi.org/10.1080/01496395.2014.978461
Zhao R, Wang Y, Li X, Sun BL, Wang C (2015) Synthesis of beta-cyclodextrin-Based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene Blue. ACS Appl Mat Int 7:26649–26657. https://doi.org/10.1021/acsami.5b08403
Zhou JW, Ritter H (2010) Cyclodextrin functionalized polymers as drug delivery systems. Polym Chem 1:1552–1559. https://doi.org/10.1039/c0py00219d
Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Bjorkhem I, Gustafsson JA, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lutjohann D, Latz E (2016) Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Translation Medicine. https://doi.org/10.1126/scitranslmed.aad6100
Author information
Authors and Affiliations
Corresponding author
Additional information
This review is dedicated to the memory of Professor Benito Casu (Istituto di Chimica e Biochimica G. Ronzoni, Milan, Italy).
Rights and permissions
About this article
Cite this article
Crini, G., Fourmentin, S., Fenyvesi, É. et al. Cyclodextrins, from molecules to applications. Environ Chem Lett 16, 1361–1375 (2018). https://doi.org/10.1007/s10311-018-0763-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0763-2