Abstract
Cyclodextrins are natural oligosaccharides obtained from starch. They were discovered in 1891 by Villiers, and attracted major scientific and industrial interests from the late 1970s. Actually, cyclodextrins are among the most remarkable macrocyclic molecules with major theoretical and practical interest for chemistry and biology. Cyclodextrins belong to the family of cage molecules due to their structure, which is composed of a hydrophobic cavity that can encapsulate other molecules. Indeed, the most characteristic feature of cyclodextrins is their ability to form inclusion complexes with various molecules through host-guest interactions. Cyclodextrins and their derivatives have a wide variety of practical applications including pharmacy, medicine, foods, cosmetics, toiletries, catalysis, chromatography, biotechnology, nanotechnology, and textile production. Cyclodextrins are also the object of numerous fundamental studies. Between 2011 and 2015, 18,430 cyclodextrin-related publications have been published. In this chapter, after a brief description of cyclodextrin basics, we highlight selected works on cyclodextrins published over the last 5 years by various research groups.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- AHLs:
-
N-acyl-L-homoserine lactones
- AIP:
-
Auto-inducing peptides
- AIT:
-
Allyl-isothiocyanate
- BBB:
-
Blood-brain barrier
- CC:
-
Cholesterol crystals
- CD:
-
Cyclodextrin
- CD-ICs:
-
Cyclodextrin inclusion complexes
- CNS:
-
Central nervous system
- EFSA:
-
European Food Safety Administration
- ES:
-
Electrospinning
- GC:
-
Gas chromatography
- HP-β-CD:
-
Hydroxypropyl-β-cyclodextrin
- IT:
-
Imprinting technique
- LC:
-
Liquid chromatography
- MIP:
-
Molecularly imprinted polymers
- MOF:
-
Metal organic frameworks
- NF:
-
Nanofibers
- NMR:
-
Nuclear magnetic resonance
- NPC:
-
Niemann-Pick type C disease
- PAH:
-
Polycyclic aromatic hydrocarbons
- PAN:
-
Polyacrylonitrile
- PCB:
-
Polychlorinated biphenyls
- PDT:
-
Photodynamic therapy
- QS:
-
Quorum sensing
- RAMEB:
-
Randomly methylated-β-CD
- SFC:
-
Supercritical fluid chromatography
- VOC:
-
Volatile organic compounds
1.1 Cyclodextrins – An Introduction
1.1.1 Cyclodextrin Description
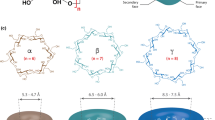
Cyclodextrins (CDs) are cyclic oligomers obtained from the enzymatic degradation of starch, one of the most essential polysaccharide in the nature. Cyclodextrins make up a family of cyclic oligosaccharides. They are composed of six or more D-glucopyranoside units linked in α(1–4), like in amylose, a component of starch. They are also called cycloamyloses. Typical native cyclodextrins contain six, seven or eight glucose units and are denoted α-, β- and γ-CDs, respectively. They are produced in a highly pure form at an industrial scale.
Cyclodextrins are hollow, truncated-cone-shaped molecules made up of several glucose units linked together covalently by oxygen atoms and held in shape via hydrogen bonding between the secondary hydroxyl groups on adjacent units at the wider rim of the cavity. Indeed, cyclodextrins are ring molecules and they are toroidal or cone-shaped. They are not cylindrical due to the lack of free rotation at the level of bonds between glucopyranose units. The primary hydroxyl groups are located on the narrow side and the secondary groups on the wider side. The molecular structures and characteristics of the three analogues are schematized in Fig. 1.1 and their main characteristics in Table 1.1. Other cyclodextrins, with less than six and more than eight units, denoted large CDs, may also be isolated at the laboratory scale. Indeed, depending on the particular transferase used during enzymatic degradation of starch, the nature of starch and the reaction conditions, different types of cyclodextrins result.
β-Cyclodextrin (β-CD) is the most studied and most frequently used, based on its cheapness, availability, and complex-forming capacities towards a large range of substances. Indeed, as others cyclodextrins, the most characteristic feature of the β-CD molecule is its ability to form inclusion compounds with various substances through host-guest interactions (Fig. 1.2). Its central cavity which is composed of seven glucose units is hydrophobic when the external part is hydrophilic because the presence of 21 hydroxyl groups. The core of its structure can trap or encapsulate other substances. This remarkable encapsulation properties can modify and/or improve the physical, chemical, and/or biological characteristics of the guest molecule (Szejtli 1988; Morin-Crini et al. 2015).
Formation of an inclusion complex is the result of an association/dissociation equilibrium between the free guest and the free host and the complex. This is governed by a constant Kf, denoted formation or stability constant. The higher the Kf value is, the more stable the inclusion is, and the less dissociation that occurs. The driving forces for complex formation include release of high-energy water, solvent effects, hydrophobic interactions, hydrogen bonds, van der Waals interactions, and dipole-dipole interactions. As a general rule stated by Cramer in the 1950s, the complex is strong when there is size complementarity between the guest and the cyclodextrin cavity (Crini 2014). Depending on the respective size of the guest and host molecules, one guest molecule can interact with one or two (or more) cyclodextrin (i.e. host:guest complexes 1:1 and 2:1), or two (or more) guest molecules can interact with one cyclodextrin or two (or more) (i.e. host:guest complexes 1:2 and 2:2) as showed in Fig. 1.3. Because of their conformation, some guests can be included in one or two cyclodextrin molecules, and depending on the CD size, it is a different part of the guest molecule that can be included. Cyclodextrins themselves can self-associate to form aggregates or micelles in aqueous solutions. So, in solution, multiple inclusion equilibria can coexist. While stoichiometry is often characterized by means of Job plots, affinities are generally determined by titration, competition or solubility procedures (Connors 1997; Landy et al. 2000; Decock et al. 2006; Brewster and Loftsson 2007). Many techniques including NMR, calorimetry, fluorescence, circular dichroism and headspace gas chromatography have been used for complexation studies (Decock et al. 2008; Morin-Crini et al. 2015).
Various methods such as co-precipitation and derivatives methods such as spray drying and freeze-drying, kneading, slurry complexation, paste complexation, damp mixing, heating method (sealed-heating), extrusion method, dry mixing, microwave treatment, and supercritical carbon dioxide can be used in the formation of CD complexes. The books published by Szejtli (1982a, 1988) can be consulted on the preparation of inclusion complexes. Evidence for a guest inclusion into the cyclodextrin cavity may be proved by various analytical techniques including nuclear magnetic resonance (NMR) spectroscopy, UV-visible absorption spectroscopy, optical rotatory dispersion and circular dichroism, fluorescence, infrared (FT-IR) spectroscopy, thermo-analysis, mass spectrometry, and powder X-ray diffractometry (Morin-Crini et al. 2015).
The structure of cyclodextrins is stabilized by the formation of hydrogen bonds between C-2 and C-3 hydroxyl groups of adjacent glucose units. This phenomenon widely affects, in addition to molecular dimensions, the water solubility of cyclodextrins. The formation of a complete ring of intramolecular hydrogen bonds in β-CD counteracts its hydration and reduces its solubility as compared to other native cyclodextrins (Table 1.1) (Szejtli 1998). Disruption of intermolecular hydrogen bonds (i.e. substitution of highly reactive hydroxyl groups with either polar or apolar moieties) generally produces cyclodextrin derivatives with anomalous increased solubility. Synthesis of derivatives aims also to construct CD polymers or CDs with enhanced complexation ability and selectivity. Over than 15,000 cyclodextrin derivatives have been obtained by alkylation, hydroxyalkylation, sulfatation, phosphatation, acetylation, amination, esterification and etherification reactions (Khan et al. 1998; Szejtli 2004a; b) but only some particular one such as hydroxypropylated, methylated and sulfobutylated derivatives are marketed and widely used.
1.1.2 Fields of Application
As already stated, although they have been known for 126 years, cyclodextrins only really took off in the 1980s with the first applications in the chromatography, pharmaceutical and food industries. Cyclodextrins were used as host molecules for molecular recognition, as solubilizing agents for lipophilic drugs, excipients in formulation development, as delivery systems and complexing agents in the food and drug industries, or for masking of undesired odor and taste. Actually, applications are found in practically all sectors of industry but especially, in cosmetics, hygiene and personal care, textiles including textile finishing, functional textiles and cosmetotextiles, medicine and biomedicine, agrochemistry or pesticides, biotechnology, and catalysis (Morin-Crini et al. 2015). Cyclodextrins and their derivatives have also other practical applications in microencapsulation including aroma and fragrances, oils, analytical chemistry, organic chemistry (synthesis), macromolecular chemistry (materials), click chemistry, supramolecular chemistry, membranes, enzyme technology, environment such as remediation, decontamination and extraction, and nanotechnology (nanoparticles/nanosponges for various domains). Research on cyclodextrins is also very active in fields such as the formulation of detergents, glues and adhesives, the sector of plastics (packaging) and the industry of fibers and paper. However, the main market remains the pharmaceutical, food and cosmetic industries. It is interesting to note that the vast majority of these chemical and biological applications are based on the ability to form inclusion complexes (Szejtli 1982a, 1988; Szejtli 2004a; b; Duchêne 1987, 1991; Dodziuk 2006; Morin-Crini et al. 2015).
1.1.3 Literature Data
The literature on cyclodextrins is vast and spread across different disciplines: Chemistry, Biochemistry, Health Science, Agriculture, etc. Interesting books and book chapters on the different aspects of cyclodextrins such as the preparation, description, characterization, properties, chemistry, and derivatives for these disciplines can be consulted (Table 1.2). In the last two decades, a large number of generalist reviews has been also published in the literature on practically all these aspects of cyclodextrins (Table 1.3), so many that it would not be feasible to cite them all. Selected books and reviews are given in Tables 1.2 and 1.3, respectively, which contain the original sources of the data displayed, and also selected recent works discussed in this chapter.
Figure 1.4 demonstrates how the number of publications increased using cumulative numbers of 5-year periods. In 2015, with an average of 4.5 papers and 2.5 patent applications daily, the literature data shows that cyclodextrin research and development is still in the focus (Source: Cyclodextrin News, Cyclolab). Recently, Deorsola et al. (2014) have performed a thorough patent search on cyclodextrin-related patents using free databases. The period they evaluated was 1981-2011. The survey showed the leading role of Asia on the number patent applications filed in patent offices of different countries, being Japan and China on the first and second positions, followed by the US and the European Patent offices. The top 10 inventors among the total of 20,198 inventors have all Chinese names. The three leading companies are Procter & Gamble Co., Kao Corporation and Schering AG.
1.1.4 The Cyclodextrin Scientific Community
In 1981, Professor Szejtli organized the First International Cyclodextrin Symposium in Budapest, Hungary. This symposium was a great success, with participants coming from all over the world (more than 180 participants from 17 countries) while Professor Szejtli “expected 25-30 participants outside Hungary” (Szente and Szejtli 1996). The submitted manuscripts filled a 544-page volume of proceedings published by Akademiai Kiado. The second cyclodextrin symposium was organized by Professor Nagai in 1984, in Tokyo. Since 1984 and Szejtli’s initiative, a broad community of researchers has met every two years to exchange and share their works on cyclodextrins. These well-established symposiums provide opportunities for scientists who work in several aspects of cyclodextrin research to meet and discuss recent advances in all cyclodextrin fields. The 19th International Cyclodextrin Symposium organized by Professor Keiko Takahashi and Professor Takashi Hayashita will be held on April 2018 in Tokyo, Japan (the next will be organized in Sicily, Italy in 2020).
In Europe, the cyclodextrin scientific community is also very active. After Aalborg in 2009, Asti in 2011, Antalya in 2013 and Lille in 2015, Lisbon will welcome the 5th European conference in 2017. This is a biennial meeting where academic researchers and industrials come together to present the latest achievements in the field of cyclodextrin science and technology. The 9th and most recent Asian cyclodextrin Conference was held in 2017 in Singapore.
There are also several national groups that are very active (e.g. in Japan, France, Italy…) which enables a good quality of exchange on current research into cyclodextrins in each country. In France, the 19th annual colloquium of the Société Française des Cyclodextrines will be organized by Nadia Morin-Crini and Grégorio Crini in Besançon, in October 2018. This colloquium aims to provide an enabling environment for PhD students from numerous French Universities and Research Institutes, so that they can create new research collaborations. The 34th annual meeting of the Japanese community was held in 2017.
1.1.5 Szejtli Prize
The list of prestigious researchers who have contributed to the development of cyclodextrins includes Professor József Szejtli (1933–2004). Professor Szejtli made an important contribution to the chemistry of cyclodextrins, to the dissemination of results and in their industrial applications, notably by the creation of a laboratory in 1972, which became an independent company in 1992 (CYCLOLAB Ltd.) totally devoted to cyclodextrins (“From toy to tool with industrial interest”) and by the publication of numerous general reviews. CYCLOLAB was the first private research institute for the technological transfer between cyclodextrin research and industry (Crini 2014).
CYCLOLAB recently established Szejtli Prize (Fig. 1.5) to preserve his legacy, keep his memory alive and recognize his ground-breaking achievements in the area of cyclodextrin research, development and commercialization of related technologies. This prize aimed to award young researchers demonstrating outstanding results in the cyclodextrin science and technology. The first award was assigned to Professor Keiichi Motoyama, Kumamoto University, in Saarbrücken, at the 17th International Cyclodextrin Symposium in 2014 for his works in the design of new active pharmaceutical ingredients as anticancer agents. The winner of Szejtli Prize 2016 is Professor Tamer Uyar, Bilkent University, for his studies on electrospinning of functional nanofibers with cyclodextrins.
1.1.6 Nobel Prize 2016
In 2016, the Royal Swedish Academy of Sciences has decided to award Jean-Pierre Sauvage (University of Strasbourg, France), Sir James Fraser Stoddart (Weinberg College of Arts and Sciences of Northwestern, Scotland) and Bernard Lucas Feringa (University of Groningen, The Netherlands) the Nobel Prize in Chemistry “for the design and synthesis of molecular machines”. This was a high recognition for supramolecular chemistry utilizing building blocks of host-guest complexes including also cyclodextrin complexes.
1.2 Cyclodextrins: An update 2012-2016 – Present Situation, Trends and Outlook
Recent and continuing interest in cyclodextrins is evident from the number of papers that appears each year in the literature on various chemical and biological topics for both academic and industrial applications as showed in Fig. 1.4. In this section, we chose to highlight selected works on cyclodextrins published over the last 5 years by different research groups in order to have an update overview. The main objective is to summarize some of the developments related to the applications of cyclodextrins, based on a substantial number of relevant references published recently. Of course, this review is not exhaustive. Readers interested in cyclodextrin topics should refer to the library database “Cyclodextrin News” from CYCLOLAB, Ltd. (Hungary), which is a periodical collecting all of the cyclodextrin papers, proceedings, patents, conferences and lectures.
1.2.1 Cyclodextrins: From 1891 Until Now
Recently, Crini (2014) published a historical review of cyclodextrins, molecules discovered serendipitously by the French pharmacist Antoine Villiers in 1891 and then called “cellulosines”. They were subsequently named “Schardinger dextrins” at the beginning of the 20th century, in tribute to Franz Schardinger, an Austrian chemist and microbiologist who was the first to describe their basic chemistry. Schardinger is considered the “Founding Father” of cyclodextrin chemistry. After that, they were termed “cycloamyloses” by the American chemist Dexter French at the beginning of the 1940s and finally “cyclodextrins” by the German chemist Friedrich Cramer towards the end of the 1940s. Crini’s review reported their history, divided into 5 more or less clearly defined periods: The early days around their discovery from 1891 to 1911, a period of doubt between 1911 and 1935, reaching maturity from 1935 to 1950, exploration from 1950 to 1970, and the uses to which they have been put from 1970 to the present day. A series of other prestigious names has marked their history, names such as Hans Pringsheim, Paul Karrer, Karl Johann Freudenberg, Benito Casu, Wolfram Saenger, Myron Lee Bender, and József Szejtli who is considered as the “Godfather” of cyclodextrins. Each of the main periods was illustrated by precise examples from among the works published in the literature. Although this review is not exhaustive, it highlights the work of those researchers who have contributed to the development of our knowledge of cyclodextrins throughout the 120 years of their history. An historical examination of cyclodextrins can be found elsewhere (Szejtli 1998; Loftsson and Duchêne 2007; Loeve and Normand 2011).
1.2.2 Self-association of Cyclodextrins
It is now well-known that cyclodextrin molecules can adopt various types of assembly modes in aqueous solution as well as crystal structures (Gonzalez-Gaitano et al. 2003). New micro- and nanostructures including aggregates, formed by the self-assembly of cyclodextrins, have been useful, particularly in the fields of supramolecular chemistry, materials science, pharmacy including formulation and drug delivery, and medicine as reported by Kurkov and Loftsson (2013), Simoes et al. (2014), Ryzhakov et al. (2016), and Oliveri and Vecchio (2016).
The most important property of the cyclodextrins is the ability to establish specific interactions (molecular encapsulation) with various types of molecules through the formation of non-covalently bonded entities, either in the solid phase or in aqueous solution. However, cyclodextrins are not only able to form these host/guest inclusion complexes but also non-inclusion complexes (Gonzalez-Gaitano et al. 2003; Loftsson et al. 2004; Messner et al. 2010; Moya-Ortega et al. 2012; Kurkov and Loftsson 2013). The hydroxyl groups present on the outer surface can form hydrogen bonds with other molecules which makes them able, just like dextrins, non-cyclic oligosaccharides and polysaccharides, to form complexes (molecular structures) with lipophilic substances insoluble in water. In pure aqueous cyclodextrin solutions, CD molecules self-assemble to form nanoparticles with diameter from about 20 to 200 nm (Loftsson et al. 2004; He et al. 2008). At low concentrations (below 1% w/v), the fraction of CD molecules forming such aggregates is insignificant but the aggregation increases with increasing cyclodextrin concentration. Another possibility that is mentioned is the formation of aggregates able to dissolve water-insoluble lipophilic molecules (structures similar to micelles). The CD/drug complex aggregates are frequently from 100 to 4000 nm in diameter or in the nanoparticle and small microparticle size range. The complexes are kept together by weak hydrogen bonds and hydrophobic forces, and dissociate readily upon media dilution. Such systems are called cyclodextrin nanostructures, nanoassemblies or self-aggregates.
In recent years, it has been observed, in pharmaceutical applications, that other types of cyclodextrin complexes such as non-inclusion complexes, are also participating in CD solubilization of poorly soluble drugs (Bilensoy 2011; Kurkov and Loftsson 2013; Morin-Crini et al. 2015). There are some indications that formation of CD/drug complex aggregates might play an important role in CD enhancement of drug bioavailability. The cyclodextrin aggregates present the ability to form complexes, and nanosized aggregates and nanotube-type host/guest architectures can be envisaged. This is a generally unexplored domain and often causes controversies since the results obtained are closely dependent on the technique used, as pointed out by Ryzhakov et al. (2016). Another problem discussed in the literature is the stability of the cyclodextrin aggregates, in particular when these systems are used as drug delivery systems. The publications of Loftsson’s group summarized the most important features and the main conclusions of these cyclodextrin-based aggregates (Fülöp et al. 2012; Kurkov and Loftsson 2013; Ryzhakov et al. 2016; Saokham and Loftsson 2017). The use of CD-based nanoaggregates in oral drug delivery could be a promising strategy to improve the bioavailability of poorly soluble drugs. Much work is necessary to study the behavior of these nanoaggregates under conditions that are representative for the gastrointestinal tract and the effects which may cause disaggregation (Ryzhakov et al. 2016).
1.2.3 Cyclodextrins as Drug Delivery Vehicles
Although cyclodextrins can be found in 56 pharmaceutical products (source: Cyclolab), they are still regarded as novel pharmaceutical excipients, drug delivery vehicles, and anti-aggregation agents (Duchêne 1987, 1991; Frömming and Szejtli 1994; Uekama and Irie 1996; Thompson 2006; Brewster and Loftsson 2007; Van de Manakker et al. 2009; Loftsson and Brewster 2010, 2012; Bilensoy 2011; Ahuja et al. 2011; Kurkov and Loftsson 2013; Chilajwar et al. 2014; Morin-Crini et al. 2015). In the last decade, as already mentioned, it has been observed that cyclodextrins and cyclodextrin complexes in particular self-assemble to form nanoparticles and that, under certain conditions, these nanoparticles can self-assemble to form microparticles, a tendency that increases upon formation of inclusion complexes with lipophilic drugs. These properties have changed the way we perform cyclodextrin pharmaceutical research and have given rise to new cyclodextrin formulation opportunities as summarized by Bilensoy (2011), and Kurkov and Loftsson (2013). The design of functional cyclodextrin nanoparticles formed by self-assembly is also a developing area in the field of nanomedicine (cancer therapy) as reported by Fülöp et al. (2012). Nanoparticle-based systems can improve bioavailability, reduce immunogenicity, modify drug metabolism, reduce toxicity, and increase the biological half-life of drugs after systemic administration (Bilensoy 2011; Fülöp et al. 2012; Kurkov and Loftsson 2013; Brandariz and Iglesias 2013; Tejashri et al. 2013; Concheiro and Alvarez-Lorenzo 2013; Simoes et al. 2014; Morin-Crini et al. 2015; Sharma and Baldi 2016).
The field of medicine using cyclodextrins and their derivatives is vast including biomedicine (wound dressing, biosensors, medical devices), nanomedicine (nanoparticles for drug delivery), antifungal treatment, antimicrobial therapy, gene therapy, tissue engineering, cancer treatment, and magnetic resonance imaging (Van de Manakker et al. 2009; Yang and Keam 2009; Caliceti et al. 2010; Dong et al. 2011; Bilensoy 2011; Kanwar et al. 2011; Moya-Ortega et al. 2012; Macaev et al. 2013; Zhang and Ma 2013; Hirakawa and Tomita 2013; Morohoshi et al. 2013; Miller et al. 2015; Okano et al. 2016; Brackman et al. 2016; Silva et al. 2016).
Interesting recent studies were devoted to the physicochemical properties of local anesthetics (LA) and their inclusion complexes with cyclodextrins in order to understand their behavior (Brandariz and Iglesias 2013). Their capacity to reach and to block sodium channels and act as anesthetics depends on their protonation state. Different studies showed that the extent of complexation with CDs varies greatly with the protonation state of the involved molecules, an interesting fact in the administration of LA, as recently reported by Brandariz and Iglesias (2013).
Another interesting example concerns the preparation of vaccines Daptacel® (Sanofi Group, Pasteur) for protection against diphtheria, tetanus and whooping cough which contain dimethyl-β-cyclodextrin.
Cyclodextrins can be used in antifungal formulations as auxiliary substances to modify and to improve physicochemical properties of the active compound such as solubility and stability. The biological effects of cyclodextrins, important for their use within antimycotic formulations, can be divided into: (i) effects based on the ability of cyclodextrins to form inclusion complexes with endogenous substances (e.g. membrane lipids, cellular cholesterol), (ii) effects based on formation of inclusion complexes with component parts of fungi cells, and (iii) effects based on the chemical nature of cyclodextrins and their derivatives. The advances in research of biological activity of CDs with focus on their properties responsible for their synergistic effect with antimycotic compounds were recently discussed by Macaev et al. (2013). The number of publications on the use of cyclodextrins in antifungal formulations is still growing.
Oliveri and Vecchio (2016) reviewed the use of cyclodextrins and their derivatives as anti-aggregation agents in a number of proteins such as insulin, prion protein, and amyloid-beta, and some multimeric enzymes. There are many diseases that are correlated to protein misfolding and amyloid formation processes affecting numerous organs and tissues. There are over 30 different amyloid proteins and a number of corresponding diseases including Alzheimer’s diseases the most common neurodegenerative disease. Treatment of these diseases is still a goal to reach, and many molecules including cyclodextrins were studied in this perspective.
Onchocerciasis, also known as River Blindness, is a disease caused by infection with the parasitic worm Onchocerca volvulus. The parasite worm is spread by the bites of black fly. These flies live near to rivers in sub-Saharan Africa, hence the name of the disease. There is no vaccine against it. Insecticides are used to decrease the fly population. People infected are treated with ivermectin. The drug kills the larvae but not the adult worm therefore the treatment should be repeated once or twice a year. However, ivermectin is not compatible with conventional excipients (Shaw et al. 1999; Shen et al. 2014). Recently, novel formulations have been proposed: Intraperitoneal injection of cyclodextrin-conjugated ivermectin proved to be effective in inhibition of transcription factor-dependent human colon cancer xenograft in vivo (Melotti et al. 2014).
Actually, cyclodextrins are also perceived as dream molecules for the development of applications in biomedicine and nanomedicine including nanovectorization, such as, for instance, nanoparticles for drug delivery, innovative biosensors for detection of biological targets, bio-recognition events, molecular diagnosis and medical imaging, gene therapy or tissue engineering (Concheiro and Alvarez-Lorenzo 2013; Zhang and Ma 2013; Tan et al. 2014; Simoes et al. 2014; Dong et al. 2015; Wu et al. 2015b; Yuan and Zhang 2016).
Medical devices including catheters, prosthesis, vascular grafts, and bone implants can also benefit from surface grafting or thermofixation of cyclodextrins. This explains the recent increase in the number of research papers dealing with these topics (Concheiro and Alvarez-Lorenzo 2013). However, most of these studies are in the proof-of-concept stage, and only a few therapeutic nanosystems have been comprehensively investigated. The successful translation of these laboratory innovations to clinical reality remains challenging. The cost is an important factor that limits the successful translation of new materials containing cyclodextrins. Another problem concerns the lack of data on the biocompatibility evaluation and on the toxicity of these nanomaterials. In spite of these, Zhang and Ma (2013) concluded that the future of cyclodextrin-based supramolecular systems mainly in drug and gene delivery is promising in view of the notable clinical success. The cyclodextrin-based nanomaterials have found applications not only in biomedical sector, pharmacy, pharmacotherapy, biology, and biotechnology, but also in the textile industry by providing clothes for transdermal delivery.
A new generation of drug eluting stents based on the strong anchorage of a biocompatible and bioresorbable cyclodextrin-based polymer onto metallic devices has been elaborated (Perez-Anes et al. 2015). Polydopamine, a strong adhesive polymer, was applied as a first coated layer. Cyclodextrin was fixed by in situ polycondensation with citric acid (polycyclodextrins formation). As a third layer an amine-rich polymer, polyethyleneimine was used to stabilize the anionic cyclodextrin layer. As an alternative polycyclodextrins and chitosan layers were applied using layer-by-layer deposition technique.
Junthip et al. (2016) proposed new textiles modified with multilayer cyclodextrin polyelectrolytes (e.g. citric acid-cyclodextrin polymer and quaternary amino cyclodextrin polymer) using layer-by-layer technique to obtain textiles with antibacterial properties to be applied as wound coatings or prostheses. Sustained release of three model compounds such as tert-butyl-benzoic acid, methylene blue and triclosan, was presented. Biological and microbiological tests were performed to investigate the cytocompatibility and the intrinsic antibacterial activity of multilayer assemblies.
Functionalized cyclodextrins are interesting scaffolds for contrast agents used in magnetic resonance imaging as recently reported by Gouhier’s group (Idriss et al. 2013; Zgani et al. 2017). For the further improvement of the sensitivity of this medical diagnostic tool, it is necessary to fully understand the role of the cyclodextrins in the efficiency of the contrast agents. The review by Mavridis and Yannakopoulou (2015) can be also consulted on the same topic.
Different methods of immobilizing cyclodextrin onto polysaccharide were discussed by Yang and Yang (2013). These materials possess the cumulative effects of inclusion, size specificity, controlled release ability and transport properties of cyclodextrin as well as the biocompatibility, non-toxicity, and biodegradability of polysaccharide. Their nanoscale association could also lead to the formation of original particles and films which pave the way to new applications in tissue engineering.
Wound dressings should be capable of mechanical wound protection and should also facilitate the healing process via maintenance of suitable environmental conditions and controlled delivery of bioactive molecules. It is known that, among macromolecular systems, hydrogels present suitable properties for wound-dressing applications such as cost-effectiveness, easy to use, good biocompatibility, together with a high-water content, the latter of which is important for the maintenance of a moist environment and ready removal from the wound with a minimal level of associated pain. As reported by Pinho et al. (2014), their properties as drug delivery systems can be improved by the use of cyclodextrins as cross-linking agents. The conjugation of cyclodextrin molecules with hydrogels may allow the achievement of an optimal wound-dressing material, because the hydrogel component will maintain the moist environment required for the healing process, and the cyclodextrin moiety has the ability to protect and modulate the release of bioactive molecules. Pinho et al. (2014) concluded that cyclodextrin-based hydrogels with target characteristics as interesting new materials for wound-dressing applications due to their powerful complexation abilities and biocompatibilities.
1.2.4 Cyclodextrins as Active Ingredients
Currently the field of medicine is closely concerned with cyclodextrin inclusion complexes. These complexes are preformed prior to be administrated. There are, however, some cases when the complexes are formed within the body. The best-known example is the one containing the active compound sugammadex (Bridion®): it is a modified γ-CD (Fig. 1.6; Table 1.4) used as an antidote to certain curare-like muscle relaxants in anesthesia since 2008 (Booij 2009; Yang and Keam 2009; Ozbilgin et al. 2016). After intravenous administration, it neutralizes steroid curare-like agents (rocuronium, vecuronium) by forming an inactive complex in the plasma which is then eliminated in the urine. Sugammadex has improved effectiveness compared with currently available methods of accelerating reversal of neuromuscular blockade. Its mechanism of action also differs from that of other commonly used reversal agents e.g. neostigmine and edrophonium. Sugammadex is biologically inactive, does not bind to plasma proteins, and appears to be safe and well tolerated although it has few side effects. Its cost is markedly higher than that of any of the other drugs used in anesthesia (Donati 2011). The literature data are actually abundant with a matter of considerable debate. Sugammadex shows an unexpected rise from 17 papers in 2000-2005 to 780 in 2010-2015 (Source: Cyclodextrin News, Cyclolab).
Application of hydroxypropyl-β-cyclodextrin (HP-β-CD) against Niemann-Pick C disease (NPC) started with a surprising observation in 2007 that this cyclodextrin aimed as excipient was more effective than allopregnanolone, the active (Walkley et al. 2016). As no alternative treatment existed at that time the NPC1 disease families pushed FDA for approval for individual access for their children and as a result of a large collaborative work in all areas of drug development, including chemistry and manufacturing, formulation, pharmacology, pharmacokinetics, toxicology, and regulatory affairs, the preclinical clinical Phase 1 and Phase 2 studies have been concluded to start Phase 3 studies in 2015. In the meantime, the research focuses on the still not completely understood mechanism how HP-β-CD can help to NPC patients (Tanaka et al. 2015; Davidson et al. 2016).
Cyclodextrins are used in autophagy, a catabolic process with an essential function in the maintenance of cellular and tissue homeostasis. It is primarily recognized for its role in the degradation of dysfunctional proteins and unwanted organelles. Actually, the range of substrates also includes lipids. Autophagy is a self-degradative process that is important for balancing sources of energy at critical times in development and in response to nutrient stress. It also plays a housekeeping role in removing misfolded or aggregated proteins, clearing damaged organelles, such as mitochondria, endoplasmic reticulum and peroxisomes, as well as eliminating intracellular pathogens. Thus, autophagy is generally thought of as a survival mechanism, although its deregulation has been linked to non-apoptotic cell death (Glick et al. 2010). As cellular membranes play important role in autophagy, their modulation by cyclodextrins modifies this important house-keeping process of the cells. However, the results of various studies seem to be controversial partly because different cell types, different cyclodextrins were used at different concentrations. The accumulation of autophagosomes, the intermediary products of autophagy, was interpreted both as a sign of activated and impaired autophagy. Hydroxypropyl-β-cyclodextrin treatment at high concentration leads to cholesterol depletion in an extent which leads to hindered fusion of the cellular membranes and this way to the diminished fusion of autophagosomes with lysosomes or to reduced expulsion of the autophagolysosomes. At low HP-β-CD concentrations, however, the autophagy is not perturbed or might be even improved. A combination of cholesterol removal by HP-β-CD and autophagy stimulation by rapamycin or carbamazepine seems to be a promising strategy in the treatment of impaired autophagy in lysosomal storage disorders, such as Niemann-Pick type C disease (Maetzel et al. 2014; Ward et al. 2016). Other interesting works have been published on cyclodextrin role (Yokoo et al. 2015; Tamura and Yui 2015; Motoyama et al. 2015, 2016; Manchon et al. 2016). Hydroxypropyl-β-cyclodextrin was found effective in inhibition of leukemic cell proliferation at various leukemic cell lines as recently reported by Arima’s group (Yokoo et al. 2015). Their results demonstrated that hydroxypropyl-β-cyclodextrin was a potential anticancer agent in leukemia. Further studies are needed to understand the effect of cyclodextrins on autophagy and specially to learn how these effects can be utilized in the therapy of various illnesses including neurodegenerative diseases and cancers.
Cyclodextrins are also used for prevention and treatment of atherosclerosis as recently reported by Zimmer et al. (2016). These authors published a comprehensively study on the anti-atherosclerotic and anti-inflammatory effects of hydroxypropyl-β-cyclodextrin used as solubilizing agent to increase cholesterol solubility. Atherosclerosis is a chronic inflammatory disease driven primarily by a continuous retention of cholesterol within the subendothelial space where it precipitates to form cholesterol crystals (CC). Despite ongoing advances in the prevention and treatment of atherosclerosis, cardiovascular disease remains the leading cause of death worldwide. Continuous retention of apolipoprotein B-containing lipoproteins in the subendothelial space causes a local overabundance of free cholesterol. Cyclodextrin treatment of murine atherosclerosis reduced atherosclerotic plaque size and CC load and promoted plaque regression even with a continued cholesterol-rich diet. Mechanistically, cyclodextrins increased oxysterol production in both macrophages and human atherosclerotic plaques and promoted liver X receptor-mediated transcriptional reprogramming to improve cholesterol efflux and exert anti-inflammatory effects. In vivo, this cyclodextrin-mediated liver X receptor agonist was required for the anti-atherosclerotic and anti-inflammatory effects of cyclodextrins as well as for augmented reverse cholesterol transport. Because cyclodextrin treatment in humans is safe and cyclodextrin beneficially affects key mechanisms of atherogenesis, it may therefore be used clinically to prevent or treat human atherosclerosis. The authors are optimistic as hydroxypropyl-β-cyclodextrin is a drug (it has received the orphan drug status against NPC).
A recent review on the effect of cyclodextrins on blood brain barrier (BBB) summarizes the findings of several research groups on various animal models of the central nervous system (CNS) diseases (Vecsernyés et al. 2014). The CD-mediated cholesterol modulations change the action of various proteins in the membrane of epithelial cells in BBB. These proteins play significant role in pathogenesis of stroke, cerebral hypoxia and ishemia, Alzheimer, Parkinson and Huttington disease, epilepsia, CNS infections, and brain tumor. Extensive research is going on to translate these effects into therapy.
The alarming spread of bacterial resistance to traditional antibiotics has warranted the study of alternative antimicrobial agents. Quorum sensing (QS) is a chemical cell-to-cell communication mechanism utilized by bacteria to coordinate group behaviors and establish infections (Hirakawa and Tomita 2013; Morohoshi et al. 2013; Miller et al. 2015; Okano et al. 2016). It is known that cyclodextrins can interact with N-acyl-L-homoserine lactones (AHLs), the main signaling molecules for the bacterial cell-to-cell communication QS system. Many bacteria regulate their cooperative activities through releasing, sensing and responding to small signaling molecules. This mechanism called quorum sensing makes possible for a population of bacteria to behave as a multi-cellular organism in host colonization, formation of biofilms, defense against competitors and adaptation to changing environment. Quorum sensing is a new target for the development of antibiotic agents. The QS system is regulated by three different-type of signals: AHLs and small peptides (called auto-inducing peptides, AIP) are produced in gram-negative and gram-positive bacteria, respectively, while production of the autoinducer-2 (AI-2) was known as a universal signal for responding to bacterial populations in both gram-negative and gram-positive bacteria. Inclusion complexes between cyclodextrins and bacterial signal molecules are responsible for inhibitory effects on quorum sensing. Since many bacteria have QS system for controlling gene expression in response to cell population density by means of signal molecules, an intercept of the quorum sensing signal onto the cyclodextrins can be a general method to control transcription of the QS-regulated genes. The concept of complexation of signal molecules (AHLs or peptides) by cyclodextrins does not aim to kill the bacteria but to control their growth and to decrease their virulence (Hirakawa and Tomita 2013). The development of novel strategies in the prevention and treatment of biofilm infections are expected in the next years. This will be useful not only in medicine but also in cosmetic, textile and packaging fields (Brackman et al. 2016; Silva et al. 2016).
Fluorophore-functionalized cyclodextrins have been extensively studied and successfully utilized in photodynamic therapy (PDT) (Mazzaglia et al. 2012). This minimally invasive therapeutic approach has proven to be efficient for the treatment of cancer and bacterial infections. In PDT, the photosensitizer gets excited and transfers the energy to nearby molecular oxygen photodonor. This leads to an in situ generation of singlet oxygen (1O2) offering advantages over conventional drugs (e.g. no drug resistance is developed). In the combined chemotherapy and PDT, the cavity of cyclodextrin is utilized as carrier for a drug with anticancer or antibiotic effect while the cavity entrance serves as molecular scaffold for attaching the photosensitizer (porphyrin or xanthene dye), nitric oxide releasing moiety, etc. (Králová et al. 2010; Fraix et al. 2015, 2016). The fluorescence of such nanoassemblies is utilized in in vitro experiments for imaging (Kirejev et al. 2014).
1.2.5 Cyclodextrins and Nanotechnology
Cyclodextrins and their derivatives have been successfully employed to create novel nanomaterials, often called nanosponges (Bilensoy and Hincal 2009; Goyal et al. 2011; Davis and Higson 2011; Tejashri et al. 2013; Chilajwar et al. 2014; Trotta et al. 2014, 2015; Mavridis and Yannakopoulou 2015). A broad spectrum of cyclodextrin-containing materials with versatile supramolecular architectures such as nanoparticles, nanosponges, nanomicelles, and nanovesicles has been synthetized to assemble functional platforms. For pharmaceutical and biomedical applications (Bilensoy and Hincal 2009; Goyal et al. 2011), nanomaterials can be formulated as oral, parenteral, topical or inhalation dosage forms. These materials have also found applications in nanomedicine.
At present, nanotechnology is receiving considerable acknowledgment due to its potential to combine features that are difficult to achieve by making use of a drug alone. Cyclodextrin-based nanomaterials are a contemporary approach for highlighting the advancements which could be brought about in a drug delivery system. Chilajwar et al. (2014) recently reported that statistical analyses have shown that around 40% of currently marketed drugs and about 90% of drugs in their developmental phase encounter solubility-related problems. Cyclodextrin-based nanosponges have the capacity to emerge as an innovative approach over conventional cycodextrins by overcoming the disadvantages associated with the latter. These novel class structures have been also developed since their use can improve a drug’s bioavailability by modifying the pharmacokinetic parameters of actives. Nanosponges offer high drug loading compared to other nanocarriers and are thus suitable for solving issues related to solubility, stability, and delayed release of actives (Tejashri et al. 2013). Although the methods of preparation are well-known, more information on their characterization is required to optimize their performance for therapeutic purposes and to demonstrate the role of the nanocavity in the complexation. Neutral, cationic and/or anionic amphiphilic cyclodextrins have been also proposed to increase interactions of cyclodextrins with biological membranes.
Cyclodextrin-based nanosponges have been also developed as tool for the delivery of anticancer drugs, e.g. paclitaxel, doxorubicin, 5-fluorourcil and tamoxifen, as reported by Trotta et al. (2014, 2015). These innovative materials can be considered as a challenging technology for the development of innovative formulations, suitable for various administration routes for anti-cancer drugs.
Metal organic frameworks (MOF) based on cyclodextrin have been developed by the group of 2016 Nobel laureate, Sir Stoddart (Smaldone et al. 2010). Mixing γ-cyclodextrin with alkali metal salts and alcohol (all edible constituents) robust, nanoporous MOF are obtained. On the other hand, nanoMOF of iron polycarboxylates were coated with cyclodextrin phosphates to obtain engineered nanoMOF for targeted drug delivery, catalysis, and sensing (Agostoni et al. 2015).
1.2.6 Cyclodextrins and Foods
In recent years, the growth of the functional foods industry has increased research into new compounds with high added value for use in the fortification of traditional products (Martina et al. 2013; López-Nicolás et al. 2014; Calo et al. 2015; Sharma and Baldi 2016; Kfoury et al. 2016; Fenyvesi and Szente 2016; Zarzycki et al. 2016). One of the most promising functional food groups is those enriched in antioxidant compounds of a lipophilic nature. In spite of the numerous advantages reported for such antioxidant molecules, they may also have disadvantages that impede their use in functional foods, although these problems may be avoided by the use of encapsulant agents such as cyclodextrins (Hedges et al. 1995; Appendini and Hotchkiss 2002; Szejtli 2004b; Szente and Szejtli 2004; Hashimoto 2006; Astray et al. 2009; Fang and Bhandari 2010; Fenyvesi et al. 2016).
It has been recently recognized that α-cyclodextrin being non-digestible is a dietary fiber with beneficial effects on digestion of fat and carbohydrates (Artiss et al. 2006). It has been marketed for body weight control in several countries. The antidiabetic effect has been recognized by European Food Safety Administration (EFSA) approving the health claim: “Consumption of alpha-cyclodextrin contributes to the reduction of the blood glucose rise after starch-containing meals” (EFSA 2012). The role of cyclodextrin molecules played in food technology and human nutrition have been recently reviewed (Fenyvesi et al. 2016).
An excellent review of the most recent studies on the complexes formed between several important types of antioxidant compounds and cyclodextrins was published by López-Nicolás et al. (2014). This comprehensive review focus on the contradictory data reported in the literature concerning the antioxidant activity of the host/guest molecule complexes, the different complexation constants reported for identical complexes, the bioavailability of the antioxidant compound in the presence of cyclodextrins and the recommendations concerning the use of natural or modified cyclodextrins. The authors also concluded that cyclodextrins will act as secondary antioxidants, enhancing the ability of traditional antioxidants to prevent enzymatic browning in different foods. Another interesting review on the encapsulation of antioxidants such as flavonoids and phenolic acids and their applications in food products, food supplements and also packaging has been published by Zarzycki et al. (2016).
Effects of encapsulation, such as solubility enhancement, protection, controlled release, improved organoleptic behavior and masked off-flavors, active packaging as well as improved handling and dosage were recently discussed in details in the review by Kfoury et al. (2016). The authors also gave a detailed evaluation of the experimental techniques used for studying the cyclodextrin/flavor complexes including static headspace gas chromatography, UV-visible and fluorescence spectroscopy, isothermal titration calorimetry, phase solubility studies, NMR spectroscopy, thermoanalytical methods and microscopy. There was an interesting large table listing the methods of complexation and analysis for over 100 aroma compounds and essential oils with significant references.
A general overview on the nano-encapsulation of flavors and aromas was also given in a chapter published by Fenyvesi and Szente (2016). Starting with the history dating back to the sixties of the last century, the advantages were summarized, the approval status of cyclodextrins in food was evaluated, and the methods of preparation and analysis were shortly outlined. Flavor complexes in food processing to reduce the loss in color, odor and taste were revealed. Several examples were given to illustrate the advantages of nanoencapsulation of flavors in aroma preserving food packaging.
An interesting effect of cyclodextrin encapsulation is in the container and wrapping materials for foods. Wasabi (Japanese horseradish) is a plant extract used for commercial antimicrobial packaging (Ko et al. 2012). The main active antimicrobial ingredient in wasabi extract is allyl-isothiocyanate (AIT). This substance is volatile and decomposes rapidly by oxidation. The AIT gas inhibits many fungi and bacteria. This extract has been encapsulated in cyclodextrin to control the volatility of AIT and to stabilize it in wrapping materials. The AIT in the encapsulated powder becomes volatile when the AIT-CD complex is exposed to high humidity conditions after the packaging of the food product. The evaporated AIT then migrates to the food surface, and inhibits the growth of aerobic bacteria. The AIT-CD complex powder has been incorporated in packaging materials of drip sheets, polymer films (polyethylene, nylon), and tablets (Han 2005; Sun 2012). These products are used for rice lunch boxes, meats, fresh produce, and raw fish (Sashimi) to keep them fresh, and are commercially available in Japan (under trade name Wasapower®).
1.2.7 Cyclodextrins and Cosmetics
Encapsulation techniques using cyclodextrins as (nano)-encapsulating agents are increasingly used not only by food, aroma, and pharmaceutical industries but also by cosmetic, fragrance and flavor industries, toiletry and personal care sectors for improving the efficiency of odorant and aroma substances, odor control in perfumes, masking unpleasant smells and tastes of some compounds, improving the physical and/or stability of essential oils and volatile compounds, stabilizing volatiles by reducing or eliminating any losses through evaporation, modifying the physicochemical and/or biological properties of the guest to afford a protective effect, or transforming of liquid compounds into crystalline form (Bilensoy 2011; Ammala 2013; Hougeir and Kircik 2012; Kfoury et al. 2016; Zarzycki et al. 2016; Fenyvesi and Szente 2016). The fragrance and flavor industry is a large and innovate sector of the chemical industry. Fragrance chemicals are added to consumer products such as personal care products, perfumes, deodorants, laundry detergents, etc. Encapsulation techniques using cyclodextrins are increasingly used by this industry for protecting fragile molecules (eye-drop solutions) and improving the efficiency of odorant and aroma substances, but also to avoid the degradation of flavors by processing or, on storage, allowing the use of minor amounts of flavors. Encapsulation of flavors by cyclodextrins is also an essential process that ensure controlled release, reduce volatility, increase solubility, dissolution and bioavailability, and decrease the allergic reactions (Szejtli 1982a, 1982b, 1996; Buschmann and Schollmeyer 2002, 2004; Hashimoto 1996, 2006; Venturini et al. 2008; Cabral Marques 2010; Bhaskara-Amrit et al. 2011; Bilensoy 2011; Tarimci 2011; Auzely-Vélty 2011). The use of these host compounds is also promising for various emerging fields such as aromatherapy and cosmeto-textiles – these new products also called ‘cosmeceuticals’ (Bilensoy 2011).
1.2.8 Cyclodextrins and Textiles
Textile finishing is crucial for giving textiles new functionalities and making them appropriate for special applications such as antimicrobial resistance and flame retardancy (Citernesi and Sciacchitano 1995; Buschmann et al. 1998; Hashimoto 2006; Grigoriu et al. 2008; Ammayappan and Moses 2009; Ripoll et al. 2010; Andreaus et al. 2010). In recent years, the use of low-environmental impact technologies based on sustainable substances such as polysaccharides (chitosan, alginate), cyclodextrins and others compounds (sericin protein) has been proposed as a novel possible route for large scale development of bioactive textiles (Bhaskara-Amrit et al. 2011; Voncina 2011; Voncina and Vivod 2013; Islam et al. 2013). Traditionally, chemicals such as inorganic salts (zinc pyrithione), phenolic derivatives, antibiotics, formaldehyde derivatives, dyes, and other compounds (triclosan) have been employed to impart antimicrobial activity to textile fibers. However, these compounds are not environmental friendly and some have toxic effects. With the public’s enhanced awareness of ecosafety, it is necessary to develop new strategies including both the use of natural products and the concept of green chemistry. Cyclodextrin molecules can be an ecofriendly alternative and used as finishing agents. In this sector, their main characteristics are: ecofriendly nature, cost-effectiveness and ease of production at large-scale, physicochemical (e.g. inclusion complex forming ability, solubilizing activity, chelating activity, slow release of fragrances) and biological (e.g. biocompatibility, biodegradability, drug carrier ability, insecticidal delivery) properties. These properties can be applied to different areas of applications such as deodorant (odor absorption, stabilization of active ingredients), fragrance/aroma, UV protection, water resistance, antimicrobial resistance, flame retardancy, and also insect repellent. In general, cyclodextrins are grafted in materials using binding and cross-linking agents. The comprehensive reviews of Islam et al. (2013) and Voncina and Vivod (2013), and the previous works by Hebeish and El-Hilw (2001), Martel et al. (2002), Romi et al. (2005), and Abdel-Halim et al. (2010) can be consulted. Cyclodextrin is considered as a promising reagent in textile finishing.
More and more research and practical use results also indicate that cyclodextrins might also act as active compounds in functionalized textiles called cosmeto-textiles. A cosmeto-textile allows the administration of active natural substances, like vitamins, oils and therapeutic extracts, simply and controllably. Neither in cosmetics nor textiles, the microencapsulated ingredients on cosmeto-textiles ensure their slimming, hydrating or perfuming progressive effect on the skin. It can also be used to change the surface properties of a fabric in order to make it self-cleaning, hydrophobic or lipophobic. Cyclodextrins may function as encapsulating, dispersing and levelling agents in the dyeing and washing of materials. Furthermore, as already mentioned, they may be anchored to polymers and fibers in order to impart target properties such as odor reduction, UV protection, or for the controlled release of perfumes, aromas, substances with therapeutical effects like antimicrobial properties, and also mosquito repellents. The techniques used to functionalize materials include microencapsulation, plasma, and sol-gel. The adhesion of the substances that functionalize textiles can be achieved physically or chemically. Voncina and Vivod (2013) reviewed the current state of the art concerning functionalization techniques and the methods used to characterize a functionalized fabric.
Cosmeto-textiles meet an increasing demand on the market and the cosmetic and textile industries are on the forefront of the research on this topic. These innovative materials are increasingly used not only in these sectors but also in pharmaceutical and medical industries, and in food packaging. Development of textiles with an antimicrobial activity can be also useful for water and air treatment.
1.2.9 Cyclodextrins and Separation Techniques
Chirality is an important modulator of the effects and properties of chiral substances not only in pharmacology, agrochemistry, food and environmental chemistry but also in all biological systems. As additives and/or chiral selectors, cyclodextrins and their derivatives have been used extensively in separation science because they have been shown to discriminate between positional isomers, functional groups, homologues and enantiomers. This property makes them one of the most useful agents for a wide variety of separations (Smolková-Keulemansová 1982; Ward and Armstrong 1988; Han and Armstrong 1989; Sybilska and Zukowski 1989; Menges and Armstrong 1991; Hilton and Armstrong 1991; Fanali 1993; Fanali et al. 1994; Schneiderman and Stalcup 2000; He and Beesley 2005; Vetter and Bester 2006). Cyclodextrin derivatives are more interesting than natives due to their higher solubility and increased selectivity introduced by the presence of non-ionic or ionic substituents on the cyclodextrin molecules. Cyclodextrins are used in liquid chromatography (LC), gas chromatography (GC), thin-layer chromatography, gel electrophoresis, capillary electrophoresis, electrokinetic chromatography, dialysis, separation on hollow fibers, solid- and liquid-phases extractions, liquid, gas and supercritical fluid chromatography, separation through liquid and composite membranes, and also molecularly imprinted polymers.
Although an important number of works have been published, the sector of cyclodextrin-based chromatography and electrophoresis continues to interest the scientific community (Hongdeng et al. 2011; Xiao et al. 2012; West 2014; Scriba 2016). The number of publications continues to grow not only in high performance LC, ultra-high performance LC, capillary chromatography and GC, but also in supercritical fluid chromatography (SFC). Actually, SFC is considered as a green separation technique, as it avoids the use of organic mobile phase, and is an ideal alternative technique with fast and efficient separation for the preparation and separation of pure substances e.g. enantioselective separation, chiral extraction, etc.).
Xiao et al. (2012) reported various synthetic and functional groups immobilization strategies of novel cyclodextrin chiral stationary phases for chromatography, in particular for supercritical fluid chromatography. The authors concluded that the use of high performance CD-based stationary phases for preparative supercritical fluid chromatography will likely play an important role for future pharmaceutical industry. The interest of supercritical fluid chromatography for screening methods and for the preparative scale was also showed by West (2014). While the pharmaceutical sector is the main application area of enantioselective separations, other industries such as agrochemicals and fragrances are also concerned.
Scriba (2016) recently published an interesting update review on the contributions to the understanding of the binding mechanism between chiral selectors and cyclodextrins in analytical enantioseparations dating between 2012 and early 2016. The author showed that many tools are available nowadays to study the mechanism of enantiorecognition including spectroscopic techniques (NMR) as well as molecular modeling for the visualization and analysis of the dynamics of the process. Selectors such as cyclodextrins appeared advantageous due to the much broader range of applications for structurally diverse analytes. Either random substituted cyclodextrins, which are mixtures of isomers with similar structure, or single isomer cyclodextrins are used. This high versatility makes cyclodextrins the first-choice selectors. The single isomers have the advantage of uniform structure, while the random substituted cyclodextrins often suffer from the batch-to-batch reproducibility (Li and Vigh 2004; Benkovics et al. 2016).
Lay et al. (2016) illustrated the exotic applications of imprinting techniques (IT) employing cyclodextrins. The exploitation of IT could produce products of molecularly imprinted polymers (MIP), which are very robust with long-term stability, reliability, cost-efficiency, and selectivity. MIP containing either cyclodextrin or its derivatives demonstrate superior binding effects for a target molecule e.g. steroidals, amino acids, polysaccharides, drugs, plant hormones, proteins, pesticides, and plastic additives. The authors concluded that cyclodextrins and their derivatives as emerging single or binary functional monomers are a versatile tool in separation science.
1.2.10 Cyclodextrins and Catalysis
Cyclodextrins have long been known to be good contributors to the development of catalytic processes. They have been used for mass-transfer catalytic reactions and for the production of new catalysts mimicking enzymatic activity (Macaev and Boldescu 2015; Hong et al. 2015). The following classification for catalytic applications of cyclodextrins was proposed by Monflier’s group (Hapiot et al. 2006, 2011, 2014): (i) to significantly increase the rate and selectivity of reactions catalyzed by water-soluble organometallic complexes; (ii) to design new water-soluble ligands for aqueous organometallic catalysis; (iii) to stabilize catalytically-active noble metal nanoparticles in water; and (iv) to facilitate reactions catalyzed by supported metals or metallic powder in water. Cyclodextrins were used in organic synthesis as promoters or catalysts of different reactions, as components of artificial enzymatic systems, as stabilizers for the nanodimensional metallic catalysts. A comprehensive collection of recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis can be found in the review by Hapiot et al. (2014).
As already mentioned, cyclodextrins have been used as separating agents for racemic mixtures or in chiral resolution of enantiomers. The formation of an inclusion complex between the β-CD unit of a catalytic system and a substrate leads to an increase of the local concentration of the latter and immobilizes it near the catalytically-active center. As a result, acceleration in the reaction rate, higher substrate selectivity, enantioselectivity and regioselectivity can often be achieved. Recently, Macaev and Boldescu (2015) published a state-of-the-art of cyclodextrins in asymmetric and stereospecific synthesis. Three topics were summarized: (1) cyclodextrins’ complexes with transition metals as asymmetric and stereospecific catalysts; (2) cyclodextrins’ non-metallic derivatives as asymmetric and stereospecific catalysts; and (3) cyclodextrins promoting asymmetric and stereospecific catalysis in water. The authors concluded that cyclodextrins and their derivatives can be a feasible alternative to traditional catalysts in a variety of reactions. Particularly, cyclodextrins can play the role of nanoreactors in which the asymmetric or stereospecific synthesis is facilitated. A wide range of stereospecific and asymmetric reactions can be performed with the application of non-metallic derivatives of cyclodextrins as catalysts. These include reactions of halogenation and hydrohalogenation, oxidation, reduction, hydrogenation, aldol reactions, photolysis, addition and substitution reactions. Finally, an interesting discussion was made on the effects of different factors such as correlation between the substrate molecular dimensions and the cyclodextrin cavity size, geometry in the approach of the substrate to the “active site”, influence of pH and temperature, on the enantioselectivity of these reactions.
1.2.11 Cyclodextrin-based Supramolecular Architectures
Supramolecular chemistry was defined as “the chemistry of non-covalent interactions” by the 1987 Nobel Prize Jean-Marie Lehn. These interactions can be used to form new nanoassemblies for multiple applications. The state of the art reviews of the design of complex macromolecular architectures based on cyclodextrin were presented by Schmidt et al. (2014) and Dong et al. (2015), and comprehensively discussed. In particular, by an elegant combination of dynamic/reversible structures with exceptional functions, functional supramolecular polymers are attracting increasing attention in various fields such as biomedical, e.g. gene transfection, protein delivery, bio-imaging and diagnosis, tissue engineering, and biomimetic chemistry, and material science (polymer science, nanotechnology). Although extensive work has been done on cyclodextrin-based supramolecular architectures, future research needs to take into account their precise physicochemical characterization Wenz et al. 2006; Wenz 2009; Li 2009; Hapiot et al. 2006; Harada et al. 2009a, 2009b; Dong et al. 2011, 2015; Schmidt et al. 2014; Agostoni et al. 2015; Karim and Loh 2016; Gontero et al. 2017; Valetti et al. 2017). Indeed, the understanding and the design of supramolecular systems require a detailed characterization with respect to stoichiometry, affinity, structure, heterogeneity, and supramolecular dynamics.
1.2.12 Cyclodextrins and Sugar-based Surfactants
Carbohydrate-based surfactants are today an important class of amphiphilic compounds which play an important role with both fundamental and practical applications. The growing interest in such compounds is due to, inter alia, their preparation from renewable raw materials, their ready biodegradability and biocompatibility, as well as other more basic reasons of practical, economic and environmental order. When complexed with cyclodextrins, carbohydrate-based surfactants considerably increase their performance and potential application range (Dodziuk 2006; Villalonga et al. 2007; Li et al. 2011). The formation of CD/surfactant host-guest compounds leads to an increase in the critical micelle concentration and in the solubility of surfactants. The use of these new systems is promising as reported by Valente and Söderman (2014).
1.2.13 Cyclodextrins and Click Chemistry
Click chemistry describes a family of modular, efficient, versatile and reliable reactions which have acquired a pivotal role as one of the most useful synthetic tools for functionalization of cyclodextrins with a potentially broad range of applications (Faugeras et al. 2012; Dondoni and Marra 2012; Schmidt et al. 2014; Hou et al. 2016). Cyclodextrins modified by the click reaction are building blocks of superstructures used for drug delivery systems (Venturini et al. 2008; Zhou and Ritter 2010; Nielsen et al. 2010), for the modification of macromolecular surfaces (Celebioglu et al. 2014a), for the preparation of cyclodextrin dimers and trimers (Mourer et al. 2008; Tungala et al. 2013), and for the generation of various glycoconjugates (glycopeptides, glycodendrimers, etc.) (Kushwaha et al. 2013; Dodziuk 2006; Bilensoy 2011). A tutorial review with the recent development in thiol-ene coupling for peptide glycosylation was published by Dondoni and Marra (2012). Cyclodextrins will play a very important role in all these new developments.
1.2.14 Cyclodextrins and Agrochemistry
An interesting sector for cyclodextrins is agrochemistry (Ho et al. 2014; Campos et al. 2015; Yusoff et al. 2016). Development of environmentally-friendly pesticides for sustainable agriculture is a key focus in the agrochemical industry. Identifying novel active ingredients and improving the delivery system of an active ingredient are the main challenges in developing new agrochemical formulations. Conventional formulations of agrochemicals are likely to contaminate the environment and there is a need for controlled-release formulations of agrochemicals to reduce pollution and health hazards. Strategies for the control of delivery systems for slow and sustained release of agrochemicals are of great interest to environmental scientists. In this context, the selection of materials to be used as carrier agents in agrochemical formulations is crucial. In recent years, the efficacy of cyclodextrins for pesticide formulations has been evaluated. Cyclodextrins can effectively encapsulate or bind the pesticide’s active ingredients in the material’s matrix with a sustained release profile and slow mobility in soil (Yusoff et al. 2016). It is well-known that cyclodextrin molecules form complexes with a wide variety of agricultural substances such as insecticides, fungicides, herbicides, repellents, pheromones, and growth regulators (Szente and Szejtli 1996; Morillo 2006; Luca and Grigoriu 2007; Venturini et al. 2008). This is at the origin of many benefits: modification of the physicochemical properties of the included guest, i.e., physical state, stability, solubility, and bioavailability, stabilization against the effects of light or biochemical degradation, and reduction of volatility. All these benefits are interesting during the preparation of the commercial formulations. It is important to note that most of the pesticides-CD inclusion complexes studied in the literature have used β-CD because of its lower price, and most of the published papers related to pesticides are not directly practice-oriented (Garrido et al. 2014; Fernandes et al. 2014).
1.2.15 Electrospinning of Functional Nanofibers with Cyclodextrins
Electrospinning (ES) is one of the most useful techniques for nanofibers (NF) production due to its versatility and cost-effectiveness. Discovered in the early 1900’s, ES is a long-known polymer processing technique that has recently been rediscovered to produce nanofibers/nanowebs in order to create many products for medical (medical devices, tissue engineering scaffolds), textile (clothing) and environmental (filtration media, membranes) applications. Nanofibers also represent high potential in other fields such as sensors, electronics, energy and biotechnology. The principle is simple: ES is an electro-hydrodynamic process in which a charged polymer jet is collected on a grounded collector; a rapidly rotating collector results in aligned nanofibers while stationary collectors result in randomly oriented fiber mats. The polymer jet is formed when an applied electrostatic charge overcomes the surface tension of the solution. There is a minimum concentration for a given polymer, termed the critical entanglement concentration, below which a stable jet cannot be achieved and no nanofiber will form - although nanoparticles may be achieved (electrospray). The characteristics of nanofibers such as high surface-to-volume, porous structure, controllable fiber diameter and variety of morphologies (core-shell, hollow, porous) can be manipulated by changing the parameters of the process (i.e. viscosity, conductivity, flow rate, concentration, solvent and needle type). The ultra-fine fibers are 1000 times smaller than a single human hair and range from about ten nanometers to few microns in diameter. ES nanofibers possess several remarkable characteristics such as very large surface-to-volume ratio, high porosity within the nanoscale, and unique physical and mechanical properties along with the flexibility for chemical/physical (multi-)functionalization. These properties and multi-functionality make them favorable candidates in many areas including textiles, energy, sensors, electronics, healthcare, environmental (filtration), food, packaging, and also agriculture.
A literature data survey shows that studies mostly focused on polymeric nanofibers and their functionalization. Indeed, electrospinning of nanofibers from polymer solution is very common since entanglements and overlapping between macromolecular chains play crucial role for uniform fiber formation. Polymers have to possess high molecular weight and high polymer concentrations are desirable. Numerous polymers including polystyrene, polyester, cellulose acetate, polylactic acid, polycaprolactone, and polyethylene oxide were studied. However, polymer-based nanofibers production requires organic solvents and this causes some problems in specific applications.
Recently, electrospinning of functional nanofibers with cyclodextrin molecules was proposed, and an important contribution was made by Uyar’s group on this topic (Celebioglu and Uyar 2012, 2013a, 2013b; Kayaci and Uyar 2012, 2014; Kayaci et al. 2013a, 2013b, 2013c, 2014, 2015; Celebioglu et al. 2014a, 2014b, 2016; Keskin et al. 2015a, 2015b; Aytac et al. 2015, 2016a, 2016b; Aytac and Uyar 2016, 2017; Senthamizhan et al. 2016). Functional nanofibers incorporating cyclodextrin were developed via ES using different polymers and reactions (cross-linking, grafting, click chemistry). Cyclodextrin molecules were also proposed to produce nanofibers without using any polymer carrier because cyclodextrin can form aggregates via intermolecular hydrogen bonding in their concentrated solutions or polymeric structures through cross-linked reactions. The functionalization of nanofibers with cyclodextrins and cyclodextrin inclusion complexes (denoted CD-ICs) is extremely attractive since electrospun nanofibers/nanowebs containing cyclodextrins and/or CD-ICs have unique characteristics that can potentially improve and broaden the application areas of cyclodextrins and electrospun nanofibers. Uyar and his research team proposed various materials: CD functionalized electrospinning polymeric nanofibers, molecular filters based on cyclodextrin functionalized electrospinning polymeric nanofibers, electrospinning of nanofibers from cyclodextrins, and electrospinning of CD-ICs nanofibers. Various applications were target of including functional materials for textile and medical textile, drug delivery, control release of antibacterials, packaging and food applications, complexation and microencapsulation (essential oils, antioxidants), and environmental purposes (e.g. filtration, purification and separation processes).
By means of the electrospinning technique, Uyar’s group have successfully produced cyclodextrin functionalized polymeric nanofibers using polymers such as polystyrene, polymethylmethacrylate and polyethylene oxide. These polymers were blended with α-, β- and γ-cyclodextrins and electrospinning into uniform nanofibers. The authors noted that the addition of cyclodextrin in the polymer solutions facilitated the ability to produce electrospun nanofibers from polymer solutions at low polymer concentration yielding in bead-free and uniform nanofibers. Cyclodextrin molecules were homogeneously distributed within the polymer matrix without forming phase separated crystalline aggregates. However, the choice of polymeric matrix is important in which polymer chains should not make inclusion complexation with cyclodextrin cavity and therefore cyclodextrin will be available for further complexation depending on the application target. Uyar’s group also demonstrated that the combination of cyclodextrins and the electrospinning nanofibers can potentially increase the efficiency of filters by facilitating complex formation with organic compounds and the very high surface area of the nanofibers.
1.2.16 Cyclodextrins and Remediation
In the last two decades, cyclodextrins as complexing agents have attracted considerable attention in environmental science in terms of removal of pollutants present in all environmental compartments, soils, air, waters and wastewaters, and sediments. Cyclodextrins can be used in soluble or insoluble form. In general, the removal of pollutants is mainly accounted for by the formation of inclusion processes due to cyclodextrin molecules and the specific structure of the material containing CDs used. Different materials have been proposed such as insoluble polymers, e.g. cross-linked structures, hydrogels and nanosponges, functionalized materials, e.g. polymers, silica and organic resins, organic-inorganic systems, membranes, and nanofibers (Crini and Morcellet 2002; Fakayode et al. 2007; Kozlowski and Sliwa 2010; Landy et al. 2012a, 2012b; Atteia et al. 2013; Morin-Crini and Crini 2014; Morin-Crini et al. 2015).
Soil flushing using cyclodextrin-based aqueous solutions was employed to solubilize pollutants. Cyclodextrin molecules are used as additives to enhance efficiencies and reduce the treatment time compared to the use of water alone or conventional surfactants. The review by Atteia et al. (2013) can be consulted on this topic.
Cyclodextrins found their role in the environmental risk management. Both risk assessment and risk reduction technologies may benefit from the CD’s ability of forming inclusion complexes with the typical organic contaminants in soils as reported by Gruiz et al. (2011) and Fenyvesi et al. (2016). The solubility enhancement of these compounds of usually poor water solubility can be utilized. Extraction of soils with aqueous solutions of well soluble cyclodextrin derivatives (HP-β-CD or RAMEB, randomly methylated-β-CD) gives information on the contaminant fraction easy to mobilize. As this fraction is the most available for the soil microbes, extracting the soil with HP-β-CD solution is considered as a measure of the bioavailable, biodegradable fraction. Nowadays, this method has become a part of the everyday protocol to predict the microbial bioavailability of organic pollutants of soils.
The aim of the risk reduction technologies is not only the removal of the contaminant from the soil but also destroying the polluting compounds by chemical or biological means. These technologies have improved economic parameters when performed in situ (no need of transporting the soil or its extract). Both in situ chemical oxidation and microbial degradation show improved efficiency when combined with CD flushing. The lab-scale experiments with various pollutants including monoaromatics such as benzene, toluene, ethylbenzene and xylenes, and BTEX, polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls (PCB), and motor oil proved that the bioavailability of the contaminants in bioremediation technologies is enhanced. RAMEB has a catalytic effect in in situ chemical oxidation of chlorinated solvents such as trichloroethylene. Several strictly controlled field demonstrations of the technologies showed that CD flushing can be adjusted to the current running technologies without special technical difficulties. The benefits are clear: saving time compared to the traditional technologies which may pay off the price of cyclodextrin. The vivid soil life proved that cyclodextrins were nontoxic adjuvants enhancing the bioavailability of the nutrients as well. The biodegradability of the cyclodextrin derivatives used in soil remediation is pre-requisite of their application. The cyclodextrin derivatives were degraded in a lower rate compared to the underivatized ones. However, even the less degradable RAMEB was slowly degraded by the microflora of contaminated soils.
Cyclodextrin-based materials for water and wastewater treatment include cross-linked polymers and nanosponges, membranes, nanofibers, and functionalized systems such as polymers, silica, and organic resins. Morin-Crini and Crini (2013) reviewed the developments in the use of cross-linked cyclodextrin-based polymers as complexing polymeric matrices for pollutant removal by oriented-adsorption processes. The authors summarized the features of these polymers and how they were used in decontamination applications. Numerous interesting studies on the treatment of real effluents can be found in the literature (Nagy et al. 2014; Khaoulani et al. 2015; Morin-Crini et al. 2015; Charles et al. 2016; Euvrard et al. 2016; Han et al. 2016; Alsbaiee et al. 2016). Imprinting technology (IT) makes possible to develop sorbents with extremely high specificity (Lay et al. 2016). Cyclodextrin-containing molecular imprinting polymers and cyclodextrins grafted to the surface of silica or other support via IT offer multiple binding sites with tunable surface properties for sorption of selected chemicals, e.g. perfluorinated compounds (Karoyo and Wilson 2015).
Nanofibers containing cyclodextrin molecules are another interesting material for pollutant removal. All the results published by Uyar’s group demonstrated that these new materials may have high potential to be used as air filters for the removal of organic vapor wastes, adsorbents/filters for pollutant removal from aqueous solutions, and also drug delivery system. The pollutants studied include dyes (Keskin et al. 2015a, 2015b), volatile organic compounds (VOC) (Celebioglu et al. 2016; Kayaci et al. 2015; Kayaci and Uyar 2014), aromatic derivatives such as PAH (Celebioglu et al. 2014a; Kayaci et al. 2013a; b; c), triclosan (Celebioglu et al. 2014b), metals (Senthamizhan et al. 2016) and drugs (Aytac et al. 2015). Electrospun nanofibers without having cyclodextrin were ineffective for entrapment of pollutants whereas cyclodextrin-based nanofibrous membranes can effectively entrap pollutants by taking advantage of the high surface-volume ratio of nanofibers with the added advantage of inclusion complexation capability of cyclodextrins presenting on the nanofiber surface. Uyar’s group proposed cyclodextrin functionalized cellulose acetate nanofibers prepared by combining electrospinning and click reaction as efficient adsorbent for the purpose of water purification and wastewater treatment containing PAH molecules (Celebioglu et al. 2014a). First, cyclodextrin molecules and cellulose acetate nanofibers were modified to obtain azide-CD and propargyl-terminated nanofibers, respectively. Then, click chemistry reaction was performed between these two derivatives to obtain permanent grafting of cyclodextrin molecules onto nanofibers surface. Click chemistry can be an interesting alternative to modify the surface of nanofibers because these reactions showed high yields. The adsorption capacity of cyclodextrin-grafted electrospun cellulose acetate nanofibers were determined by removing phenanthrene as guest model present in aqueous solutions. The material exhibited high adsorption properties and all the results were interpreted using adsorption surface due to the high surface area of nanofibers and chemisorption via inclusion complexation. Another alternative proposed was to pre-polymerize cyclodextrin onto electrospun polyester nanofibers using citric acid as cross-linking agent. However, although, nanofibers have shown enhanced mechanical and thermal properties, their surface areas were less important (Kayaci and Uyar 2012). Uyar’s group also published a series of papers on the electrospinning of nanofibers from non-polymeric systems such as native cyclodextrin molecules (Celebioglu and Uyar 2013a, 2013b, Celebioglu and Uyar 2012; Celebioglu et al. 2016). They have successfully performed electrospinning of nanofibers from α-CD, β-CD and γ-CD molecules and their derivatives (such as hydroxypropyl or methyl derivatives) without using a carrier polymer matrix. Similar to polymeric systems, the electrospinning of cyclodextrin solutions resulted in different morphologies and average fiber diameters depending on the cyclodextrin type and cyclodextrin concentration. Also, the success of the electrospinning of the cyclodextrin-nanofibers strongly depends on type of solvent (water, DMF) and intermolecular interactions between the cyclodextrin molecules. The existence of cyclodextrin aggregates via hydrogen bonding and very high solution viscosity and viscoelastic solid-like behavior of cyclodextrin solutions were found to be the key factors for obtaining bead-free nanofibers from cyclodextrin. The addition of urea disrupted cyclodextrin aggregates and lowered the viscosity significantly, and therefore, the urea-added cyclodextrin solutions yielded beaded fibers and/or beads. Using XRD data, the authors showed that electrospun cyclodextrin-nanofibers have amorphous characteristic without showing any particular orientation or crystalline aggregation of cyclodextrin molecules. Celebioglu and Uyar (2013b) demonstrated that γ-CD nanofibers were efficient for entrapping of aniline and toluene as guest VOC models by inclusion complexation whereas γ-CD in powder form did not show any entrapment ability. The driving forces of inclusion complex formation proposed were electrostatic interactions, Van der Waals contributions and hydrogen bonding. The inclusion complexation ability of cyclodextrin molecules was combined with very high surface area and versatile features of nanofibers. The authors concluded that electrospun cyclodextrin-nanofibers could serve as useful filtering material for air filtration purposes due to their molecular entrapment capability of volatile organic compounds.
There are several other interesting works for synthesizing membranes and nanofibers containing cyclodextrins (Meng et al. 2014; Xiao et al. 2014; Ghemati and Aliouche 2014; Zhao et al. 2015; Zhang et al. 2015; Wu et al. 2015a; Norena-Caro and Alvarez-Lainez 2016; Wei et al. 2016; Costoya et al. 2017). For example, Norena-Caro and Alvarez-Lainez (2016) proposed two different methods for the production of electrospun polyacrylonitrile (PAN) nanofibers containing cyclodextrin capable of capturing formaldehyde, a common indoor pollutant. The former comprised the addition of cyclodextrins to PAN/dimethyl sulfoxide solutions and the subsequent electrospinning of the mixture. The latter involved the crosslinking of cyclodextrins on electrospun PAN fibers by alkaline hydrolysis and esterification with citric acid. The results showed that both functionalized nanofibers might be used for indoor air purification. However, functionalized fibers obtained by addition of cyclodextrins were more effective for capturing formaldehyde than fibers obtained by crosslinking of cyclodextrins.
Cyclodextrin-functionalized silica networks used as adsorbents/filters for environmental applications has recently received a lot of attention as reported by Gibson (2014), Samiey et al. (2014), Lee and Park (2015), Dinker and Kulkarni (2015), Vunain et al. (2016), Mahmud and Wilson (2016), and Yamamoto and Kuroda (2016). Two main class of materials have been proposed: cyclodextrin-functionalized silicas prepared through grafting reactions and cyclodextrin-silica hybrid systems prepared through sol-gel or self-assembly process. These materials could be applied in the elimination, enrichment and detection of environmental pollutants in air and water samples.
Actually, fundamental research is also focusing on cyclodextrin-based nanoparticles for environmental applications (Taka et al. 2017). Indeed, nanosponges have not only been explored for their pharmaceutical applications but for water purification and wastewater treatment (Landy et al. 2012a; Tong and Chen 2013; Taka et al. 2017). This emerging technology of cyclodextrin-based nanosponges is expected to provide technical solutions to water treatment.
To conclude, the environmental application of cyclodextrins was one of the focuses of the 18th International Cyclodextrin Symposium held in Gainesville on May 19-21, 2016. Two of the 4 invited lectures, 7 of 24 oral presentations and 6 of 31 posters were related to this topic. However, in spite of numerous results, publications, and patents, cyclodextrins are still not used for soil remediation and water treatment. Why? It is difficult to respond.
1.2.17 Cyclodextrins: Other Interesting Selected Reviews and Works
Szente et al. (2016) published a comprehensive overview on the methods used for analysis of cyclodextrins and their derivatives. The paper intends to act as a guide for the readers in looking around the classical and modern instrumental analytical methods suitable for identification, characterization and determination of cyclodextrins themselves, cyclodextrins in finished products or even in biological samples. A thorough analytical characterization of cyclodextrin complexes is of fundamental importance to provide an adequate support in selection of the most suitable cyclodextrin for each guest molecule, and also in view of possible future marketing of drug-cyclodextrin formulations for example. However, the analytical characterization of drug-cyclodextrin solid systems and the assessment of the actual inclusion complex formation is not a simple task and involves the combined use of several analytical techniques as reported by Mura (2015). In this review, the author also presented a general prospect of the principal analytical techniques which can be employed for a suitable characterization of drug-cyclodextrin systems in the solid state, evidencing their respective potential advantages and limits. The applications of each examined technique were described and discussed by pertinent examples from literature.
Curcumionoids such as curcumin and related compounds are well-known, in particular in South Asia for their antioxidant, antibacterial, antifungal, anticancer and anti-inflammatory effects. Curcumin/CD formulations for application as food supplement are on the market. Recently, the benefits in cystic fibrosis and Alzheimer disease have also been proved in cellular and animal models (Higdon and Drake 2014). Curcumin is an oil-soluble polyphenol pigment, practically insoluble in water at acidic and neutral pH, but soluble in alkali. It has low bioavailability owing to the poor aqueous solubility. Recent studies showed that natural cyclodextrin molecules can enhance notably the solubility of curcumin. This result in enhanced bioavailability and improved clinical effects (e.g. aggregation of curcumin/CD complex in spherical nanoparticles form helps the cell penetration). Complexation with cyclodextrins also protects from decomposition upon UV-exposure. Further results on prostate cancers are expected in the near future (Boztas et al. 2013; Zhang et al. 2016).
Rezanka (2016) recently reviewed monosubstituted cyclodextrin derivatives with particular focus on the synthesis of allyl, cinnamyl, propargyl, formylmethyl, carboxymethyl, azido and amino derivatives. Synthesis and modification methods of magnetic chitosan materials containing cyclodextrins were reviewed along with some interesting applications in analytical separations by Tong and Chen (2013). Magnetic nanoparticles grafted with cyclodextrin were developed for various applications, such as solid phase extraction that is for sample concentration prior analysis, for magnetoresponsive drug delivery (Banerjee and Chen 2008) and for magnetic manipulation of cholesterol removal (Horák et al. 2017).
The advances in the developments of supramolecular hydrogels based on the polypseudorotaxanes and polyrotaxanes formed by inclusion complexes of cyclodextrins threading onto polymer chains were detailed by Li (2009). Both physical and chemical hydrogels of many different types were discussed with respect to their preparation, structure, property, and gelation mechanism. A large number of physical supramolecular hydrogels were formed induced by self-assembly of densely packed cyclodextrin rings threaded on polymer or copolymer chains acting as physical crosslinking points. The thermo-reversible and thixotropic properties of these hydrogels had inspired their applications as injectable drug delivery systems. Slide-ring gels of extreme swelling capacity are formed by threading cyclodextrin polymers onto polymer backbones (Kali et al. 2015; Murakami et al. 2017). Chemical supramolecular hydrogels synthesized from polypseudorotaxanes and polyrotaxanes were based on the chemical crosslinking of either the cyclodextrin molecules or the included polymer chains. The chemical supramolecular hydrogels were often made biodegradable through incorporation of hydrolyzable threading polymers, end caps, or cross-linkers, for their potential applications as biomaterials.
While interactions of cyclodextrins with metal ions have been studied for decades, structurally well-defined systems are relatively rare and the application of cyclodextrin-based functional materials is in its initial phase. The review published by Prochowicz et al. (2016) focuses on the synthesis, reactivity and structural diversity of well-defined metal complexes derived essentially from native cyclodextrins. Various structural motifs for metal complexes based on cyclodextrins were delineated ranging from monomeric species, dinuclear systems, homo- and heterometallic sandwich-type complexes to cylindrical, extended structures. The reported examples are discussed with an emphasis placed on how the character of used metal or auxiliary ions, and the formation of intra- and/or intermolecular hydrogen bonds can influence the mode of aggregation and supramolecular arrangement of the resulting metal complexes.
Letort et al. (2016) provided an update on the current use of cyclodextrins against organophosphorus compound intoxications. Organophosphorus pesticides and nerve agents play a determinant role in the inhibition of cholinesterases. The cyclic structure of cyclodextrin molecules and their toroidal shape are perfectly suitable to design new chemical scavengers able to trap and hydrolyze the organophosphorus compounds before they reach their biological target.
Cyclodextrin-based nanosponges found a broad range of applications. However, their structural and dynamic characterization is still a challenge, mainly due to their amorphous state. Mele’s group demonstrated that NMR spectroscopy, especially high resolution magic angle spinning techniques, is an interesting tool to obtain information not only on their structure but also on the dynamics of organic molecules encapsulated in these polymeric hydrogels (Mallard et al. 2015; Ferro et al. 2014, 2016).
1.3 Conclusion
This chapter gives an overview of recent selected works on cyclodextrins used in various fields. Large-scale applications of cyclodextrins have been made possible by their ease of production and low cost, in particular for the native molecules, non-toxicity, biocompatibility and their ability to form inclusion complexes. This is the basis for their applications in the pharmaceutical, food, and cosmetic industries. Other practical and industrial applications have been reported in other sectors such as chromatography and biomedicine. The increasing number of publications on cyclodextrins shows that there is always an increasing interest in these molecules and their applications from academic and practical point of views. The emergence of cyclodextrins as active and smart molecules rather than complexing molecules in numerous cosmetic, textile, therapeutic and biomedical products seems to be the next step in the development of cyclodextrin technology. Further studies, contributions, and industrial developments are expected in the near future in the following domains: catalysis, bacterial resistance, anticancer drugs (chemotherapy), magnetic resonance imaging, biotechnology, e.g. biotransformation, fermentation processing, enzyme models, peptide and protein delivery, material science, e.g. wrapping materials and packaging, cosmeto-textiles, agrochemistry, and sensor applications.
References
Abdel-Halim ES, Fouda MMG, Hamdy I, Abdel-Mohdy FA, El-Sawy SM (2010) Incorporation of chlorohexidin diacetate into cotton fabrics grafted with glycidyl methacrylate and cyclodextrin. Carbohydr Polym 79:47–53. https://doi.org/10.1016/j.carbpol.2009.07.050
Agostoni V, Horcajada P, Noiray M, Malanga M, Aykaç A, Jicsinszky L, Vargas-Berenguel A, Semiramoth N, Daoud-Mahammed S, Nicolas V, Martineau C, Taulelle F, Vigneron J, Etcheberry F, Serre C, Gref R (2015) A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci Rep 5:7925. https://doi.org/10.1038/srep07925
Ahuja A, Baboota S, Ali J, Mustafa G (2011) Cyclodextrins as potential excipients in pharmaceutical formulations: solubilizing and stabilizing effects. In: Bilensoy E (ed) Cyclodextrins in pharmaceutics, cosmetics and biomedicine: current and future industrial. John Wiley. Chapter 2, Hoboken, pp 19–43. https://doi.org/10.1002/9780470926819
Alsbaiee A, Smith BJ, Xiao L, Ling YH, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 529:190–U146. https://doi.org/10.1038/nature16185
Ammala A (2013) Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. Int J Cosmet Sci 35:113–124. https://doi.org/10.1111/ics.12017
Ammayappan L, Moses JJ (2009) An overview on application of cyclodextrins in textile product enhancement. J Text Assoc 70:9–18
Andreaus J, Dalmolin MC, De Oliveira IB, Barcellos IO (2010) Application of cyclodextrins in textile processes. Quim Nova 33:929–937. https://doi.org/10.1590/S0100-40422010000400031
Appendini P, Hotchkiss JH (2002) Review of antimicrobial food packaging. Innovative Food Sci Emerg Technol 3:113–126. https://doi.org/10.1016/S1466-8564(02)00012-7
Armspach D, Gattuso G, Koeniger R, Stoddart JF (1999) Cyclodextrins. In: Hecht SM (ed) Bioorganic chemistry: carbohydrates. Oxford University Press, New York, pp 458–488
Artiss JD, Brogan K, Brucal M, Moghaddam M, Jen KL (2006) The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism 55:195–202
Assaf KI, Gabel D, Zimmermann W, Nau WM (2016) High-affinity host-guest chemistry of large-ring cyclodextrins. Org Biomol Chem 14:7702–7706. https://doi.org/10.1039/c6ob01161f
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gandara J (2009) A review on the use of cyclodextrins in foods. Food Hydrocoll 23:1631–1640. https://doi.org/10.1016/j.foodhyd.2009.01.001
Atteia O, Estrada ED, Bertin H (2013) Soil flushing: a review of the origin of efficiency variability. Rev Environ Sci Biotechnol 12:379–389. https://doi.org/10.1007/s11157-013-9316-0
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry. Marcel Dekker, Inc., New-York
Atwood JL, Steed JW (2009) Supramolecular chemistry, 2nd edn. John Wiley, Chichester
Atwood JL, Davies EE, MacNicol DD (1984) Inclusion compounds. Academic Press, Michigan
Auzely-Vélty R (2011) Self-assembling polysaccharide systems based on cyclodextrin complexation: synthesis, properties and potential applications in the biomaterials field. CR Chim 14:167–177. https://doi.org/10.1016/j.crci.2010.04.019
Aytac Z, Uyar T (2016) Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur Polym J 79:140–149. https://doi.org/10.1016/j.eurpolymj.2016.04.029
Aytac Z, Uyar T (2017) Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: enhanced water solubility and slow release of curcumin. Int J Pharm 518:177–184. https://doi.org/10.1016/j.ijpharm.2016.12.061
Aytac Z, Sen HS, Durgun E, Uyar T (2015) Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B Biointerfaces 128:331–338. https://doi.org/10.1016/j.colsurfb.2015.02.019
Aytac Z, Yildiz ZI, Kayaci F, San NO, Kusku SI, Durgun E, Tekinay T, Uyar T (2016a) Fast-dissolving, prolonged release and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J Agric Food Chem 64:7325–7334. https://doi.org/10.1021/acs.jafc.6b02632
Aytac Z, Yildiz ZI, Kayaci F, San NO, Tekinay T, Uyar T (2016b) Electrospinning of polymer-free cyclodextrin/geraniol-inclusion complex nanofibers: enhanced shelf-life of geraniol with antibacterial and antioxidant properties. RSC Adv 6:46089–46099. https://doi.org/10.1039/C6RA07088D
Banerjee SS, Chen DH (2008) Cyclodextrin conjugated magnetic colloidal nanoparticles as a nanocarrier for targeted anticancer drug delivery. Nanotechnology 19:265602. https://doi.org/10.1088/0957-4484/19/26/265602
Bar R (1996) Applications of cyclodextrins in biotechnology. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, Volume 3. Pergamon Oxford, London, pp 423–440
Bender H (1986) Production, characterization and applications of cyclodextrins. In: Liss AR (ed) Advances in biotechnological processes. Wiley. Chapter 6, New York, pp 31–71
Bender ML, Komiyama M (1978) Cyclodextrins chemistry. Springer Verlag, Berlin
Benkovics G, Fejős I, Darcsi A, Varga E, Malanga M, Fenyvesi É, Sohajda T, Szente L, Sz B, Szemán J (2016) Single-isomer carboxymethyl-γ-cyclodextrin as chiral resolving agent for capillary electrophoresis. J Chromatogr A 1467:445–453. https://doi.org/10.1016/j.chroma.2016.06.083
Berendt RT, Sperger DM, Isbester PK, Munson EJ (2006) Solid-state NMR spectroscopy in pharmaceutical research and analysis. TrAC Trends Anal Chem 25:977–984. https://doi.org/10.1016/j.trac.2006.07.006
Bergeron RJ (1984) Cycloamylose-substrate binding. In: Inclusion compounds - physical properties and applications. Atwood JL, Davies JED, MacNicol DD, eds. London: Academic Press. Volume 3, chapter 12, pp 391-443. ISBN-13: 978-0120671038
Bhaskara-Amrit UR, Agrawal PB, Warmoeskerken MMCG (2011) Applications of β-cyclodextrins in textiles. Autex Res J 11:94–101. http://autexrj.org/No4-2011-/0020_11.pdf
Bhosale SV, Bhosale SV (2007) Beta-cyclodextrin as a catalyst in organic synthesis. Mini-Rev Org Chem 4:231–242. https://doi.org/10.2174/157019307781369922
Bilensoy E (2011) Cyclodextrins in pharmaceutics, cosmetics and biomedicine: current and future industrial applications. John Wiley, Hoboken, 395 p. https://doi.org/10.1002/9780470926819
Bilensoy E, Hincal AA (2009) Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opin Drug Deliv 6:1161–1173. https://doi.org/10.1517/17425240903222218
Booij LHDJ (2009) Cyclodextrins and the emergence of Sugammadex. Anaesthesia 64:31–37. https://doi.org/10.1111/j.1365-2044.2008.05868.x
Boztas AO, Karakuzu O, Galante G, Ugur Z, Kocabas F, Altuntas CZ, Yazaydin AO (2013) Synergistic interaction of paclitaxel and curcumin with cyclodextrin polymer complexation in human cancer cells. Mol Pharm 10:2676–2683. https://doi.org/10.1021/mp400101k
Brackman G, Garcia-Fernandez MJ, Lenoir J, De Meyer L, Remon JP, De Beer T, Concheiro A, Alvarez-Lorenzo C, Coenye T (2016) Dressings loaded with cyclodextrin-hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol Biosci 16:859–869. https://doi.org/10.1002/mabi.201500437
Brandariz I, Iglesias E (2013) Local anesthetics: acid-base behavior and inclusion with cyclodextrins. Curr Org Chem 10:3050–3063. https://doi.org/10.2174/13852728113179990023
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 59:645–666. https://doi.org/10.1016/j.addr.2007.05.012
Buschmann HJ, Schollmeyer EJ (2002) Applications of cyclodextrins in cosmetic products: a review. J Cosmet Sci 53:185–191
Buschmann HJ, Schollmeyer E (2004) Cosmetic textiles: A new functionality of clothes. Cosmet Toiletries 11:105–112
Buschmann HJ, Denter U, Knittel D, Schollmeyer E (1998) The use of cyclodextrins in textile processes - an overview. J Text Inst 89:554–561. https://doi.org/10.1080/00405009808658641
Cabral Marques HM (2010) A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J 25:313–326. https://doi.org/10.1002/ffj.2019
Cairo MR (2011) Structural aspects of crystalline derivatized cyclodextrins and their inclusion complexes. Curr Org Chem 15:815–830. https://doi.org/10.2174/138527211794518862
Caliceti P, Salmaso S, Bersani S (2010) Polysaccharide-based anticancer prodrugs. In: Reddy LH, Couvreur P (eds) Macromolecular anticancer therapeutics. Springer, New York, pp 163–166
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems - a review. Food Control 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Campos EVR, de Oliveira JL, Fraceto LF, Singh B (2015) Polysaccharides as safer release systems for agrochemicals. Agron Sustain Dev 35:47–66. https://doi.org/10.1007/s13593-014-0263-0
Celebioglu A, Uyar T (2012) Electrospinning of nanofibers from non-polymeric systems: polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 4:621–631. https://doi.org/10.1039/c1nr11364j
Celebioglu A, Uyar T (2013a) Electrospinning of nanofibers from non-polymeric systems: electrospun nanofibers from native cyclodextrins. J Colloid Interface Sci 404:1–7. https://doi.org/10.1016/j.jcis.2013.04.034
Celebioglu A, Uyar T (2013b) Electrospun gamma-cyclodextrin (gamma-CD) nanofibers for the entrapment of volatile organic compounds. RSC Adv 3(45):22891–22895. https://doi.org/10.1039/C3RA44870C
Celebioglu A, Demirci S, Uyar T (2014a) Cyclodextrin-grafted electrospun cellulose acetate nanofibers via click reaction for removal of phenanthrene. Appl Surf Sci 305:581–588. https://doi.org/10.1016/j.apsusc.2014.03.138
Celebioglu A, Umu OCO, Tekinay T, Uyar T (2014b) Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloids Surf B Biointerfaces 116:612–619. https://doi.org/10.1016/j.colsurfb.2013.10.029
Celebioglu A, Sen HS, Durgun E, Uyar T (2016) Molecular entrapment of volatile organic compounds (VOCs) by electrospun cyclodextrin nanofibers. Chemosphere 144:736–744. https://doi.org/10.1016/j.chemosphere.2015.09.029
Charles J, Bradu C, Morin-Crini N, Sancey B, Winterton P, Torri G, Badot PM, Crini G (2016) Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: Chemical abatement. J Saudi Chem Soc 20:185–194. https://doi.org/10.1016/j.jscs.2013.03.007
Chen G, Jiang M (2001) Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem Soc Rev 40:2254–2266. https://doi.org/10.1039/c0cs00153h
Chilajwar SV, Pednekar PP, Jadhav KR, Gupta GJC, Kadam VJ (2014) Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin Drug Deliv 11:111–120. https://doi.org/10.1517/17425247.2014.865013
Citernesi U, Sciacchitano M (1995) Cyclodextrins in functional dermocosmetics. Cosmetics Toiletries 110:53–61
Clarke RJ, Coates JH, Lincoln SF (1988) Inclusion complexes of the cyclomalto-oligosaccharides (cyclodextrins). Adv Carbohydr Chem Biochem 46:205–249. https://doi.org/10.3998/ark.5550190.0006.e14
Concheiro A, Alvarez-Lorenzo C (2013) Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv Drug Deliv Rev 65:1188–1203. https://doi.org/10.1016/j.addr.2013.04.015
Connors KA (1997) The stability of cyclodextrin complexes in solution. Chem Rev 97:1325–1357. https://doi.org/10.1021/cr960371r
Costoya A, Concheiro A, Alvarez-Lorenzo C (2017) Electrospun fibers of cyclodextrins and poly(cyclodextrins). Molecules 22:230. https://doi.org/10.3390/molecules22020230
Cram DJ (1988) The design of molecular hosts, guests and their complexes. Angew Chem Int Ed 27(8):1009–1020. https://doi.org/10.1002/anie.198810093
Crini G (2006) Non-conventional adsorbents for dye removal: A review. Bioresour Technol 97:1061–1085. https://doi.org/10.1016/j.biortech.2005.05.001
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. https://doi.org/10.1021/cr500081p
Crini G, Morcellet M (2002) Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci 25:789–813. https://doi.org/10.1002/1615-9314(20020901)25:13<789::AID-JSSC789>3.0.CO;2-J
Davidson CD, Fishman YI, Puskás I, Szemán J, Sohajda T, McCauliff LA, Sikora J, Storch J, Vanier MT, Szente L, Walkley SU, Dobrenis K (2016) Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann Clin Transl Neuro 3:366–380. https://doi.org/10.1002/acn3.306
Davis ME, Brewster ME (2004) Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 3:1023–1035. https://doi.org/10.1038/nrd1576
Davis F, Higson S (2011) Cyclodextrins. In: Davis F, Higson S (eds) Macrocycles: construction, chemistry and nanotechnology applications. John Wiley, United Kingdom. Chapter 6
Decock G, Fourmentin S, Surpateanu GG, Landy D, Decock P, Surpateanu G (2006) Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins. Supramol Chem 18:477. https://doi.org/10.1080/10610270600665749
Decock G, Landy D, Surpateanu G, Fourmentin S (2008) Study of the retention of aroma components by cyclodextrins by static headspace gas chromatography. J Incl Phenom Macrocycl Chem 62:297. https://doi.org/10.1007/s10847-008-9471-z
Deorsola AC, Mothé CG, de Oliviera LG, Deorsola AB (2014) Technological monitoring of cyclodextrin – World panorama. World Patent Inf 39:41–49. https://doi.org/10.1016/j.wpi.2014.06.004
Dinker MK, Kulkarni PS (2015) Recent advances in silica-based materials for the removal of hexavalent chromium: a review. J Chem Eng Data 60:2521–2540. https://doi.org/10.1021/acs.jced.5b00292
Dodziuk H (2002) Introduction to supramolecular chemistry. Kluwer Academic Publishers, Dordrecht. 10.1007/0-306-47587-1 (e-book)
Dodziuk H (ed) (2006) Cyclodextrins and their complexes: chemistry, analytical methods, applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 489p. https://doi.org/10.1002/3527608982
Donati F (2011) Sugammadex: a cyclodextrin-based novel formulation and marketing story. In: Bilensoy E (ed) Cyclodextrins in pharmaceutics, cosmetics and biomedicine: current and future industrial applications. John Wiley. Chapter 19, Hoboken, pp 636–370. https://doi.org/10.1002/9780470926819
Dondoni A, Marra A (2012) Recent applications of thiol-ene coupling as a click process for glycoconjugation. Chem Soc Rev 41:573–586. https://doi.org/10.1039/c1cs15157f
Dong HQ, Li YY, Li L, Shi DL (2011) Cyclodextrins/polymer based (pseudo)polyrotaxanes for biomedical applications. Prog Chem 23:914–922
Dong RJ, Zhou YF, Huang XH, Zhu XY, Lu YF, Shen J (2015) Functional supramolecular polymers for biomedical applications. Adv Mater 27:498–526. https://doi.org/10.1002/adma.201402975
Duchêne D (ed) (1987) Cyclodextrins and their industrial uses. Éditions de Santé, Paris
Duchêne D (ed) (1991) New trends in cyclodextrins and derivatives. Éditions de Santé, Paris
Duchêne D, Vaution C, Glomot F (1986) Cyclodextrins, their value in pharmaceutical technology. Drug Dev Ind Pharm 12:2193–2215
EFSA (2012) Scientific Opinion on the substantiation of health claims related to alpha-cyclodextrin and reduction of post prandial glycaemic responses (ID 2926, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 10:2713–2730. https://doi.org/10.2903/j.efsa.2012.2713
Endo T (2011) Large-ring cyclodextrins. Trends Glycosci Glycotechnol 23:79–92. https://doi.org/10.4052/tigg.23.79
Endo T, Ueda H (2004) Large ring cyclodextrins - recent progress. Fabad. J Pharm Sci 29:27–38. https://doi.org/10.1002/chin.200638261
Euvrard E, Morin-Crini N, Druart C, Bugnet J, Martel B, Cosentino C, Moutarlier V, Crini G (2016) Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein J Org Chem 12:1826–1838. https://doi.org/10.3762/bjoc.12.172
Fakayode SO, Lowry M, Fletcher KA, Huang XD, Powe AM, Warner IM (2007) Cyclodextrins host-guest chemistry in analytical and environmental chemistry. Curr Anal Chem 3:171–181. https://doi.org/10.2174/157341107781023811
Fanali S (1993) Use of cyclodextrins in capillary electrophoresis. In: Capillary electrophoresis technology. Guzman NA, ed. New York: Marcel Dekker Inc. Chromatographic Science Series, Volume 64, part V, pp. 731–752. ISBN: 0-8247-9042-1
Fanali S, Cristalli M, Vespalec R, Bocek P (1994) Chiral separations in capillary electrophoresis. In: Chrambach A, Dunn MJ, Radola BJ (eds) Advances in electrophoresis. VCH Verlagsgesellschaft mbH. Chapter 7, Weinheim, pp 1–88
Fang Z, Bhandari B (2010) Encapsulation of polyphenols- A review. Trends Food Sci Technol 21:510–523. https://doi.org/10.1016/j.tifs.2010.08.003
Faugeras PA, Boens B, Elchinger PH, Brouillette F, Montplaisir D, Zerrouki R, Lucas R (2012) When cyclodextrins meet click chemistry. Eur J Org Chem (22):4087–4105. https://doi.org/10.1002/ejoc.201200013
Fenyvesi É (1988) Cyclodextrin polymers in the pharmaceutical industry. J Incl Phenom 6:537–545. https://doi.org/10.1007/BF00660751
Fenyvesi É, Szente (2016) Nanoencapsulation of flavors and aromas by cyclodextrins. In: Encapsulations: nanotechnology in the agri-food industry. Grumezescu A, ed. 1st edition. Volume 2, chapter 18, pp. 769–792. ISBN: 978-0-12-804378-3
Fenyvesi É, Vikmon MA, Szente L (2016) Cyclodextrins in food technology and human nutrition: benefits and limitations. Crit Rev Food Sci Nutr 56:1981–2004. https://doi.org/10.1080/10408398.2013.809513
Fernandes C, Encarnação I, Gaspar A, Garrido J, Borges F, Garrido EM (2014) Influence of hydroxypropyl-cyclodextrin on the photostability of fungicide pyrimethanil. Int J Photoenergy 2014:1–8. https://doi.org/10.1155/2014/489873
Ferro M, Castiglione F, Punta C, Meloni L, Panzeri W, Rossi B, Trotta F, Mele A (2014) Anomalous diffusion of ibuprofen in cyclodextrin nanosponge hydrogels: an HRMAS NMR study. Beilstein J Org Chem 10:2715–2723. https://doi.org/10.3762/bjoc.10.286
Ferro M, Castiglione F, Punta C, Meloni L, Panzeri W, Rossi B, Trotta F, Mele A (2016) Transport properties of ibuprofen encapsulated in cyclodextrin nanosponge hydrogels: a proton HR-MAS NMR spectroscopy study. J Vis Exp 114:53769. https://doi.org/10.3791/53769
Fraix A, Marino N, Sortino S (2015) Phototherapeutic release of nitric oxide with engineered nanoconstructs. In: Sortino S (ed) Light-responsive nanostructured systems for applications in nanomedicine. Springer, New York. https://doi.org/10.1007/978-3-319-22942-3_8
Fraix A, Marino N, Sortino S (2016) Phototherapeutic release of nitric oxide with engineered nanoconstructs. Top Curr Chem 370:225–257. https://doi.org/10.1007/978-3-319-22942-3_8
Frömming KH, Szejtli J (1994) Cyclodextrins in pharmacy. Kluwer Academic Publishers, Dordrecht
Fülöp Z, Kurkov SV, Nielsen TT, Larsen KL, Loftsson T (2012) Self-assembly of cyclodextrins: formation of cyclodextrin polymer based nanoparticles. J Drug Delivery Sci Technol 22:215–221. https://doi.org/10.1016/S1773-2247(12)50032-8
Garrido J, Cagide F, Melle-Franco M, Borges F, Garrido EM (2014) Microencapsulation of herbicide MCPA with native β-cyclodextrin and its methyl and hydroxypropyl derivatives: an experimental and theoretical investigation. J Mol Struct 1061:76–81. https://doi.org/10.1016/j.molstruc.2013.12.067
Ghemati D, Aliouche D (2014) Dye adsorption behavior of polyvinyl alcohol/glutaraldehyde/beta-cyclodextrin polymer membranes. J Appl Spectrosc 81(2):257–263. https://doi.org/10.1007/s10812-014-9919-4
Gibson LT (2014) Mesosilica materials and organic pollutant adsorption: Part B - Removal from aqueous solution. Chem Soc Rev 43(15):5173–5182. https://doi.org/10.1039/C3CS60095E
Glick D, Barth S, MacLeod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Gontero D, Lessard-Viger M, Brouard D, Bracamonte AG, Boudreau D, Veglia AV (2017) Smart multifunctional nanoparticles design as sensors and drug delivery systems based on supramolecular chemistry. Microchem J 130:316–328. https://doi.org/10.1016/j.microc.2016.10.007
Gonzalez-Gaitano G, Rodriguez P, Isasi JR, Fuentes M, Tardajos G, Sanchez M (2003) The aggregation of cyclodextrins as studied by photon correlation spectroscopy. J Incl Phenom Macrocycl Chem 44:101–105. https://doi.org/10.1023/A:1023065823358
Gould S, Scott RC (2005) 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol 43:1451–1459. https://doi.org/10.1016/j.fct.2005.03.007
Goyal AK, Johal ES, Rath G (2011) Nanotechnology for water treatment. Curr Nanosci 7:640–654. https://doi.org/10.2174/157341311796196772
Grigoriu AM, Luca C, Grigoriu A (2008) Cyclodextrins applications in the textile industry. Cellulose. Chem Technol 42:103–112
Gruiz K, Molnár M, Fenyvesi É, Cs H, Atkári Á, Barkács K (2011) Cyclodextrins in innovative engineering tools for risk-based environmental management. J Incl Phenom Macrocycl Chem 70:299–306. https://doi.org/10.1007/s10847-010-9909-y
Grumezescu AM (2016) Encapsulations. In: Nanotechnology in the agri-food industry. Academic Press Elsevier. 1st edition. vol 2, 924 p. ISBN: 978-0-12-804378-3
Guo R, Wilson LD (2013) Cyclodextrin-based microcapsule materials - their preparation and physiochemical properties. Curr Org Chem 17:14–21. https://doi.org/10.2174/138527213805289204
Han JH (2005) Innovations in food packaging. Food science and technology, international series. ISBN: 0-12-311632-5. 503 p
Han SM, Armstrong DW (1989) HPLC separation of enantiomers and other isomers with cyclodextrin-bonded phases: rules for chiral recognition. In: Krstulovic AM (ed) Chiral separations by HPLC. Ellis Horwood Limited, John Wiley & Sons. Chapter 10, New York, p 208
Hapiot F, Tilloy S, Monflier E (2006) Cyclodextrins as supramolecular hosts for organometallic complexes. Chem Rev 106:767–781. https://doi.org/10.1021/cr050576c
Hapiot F, Ponchel A, Tilloy S, Monflier E (2011) Cyclodextrins and their applications in aqueous-phase metal-catalyzed reactions. CR Chimie 14:149–166. https://doi.org/10.1016/j.crci.2010.04.003
Hapiot F, Bricout H, Menuel S, Tilloy S, Monflier E (2014) Recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis. Cat Sci Technol 4:1899–1908. https://doi.org/10.1039/C4CY00005F
Harada A, Takashima Y, Yamaguchi H (2009a) Cyclodextrin-based supramolecular polymers. Chem Soc Rev 38:875–882. https://doi.org/10.1039/B705458K
Harada A, Takashima Y, Yamaguchi H (2009b) Polymeric rotaxanes. Chem Rev 109:5974–6023. https://doi.org/10.1021/cr9000622
Harada A, Takashima Y, Nakahata M (2014) Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc Chem Res 47:2128–2140. https://doi.org/10.1021/ar500109h
Hashimoto HJ (1996) Cyclodextrins in foods, cosmetics, and toiletries. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 483–502
Hashimoto H (2002) Present status of industrial application of cyclodextrins in Japan. J Incl Phenom 44:57–62. https://doi.org/10.1023/A:1023036406829
Hashimoto H (2006) Cyclodextrin applications in food, cosmetic, toiletry, textile and wrapping materiel fields. In: Dodziuk H (ed) Cyclodextrins and their Complexes. Chemistry, Analytical Methods, Applications. Wiley-VCH, Verlag GmbH & Co. KGaA, Weinheim., Chapter 16, pp 452–459. https://doi.org/10.1002/3527608982.ch16
He LF, Beesley TE (2005) Applications of enantiomeric gas chromatography: A review. J Liq Chromatogr Relat Technol 28:1075–1114. https://doi.org/10.1081/JLC-200052997
He Y, Fu P, Shen X, Gao H (2008) Cyclodextrin-based aggregates and characterization by microscopy. Micron 39:495–516. https://doi.org/10.1016/j.micron.2007.06.017
Hebeish A, El-Hilw ZH (2001) Chemical finishing of cotton using reactive cyclodextrin. Color Technol 117:104–110. https://doi.org/10.1111/j.1478-4408.2001.tb00343.x
Hedges AR (1998) Industrial applications of cyclodextrins. Chem Rev 98:2035–2044. https://doi.org/10.1021/cr970014w
Hedges AR, Shieh WJ, Sikorski CT (1995) Use of cyclodextrins for encapsulation in the use and treatment of food products. In: Risch SJ, Reineccius GA. (eds) ACS Sym SerEncapsulation and controlled release of food ingredients, vol 590. American Chemical Soc, Washington, DC, pp 60–71
Herbstein FH (2005) Cyclodextrins, and some analogs, as hosts. In: Crystalline molecular complexes and compounds. Oxford Science Publications, vol 1. Oxford University Press, New York, p 73
Higdon J, Drake JV (2014) Curcumin. Available from: Linus Pauling Institute, Micronutrient Research for Optimum Health, Oregon State University. http://lpi.oregonstate.edu/infocenter/phytochemicals/curcumin
Hilton ML, Armstrong DW (1991) Contribution of cyclodextrins and derivatives to liquid chromatography. In: Duchêne D (ed) New trends in cyclodextrins and derivatives. Éditions de Santé, Paris, pp 517–549
Hirakawa H, Tomita H (2013) Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front Microbiol 4:114. https://doi.org/10.3389/fmicb.2013.00114
Ho TM, Howes T, Bhandari BR (2014) Encapsulation of gases in powder solid matrices and their applications: a review. Powder Technol 259:87–108. https://doi.org/10.1016/j.powtec.2014.03.054
Hong SB, Liu MY, Zhang W, Deng W (2015) Organic reactions catalyzed by cyclodextrin and its derivatives. Chin J Org Chem 35:325–336. https://doi.org/10.6023/cjoc201409001
Hongdeng Q, Xiaojing L, Min S, Shengxiang J (2011) Development of silica-based stationary phases for high-performance liquid chromatography. Anal Bioanal Chem 399:3307–3322. https://doi.org/10.1007/s00216-010-4611-x
Horák D, Beneš M, Procházková Z, Trchová M, Borysov A, Pastukhov A, Paliienko K, Borisova T (2017) Effect of O-methyl-β-cyclodextrin-modified magnetic nanoparticles on the uptake and extracellular level of l-glutamate in brain nerve terminals. Colloids Surf B Biointerfaces 149:64–71. https://doi.org/10.1016/j.colsurfb.2016.10.007
Hou XS, Ke CF, Stoddart JF (2016) Cooperative capture synthesis: yet another playground for copper-free click chemistry. Chem Soc Rev 45:3766–3780. https://doi.org/10.1039/c6cs00055j
Hougeir EG, Kircik L (2012) A review of delivery systems in cosmetics. Dermatol Therm 25:234–237. https://doi.org/10.1111/j.1529-8019.2012.01501.x
Iacovino R, Caso JV, Di Donato C, Malgieri G, Palmieri M, Russo L, Isernia C (2017) Cyclodextrins as complexing agents: preparation and applications. Curr Org Chem 21:1–15. https://doi.org/10.2174/1385272820666160909111842
Idriss H, Estour F, Zgani I, Barbot C, Biscotti A, Petit S, Galaup C, Hubert-Roux M, Nicol L, Mulder P, Gouhier G (2013) Effect of the second coordination sphere on new contrast agents based on cyclodextrin scaffolds for MRI signals. RSC Adv 3:4531. https://doi.org/10.1039/C3RA40314A
Irie T, Uekama K (1997) Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci 86:147–162. https://doi.org/10.1021/js960213f
Islam SU, Shahid M, Mohammad F (2013) Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers - a review. Ind Eng Chem Res 52:5245–5260. https://doi.org/10.1021/ie303627x
Jicsinszky L, Fenyvesi É, Hashimoto H (1996) Cyclodextrin derivatives. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3, London, Pergamon Oxford, pp 57–188
Junthip J, Tabary N, Chai F, Leclercq L, Maton M, Cazaux F, Neut C, Paccou L, Guinet Y, Staelens JN, Bria M, Landy D, Hedoux A, Blanchemain N, Martel B (2016) Layer-by-layer coating of textile with two oppositely charged cyclodextrin polyelectrolytes for extended drug delivery. J Biomed Mater Res A 104:408–424. https://doi.org/10.1002/jbm.a.35674
Kainuma K (1984) Starch oligosaccharides: Linear, branched, and cyclic. In: Whistler RL, JN BM, Paschall EF (eds) Starch: chemistry and technology, 2nd edn. Academic Press, London, pp 125–152. 978-0-12-746270-7
Kali G, Eisenbarth H, Wenz G (2015) One pot synthesis of a polyisoprene polyrotaxane and conversion to a slide-ring gel. Macromol Rapid Commun. https://doi.org/10.1002/marc.201500548
Kano K (1993) Selectivities in cyclodextrin chemistry. In: Dugas H, Schmidtchen FP (eds) Bioorganic Chemistry Frontiers, vol 3. Springer-Verlag, Berlin, pp 1–23. 978-3-642-78110-0
Kanwar JR, Long BM, Kanwar RK (2011) The use of cyclodextrins nanoparticles for oral delivery. Curr Med Chem 18:2079–2085
Karim AA, Loh XJ (2016) Towards cyclodextrin-based supramolecular materials. In: Polymers for personnal care products and cosmetics. Chapter 9, pp 154–177. https://doi.org/10.1039/9781782623984-00154
Karoyo AH, Wilson LD (2015) Nano-sized cyclodextrin-based molecularly imprinted polymer adsorbents for perfluorinated compounds - a mini-review. Nanomaterials 5:981–1003. https://doi.org/10.3390/nano5020981
Kayaci F, Uyar T (2012) Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem 133:641–649. https://doi.org/10.1016/j.foodchem.2012.01.040
Kayaci F, Uyar T (2014) Electrospun polyester/cyclodextrin nanofibers for entrapment of volatile organic compounds. Polym Eng Sci 54(12):2970–2978. https://doi.org/10.1002/pen.23858
Kayaci F, Aytac Z, Uyar T (2013a) Surface modification of electrospun polyester nanofibers with cyclodextrin polymer for the removal of phenanthrene from aqueous solution. J Hazard Mater 261:286–294. https://doi.org/10.1016/j.jhazmat.2013.07.041
Kayaci F, Umu OCO, Tekinay T, Uyar T (2013b) Antibacterial electrospun polylactic acid (PLA) nanofibrous webs incorporating triclosan/cyclodextrin inclusion complexes. J Agric Food Chem 61:3901–3908. https://doi.org/10.1021/jf400440b
Kayaci F, Ertas Y, Uyar T (2013c) Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J Agric Food Chem 61:8156–8165. https://doi.org/10.1021/jf402923c
Kayaci F, Sen HS, Durgun E, Uyar T (2014) Functional electrospun polymeric nanofibers ıncorporating geraniol-cyclodextrin ınclusion complexes: high thermal stability and enhanced durability of geraniol. Food Res Int 62:424–431. https://doi.org/10.1016/j.foodres.2014.03.033
Kayaci F, Sen HS, Durgun E, Uyar T (2015) Electrospun nylon 6,6 nanofibers functionalized with cyclodextrins for removal of toluene vapor. J Appl Polym Sci 132:41941. https://doi.org/10.1002/app.41941
Keskin NOS, Celebioglu A, Uyar T, Tekinay T (2015a) Microalgae immobilized by nanofibrous web for removal of reactive dyes from wastewater. Ind Eng Chem Res 54:5802–5809. https://doi.org/10.1021/acs.iecr.5b01033
Keskin NOS, Celebioglu A, Sarioglu OF, Ozkan AD, Uyar T, Tekinay T (2015b) Removal of a reactive dye and hexavalent chromium by a reusable bacteria attached electrospun nanofibrous web. RSC Adv 5:86867–86874. https://doi.org/10.1039/C5RA15601G
Kfoury M, Hădărugă NG, Hădărugă DI, Fourmentin S (2016) Cyclodextrins as encapsulation material for flavors and aroma. In: Encapsulations: nanotechnology in the agri-food industry, vol 2, 1st edn, pp 127–192. 978-0-12-804378-3
Khan AR, Forgo P, Stine KJ, D’Souza VT (1998) Methods for selective modifications of cyclodextrins. Chem Rev 98:1977–1996. https://doi.org/10.1021/cr970012b
Khaoulani S, Chaker H, Cadet C, Bychkov E, Cherif L, Bengueddach A, Fourmentin S (2015) Wastewater treatment by cyclodextrin polymers and noble metal/mesoporous TiO2 photocatalysts. CR Chimie 18:23–31. https://doi.org/10.1016/j.crci.2014.07.004
Kirejev V, Gonçalves AR, Aggelidou C, Manet I, Mårtensson J, Yannakopoulou K, Ericson MB (2014) Photophysics and ex vivo biodistribution of β-cyclodextrin-meso-tetra(m-hydroxyphenyl)porphyrin conjugate for biomedical applications. Photochem Photobiol Sci 13:1185–1191. https://doi.org/10.1039/c4pp00088a
Ko JA, Jeon JY, Park HJ (2012) Preparation and characterization of allyl isothiocyanate microcapsules by spray drying. J Food Biochem 36:255–261. https://doi.org/10.1111/j.1745-4514.2010.00533.x
Komiyama M (1996) Cyclodextrins as enzyme models. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 401–422
Komiyama M, Monflier E (2006) Cyclodextrin catalysis. In: Dodziuk H (ed) Cyclodextrins and their complexes: chemistry, analytical methods, applications. Wiley-VCH, Verlag GmbH & Co. KGaA, Weinheim. Chapter 4, pp 93–105
Kozlowski CA, Sliwa W (2008) The use of membranes with cyclodextrin units in separation processes: recent advances. Carbohydr Polym 74:1–9. https://doi.org/10.1016/j.carbpol.2008.01.010
Kozlowski CA, Sliwa W (2010) Use of cyclodextrin polymers in separation of organic species. In: Polymer science and technology series. Nova Science Publishers, New York
Králová J, Kejík Z, Bríza T, Poucková P, Král A, Martásek P, Král V (2010) Porphyrin-cyclodextrin conjugates as a nanosystem for versatile drug delivery and multimodal cancer therapy. J Med Chem 53:128–138. https://doi.org/10.1021/jm9007278
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453:167–180. https://doi.org/10.1016/j.ijpharm.2012.06.055
Kushwaha D, Dwivedi P, Kuanar SK, Tiwari VK (2013) Click reaction in carbohydrate chemistry: recent developments and future perspective. Curr Org Synth 10:90–135. https://doi.org/10.2174/1570179411310010005
Landy D, Mallard I, Ponchel A, Monflier E, Fourmentin S (2012a) Cyclodextrins for remediation technologies. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry for a sustainable world: nanotechnology and health risk, vol 1. Springer Verlag, Berlin, pp 47–81
Landy D, Mallard I, Ponchel A, Monflier E, Fourmentin S (2012b) Remediation technologies using cyclodextrins: an overview. Environ Chem Lett 10:225–237. https://doi.org/10.1007/s10311-011-0351-1
Larsen KL (2002) Large cyclodextrins. J Incl Phenom 43:1–13. https://doi.org/10.1023/A:1020494503684
Lay S, Ni XF, Yu HN, Shen SR (2016) State-of-the-art applications of cyclodextrins as functional monomers in molecular imprinting techniques: a review. J Sep Sci 39:2321–2331. https://doi.org/10.1002/jssc.201600003
Lee SY, Park SJ (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11. https://doi.org/10.1016/j.jiec.2014.09.001
Letort S, Balieu S, Erb W, Gouhier G, Estour F (2016) Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds. Beilstein J Org Chem 12:204–228. https://doi.org/10.3762/bjoc.12.23
Li J (2009) Cyclodextrin inclusion polymers forming hydrogels. In: Wenz G (ed) Inclusion polymers, Advances in polymer science, vol 222, pp 79–112. https://doi.org/10.1007/12_2008_9
Li S, Purdy WC (1992) Cyclodextrins and their applications in analytical chemistry. Chem Rev 92:1457–1470. https://doi.org/10.1021/cr00014a009
Li S, Vigh G (2004) Single-isomer sulfated alpha-cyclodextrins for capillary electrophoresis: Hexakis(2,3-di-O-methyl-6-O-sulfo)-alpha-cyclodextrin, synthesis, analytical characterization, and initial screening tests. Electrophoresis 25:2657–2670. https://doi.org/10.1002/elps.200405839
Li ZF, Wang M, Wang F, Gu ZB, Du GC, Wu J, Chen J (2007) gamma-Cyclodextrin: a review on enzymatic production and applications. Appl Microbiol Biotechnol 77:245–255. https://doi.org/10.1007/s00253-007-1166-7
Li JJ, Zhao F, Li J (2011) Polyrotaxanes for applications in life science and biotechnology. Appl Microbiol Biotechnol 90:427–443. https://doi.org/10.1007/s00253-010-3037-x
Lincoln SF, Easton CJ (1998) Inclusion complexes of the cyclodextrins. In: Polysaccharides: structural diversity and functional versatility. Dumitriu S, ed. New York: Marcel Dekker, Inc.. Chapter 14, pp 473–521. ISBN: 0-8247-0127-5
Loeve S, Normand M (2011) How to trust a molecule? The case of cyclodextrins entering the naorealm. In: Zülsdorf TB, Coenen C, Fiedeler U, Ferrari A, Milburn C, Wienroth M (eds) Quantum engagements: social reflections of nanoscience and emerging techologies. IOS Press AKA Verlag, Heidelberg, pp 195–216
Loftsson T, Brewster ME (2010) Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol 62:1607–1621. https://doi.org/10.1111/j.2042-7158.2010.01030.x
Loftsson T, Brewster ME (2012) Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci 101:3019–3032. https://doi.org/10.1002/jps.23077
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications: historical perspectives. Int J Pharm 329:1–11. https://doi.org/10.1016/j.ijpharm.2006.10.044
Loftsson T, Masson M, Brewster ME (2004) Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93:1091–1099. https://doi.org/10.1002/jps.20047
López-Nicolás JM, Rodríguez-Bonilla P, García-Carmona F (2014) Cyclodextrins and antioxidants. Crit Rev Food Sci Nutr 54:251–276. https://doi.org/10.1080/10408398.2011.582544
Luca C, Grigoriu AM (2006) Cyclodextrins inclusion compounds. Rev Chim 57:248–252
Luca C, Grigoriu AM (2007) Cyclodextrins inclusion compounds in macromolecular chemistry. Cellul Chem Technol 41:1–12
Macaev F, Boldescu V (2015) Cyclodextrins in asymmetric and stereospecific synthesis. Symmetry 7:1699–1720. https://doi.org/10.3390/sym7041699
Macaev F, Boldescu V, Geronikaki A, Sucman N (2013) Recent advances in the use of cyclodextrins in antifungal formulations. Curr Top Med Chem 21:2677–2683. https://doi.org/10.2174/15680266113136660194
Maciollek A, Ritter H, Beckert R (2013) Superstructures of fluorescent cyclodextrin via click-reaction. Beilstein J Org Chem 9:827–831. https://doi.org/10.3762/bjoc.9.94
Maetzel D, Sarkar S, Wang H, Abi-Mosleh L, Xu P, Cheng AW, Gao Q, Mitalipova M, Jaenisch R (2014) Genetic and chemical correction of cholesterol accumulation and impaired autophagy in hepatic and neural cells derived from Niemann-Pick Type C patient-specific iPS cells. Stem Cell Rep 2:866–880. https://doi.org/10.1016/j.stemcr.2014.03.014
Mahmud ST, Wilson LD (2016) Synthesis and characterization of surface-modified mesoporous silica materials with beta-cyclodextrin. Cogent Chem 2:1132984. https://doi.org/10.1080/23312009.2015.1132984
Malanga M, Bálint M, Puskás I, Tuza K, Sohajda T, Jicsinszky L, Szente L, Fenyvesi É (2014) Synthetic strategies for the fluorescent labeling of epichlorohydrin-branched cyclodextrin polymers. Beilstein J Org Chem 10:3007–3018. https://doi.org/10.3762/bjoc.10.319
Mallard I, Baudelet D, Castiglione F, Ferro M, Panzeri W, Ragg E, Mele A (2015) Polydisperse methyl beta-cyclodextrin-epichlorohydrin polymers: variable contact time C-13 CP-MAS solid state NMR characterization. Beilstein J Org Chem 11:2785–2794. https://doi.org/10.3762/bjoc.11.299
Manchon JFM, Uzor NE, Kesler SR, Wefel JS, Townley DM, Nagaraja AS, Pradeep S, Mangala LS, Sood AK, Tsvetkov AS (2016) TFEB ameliorates the impairment of the autophagy-lysosome pathway in neurons induced by doxorubicin. Aging 8:3507–3519. https://doi.org/10.18632/aging.101144
Martel B, Morcellet M, Ruffin D, Vinet F, Weltrowski L (2002) Capture and controlled release of fragrances by CD finished textiles. J Incl Phenom Macrocycl Chem 44:439–442. https://doi.org/10.1023/A:1023028105012
Martin Del Valle EM (2004) Cyclodextrins and their uses. Process Biochem 39:1033–1046. https://doi.org/10.1016/S0032-9592(03)00258-9
Martina K, Binello A, Lawson D, Jicsinszky L, Cravotto G (2013) Recent applications of cyclodextrins as food additives and in food processing. Curr Nutr Food Sci 9:167–179. https://doi.org/10.2174/1573401311309030001
Mavridis IM, Yannakopoulou K (2015) Anionic cyclodextrins as versatile hosts for pharmaceutical nanotechnology: synthesis, drug delivery, enantioselectivity, contrast agents for MRI. Int J Pharma 492:275–290. https://doi.org/10.1016/j.ijpharm.2015.06.004
Mazzaglia A, Sciortino T, Kandoth N, Sortino S (2012) Cyclodextrin-based nanoconstructs for photoactivated therapies. J Drug Deliv Sci Technol 22:235–242. https://doi.org/10.1016/S1773-2247(12)50034-1
Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Ruiz i, Altaba A (2014) The river blindness drug ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med 6:1263–1278. https://doi.org/10.15252/emmm.201404084
Meng QR, Bai J, Li CP, Huang YR, Liu H, Li HQ (2014) Electrospun functional cyclodextrins/polystyrene (PS) composite nanofibers and their applications for sorption of Cu (II) ions under aqueous solution. Nanosci Nanotechnol Lett 6:289–294. https://doi.org/10.1166/nnl.2014.1768
Menges RA, Armstrong DW (1991) Chiral separations using native and functionalized cyclodextrin-bonded stationary phases in high-pressure liquid chromatography. In: Ahuja S (ed) Chiral Separations by Liquid Chromatography, ACS symposium series 471. Chapter 4, Washington, p 67. isbn:0841221162
Messner M, Kurkov SV, Jansook T, Loftsson T (2010) Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm 387:199–208. https://doi.org/10.1016/j.ijpharm.2009.11.035
Mikus P, Sebesta R, Kaniansky D, Salisova M (2002) Cyclodextrins and their complexes - structure and interactions. Chem List 96:693–697
Miller KP, Wang L, Chen YP, Pellechia PJ, Benicewicz BC, Decho AW (2015) Engineering nanoparticles to silence bacterial communication. Front Microbiol 6:189. https://doi.org/10.3389/fmicb.2015.00189
Mocanu G, Vizitiu D, Carpov A (2001) Cyclodextrins polymers. J Bioact Compat Polym 16:315–342
Morillo E (2006) Application of cyclodextrins in agrochemistry. In: Dodziuk H (ed) Cyclodextrins and their Complexes: chemistry, analytical methods, applications. Wiley-VCH, Verlag GmbH & Co. KGaA. Chapter 16, Weinheim, pp 459–467
Morin-Crini N, Crini G (2013) Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog Polym Sci 38:344–368. https://doi.org/10.1016/j.progpolymsci.2012.06.005
Morin-Crini N, Fourmentin S, Crini G (2015) Cyclodextrines (in French). Besançon: PUFC. 370 p. ISBN: 978-2-84867-520-6
Morohoshi T, Tokita K, Ito S, Saito Y, Maeda S, Kato K, Ikeda T (2013) Inhibition of quorum sensing in gram-negative bacteria by alkylamine-modified cyclodextrins. J Biosci Bioeng 116:175–179. https://doi.org/10.1016/j.jbiosc.2013.01.022
Mosher G, Thompson DO (2002) Complexation and cyclodextrins. In: Swarbrick J, JCE B (eds) Encyclopedia of pharmaceutical technology, 2nd edn. Marcel Dekker, New York, pp 531–558
Mosinger J, Tomankova V, Nemcova I, Zyka J (2001) Cyclodextrins in analytical chemistry. Anal Lett 34:1979–2004. https://doi.org/10.1081/AL-100106834
Motoyama K, Hirai Y, Nishiyama R, Maeda Y, Higashi T, Ishitsuka Y, Kondo Y, Irie T, Era T, Arima H (2015) Cholesterol lowering effects of mono-lactose-appended beta-cyclodextrin in Niemann-Pick type C disease-like HepG2 cells. Beilstein J Org Chem 11:2079–2086. https://doi.org/10.3762/bjoc.11.224
Motoyama K, Nishiyama R, Maeda Y, Higashi T, Kawaguchi Y, Futaki S, Ishitsuka Y, Kondo Y, Irie T, Era T, Arima H (2016) Cholesterol-lowering effect of octaarginine-appended beta-cyclodextrin in Npc1-Trap-CHO cells. Biol Pharm Bull 39:1823–1829. https://doi.org/10.1248/bpb.b16-00369
Mourer M, Hapiot F, Monflier E, Menuel S (2008) Click chemistry as an efficient tool to access β-cyclodextrin dimers. Tetrahedron 64:7159–7163. https://doi.org/10.1016/j.tet.2008.05.095
Moya-Ortega M, Alvarez-Lorenzo C, Concheiro A, Loftsson T (2012) Cyclodextrin-based nanogels for pharmaceuticals and biomedical applications. Int J Pharm 428:152–163. https://doi.org/10.1016/j.ijpharm.2012.02.038
Mura P (2015) Analytical techniques for characterization of cyclodextrin complexes in the solid state: a review. J Pharm Biomed Anal 113:226–238. https://doi.org/10.1016/j.jpba.2015.01.058
Murakami T, Schmidt BVKJ, Brown HR, Hawker CJ (2017) Structural versatility in slide-ring gels: influence of co-threaded cyclodextrin spacers. J Polym Sci A Polym Chem 55:1156–1165. https://doi.org/10.1002/pola.28490
Nagy ZM, Molnár M, Fekete-Kertész I, Molnár-Perl I, Fenyvesi É, Gruiz K (2014) Removal of emerging micropollutants from water using cyclodextrins. Sci Total Environ 485-486:711–719. https://doi.org/10.1016/j.scitotenv.2014.04.003
Nepogodiev S, Fraser Stoddart J (1998) Cyclodextrin-based catenanes and rotaxanes. Chem Rev 98:1959–1576. https://doi.org/10.1021/cr970049w
Nielsen TT, Wintgens V, Amiel C, Wimmer R, Larsen KL (2010) Facile synthesis of β-cyclodextrin-dextran polymers by “click” chemistry. Biomacromol 11:1710–1715. https://doi.org/10.1021/bm9013233
Norena-Caro D, Alvarez-Lainez M (2016) Functionalization of polyacrylonitrile nanofibers with beta-cyclodextrin for the capture of formaldehyde. Mater Des 95:632–640. https://doi.org/10.1016/j.matdes.2016.01.106
Okano C, Nasuno E, Iimura K, Kato N (2016) Cyclodextrin-immobilized microspheres for uptake of the quorum-sensing signaling molecule N-acylhomoserine lactone. J Appl Polym Sci 133:43198. https://doi.org/10.1002/app.43198
Oliveri V, Vecchio G (2016) Cyclodextrins as protective agents of protein aggregation: an overview. Chem Asian J 11:1648–1657. https://doi.org/10.1002/asia.201600259
Ozbilgin S, Yilmaz O, Ergur BU, Hanci V, Ozbal S, Yurtlu S, Gunenc SF, Kuvaki B, Kucuk BA, Sisman AR (2016) Effectiveness of sugammadex for cerebral ischemia/reperfusion injury. Kaohsiung J Med Sci 32:292–301. https://doi.org/10.1016/j.kjms.2016.05.002
Perez-Anes A, Gargouri M, laure W, Van Den Berghe H, Courcot E, Sobocinski J, Tabary N, Chai F, Blach JF, Addad A, Woisel P, Douroumis D, Martel B, Blanchemain N, Lyskawa J (2015) Bioinspired titanium drug eluting platforms based on a poly-beta-cyclodextrin-chitosan layer-by-layer self-assembly targeting infections. ACS Appl Mater Interfaces 7:12882–12893. https://doi.org/10.1021/acsami.5b02402
Pessine FBT, Calderini A, Alexandrino GL (2012) Review: cyclodextrin inclusion complexes probed by NMR techniques. In: Dong-Hyun K. (ed) Magnetic resonance spectroscopy. In TECH, Rijeak, chapter 2, pp 237–264. https://doi.org/10.5772/32029
Pinho E, Grootveld M, Soares G, Henriques M (2014) Cyclodextrin-based hydrogels toward improved wound dressings. Crit Rev Biotechnol 34:328–337. https://doi.org/10.3109/07388551.2013.794413
Potrzebowski MJ, Kazmierski S (2005) High-resolution solid-state NMR studies of inclusion complexes. In: Klinowski J (ed) New Techniques in solid-state NMR, Topics in current chemistry, vol 246, pp 91–140. https://doi.org/10.1007/b98649
Prochowicz D, Kornowicz A, Justyniak I, Lewinski J (2016) Metal complexes based on native cyclodextrins: synthesis and structural diversity. Coord Chem Rev 306:331–345. https://doi.org/10.1016/j.ccr.2015.07.016
Rezanka M (2016) Monosubstituted cyclodextrins as precursors for further use. Eur J Org Chem (32):5322–5334. https://doi.org/10.1002/ejoc.201600693
Ripoll L, Bordes C, Etheve S, Elaissari A, Fessi H (2010) Cosmeto-textile from formulation to characterization: an overview. e-Polymers:040. https://doi.org/10.1515/epoly.2010.10.1.409
Robyt JF (1998) Cyclodextrins. In: Essentials of carbohydrate of chemistry. New York: Springer-Verlag, p. 245–261. ISBN: 978-1-4612-7220-5 (print). DOI https://doi.org/10.1007/978-1-4612-1622-3
Romi R, Nostro PL, Bocci E, Ridi F, Baglioni P (2005) Bioengineering of a cellulosic fabric for insecticide delivery via grafted cyclodextrin. Biotechnol Prog 21:1724–1730. https://doi.org/10.1021/bp050276g
Ryzhakov A, Thi TD, Stappaerts J, Bertoletti L, Kimpe K, Couto ARS, Saokham P, Van den Mooter G, Augustijns P, Somsen GW, Kurkov S, Inghelbrecht S, Arien A, Jimidar MI, Schrijnemakers K, Loftsson T (2016) Self-assembly of cyclodextrins and their complexes in aqueous solutions. J Pharm Sci 105:2556–2569. https://doi.org/10.1016/j.xphs.2016.01.019
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed 19:344–362. https://doi.org/10.1002/anie.198003441
Saenger W (1984) Structural aspects of cyclodextrins and their inclusion complexes. In: Inclusion compounds - structural aspects of inclusion compounds formed by organic host lattices. Atwood JL, Davies JED, MacNicol DD, eds. London: Academic Press. Volume 2, pp. 231–259. ISBN-13: 978-0120671021
Samiey B, Cheng CH, Wu JG (2014) Organic-inorganic hybrid polymers as adsorbent for removal of heavy metal ions from solutions: a review. Materials 7:673–726. https://doi.org/10.3390/ma7020673
Saokham P, Loftsson T (2017) gamma-Cyclodextrin. Int J Pharm 516:278–292. https://doi.org/10.1016/j.ijpharm.2016.10.062
Schmid G (1996) Enzymology of cyclodextrins. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 615–626
Schmidt BVKJ, Hetzer M, Ritter H, Barner-Kowollik C (2014) Complex macromolecular architecture design via cyclodextrin host/guest complexes. Prog Polym Sci 39:235–249. https://doi.org/10.1016/j.progpolymsci.2013.09.006
Schneider HJ (2012) Applications of supramolecular chemistry. CRC Press, Boca Raton
Schneider HJ, Yatsimirsky AK (2000) Principles and methods in supramolecular chemistry. John Wiley, Chichester
Schneider HJ, Hacket F, Rüdiger V, Ikeda H (1998) NMR Studies of cyclodextrins and cyclodextrin complexes. Chem Rev 98:1755–1785. https://doi.org/10.1021/cr970019t
Schneiderman E, Stalcup AM (2000) Cyclodextrins: a versatile tool in separation science. J Chromatogr B 745:83–102. https://doi.org/10.1016/S0378-4347(00)00057-8
Scriba GKE (2016) Chiral recognition in separation science - an update. J Chromatogr A 1467:56–78. https://doi.org/10.1016/j.chroma.2016.05.061
Senthamizhan A, Balusamy B, Celebioglu A, Uyar T (2016) Nanotraps in porous electrospun fibers for effective removal of lead(II) in water. J Mater Chem A 4:2484–2493. https://doi.org/10.1039/C5TA09166G
Sharma N, Baldi A (2016) Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 23:739–757. https://doi.org/10.3109/10717544.2014.938839
Shaw NO, De Villiers MM, Lotter AP (1999) Preformulation stability screening of ivermectin with non-ionic emulsion excipients. Pharmazie 54:372–376
Shen H, Ji H (2011) Application of cyclodextrin derivatives in liquid-phase organic synthesis. Chin J Org Chem 31:791–803
Shen HM, Ji HB, Wu HK, Shi HX (2014a) Recent advances in the immobilization of beta-cyclodextrin and their application. Chin J Org Chem 34:1549–1572. https://doi.org/10.6023/cjoc201402024
Shen W, Zhang GH, Guo N, Li YT (2014b) Study on the inclusion compound of avermectin by infrared spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 34:1201–1205. https://doi.org/10.3964/j.issn.1000-0593(2014)05-1201-05
Shieh WJ, Hedges AR (1996) Properties and applications of cyclodextrins. J Macromol Sci Pure Appl Chem A33:673–683. https://doi.org/10.1080/10601329608010886
Silva A, Duarte A, Sousa S, Ramos A, Domingues FC (2016) Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT-Food Sci Technol 73:481–489. https://doi.org/10.1016/j.lwt.2016.06.043
Simoes SMN, Veiga F, Torres-Labandeira JJ, Ribeiro ACF, Concheiro A, Alvarez-Lorenzo C (2014) Syringeable self-assembled cyclodextrin gels for drug delivery. Curr Top Med Chem 14:494–509. https://doi.org/10.2174/1568026613666131219124308
Singh M, Sharma R, Banerjee UC (2002) Biotechnological applications of cyclodextrins. Biotechnol Adv 20:341–359. https://doi.org/10.1016/S0734-9750(02)00020-4
Smaldone RA, Forgan RS, Furukawa H, Gassensmith JJ, Slawin AMZ, Yaghi OM Stoddart JF (2010) Metal-organic frameworks from edible natural products. Angew Chem Int Ed 49:8630–8634. https://doi.org/10.1002/anie.201002343
Smolková-Keulemansová E (1982) Cyclodextrins as stationary phases in chromatography. J Chromatogr 251:17–34. https://doi.org/10.1002/jhrc.1240120113
Stella VJ, He Q (2008) Cyclodextrins. Toxicol Pathol 36:30–42. https://doi.org/10.1177/0192623307310945
Sun DW (2012) Handbook of frozen food processing and packaging. Boca Raton: CRC Press. ISBN: 978-1-4398-3604-0. 936 p
Sybilska D, Zukowski J (1989) Cyclodextrin additives. In: Krstulovic AM (ed) Chiral separations by HPLC. Ellis Horwood Limited, John Wiley. Chapter 7, New York, p 147
Szejtli J (1982a) Cyclodextrins and their inclusion complexes. Akademiai Kiado, Budapest
Szejtli J (1982b) Cyclodextrins in food, cosmetics and toiletries. Starch/Stärke 34:379–385. https://doi.org/10.1002/star.19820341106
Szejtli J (1984) Industrial applications of cyclodextrins. In: Atwood JL, JED D, DD MN (eds) Inclusion compounds, vol 3. Academic Press, London, pp 331–390
Szejtli J (1987) The metabolism, toxicity and biological effects of cyclodextrins. In: Duchêne D (ed) Cyclodextrins and their industrial uses. Editions de Santé, Paris. Chapter 5, pp 173–210
Szejtli J (1988) Cyclodextrin technology. Kluwer Academic Publishers, Dordrecht
Szejtli J (1990) The cyclodextrins and their applications in biotechnology. Carbohydr Polym 12:375–392. https://doi.org/10.1016/0144-8617(90)90088-A
Szejtli J (1991) The use of cyclodextrins in biotechnological operations. In: Duchêne D (ed) New trends in cyclodextrins and derivatives. Éditions de Santé, Paris, pp 595–626
Szejtli J (1996) Use of cyclodextrins in chemical products and processes. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 603–614
Szejtli J (1997) Utilization of cyclodextrins in industrial products and processes. J Mater Chem 7:575–587. https://doi.org/10.1039/A605235E
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1753. https://doi.org/10.1021/cr970022c
Szejtli J (2004a) Past, present and future of cyclodextrin research. Pure Appl Chem 76:1825–1845
Szejtli J (2004b) Cyclodextrins. In: Chemical and functional properties of food saccharides. Tomasik P, ed. New York: CRC Press. Chapter 17, p. 272–290. ISBN 0-8493-1486-0
Szente L (1996a) Analytical methods for cyclodextrins, cyclodextrin derivatives, and cyclodextrin complex. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 253–278
Szente L (1996b) Preparation of cyclodextrin complexes. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 243–252
Szente L, Szejtli J (1996) Cyclodextrins in pesticides. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 503–514
Szente L, Szejtli J (2004) Cyclodextrins as food ingredients. Trends Food Sci Technol 15:137–142. https://doi.org/10.1016/j.tifs.2003.09.019
Szente L, Szeman J, Sohajda T (2016) Analytical characterization of cyclodextrins: history, official methods and recommended new techniques. J Pharm Biomed Anal 130:347–365. https://doi.org/10.1016/j.jpba.2016.05.009
Taka AL, Pillay K, Mbianda XY (2017) Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: a review. Carbohydr Polym 159:94–107. https://doi.org/10.1016/j.carbpol.2016.12.027
Tamura A, Yui N (2015) beta-Cyclodextrin-threaded biocleavable polyrotaxanes ameliorate impaired autophagic flux in Niemann-Pick type C disease. J Biol Chem 290:9442–9454. https://doi.org/10.1074/jbc.M114.636803
Tan S, Ladewig K, Fu Q, Blencowe A, Qiao GG (2014) Cyclodextrin-based supramolecular assemblies and hydrogels: recent advances and future perspectives. Macromol Rapid Commun 35:1166–1184. https://doi.org/10.1002/marc.201400080
Tanaka Y, Yamada Y, Ishitsuka Y, Matsuo M, Shiraishi K, Wada K, Uchio Y, Kondo Y, Takeo T, Nakagata N, Higashi T, Motoyama K, Arima H, Mochinaga S, Higaki K, Ohno K, Irie T (2015) Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann-pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol Pharm Bull 38:844–851. https://doi.org/10.1248/bpb.b14-00726
Tarimci N (2011) Cyclodextrins in the cosmetic field. In: Bilensoy E (ed) Cyclodextrins in pharmaceutics, cosmetics and biomedicine. John Wiley, London., Chapter 7, pp. 131–144. https://doi.org/10.1002/9780470926819.ch7
Tejashri G, Amrita B, Darshana J (2013) Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm 63:335–358. https://doi.org/10.2478/acph-2013-0021
Terekhova IV, Kulikov OV (2005) Cyclodextrins: physical-chemical aspects of formation complexes host-guest and molecular selectivity in relation to biologically active compounds. In: Chemistry of polysaccharides. Zaikov GE, ed. London: CRC Press. Chapter 2, pp. 38–70. ISBN: 9789067644198
Thompson DO (2006) Cyclodextrins – Enabling excipients: a case study of the development of a new excipient – Sulfobutylether β-cyclodextrin (CAPTISOL®). In: Excipient development for pharmaceutical, biotechnology, and drug delivery systems. Katdare A, Chaubal MV, eds. New York: CRC Press. pp. 51–68. ISBN:978-1-4200-0413-7. (e-book - pdf)
Tong J, Chen LG (2013) Review: preparation and application of magnetic chitosan derivatives in separation processes. Anal Lett 46:2635–2656. https://doi.org/10.1080/00032719.2013.807815
Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R (2014) The application of nanosponges to cancer drug delivery. Expert Opin Drug Deliv 11(6):931–941. https://doi.org/10.1517/17425247.2014.911729
Trotta F, Caldera F, Dianzani C, Argenziano M, Barrera G, Cavalli R (2015) New glutathione bio-responsive cyclodextrin nanosponges. ChemPlusChem 81:439–443. https://doi.org/10.1002/cplu.201500531
Tungala K, Adhikary P, Krishnamoorthi S (2013) Trimerization of β-cyclodextrin through the click reaction. Carbohydr Polym 95:295–298. https://doi.org/10.1016/j.carbpol.2013.02.074
Ueda H (2002) Physicochemical properties and complex formation abilities of large-ring cyclodextrins. J Incl Phenom Macrocycl Chem 44:53–56. https://doi.org/10.1023/A:1023055516398
Uekama K, Irie T (1996) Pharmaceutical use of cyclodextrins in various drug formulations. In: Szejtli J, Osa T (eds) Comprehensive supramolecular chemistry, vol 3. Pergamon Oxford, London, pp 451–482
Ueno A (1996) Review: fluorescent cyclodextrins for molecule sensing. Supramol Sci 3:31–36. https://doi.org/10.1016/0968-5677(96)00016-8
Valente AJM, Söderman O (2014) The formation of host-guest complexes between surfactants and cyclodextrins. Adv Colloid Int Sci 205:156–176. https://doi.org/10.1016/j.cis.2013.08.001
Valetti S, Xia X, Costa-Gouveia J, Brodin P, Bernet-Camard MF, Andersson M, Feiler A (2017) Clofazimine encapsulation in nanoporous silica particles for the oral treatment of antibiotic-resistant mycobacterium tuberculosis infections. Nanomedicine (Lond) 12(8):831–844. https://doi.org/10.2217/nnm-2016-0364
Van de Manakker F, Vermonden T, Van Nostrum CF, Hennink WE (2009) Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications. Biomacromol 10:3157–3175. https://doi.org/10.1021/bm901065f
Vaution C, Hutin M (1987) The use of cyclodextrins in various industries. In: Duchêne D (ed) Cyclodextrins and their industrial uses. Éditions de Santé, Paris, pp 297–350
Vecsernyés M, Fenyvesi F, Bácskay I, Deli MA, Szente L, Fenyvesi É (2014) Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch Med Res 45:711–729. https://doi.org/10.1016/j.arcmed.2014.11.020
Venturini CDG, Nicolini J, Machado C, Machado VG (2008) Properties and recent applications of cyclodextrins. Quim Nova 31:360–368. https://doi.org/10.1590/S0100-40422008000200032
Vetter W, Bester K (2006) Gas chromatographic enantioseparation of chiral pollutants - techniques and results. In: Busch KW, Busch MA (eds) Chiral separation. Elsevier, The Netherlands. Chapter 6, pp 131–228
Villalonga R, Cao R, Fragoso A (2007) Supramolecular chemistry of cyclodextrin in enzyme technology. Chem Rev 107:3088–3116. https://doi.org/10.1021/cr050253g
Voncina, B (2011). Application of cyclodextrins in textile dyeing. In: Textile dyeing, Hauser P, ed. Rijeka: InTech. Chapter 17, pp 373–392. ISBN: 978-953-307-565-5
Voncina B, Vivod V (2013) Cyclodextrins in textile finishing. In: Günay M (ed) Textile dyeing. InTech. Chapter 3, Rijeka, pp 53–75. https://doi.org/10.5772/53777
Vunain E, Mishra AK, Mamba BB (2016) Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: a review. Int J Biol Macromol 86:570–586. https://doi.org/10.1016/j.ijbiomac.2016.02.005
Walkley SU, Davidson CD, Jacoby J, Marella PD, Ottinger EA, Austin CP, Porter FD, Vite CH, Ory DS (2016) Fostering collaborative research for rare genetic disease: the example of niemann-pick type C disease. Orphanet J Rare Dis 11:161. https://doi.org/10.1186/s13023-016-0540-x
Wang HF, Zhang LM (2010) Molecularly imprinted functional materials based on polysaccharides. Prog Chem 22:2165–2172
Ward TJ, Armstrong DW (1988) Cyclodextrin-stationary phases. In: Zief M, Crane LJ (eds) Chromatographic chiral separations. Marcel Dekker Inc., Chapter 5, New York, p 131
Ward C, Martinez-Lopez N, Otten EG, Carroll B, Maetzel D, Singh R, Sarkar S, Korolchuk VI (2016) Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim Biophys Acta 1861:269–284. https://doi.org/10.1016/j.bbalip.2016.01.006
Wei ZZ, Liu YL, Hu HM, Yu JY, Li FX (2016) Biodegradable poly(butylene succinate-co-terephthalate) nanofibrous membranes functionalized with cyclodextrin polymer for effective methylene blue adsorption. RSC Adv 6:108240–108246. https://doi.org/10.1039/C6RA22941G
Wenz G (2009) Recognition of monomers and polymers by cyclodextrins. Incl Polym 222:1–54. https://doi.org/10.1007/12_2008_13
Wenz G, Han BH, Müller A (2006) Cyclodextrin rotaxanes and polyrotaxanes. Chem Rev 106:782–817. https://doi.org/10.1021/cr970027+
West C (2014) Enantioselective separations with supercritical fluids – review. Curr Anal Chem 10:99–120. https://doi.org/10.2174/1573411011410010009
Wu HQ, Kong JH, Yao XY, Zhao CY, Dong YL, Lu XH (2015a) Polydopamine-assisted attachment of beta-cyclodextrin on porous electrospun fibers for water purification under highly basic condition. Chem Eng J 270:101–109. https://doi.org/10.1016/j.cej.2015.02.019
Wu ZL, Song N, Menz R, Pingali B, Yang YW, Zheng YB (2015b) Nanoparticles functionalized with supramolecular host-guest systems for nanomedicine and healthcare. Nanomedicine 10:1493–1514. https://doi.org/10.2217/NNM.15.1
Xiao Y, Ng SC, Tan TTY, Wang Y (2012) Recent development of cyclodextrin chiral stationary phases and their applications in chromatography. J Chromatogr A 1269:52–68. https://doi.org/10.1016/j.chroma.2012.08.049
Xiao N, Wen Q, Liu QW, Yang QB, Li YX (2014) Electrospinning preparation of beta-cyclodextrin/glutaraldehyde crosslinked PVP nanofibrous membranes to adsorb dye in aqueous solution. Chem Res Chin Univ 30:1057–1062. https://doi.org/10.1007/s40242-014-4203-y
Yamamoto E, Kuroda K (2016) Colloidal mesoporous silica nanoparticles. Bull Chem Soc Jpn 89:501–539. https://doi.org/10.1246/bcsj.20150420
Yang LPH, Keam SJ (2009) Sugammadex: a review of its use in anaesthetic practice. Drugs 69:919–942. https://doi.org/10.2165/00003495-200969070-00008
Yang JS, Yang L (2013) Preparation and application of cyclodextrin immobilized polysaccharides. J Mater Chem B 1:909–918. https://doi.org/10.1039/c2tb00107a
Yokoo M, Kubota Y, Motoyama K, Higashi T, Taniyoshi M, Tokommaru H, Nishiyama R, Tabe Y, Mochinaga S, Sato A, Sueoka-Aragane N, Sueoka E, Arima H, Irie T, Kimura S (2015) 2-Hydroxypropyl-β-cyclodextrin acts as a novel anticancer agent. PLoS One 10:e0141946. https://doi.org/10.1371/journal.pone.0141946
Yuan Z, Zhang L (2016) Photoinduced controlled-release drug delivery systems for applications in nanomedicine. Curr Org Chem 20:1768–1785. https://doi.org/10.2174/1385272820666160112001944
Yusoff SNM, Kamari A, Aljafree NFA (2016) A review of materials used as carrier agents in pesticide formulations. Int J Environ Sci Technol 13:2977–2994. https://doi.org/10.1007/s13762-016-1096-y
Zarzycki PK, Fenert BE, Głód BK (2016) Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. In: Encapsulations. nanotechnology in the agri-food industry. Grumezescu A (ed). Chapter 17, pp 717–767. ISBN: 978-0-12-804378-3
Zgani I, Idriss H, Barbot C, Djedaïni-Pilard F, Petit S, Hubert-Roux M, Estour F, Gouhier G (2017) Positive variation of the MRI signal via intramolecular inclusion complexation of a C-2 functionalized β-cyclodextrin. Org Biomol Chem 15:564–569. https://doi.org/10.1039/c6ob02583h
Zhang J, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233. https://doi.org/10.1016/j.addr.2013.05.001
Zhang L, Li LL, Cheng LH, Chen HL (2011) Preparation and application of cyclodextrin-based molecular tube. Prog Chem 23:1936–1944
Zhang XM, Li HZ, Cao ML, Shi L, Chen CY (2015) Adsorption of basic dyes on beta-cyclodextrin functionalized poly (styrene-alt-maleic anhydride). Sep Sci Technol 50:947–957. https://doi.org/10.1080/01496395.2014.978461
Zhang LL, Man SL, Qiu HN, Liu Z, Zhang M, Ma L, Gao WY (2016) Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ Toxicol Pharmacol 48:31–38. https://doi.org/10.1016/j.etap.2016.09.021
Zhao SP, Xu W (2010) Cyclodextrin-containing supramolecular hydrogels. Prog Chem 22:916–926
Zhao R, Wang Y, Li X, Sun BL, Wang C (2015) Synthesis of beta-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene Blue. ACS Appl Mater Interfaces 7:26649–26657. https://doi.org/10.1021/acsami.5b08403
Zhou JW, Ritter H (2010) Cyclodextrin functionalized polymers as drug delivery systems. Polym Chem 1:1552–1559. https://doi.org/10.1039/c0py00219d
Zhou ZJ, Cai RX, Liu NG, Zhang L, Chen HL (2007) Preparation and application of membrane with cyclodextrins. Prog Chem 19:1436–1442
Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Bjorkhem I, Gustafsson JA, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lutjohann D, Latz E (2016) Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med 8:333ra50. https://doi.org/10.1126/scitranslmed.aad6100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
This chapter is dedicated to the memory of Professor Benito Casu (Istituto di Chimica e Biochimica G. Ronzoni, Milan, Italy).
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Crini, G., Fourmentin, S., Fenyvesi, É., Torri, G., Fourmentin, M., Morin-Crini, N. (2018). Fundamentals and Applications of Cyclodextrins. In: Fourmentin, S., Crini, G., Lichtfouse, E. (eds) Cyclodextrin Fundamentals, Reactivity and Analysis. Environmental Chemistry for a Sustainable World, vol 16. Springer, Cham. https://doi.org/10.1007/978-3-319-76159-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-76159-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-76158-9
Online ISBN: 978-3-319-76159-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)