Abstract
The intricate interplay between the host and its microbiota has garnered increasing attention in the past decade. Specifically, the emerging recognition of microorganisms within diverse cancer tissues, previously presumed sterile, has ignited a resurgence of enthusiasm and research endeavors. Four potential migratory routes have been identified as the sources of intratumoral microbial “dark matter,” including direct invasion of mucosal barriers, spreading from normal adjacent tissue, hematogenous spread, and lymphatic drainage, which contribute to the highly heterogeneous features of intratumor microbiota. Importantly, multitudes of studies delineated the roles of intratumor microbiota in cancer initiation and progression, elucidating underlying mechanisms such as genetic alterations, epigenetic modifications, immune dysfunctions, activating oncogenic pathways, and inducing metastasis. With the deepening understanding of intratumoral microbial composition, novel microbiota-based strategies for early cancer diagnosis and prognostic stratification continue to emerge. Furthermore, intratumor microbiota exerts significant influence on the efficacy of cancer therapeutics, particularly immunotherapy, making it an enticing target for intervention in cancer treatment. In this review, we present a comprehensive discussion of the current understanding pertaining to the developmental history, heterogeneous profiles, underlying originations, and carcinogenic mechanisms of intratumor microbiota, and uncover its potential predictive and intervention values, as well as several inevitable challenges as a target for personalized cancer management strategies.
Similar content being viewed by others
Introduction
The human microbiota encompasses a vast number of interacting microorganisms, including bacteria, viruses, fungi, and archaea, inhabiting various locations both on human surfaces and within the body [1]. Thereinto, the gastrointestinal tract, particularly the colon, stands out as the largest microecosystem, harboring several trillion microbial cells [2]. Moreover, the gene set among the resident microbes surpasses that of the human host by more than 100 times [3]. Once as a “forgotten organ”, the gut microflora has been extensively demonstrated over the past decade to play a pivotal role in upholding host homeostasis, which serves as a significant determinant in the variability of disease occurrence, progression, and therapeutic response [4, 5]. Thanks to advancements in modern sequencing and metagenomics techniques, it has become evident that human tissues and organs, including tumor tissues (excluding those on body surfaces or in cavities), are not entirely sterile but rather harbor low biomass microbial communities [6]. Specifically, the tumor tissue-resident microbes in both extracellular and intracellular spaces, termed as intratumor microbiota, has gained significant attention as a novel and crucial component within the tumor microenvironment (TME) across various cancer types [7]. Despite their eminently lower abundance compared to gut microbiota, recent studies have uncovered tangible findings regarding the impact of intratumor microbiota on cancer development and treatment [8].
As of now, only 11 carcinogenic microbes have been identified in humans, including seven viruses (Epstein-Barr [EBV], Hepatitis B [HBV], and Hepatitis C [HCV] viruses; Kaposi sarcoma herpesvirus; human immunodeficiency virus-1; human papillomavirus [HPV]; human T cell lymphotropic virus type 1 [HTLV]), one bacterium (Helicobacter pylori), and three platyhelminths (Opisthorchis viverrini; Clonorchis sinensis; Schistosoma haematobium) [9]. However, it is noteworthy that an estimated 13% of global cancers could be attributable to these pro-tumorigenic “oncomicrobes” [10]. Actually, the renewed topic of intratumor microbiota has been under discussion for thousands of years (Fig. 1). As early as 2600 BC, the esteemed Egyptian physician Imhotep advocated an anti-tumor regimen that entailed applying poultices, followed by incising swellings and then causing infection [11]. In the fourteenth century, the Italian priest Peregrine Laziosi (1265–1345) earned recognition as the patron saint of cancer patients when the tumor in his tibia miraculously vanished after the malignant lesion became severely infected [12]. Likely inspired by these, several endeavors were undertaken to shrink cancers through erysipelas infection. The German physician Busch in 1868 pioneered the practice of infecting cancer patients with erysipelas, resulting in a remarkable regression of the malignancy [13]. The next notable advancement in microbiotherapy came from William Coley, now credited as the father of cancer immunotherapy. In 1893, he invented a vaccine incorporating two inactivated bacteria: Streptococcus pyogenes and Serratia marcescens, known as ‘‘Coley’s toxins’’. Coley’s vaccine demonstrated universally effectiveness against a variety of malignancies, including sarcomas, melanomas, lymphomas, myelomas, and a broad spectrum of carcinomas [14, 15]. Currently, the sole conventional analogous to Coley’s vaccine is bacillus Calmette-Guerin. It is administered directly to the tumor, representing the most effective treatment for superficial bladder cancer [16, 17].
Additionally, following Bloch’s discovery of the accumulation of phages in tumor tissue leading to the suppression of cancer growth in 1940s [18], significant progress has been achieved in identifying pro-tumorigenic viruses, such as EBV and HTLV for special lymphoma subtypes [19, 20]. Of note, EBV was the first human oncogenic virus to be discovered by Epstein and Barr in 1964 [21], leading to approximately 143,000 (1.8%) annual deaths globally attributed to EBV-related malignancies [22]. In 2015, the U.S. Food and Drug Administration approved the first oncolytic virus, talimogene laherparepvec (T-VEC), for the treatment of metastatic melanoma [23], signifying a promising emerging category of anti-tumor immunotherapy.
Nevertheless, although the significant milestone achieved with the first successful cultivation of H. pylori in 1982 by Marshall and Warren, marking a pivotal moment in human medicine, currently, only this particular carcinogenic bacterium has been firmly established causal relationships with gastric cancer [24, 25]. Notably, through employing real-time quantitative polymerase chain reaction of 16S ribosomal RNA (rRNA) in 2020, Nejman et al. investigated 1010 tumors for bacteria spanning melanoma, glioblastoma, breast, lung, ovary, bone, and pancreas cancers [6], which unveiled unique microbial compositions varying among different cancer types. Subsequently, Narunsky-Haziza [26] and Dohlman [27] have individually revealed the characteristics of cancer mycobiome and its diagnostic as well as prognostic potential in 2022.
Nowadays, technological advancements are greatly expediting the characterization of intratumoral microbial “dark matter,” fueling widespread research interest in leveraging intratumor microbiota to influence both the development and treatment of cancer. Here, we present a comprehensive overview of the heterogeneous composition and probable origins of intratumor microbiota, as well as their evolving roles in modulating cancer progression and underlying mechanisms. Furthermore, we elucidate the potential transformative influence of intratumor microbiota in clinical settings, while also highlighting the inherent challenges and future prospects it poses as an intervention target for custom-fit precision cancer management strategies.
Intratumor microbiota
While the intratumor microbiota has been a subject of discussion for a long time, recent years have seen a resurgence of enthusiasm, driven by advancements in modern technology and methodology [28], such as 16S rRNA sequencing, shotgun metagenomic sequencing, electron microscopy, immunohistochemistry, fluorescence in situ hybridization (FISH), and culturomics, allowing for the differentiation of low biomass bacterial DNA from tumor tissue (Table 1). Here, we present recent encouraging findings related to intratumor microorganisms, outlining their distinct compositions and potential migratory trajectories, with the aim to foster an in-depth understanding of the microbe-host interactions within tumors.
Heterogeneity of intratumor microbiota
The abundance, composition, and spatial distribution of intratumor microbial populations vary significantly across different cancer types (Table 2). By analyzing seven tumor types—lung, breast, pancreas, ovary, bone, melanoma, and brain tumors—using a platform with species-level resolution, Nejman and colleagues unveiled cancer type-specific microbial features [6]. Their findings highlighted that breast cancer harbors a notably rich and diverse microbiome compared to other tumor types and normal breast tissues from healthy individuals. Interestingly, tumor-adjacent normal breast samples exhibited an intermediate bacterial load and richness between those of the breast tumors and normal samples. In terms of microbial composition, Firmicutes and Bacteroidetes were the predominant bacterial species in colorectal tumors, while Proteobacteria dominated the microbiome of pancreatic cancer. Actinobacteria, including Corynebacteriaceae and Micrococcaceae families, were notably prevalent in non-gastrointestinal tumors. Following that, the research team further revealed cancer-type-specific fungal localization patterns [26]. Of note, intratumor fungi were predominantly found intracellularly in breast, pancreatic, and ovarian cancers, whereas they primarily localized to macrophages in melanoma and lung cancers. In a sense, this pronounced heterogeneity of intratumor microbiota aligns with the intricate molecular and pathological characteristics of malignancies, which could be crucial for grasping the biological behavior of the tumors and exploiting novel targets for personalized cancer therapy.
Origination of intratumor microbiota
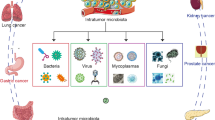
The TME provides conducive niches for microbial habitation owing to its hypoxic microenvironment, nutrients availability, and immune incompetence [72]. Although considerable research on the composition of intratumor microbes, there remains limited understanding regarding the origins of microorganisms inhabiting tumor tissue and their migratory pathways. In this context, we primarily delve into four likely originations or pathways of intratumor microbiota based on recent findings, namely direct mucosal barriers invasion, dissemination from normal adjacent tissue (NAT), hematogenous spread, and lymphatic drainage (Fig. 2).
Potential sources of intratumor microbiota. The hypoxic, nutrient-abundant, and immunoincompetent TME provides a desirable habitat for microorganisms. The initiation for microbial migratory involves direct mucosal barriers invasion, while the NAT would serve as a “transfer station” following microbes’ invasions. In addition, hematogenous spread and lymphatic drainage play significant roles as supplementary pathways for microbial migratory trajectories. Abbreviations: NAT, normal adjacent tissue; TME, tumor microenvironment
It is understandable that microorganisms can infiltrate tumors in tissues or organs that are in direct contact with the external environment, such as the gastrointestinal tract, lungs, oral cavity, nasopharynx, and genitourinary organs, due to compromised mucosal barriers caused by tumorigenesis. Among these, Fusobacterium nucleatum not only characterized the gut microbial feature of colorectal cancer (CRC) patients [73], but also exhibited enrichment in CRC tumor tissues, particularly in those with certain molecular subtypes such as Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation and microsatellite instability-high [74, 75]. Recently, Zhu et al. demonstrated that F. nucleatum could invade cancer cells by binding to DHX15, contributing to colorectal tumorigenesis in Villin-Cre/KrasG12D+/− mice [74]. Another analysis of 1697 CRC samples from Australasian revealed a close association between F. nucleatum and DNA mismatch repair deficiency (MMRd) in both hereditary and sporadic CRC cases, indicating the pivotal role of MMRd-related TME in F. nucleatum colonization [49]. Notably, genetic heterogeneity appears to play a pivotal role in determining bacterial colonization. Zepeda-Rivera et al. conducted pan-genomic analyses on 135 F. nucleatum strains and found that the F. nucleatum subspecies animalis (Fna) consists of two distinct clades [76]. Of them, Fna C1 is primarily found in the oral cavity, whereas Fna C2 predominates in human CRC tumor niches and promotes tumorigenesis by altering intestinal metabolism to increase oxidative stress.
Furthermore, efforts are underway to trace bacterial migratory pathways more accurately. Through single-nucleotide variant analysis, Qiao and colleagues unveiled that the predominance of microbes, including Corynebacterium and Staphylococcus, in nasopharyngeal carcinoma (NPC) tissues largely stemmed from the nasopharyngeal microbiota [61]. Likewise, Liao et al. identified 13 species, such as F. nucleatum and Prevotella intermedia, as oral-translocated and enriched in NPC patients through 16S rRNA sequencing on the paired nasopharyngeal-oral microbial samples, further validated by culturomics, clonal strain identification, and meta-transcriptomes [77].
Despite the discussion of the NAT as a potential source of intratumor microbiota due to the resemblance in microbial composition between NAT and tumor tissue in a number of Reviews [28, 78, 79], the NAT might function as the “transfer station” subsequent to microorganism invasion of mucosal barriers. A study involving 82 CRCs, 118 adenomas, and 149 matched adjacent normal mucosae found that the abundance of F. nucleatum in NAT from cancer cases was significantly higher than that in tumor tissue and NAT from adenoma cases [80]. In addition, the detection rate of F. nucleatum in CRC cases at stage III/IV increased gradually from superficial NAT, deep NAT, to cancer tissue. Likewise, Wong-Rolle et al. showed that the peak bacterial load was observed in the airway, followed by a lower level in tumor cells and further reduction in NAT in early-stage lung cancer [35], suggesting that intratumor microbes might originate from the lower airway through NAT. Hence, we categorized the source of spreading from NAT to be a consequence of mucosal barrier invasion.
Navigating hematogenous or lymphatic migratory routes for microbes presents more stereotypical challenges than direct invasion of mucosal barriers. But actually, the impairment of mucosal barriers consistently constitutes the initial step for microbial migratory. One notable example is the work by Bertocchi and colleagues in 2021, which reported the injured gut vascular barrier by the virulence regulator VirF from Escherichia coli could boost microbial dissemination along the gut-liver axis and further induce a premetastatic niche formation of CRC in the liver [81]. Intriguingly, the hematogenous migratory route appears to offer a promising avenue for the microbiota-based cancer modulation. Zhu et al. observed that the abundance of Akkermansia muciniphila increased in tumor tissue following the gavage of A. muciniphila in a lung cancer mouse model, which has a significant impact on the composition of intratumor microbiota [82]. Crucially, after 2 h of bacterial administration, A. muciniphila was notably elevated in blood samples as detected by 16S rDNA sequencing, suggesting a probable crosstalk between intestinal and intratumoral microbiota through systemic circulation. Similarly, the lymphatic drainage route of microbial translocation plays a pivotal role in bolstering extraintestinal anti-tumor immune responses during immune checkpoint blockade (ICB) therapy. Choi and colleagues reported that ICB treatment could stimulate the translocation of specific gut bacteria, such as Enterococcus faecalis and Lactobacillus johnsonii, to extraintestinal tissues by inducing lymph node remodeling and activating dendritic cell (DC) in a preclinical melanoma model [83], which is conducive to exert optimal anti-tumor T cell responses against extraintestinal tumors.
Impacts of intratumor microbiota on cancer occurrence and progression
The roles of microorganisms in tumorigenesis have been extensively explored, with H. pylori infection associated with gastritis, gastric ulcer, and gastric cancer serving as a paradigm of bacterium-mediated cancer formation [84]. With advances in detection techniques, accumulating data suggest the presence of tumor type-specific intratumor microbes, which are now recognized as integral components of the TME [85]. Using spatial-profiling technologies, Li and colleagues established a spatial coupling between microbes and T cells in cancer, which plays a critical role in shaping the contexture of TME [86]. Here, we mainly discuss emerging evidence regarding the mechanisms by which intratumor microbiota contribute to cancer development in recent years (Fig. 3), including genetic alterations, epigenetic modifications, immune dysfunctions, activating oncogenic pathways, inducing metastasis, and other relevant mechanisms.
Underlying mechanisms of intratumoral microbiota-mediated cancer progression. Five putative mechanisms have been proposed to illustrate how intratumor microbiota contribute to cancer formation and development, including genetic alterations, epigenetic modifications, immune dysfunctions, activating oncogenic pathways, and inducing metastasis. Abbreviations: TME, tumor microenvironment; MVs, membrane vesicles; ROS, reactive oxygen species; PD-L1, programmed cell death-ligand 1; DC, dendritic cell; MDSCs, myeloid-derived suppressor cells; TLR, Toll-like receptor; Hh pathway, Hedgehog pathway; TMAO, Trimethylamine-N-oxide; FXR, farnesoid X receptor; TMPC, surface protein of Streptococcus anginosus; MSI, microsatellite instability; BFT-1, toxic protein secreted by Bacteroides fragilis; EMT, epithelial-mesenchymal transition; Fn-Dps, F. nucleatum-DNA hunger/stationary phase protective proteins. (By Figdraw)
Genetic alterations
Genome alterations, such as DNA damage and gene mutations, play a central role in the genesis and progression of cancer. Members of the microbiota can directly cause DNA damage through the production of certain metabolites or secretions such as toxins [87, 88] and membrane vesicles (MVs) [89], which have the ability to alkylate DNA, induce DNA double-strand breaks, or generate excessive reactive oxygen species (ROS). For instance, Okuda et al. observed the associations between tumor-residing Fusobacterium and a series of altered genes in CRC, and they underscored the potential of Campylobacter in promoting carcinogenesis by inhibiting double-strand DNA break repairs [47]. Moreover, Campylobacter jejuni has been shown to induce CRC pathogenesis in a cytolethal distending toxin-dependent manner in germ-free ApcMin/+ mice [90]. MVs, which are nano- or micrometer-sized lipid-bound vesicles released from cells, serve as vehicles for the systemic delivery of a variety of molecular cargoes, including nucleic acids, sugars, lipids, and proteins, to recipient cells [91]. Miyakawa and colleagues discovered that odontolyticus secretes lipoteichoic acid-rich MVs, which can induce excessive ROS production by causing mitochondrial dysfunction in colonic epithelial cells, consequently leading to DNA damage and the initiation of CRC [89].
Epigenetic modifications
Epigenetic modifications, including DNA methylation, non-coding RNAs, and histone modifications, constitute another significant mechanism implicated in cancer development through the regulation of gene expression. F. nucleatum has been implicated in the alterations of genome-wide methylation levels in esophageal cancer [52]. Additionally, Park et al. observed an intimate relationship between high levels of F. nucleatum and hypermethylation of the CDKN2A promoter CpG island, potentially linked with M2 macrophages infiltration in CRC [75]. Apart from DNA modifications, Qiao et al. suggested an m6A-dependent mechanisms mediated by the intratumor Mycoplasma hyorhinis in boosting the initiation and progression of hepatocellular carcinoma [44]. That is, M. hyorhinis infection could facilitate the degradation of mitochondrial fusion protein 1 mRNA through increasing the level of RNA modification m6A, thereby promoting mononuclear polyploidy and cancer stemness by enhancing mitochondrial fission. Notably, microbial metabolites often engage in the complex interactions between intratumor microbiota and epigenetic changes. Ma et al. demonstrated that intratumor-bacteria-derived butyrate could fuel lung cancer development by upregulating H19 expression in tumor cells through increasing histone H3 lysine 27 acetylation at the H19 promoter and potentiating M2 macrophage polarization [92]. Interestingly, further studies have pinpointed the beneficial roles of butyrate in preventing the pathogenesis of diverse cancer types by enhancing histone acetylation and activating antitumor immunity [93,94,95], implying the existence of tumor-specific roles for microbial metabolites in different cancers.
Immune dysfunctions
The immune-oncology-microbiome axis denotes immune-mediated interactions between microbiota and host anti-tumor responses [7], where intratumor microbes and their metabolites or by-products within the TME can exert either immunosuppressive or immune-promoting effects. In pancreatic ductal adenocarcinoma (PDAC), the microbiota contributed to a tolerogenic immune microenvironment by activating Toll-like receptor (TLR) ligation in monocytic cells [96]. Strikingly, elimination of intratumor bacteria could reverse the immunosuppressive TME, lead to diminished myeloid-derived suppressor cells (MDSCs) and enhanced M1 macrophage differentiation, thereby fostering TH1 differentiation of CD4 + T cells and activating CD8 + T cells. Conversely, Kalaora et al. reported that intracellular bacteria-derived HLA-bound peptides could be presented by tumor cells and elicit immune reactivity in melanoma [97].
The tumor-associated neutrophils tend to confer a boosting effect on the microbiota-mediated tumorigenesis, but warrants further investigation. In a recent pan-cancer analysis of 4,160 metastatic tumor biopsy tissues, Battaglia et al. disclosed potential associations between microbial diversity and tumor-infiltrating neutrophils and macrophages, resulting in an immunosuppressive TME [98]. Similarly, the relationships between neutrophilic inflammation and the growth of tumor‑promoting bacteria have been observed in vulvar squamous cell carcinoma [70]. Furthermore, Tan and colleagues found that the intratumor Porphyromonas gingivalis could facilitate pancreatic cancer progression through increasing the secretion of neutrophilic chemokines and neutrophil elastase [57]. However, neutrophils have been found to hinder tumor development by restricting the numbers of bacteria and tumor-associated inflammatory responses mediated by interleukin (IL) 17 in colon cancer [99].
To date, various studies have highlighted F. nucleatum, a periodontal pathogen, as an oncogenic bacterium capable of initiating tumorigenesis [100]. Within the TME of pancreatic cancer mouse model, intratumor F. nucleatum has been shown to inhibit the infiltration of CD8 + T cells by recruiting MDSCs [58]. The virulence protein Fn-Dps (F. nucleatum-DNA hunger/stationary phase protective proteins) plays an essential role in favoring the intracellular survival of F. nucleatum within macrophages through increasing the expression of chemokine CCL2/CCL7 [101]. Additionally, Li et al. revealed that intracellular infection with F. nucleatum could suppress the proliferation and cytokine secretion of T cells and promote programmed cell death-ligand 1 (PD-L1) expression in esophageal squamous cell carcinoma (ESCC) [54]. Likewise, F. nucleatum contributed to the progression of oral squamous cell carcinoma via potentiating tumor cell proliferation and inducing M2 macrophage polarization [102]. Another periodontitis pathogen, Porphyromonas gingivalis, also exhibited close associations with the phenotype of cancer immune cell [100]. Ren and colleagues found that P. gingivalis infection robustly promoted PD-L1 expression on DCs and suppressed antigen-specific CD8 + T cells [103]. Interestingly, the co-culture of P. gingivalis and F. nucleatum in oral keratinocytes markedly increased the expressions of pro-inflammatory mediators, such as IL-1β, IL-8, IL-6, and TNF-α, implying potential linkages with the pathogenesis of oral cancer [104].
In addition to bacteria, the intratumoral mycobiome and virome also play pivotal roles in cancer progression. Among them, Alam et al. demonstrated that intratumor fungi were capable of accelerating PDAC tumor growth by activating innate lymphoid cells 2 (ILC2) through IL-33, and antifungal treatment reduced the infiltration of T helper 2 and ILC2, thereby extending survival [105]. Moreover, Aspergillus sydowii has been shown to foster lung cancer development by inducing the expansion and activation of MDSCs through IL-1β secretion [37]. Viral infections can directly regulate certain immune cells [106]. In human soft tissue sarcomas, Perry et al. reported close associations between the intratumor viral microbiome and NK cell infiltration, with a higher abundance of intratumor Respirovirus observed in patients without metastases compared to those with metastases.
Activating oncogenic pathways
Activation of oncogenic signaling pathways is a prevalent mechanism underlying cancer formation and progression. Remarkably, intratumoral F. nucleatum can promote tumor growth through modulating multiple pathways. Zhu et al. revealed that in CRC tumor tissues, F. nucleatum upregulated the oncoprotein DHX15 by activating the ERK-STAT3 pathway [74]. Similarly, F. nucleatum infection activated the mitogen-activated protein kinase (MAPK) pathway, leading to increased expression of Matrix metalloproteinase 7 protein and enhanced CRC cell migration capacity [107]. Hsueh and colleagues demonstrated that F. nucleatum facilitated the proliferation of head and neck squamous cell carcinoma through inhibiting the expression of DNA mismatch repair-related genes, including MLH1, MSH2, and MSH6, via the TLR4/MYD88/miR-205-5p signaling pathway [108]. Moreover, the progression of epithelial ovarian cancer induced by Propionibacterium acnes was associated with an elevated inflammatory response that activated the hedgehog pathway [66]. Importantly, intratumor microorganisms can regulate oncogenic pathways through their metabolites or toxins. Most recently, Fu and colleagues identified Streptococcus anginosus, apart from H. pylori, as a pathogen that facilitated gastric tumorigenesis through its surface protein TMPC, leading to activation of the MAPK pathway [51]. Administration of the microbial metabolite trimethylamine-N-oxide could promote CRC tumor cell and stem cell proliferation by activating the Wnt/β-catenin pathway [109]. In addition, intratumor enterotoxigenic Bacteroides fragilis enhanced the stemness and chemoresistance of breast cancer cell by secreting the toxic protein BFT-1, which activated the NOTCH1-HEY1 signaling pathway via directly bounding to NOD1 receptor [110].
In addition to the oncogenic pathways discussed above, several other signaling pathways involve interactions between intratumor microbiota and cancer development. For instance, intracellular bacteria residing in breast cancer suppressed the RhoA/ROCK signaling, thereby enhancing the survival of circulating tumor cells through cytoskeleton reorganization [31]. Kong and colleagues demonstrated the crucial role of the TLR4/Keap1/NRF2 pathway in which F. nucleatum regulates CRC metabolism to promote metastasis [111]. Additionally, cytokines such as IL-6 and TNF-α, stimulated by intratumor microbiota, could activate the NF-κB and STAT3 signaling and facilitate tumor proliferation [112]. Remarkably, activation of the stimulator of interferon genes (STING) pathway by intratumor microbes has been shown to confer beneficial roles in enhancing anti-tumor responses [113,114,115], such as promoting M1 macrophage polarization, as well as activating NK cells and dendritic cells, which presents a promising opportunity to improve the therapeutic outcomes of cancer patients.
Inducing metastasis
Tumor metastasis stands as a primary driver of the limited efficacy seen in cancer therapies, and emerging findings underscore the crucial involvement of intratumor microbiota in this metastatic process [116]. Detectable bacterial DNA within tumors has been noted in various cases of cancer metastasis [98]. Notably, Hilmi et al. reported that the diversity of intratumor microbiota correlated more closely with the biopsy site than with the primary cancer type [117]. Specifically, they observed lower bacterial richness in lymph node metastases compared to metastases in the lung and liver. Intratumor bacteria have the capacity to trigger the formation of a premetastatic niche in the metastatic organ, thereby facilitating the recruitment of metastatic cells [81]. Once entering the circulatory system, metastatic cancer cells can leverage intracellular bacteria to enhance their survival by rearranging the actin cytoskeleton [31]. Furthermore, the virulence proteins or metabolites produced by intracellular microbes play a critical role in the metastatic process. For instance, the virulence factor Fn-Dps from F. nucleatum can enhance its survival within macrophages and stimulate the migration of CRC cells through the epithelial-mesenchymal transition (EMT) induced by CCL2/CCL7 [101]. Additionally, low concentrations of butyrate produced by intratumor bacteria have been implicated in driving lung cancer metastasis by promoting H19 expression and enhancing M2 macrophage polarization [92].
Other relevant mechanisms
Exosomes, carrying diverse proteins, lipids, and RNAs, play a crucial role in intercellular communication and regulation [118]. Importantly, increasing evidence suggests intimate interactions between exosomes released by bacteria-infected tumor cells and cancer development (Fig. 4) [119]. Among them, F. nucleatum infection could upregulate the expression of microRNA-21 by activating TLR4/MYD88/NF-κB pathway, which indicated a higher risk of inferior outcomes in CRC patients [120]. Tang et al. reported that sustained F. nucleatum-induced expression of microRNA-31 enhanced the tumorigenicity of CRC cells by targeting eukaryotic initiation factor 4F-binding protein 1/2 [121]. In addition, a variety of researches have revealed the relationships between exosomes derived from bacteria-infected tumors, including miRNA and long non-coding RNA (lncRNA), and cancer metastasis [122,123,124,125], and these exosomes served as an important hinge joint for intratumor microbiota-mediated cancer progression. For instance, F. nucleatum has the ability to facilitate CRC metastasis by the miR-1322/CCL20 axis and M2 macrophage polarization [125]. In oral epithelial carcinomas, Zhang and colleagues demonstrated the capacity of F. nucleatum in triggering EMT through lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 pathway [126].
Roles of exosomes derived from bacteria-infected tumor cells in cancer development. Exosomes released by microbiota-infected tumor cells can promote the progression of tumor by enhancing cell proliferation, EMT, metastasis, anti-tumor drug resistance, and M2 macrophage polarization. M2, M2-type macrophages; EMT, epithelial-mesenchymal transition
Inducing resistance to anti-tumor drugs represents another critical mechanism for exosomes-induced tumor development. In a recent study, Hui et al. observed that exosomes derived from F. nucleatum-infected CRC cells transferred hsa_circ_0004085 between cells and imparting resistance to oxaliplatin/5-fluorouracil via relieving endoplasmic reticulum stress in recipient cells [127]. Likewise, Zeng and colleagues demonstrated that F. nucleatum-induced expression of miR-135b could inhibit KLF13 expression and promote cisplatin resistance in CRC [128]. While much of this research has focused on the interactions between F. nucleatum infection and CRC, these findings highlight the potential of exosomes as an intervention target for cancer patients, which deserves further exploration.
Metabolic dysregulation, such as heightened glucose metabolism, is a critical hallmark of cancer. Of note, F. nucleatum has been shown to promote CRC pathogenesis through enhancing glycolysis in tumor cells via the activation of lncRNA ENO1-IT1 transcription [129]. In oral squamous cell carcinoma, Sun et al. demonstrated that tumor-resident F. nucleatum could drive the formation of tumor-associated macrophages and a pro-tumorigenic microenvironment through regulating glycolysis and extracellular lactate deposition [130]. Apart from glucose metabolism, alterations in amino acid and nucleotide metabolism, inositol phosphate metabolism, and fatty acid biosynthesis have also been implicated in intratumor bacteria-mediated tumorigenesis [79].
Clinical implication and therapeutic potential of intratumor microbiota
Diagnostic and prognostic tools
The considerable heterogeneity within the intratumor microbiota, coupled with significant disparities in composition between normal and malignant tissue, emphasizes its potential as a target for cancer diagnosis and risk stratification. Nejman and co-authors elucidated the distinct microbial profiles across multiple cancer types [6]. Specifically, intratumor microbiota of CRC was dominated by Firmicutes and Bacteroidetes, and Proteobacteria was prevalent in pancreatic cancer, while extra-gastrointestinal tumors primarily featured the Actinobacteria phylum. Subsequently, Narunsky-Haziza et al. highlighted the diagnostic utility of cancer-type-specific fungal compositions in distinguishing diverse tumors from normal controls [26]. Among these, the most robust diagnostic performance was noted in breast cancer, with an area under receiver operating characteristic curve of 95% CI 81.40–93.53%.
Multiple studies have underscored the prognostic significance of intratumor microbiota across a spectrum of cancer types. Among these, intratumor infection by anaerobic bacteria, including Bacteroides, Peptoniphilus, and Lactobacillus, has been strongly linked to shorter survival time in PDAC [59]. Patients with a higher intratumor bacterial load, compared to those with low bacterial load, exhibited significantly inferior rates of overall survival (hazard ratio [HR], 3.41; 95% CI 1.90–6.11, P < 0.001), disease-free survival (HR, 2.90; 95% CI 1.72–4.90; P < 0.001), and distant metastasis-free survival (HR, 3.18; 95% CI 1.58–6.39; P < 0.001) in nasopharyngeal carcinoma [61]. In addition, a higher abundance of intratumor F. nucleatum is frequently indicative of poor survival in various tumor types, such as esophageal squamous cell carcinoma, cervical carcinoma, and CRC [131,132,133]. However, Neuzillet et al. noted that F. nucleatum-positive cases in oral squamous cell carcinoma exhibited a lower metastatic recurrence rate and longer overall survival when compared to F. nucleatum-negative tumors [60], implying the complexity to develop microbiota-based prognostic strategies specific to tumors.
Bracingly, intratumor microbiota-based cancer stratification models demonstrate exceptional capability in demarcating patients with particular clinic-molecular characteristics and prognosis. In an analysis of 423 patients with stage I to IV CRC, Mouradov et al. identified oncomicrobial community subtypes capable of stratifying CRCs into three distinct subgroups with unique features and outcomes, which supports the development of a framework for intratumor microbiota-based stratification of CRC [134]. Another classification system, termed hepatotype, developed through clustering analysis of microbial profiles in HCC, could serve as an independent biomarker for predicting prognosis after surgery [45]. Namely, patients with hepatotype A exhibited remarkably shorter overall survival and recurrence-free survival compared to those with hepatotype B. Furthermore, Zhang et al. constructed a virus-associated prognostic signature based on selected viral compositions in a pan-cancer level, which could classify patients into three prognostic groups, including good, intermediate, and poor survival outcomes [135].
Impacting the efficacy of cancer therapy
Chemo- and radiotherapy
Nowadays, radiotherapy and chemotherapy remain the cornerstone for cancer therapy, and ample evidence indicates the critical roles of intratumor microbiota in modulating the efficacy of chemo- and radiotherapy. According to a metagenomic analysis of biopsy tumoral tissues, five core bacteria, including Streptococcus equinus, Blautia producta, Schaalia odontolytica, Pseudomonas azotoformans, and Clostridium hylemonae, exhibited close interactions with the resistance of neoadjuvant chemoradiotherapy in locally advanced rectal cancer [136]. Moreover, Colbert et al. demonstrated that the intratumor Lactobacillus iners could trigger resistance of chemotherapy and radiation in cervical cancer cells through altering tumor metabolism and lactate signaling pathways [137].
Various intratumor “oncomicrobes” have been documented to influence the therapeutic outcomes of chemotherapy. The heightened abundance of F. nucleatum has been intimately linked to chemoresistance in CRC patients, possibly result from its role in preventing pyroptosis by the Hippo pathway [138]. Furthermore, Li et al. delineated how F. nucleatum contributed to oxaliplatin resistance in CRC by suppressing ferroptosis [139]. Interestingly, F. nucleatum levels may decrease with the administration of the chemotherapeutic agent 5-fluorouracil in CRC [140]. However, when 5-fluorouracil was metabolized by Escherichia coli, it no longer effectively curtailed cancer cell growth or the proliferation of F. nucleatum, highlighting the intricate relationships between intratumor microbes with chemoresistance. Intratumor Desulfovibrio desulfuricans has been found to exhibit increased colonization in CRC tissues among non-responders [141], and further in vivo experiments demonstrated that Desulfovibrio, along with its metabolite S-adenosylmethionine, could attenuate the effectiveness of the FOLFOX chemotherapy regimen by upregulating the expression of methyltransferase-like 3. In addition, Colibactin-producing Escherichia coli has been shown to induce chemotherapeutic resistance in CRC through facilitating EMT and cancer cell stemness [142].
Immunotherapy
Immunotherapy represents the cutting-edge in cancer treatment methods, such as ICB therapy and chimeric antigen receptor (CAR) T cell therapy. Remarkably, mounting evidence indicates the substantial influence of intratumor microbiota on regulating cancer immunotherapy response in recent years [143]. In a recent analysis of the intratumor microbiota in metastatic cancer, Battaglia et al. indicated the potential relationships between Fusobacterium and ICB resistance in lung cancer. Specifically, a higher abundance of Fusobacterium, after adjusting for genome-wide mutational burden, was significantly linked with reduced overall survival and progression-free survival in non-small cell lung cancer patients [98]. Moreover, differences in intratumoral microbial composition have been observed between responders and non-responders to ICB therapy in metastatic melanomas [6]. That is, responders were enriched in Clostridium, while Gardnerella vaginalis was more abundant in tumors of non-responders. Nevertheless, the accumulation of intratumor Bifidobacterium can convert the non-responders into responders to anti-CD47 immunotherapy by stimulating STING pathway in CRC- and lymphoma-bearing mouse models [114]. Similarly, Wu and colleagues demonstrated that the enrichment of Streptococcus in tumor tissues was conducive to a favorable outcome to anti-PD-1 treatment by inducing CD8 + T cell infiltration in an ESCC mouse model [55]. In addition, Bender et al. elucidated the interactions between intratumoral Lactobacillus reuteri and the TME in melanoma, mediated by the metabolite indole-3-aldehyde, using a preclinical mouse model, which simulated anti-tumor immunity and augmented the efficacy of ICB therapy by amplifying interferon-γ-producing CD8 + T cells [144].
Although CAR-T cell therapy stands as a monumental breakthrough in cancer treatment, demonstrating remarkable efficacy against multiple lymphohematopoietic malignancies, its application to solid tumors remains challenging due to limited effector cell infiltration into tumors and the highly immunosuppressive TME [145]. Encouragingly, emerging evidence suggests the potential to improve CAR-T efficacy through genetically engineered virus. Wang and colleagues revealed that adenovirus armed with the chemokine CXCL11 could significantly potentiate CAR-T effectiveness and establish a long-lasting anti-tumor response by reshaping the immunosuppressive TME in glioblastoma [146], including elevated infiltration of CD8 + T cells, NK cells, and M1-polarized macrophages, along with reduced frequencies of MDSCs, regulatory T cells, and M2-polarized macrophages.
Targets for cancer therapy
As a leading cause of mortality worldwide, cancer poses a significant global public health challenge. Thus, there is an urgent imperative to explore novel targets for cancer treatment [147]. Considering the profound influence of intratumor microbiota on cancer progression and treatment outcomes, harnessing intratumor microbiota as therapeutic targets would present innovative strategies to complement existing cancer treatments (Fig. 5), such as (1) modulating microbial composition to reinvigorate anti-tumor immunity; (2) engineering bacteria to deliver therapeutic payloads directly to tumors or convert the immunosuppressive TME to an immunostimulatory one; and (3) exploiting oncolytic viruses or bacteria to directly eliminate tumor cells.
Targets for intratumoral microbiota-based cancer therapeutic strategies. Modification of intratumor microbiota presents promising avenues for enhancing existing cancer therapies, such as modulating microbial composition by antibiotics or nanoparticle-coupled antibiotics to reinvigorate anti-tumor immunity, engineering bacteria to deliver therapeutic payloads directly to tumors or developing cancer vaccines to trigger immunogenic cell death, and utilizing oncolytic viruses or bacteria to directly eliminate tumor cells. PD-L1, programmed cell death-ligand 1; mAb, monoclonal antibody; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; STING, stimulating stimulator of interferon genes; ICD, immunogenic cell death; PFN, perforin; GzmB, Granzyme B; DAMPs, damage-associated molecular pattern molecules; TAAs, tumor-associated antigens; PAMPs, pathogen-associated molecular pattern molecules; TANs, tumor-associated neoantigens
Reinvigorating anti-tumor immunity
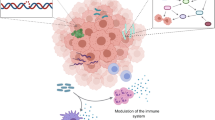
The intratumor microbiota emerges as a promising target for revitalizing the anti-tumor immune response that is crucial for advancing cancer treatments. The elimination of intratumor bacterial by antibiotics could potentially reverse the immune tolerance within the TME in PDAC, thereby enhancing the efficacy of ICB therapy [96]. Nevertheless, given the potential influence of oral antibiotics on systemic microbial flora, such as the induction of gut microbial dysbiosis, which often correlates with dampened responses to immunotherapy [148], therefore, a more targeted bacteriolytic approach within the tumor is warranted. Indeed, several studies have demonstrated the effectiveness of nanoparticle-coupled antibiotics in eliminating tumor-promoting intratumor bacteria without disrupting the body’s microbiota balance. For instance, Wang et al. devised a liposome-encapsulated antibiotic silver-tinidazole complex (LipoAgTNZ) to target intratumor bacteria in primary CRC tumors and liver metastases with the aim to prevent gut dysbiosis [149]. Significantly, LipoAgTNZ treatment contributed to the generation of microbial neoantigens, thereby potentiating anti-tumor CD8 + T cells and resulting in long-term survival in two F. nucleatum-infected CRC models. Additionally, Gao et al. reported that dual-targeting nanoparticles containing metronidazole and fluorouridine accumulated within the TME and released these drugs, achieving a synergistic anti-tumor effect by attacking intratumor bacteria and cancer cells, respectively [150]. This finding underscores the feasibility of “kill two birds with one stone” by targeting intratumor microbes in cancer therapy.
Peptides derived from intracellular bacteria has been observed to be immunogenic and can be presented by the human leukocyte antigen class (HLA)-I and HLA-II molecules on tumor cells to elicit anti-tumor immune reactivity in melanoma [97], highlighting the theoretical foundation for intratumor microbiota-mediated anti-tumor immune activation. Bacterial outer membrane vesicles (OMVs) serve as potent immune adjuvants, capable of transforming the immunosuppressive TME into an immunologically active one. Caproni et al. demonstrated that intratumor injection of OMVs derived from the E. coli BL21(DE3)Δ60 strain could induce robust anti-tumor activity, accompanied by rapid infiltrations of DCs and NK cells in a tumor-bearing mouse model [151]. In addition, intratumoral vaccination can trigger significant anti-tumor immunity. Peng and colleagues indicated that intratumoral Tissue Antigen-Cervical Intraepithelial Neoplasia vaccination arouse a stronger antigen-specific CD8 + T cell responses and anti-tumor effects when combined with anti-PD-1 blockade therapy in a preclinical HPV model [152].
Engineered bacteria
Bacteria can be genetically engineered to function as therapeutic delivery vehicles in cancer therapy [153]. Intercellular self-replication and translocation represent the primary mechanisms facilitating bacterial penetration into tumors [154]. Gurbatri et al. developed a probiotic bacteria system to meticulously produce and release nanobodies targeting PD-L1 and cytotoxic T lymphocyte-associated protein-4 within tumors [155]. They observed a significantly amplified anti-tumor effect with this probiotic E. coli Nissle 1917 (EcN) system, leading to superior tumor eradication and survival benefits compared to analogous clinically relevant antibodies. Moreover, Savage and colleagues engineered bacteria to secrete chemokines, including CXCL16 and CCL20, directly into tumors [156], which could recruit and activate innate (DCs) and adaptive (CD8 + T cells) anti-tumor immune responses, thus providing a novel approach to potentiate cancer immunotherapy. It is worth mentioning that in a phase I clinical trial involving advanced cancer patients, Luke et al. investigated the safety and efficacy of intratumoral injection of engineered EcN expressing a STING agonist, with and without ICB therapy [157]. The researchers observed the well-tolerated and effective nature of this probiotic EcN treatment, with no severe therapy-related adverse events or infections reported.
Notably, beyond serving as a platform for the intratumoral delivery of cancer therapeutics, engineered bacteria also possess the capability to activate the anti-tumor response within the immunologically “cold” TME. Zhou and colleagues demonstrated the activation of CD8 + T cells in the TME of melanoma following the treatment with genetically modified Salmonella typhimurium, which induced targeted methionine deprivation in tumor tissues [158]. Additionally, these encoded bacteria could be employed as in situ cancer vaccines with minimal systemic exposure and adverse effects. Among them, calcium carbonate biomineralized Salmonella has been shown to function as a cancer vaccine producer, inducing immunogenic cell death (ICD) in cancer cells and promoting the formation of gap junctions between tumor cells and DCs to enhance antigen presentation [159]. On the other hand, the internalization of Ca2+ into various immune cells could synergize with Salmonella to systematically regulate the immune system, including DCs maturation, macrophages polarization, and T cells activation. Likewise, Zhu et al. displayed that intratumoral delivery of a probiotic, food-grade Lactococcus lactis-based in situ vaccination (FOLactis), contributed to sustained activation of NK cells, cytotoxic T cells, and conventional-type-1-dendritic cells within tumors and tumor-draining lymph nodes by secreting a fusion protein of Fms-like tyrosine kinase 3 ligand and co-stimulator OX40 ligand in a murine colon cancer model [160]. Further, FOLactis established long-term immunological memory and protection lasting over 60 days, significantly prolonged the survival of tumor-bearing mice in combination with anti-PD-1 therapy.
Oncolytic virus and bacteria
Oncolytic viruses (OVs) refer to native viral species or genetically engineered viruses that selectively infect and preferentially lyse tumor cells while sparing non-neoplastic cells [23], making them appealing candidates for precise cancer treatment. Additionally, exposure to OVs can trigger ICD by prompting the release of damage-associated molecular pattern molecules, pathogen-associated molecular pattern molecules, tumor-associated antigens, and tumor-associated neoantigens, leading to the amplification of innate immunity and adaptive anti-tumor response [161]. So far, a host of preclinical, clinical trials, and real-world clinical studies have highlighted the promise of oncolytic virotherapy. Four commercial OVs, including H101, T-VEC, ECHO-7, and Teserpaturev, have been approved for cancer therapy globally, as extensively reviewed [23, 161,162,163]. Notably, the combination of OVs with immunotherapeutic strategies, such as ICB and adoptive cellular therapy, has yielded compelling results and manageable safety profiles in tumor intervention in recent years (Table 3). Most recently, Wang et al. evaluated the efficacy of combining an oncolytic vaccinia virus encoding hyaluronidase with diverse cancer therapies, including gemcitabine, doxorubicin, liraglutide, anti-PD-1 and anti-CD47 monoclonal antibodies, or CAR-T cells [164]. The authors found that OVs remarkably improved the therapeutic outcomes of existing cancer therapeutics by degrading hyaluronic acid in the TME, highlighting the alluring foreground of OVs in potentiating the efficacy of cancer treatments. Despite this, several concerns regarding oncolytic adenovirus should be acknowledged, such as the potential safety thereat if “off-target” and dissemination throughout the body, in vivo pre-existing neutralizing antibodies to restrain OVs, and poor targeting delivery efficacy to reach tumor tissues [162, 165].
In addition, particular bacteria have demonstrated oncolytic effects in recent years. Among these, Clostridium ghonii has shown oncolytic abilities both in in-vitro and in a mouse model of lung cancer by facilitating the apoptosis and necrosis in tumor cells [197]. Notably, Goto et al. were the first to document the safety and tumor-suppressing effects of administering tumor-isolated oncolytic bacteria via intravenous injection in various preclinical models, including CRC, sarcoma, metastatic lung cancer, and drug-resistant breast cancer [198]. Further, the tumor-derived Cutibacterium acnes has proven to be an effective anti-tumor “living drug” with oncolytic potential in a mouse model of CRC [199]. Importantly, in the pioneering phase I clinical trial investigating bacteriolytic therapy utilizing attenuated Clostridium novyi-NT (non-toxic) for refractory solid tumors [200], Janku and colleagues observed that a sole intratumoral injection of C. novyi-NT led to bacterial spore germination, resulting in tumor masse lysis in 42% of patients with manageable toxicities. Despite these findings hint at the potential feasibility of utilizing oncolytic bacteria in cancer therapy, further research is warranted to verify their safety, efficacy, and underling mechanisms of action.
Prospect and challenges
The burgeoning recognition of the roles played by intratumor microbiota in tumorigenesis presents both promising prospects and formidable challenges in cancer management. On one front, the nuanced understanding of the intricate interactions between microbial communities and the processes driving tumor initiation and progression offers novel avenues for innovative therapeutic intervention strategies. Targeting specific cancer-promoting or immunosuppressive oncomicrobes inhabiting tumors holds the potential to disrupt tumorigenic pathways and augment the efficacy of existing cancer treatments. Furthermore, leveraging the tumor microbiome as a discerning biomarker for cancer diagnosis and prognostication exhibits considerable promise for advancing personalized medicine paradigms. Lastly, exploiting microbiota-mediated cancer therapy involves harnessing engineered microorganisms as “living drugs” to rejuvenate anti-tumor immunity, deliver therapeutic payloads, or directly kill cancer cells.
Nevertheless, several challenges must be addressed to fully utilize the potential of intratumor microbiota in cancer management. Foremost among these obstacles is the highly heterogeneity within and between tumor types but low biomass characterizing tumor microbiome, which impede precise characterization and establishment of universal therapeutic approaches. Meanwhile, it is essential to refine and standardize methodologies for profiling intratumor microbial communities while eliminating host genome and environmental contamination, which is imperative for reproducibility and comparability of intratumor microbiota studies. In a recent quantitative microbiome profiling analysis of CRC, Tito et al. did not observe significant associations between the well-established microbial marker F. nucleatum and CRC diagnostic groups, including healthy individuals, adenoma patients, and those diagnosed with carcinoma, when controlling for several covariates [201], highlighting the challenges and uncertainties inherent in delineating appropriate controls for identifying biomarkers in cancer microbiome research. In addition, deciphering how host factors, including genetics, immune status, and environmental exposures, regulate microbial colonization and activity within tumors necessitates interdisciplinary collaborations and innovative research approaches. Importantly, untangling the causal relationships between specific microbial taxa and tumorigenesis warrants comprehensive longitudinal investigations spanning diverse cancer types and patient cohorts.
Furthermore, the development of cancer therapeutic strategies targeting intratumor microbiota requires meticulous attention to safety and efficacy considerations. Apart from potential off-target effects, manipulating microbial populations within tumors may inadvertently disrupt the symbiotic balance of commensal microbiota in the host, potentially precipitating adverse effects or engendering treatment resistance. Thus, refining precision medicine approaches that selectively target carcinogenic microbiota populations without altering the composition of beneficial microbial communities assumes paramount importance for the success of microbiota-based cancer therapies.
In summary, while the exploration of intratumor microbiota signifies a promisingly revitalized frontier in cancer research, navigating the complexities and uncertainties inherent in this field demands a concerted effort from researchers, clinicians, and policymakers alike. By embracing interdisciplinary collaborations, advancing technological innovations, and upholding ethical standards, we can unlock the full potential of intratumor microbiota as pivotal determinants of cancer pathogenesis and therapeutic response, ultimately improving outcomes for cancer patients.
Availability of data and materials
Not applicable.
Abbreviations
- TME:
-
Tumor microenvironment
- EBV:
-
Epstein-Barr Virus
- HBV:
-
Hepatitis B Virus
- HCV:
-
Hepatitis C Virus
- HPV:
-
human papillomavirus
- HTLV:
-
human T cell lymphotropic virus type 1
- HCC:
-
Hepatocellular carcinoma
- T-VEC:
-
Talimogene laherparepvec
- 16S rRNA:
-
16S ribosomal RNA
- FISH:
-
Fluorescence in situ hybridization
- NAT:
-
Normal adjacent tissue
- CRC:
-
Colorectal cancer
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- MMRd:
-
Mismatch repair deficiency
- Fna:
-
F. nucleatum Subspecies animalis
- NPC:
-
Nasopharyngeal carcinoma
- ICB:
-
Immune checkpoint blockade
- DC:
-
Dendritic cell
- MVs:
-
Membrane vesicles
- ROS:
-
Reactive oxygen species
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TLR:
-
Toll-like receptor
- MDSCs:
-
Myeloid-derived suppressor cells
- IL:
-
Interleukin
- PD-L1:
-
Programmed cell death-ligand 1
- ESCC:
-
Esophageal squamous cell carcinoma
- ILC2:
-
Innate lymphoid cells 2
- MAPK:
-
Mitogen-activated protein kinase
- STING:
-
Stimulating stimulator of interferon genes
- EMT:
-
Epithelial–mesenchymal transition
- lncRNA:
-
Long non-coding RNA
- HR:
-
Hazard ratio
- CAR:
-
Chimeric antigen receptor
- LipoAgTNZ:
-
Liposome-encapsulated antibiotic silver-tinidazole complex
- HLA:
-
Human leukocyte antigen class
- OMVs:
-
Outer membrane vesicles
- EcN:
-
E. coli Nissle 1917
- ICD:
-
Immunogenic cell death
- FOLactis:
-
Food-grade Lactococcus lactis-based in situ vaccination
- OVs:
-
Oncolytic viruses
References
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–40.
Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–93.
Schupack DA, et al. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol. 2022;19(1):7–25.
Nejman D, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–80.
Sepich-Poore GD, et al. The microbiome and human cancer. Science. 2021;371(6536):4552.
Meng YF, et al. Role of the intratumoral microbiome in tumor progression and therapeutics implications. Biochim Biophys Acta Rev Cancer. 2023;1878(6): 189014.
Biological agents. IARC Monogr Eval Carcinog Risks Hum, 2012. 100(Pt B): p. 1–441.
de Martel C, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–90.
Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79(938):672–80.
Turner T, Caspari T. When heat casts a spell on the DNA damage checkpoints. Open Biol. 2014;4(3): 140008.
Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92(3):421–5.
Hoption Cann SA, et al. Spontaneous regression: a hidden treasure buried in time. Med Hypotheses. 2002;58(2):115–9.
Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103.
Bassi P. BCG (Bacillus of Calmette Guerin) therapy of high-risk superficial bladder cancer. Surg Oncol. 2002;11(1–2):77–83.
Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53–6.
Budynek P, et al. Bacteriophages and cancer. Arch Microbiol. 2010;192(5):315–20.
Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16(12):789–802.
Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15(11):e517–26.
Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702–3.
Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990–2010. Infect Agent Cancer. 2014;9(1):38.
Shalhout SZ, et al. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. 2023;20(3):160–77.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–5.
Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863–73.
Narunsky-Haziza L, et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell. 2022;185(20):3789-3806.e17.
Dohlman AB, et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell. 2022;185(20):3807-3822.e12.
Lu YQ, et al. Broadening oncological boundaries: the intratumoral microbiota. Trends Microbiol. 2024. https://doi.org/10.1016/j.tim.2024.01.007.
Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516–22.
Quince C, et al. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35(9):833–44.
Fu A, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185(8):1356-1372.e26.
Massironi S, et al. Intratumor Microbiome in Neuroendocrine Neoplasms: A New Partner of Tumor Microenvironment? A Pilot Study. Cells. 2022;11(4):692.
Phelps CM, et al. Detection of viable commensal bacteria in murine melanoma tumors by culturomics. STAR Protoc. 2023;4(3): 102492.
Dumont-Leblond N, et al. Non-small cell lung cancer microbiota characterization: prevalence of enteric and potentially pathogenic bacteria in cancer tissues. PLoS ONE. 2021;16(4): e0249832.
Wong-Rolle A, et al. Spatial meta-transcriptomics reveal associations of intratumor bacteria burden with lung cancer cells showing a distinct oncogenic signature. J Immunother Cancer. 2022;10(7): e004698.
Zhang M, et al. Lung microbiota features of stage III and IV non-small cell lung cancer patients without lung infection. Transl Cancer Res. 2022;11(2):426–34.
Liu NN, et al. The intratumor mycobiome promotes lung cancer progression via myeloid-derived suppressor cells. Cancer Cell. 2023;41(11):1927-1944.e9.
Wang Y, et al. Intra-tumoral microbial community profiling and associated metabolites alterations of TNBC. Front Oncol. 2023;13:1143163.
Chang J, et al. Potential values of formalin-fixed paraffin-embedded tissues for intratumoral microbiome analysis in breast cancer. Heliyon. 2023;9(6): e16267.
Huang JH, et al. The intratumoral bacterial metataxonomic signature of hepatocellular carcinoma. Microbiol Spectr. 2022;10(5): e0098322.
Qu D, et al. Intratumoral microbiome of human primary liver cancer. Hepatol Commun. 2022;6(7):1741–52.
Chai X, et al. Intratumor microbiome features reveal antitumor potentials of intrahepatic cholangiocarcinoma. Gut Microbes. 2023;15(1):2156255.
He Y, et al. Overview of microbial profiles in human hepatocellular carcinoma and adjacent nontumor tissues. J Transl Med. 2023;21(1):68.
Qiao K, et al. Intratumor mycoplasma promotes the initiation and progression of hepatocellular carcinoma. Cell Rep. 2023;42(12): 113563.
Sun L, et al. Intratumoural microbiome can predict the prognosis of hepatocellular carcinoma after surgery. Clin Transl Med. 2023;13(7): e1331.
Liu W, et al. Microbial community heterogeneity within colorectal neoplasia and its correlation with colorectal carcinogenesis. Gastroenterology. 2021;160(7):2395–408.
Okuda S, et al. Profiling of host genetic alterations and intra-tumor microbiomes in colorectal cancer. Comput Struct Biotechnol J. 2021;19:3330–8.
Barot SV, et al. Distinct intratumoral microbiome of young-onset and average-onset colorectal cancer. EBioMedicine. 2024;100: 104980.
Joo JE, et al. Intratumoral presence of the genotoxic gut bacteria pks(+) E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer. Br J Cancer. 2024. https://doi.org/10.1038/s41416-023-02554-x.
Peng R, et al. Gastric microbiome alterations are associated with decreased CD8+ tissue-resident memory T cells in the tumor microenvironment of gastric cancer. Cancer Immunol Res. 2022;10(10):1224–40.
Fu K, et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell. 2024;187(4):882-896.e17.
Baba Y, et al. Relationship between gut microbiome Fusobacterium nucleatum and LINE-1 methylation level in esophageal cancer. Esophagus. 2023;20(4):704–12.
Kosumi K, et al. Intratumour Fusobacterium nucleatum and immune response to oesophageal cancer. Br J Cancer. 2023;128(6):1155–65.
Li Y, et al. Intracellular Fusobacterium nucleatum infection attenuates antitumor immunity in esophageal squamous cell carcinoma. Nat Commun. 2023;14(1):5788.
Wu H, et al. Intratumoral microbiota composition regulates chemoimmunotherapy response in esophageal squamous cell carcinoma. Cancer Res. 2023. https://doi.org/10.1158/0008-5472.CAN-22-2593.
Huang Y, et al. Intratumor microbiome analysis identifies positive association between megasphaera and survival of Chinese patients with pancreatic ductal adenocarcinomas. Front Immunol. 2022;13: 785422.
Tan Q, et al. Periodontitis pathogen Porphyromonas gingivalis promotes pancreatic tumorigenesis via neutrophil elastase from tumor-associated neutrophils. Gut Microbes. 2022;14(1):2073785.
Hayashi M, et al. Intratumor Fusobacterium nucleatum promotes the progression of pancreatic cancer via the CXCL1-CXCR2 axis. Cancer Sci. 2023;114(9):3666–78.
Abe S, et al. Impact of intratumoral microbiome on tumor immunity and prognosis in human pancreatic ductal adenocarcinoma. J Gastroenterol. 2024. https://doi.org/10.1007/s00535-023-02069-5.
Neuzillet C, et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci Rep. 2021;11(1):7870.
Qiao H, et al. Association of intratumoral microbiota with prognosis in patients with nasopharyngeal carcinoma from 2 hospitals in China. JAMA Oncol. 2022;8(9):1301–9.
Zeng B, et al. The oral cancer microbiome contains tumor space-specific and clinicopathology-specific bacteria. Front Cell Infect Microbiol. 2022;12: 942328.
Pratap Singh R, et al. Intratumoral microbiota changes with tumor stage and influences the immune signature of oral squamous cell carcinoma. Microbiol Spectr. 2023;11(4): e0459622.
Wang J, et al. Uncovering the microbiota in renal cell carcinoma tissue using 16S rRNA gene sequencing. J Cancer Res Clin Oncol. 2021;147(2):481–91.
Hawkins GM, et al. Differences in the microbial profiles of early stage endometrial cancers between black and white women. Gynecol Oncol. 2022;165(2):248–56.
Huang Q, et al. Endogenous propionibacterium acnes promotes ovarian cancer progression via regulating hedgehog signalling pathway. Cancers (Basel). 2022;14(21):5178.
Yuan L, et al. Tumor microbiome diversity influences papillary thyroid cancer invasion. Commun Biol. 2022;5(1):864.
Cantini G, et al. Intratumour microbiota modulates adrenocortical cancer responsiveness to mitotane. Endocr Relat Cancer. 2023. https://doi.org/10.1530/ERC-23-0094.
Perry LM, et al. Human soft tissue sarcomas harbor an intratumoral viral microbiome which is linked with natural killer cell infiltrate and prognosis. J Immunother Cancer. 2023;11(1): e004285.
Rustetska N, et al. The intratumour microbiota and neutrophilic inflammation in squamous cell vulvar carcinoma microenvironment. J Transl Med. 2023;21(1):285.
Ye L, et al. Evidence for an intra-tumoral microbiome in pituitary neuroendocrine tumors with different clinical phenotypes. J Neurooncol. 2023;163(1):133–42.
Swanton C, et al. Embracing cancer complexity: hallmarks of systemic disease. Cell. 2024;187(7):1589–616.
Kong C, et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-327156.
Zhu H, et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat Commun. 2024;15(1):1688.
Park HE, et al. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471(3):329–36.
Zepeda-Rivera M, et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature. 2024;628(8007):424–32.
Liao Y, et al. Microbes translocation from oral cavity to nasopharyngeal carcinoma in patients. Nat Commun. 2024;15(1):1645.
Yang L, et al. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther. 2023;8(1):35.
Cao Y, et al. Intratumoural microbiota: a new frontier in cancer development and therapy. Signal Transduct Target Ther. 2024;9(1):15.
Yamamoto S, et al. Heterogeneous distribution of Fusobacterium nucleatum in the progression of colorectal cancer. J Gastroenterol Hepatol. 2021;36(7):1869–76.
Bertocchi A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39(5):708-724.e11.
Zhu Z, et al. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of gut Akkermansia muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in mice. Gut Microbes. 2023;15(1):2166700.
Choi Y, et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci Immunol. 2023;8(81):eabo2003.
Malfertheiner P, Link A, Selgrad M. Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol. 2014;11(10):628–38.
Ciernikova S, et al. Tumor microbiome—an integral part of the tumor microenvironment. Front Oncol. 2022;12:1063100.
Li Y, et al. Multimodal immune phenotyping reveals microbial-T cell interactions that shape pancreatic cancer. Cell Rep Med. 2024;5:101397.
Cao Y, et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science. 2022;378(6618):eabm3233.
Pleguezuelos-Manzano C, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580(7802):269–73.
Miyakawa Y, et al. Gut Bacteria-derived membrane vesicles induce colonic dysplasia by inducing DNA damage in colon epithelial cells. Cell Mol Gastroenterol Hepatol. 2024;17(5):745–67.
He Z, et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68(2):289–300.
Srivatsav AT, Kapoor S. The emerging world of membrane vesicles: functional relevance, theranostic avenues and tools for investigating membrane function. Front Mol Biosci. 2021;8: 640355.
Ma Y, et al. Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep Med. 2024;5(4): 101488.
Sun X, Zhu MJ. Butyrate inhibits indices of colorectal carcinogenesis via enhancing α-ketoglutarate-dependent DNA demethylation of mismatch repair genes. Mol Nutr Food Res. 2018;62(10): e1700932.
Mowat C, et al. Short chain fatty acids prime colorectal cancer cells to activate antitumor immunity. Front Immunol. 2023;14:1190810.
Mirzaei R, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139: 111619.
Pushalkar S, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–16.
Kalaora S, et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature. 2021;592(7852):138–43.
Battaglia TW, et al. A pan-cancer analysis of the microbiome in metastatic cancer. Cell. 2024;187(9):2324-2335.e19.
Triner D, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. 2019;156(5):1467–82.
Chen Z, Huang L. Fusobacterium nucleatum carcinogenesis and drug delivery interventions. Adv Drug Deliv Rev. 2024;209: 115319.
Wu Y, et al. Fn-Dps, a novel virulence factor of Fusobacterium nucleatum, disrupts erythrocytes and promotes metastasis in colorectal cancer. PLoS Pathog. 2023;19(1): e1011096.
Nie F, et al. The role of CXCL2-mediated crosstalk between tumor cells and macrophages in Fusobacterium nucleatum-promoted oral squamous cell carcinoma progression. Cell Death Dis. 2024;15(4):277.
Ren J, et al. P. gingivalis infection upregulates PD-L1 expression on dendritic cells, suppresses CD8+ T-cell responses, and aggravates oral cancer. Cancer Immunol Res. 2023;11(3):290–305.
Yáñez L, et al. Co-culture of P. gingivalis and F. nucleatum synergistically elevates IL-6 expression via TLR4 signaling in oral keratinocytes. Int J Mol Sci. 2024;25(7):3611.
Alam A, et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell. 2022;40(2):153-167.e11.
Brandstadter JD, Yang Y. Natural killer cell responses to viral infection. J Innate Immun. 2011;3(3):274–9.
Ou S, et al. Fusobacterium nucleatum upregulates MMP7 to promote metastasis-related characteristics of colorectal cancer cell via activating MAPK(JNK)-AP1 axis. J Transl Med. 2023;21(1):704.
Sueh CY, et al. Fusobacterium nucleatum impairs DNA mismatch repair and stability in patients with squamous cell carcinoma of the head and neck. Cancer. 2022;128(17):3170–84.
Zhang W, et al. Microbial metabolite trimethylamine-N-oxide induces intestinal carcinogenesis through inhibiting farnesoid X receptor signaling. Cell Oncol (Dordr). 2024. https://doi.org/10.1007/s13402-024-00920-2.
Ma W, et al. Microbiota enterotoxigenic Bacteroides fragilis-secreted BFT-1 promotes breast cancer cell stemness and chemoresistance through its functional receptor NOD1. Protein Cell. 2024. https://doi.org/10.1093/procel/pwae005.
Kong C, et al. Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2 signaling. Cancer Res. 2021;81(17):4485–98.
Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80–6.
Lam KC, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184(21):5338-5356.e21.
Shi Y, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5): e20192282.
Jing W, et al. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7(1):115.
Fu A, et al. Emerging roles of intratumor microbiota in cancer metastasis. Trends Cell Biol. 2023;33(7):583–93.
Hilmi M, et al. Intratumoral microbiome is driven by metastatic site and associated with immune histopathological parameters: an ancillary study of the SHIVA clinical trial. Eur J Cancer. 2023;183:152–61.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Che S, et al. Unveiling the intratumoral microbiota within cancer landscapes. iScience. 2024;27(6): 109893.
Yang Y, et al. Fusobacterium nucleatum Increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. 2017;152(4):851-866.e24.
Tang B, et al. MicroRNA-31 induced by Fusobacterium nucleatum infection promotes colorectal cancer tumorigenesis. SiScience. 2023;26(5): 106770.
Zhang M, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis by excretion of miR-122–5p from cells via exosomes. iScience. 2023;26(9): 107686.
Guo S, et al. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. 2020. https://doi.org/10.1136/gutjnl-2020-321187.
Lu X, et al. Long non-coding RNA EVADR induced by Fusobacterium nucleatum infection promotes colorectal cancer metastasis. Cell Rep. 2022;40(3): 111127.
Xu C, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes. 2021;13(1):1980347.
Zhang S, et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. Febs j. 2020;287(18):4032–47.
Hui B, et al. Exosomes secreted by Fusobacterium nucleatum-infected colon cancer cells transmit resistance to oxaliplatin and 5-FU by delivering hsa_circ_0004085. J Nanobiotechnology. 2024;22(1):62.
Zeng W, Pan J, Ye G. miR-135b aggravates fusobacterium nucleatum-induced cisplatin resistance in colorectal cancer by targeting KLF13. J Microbiol. 2024;62(2):63–73.
Hong J, et al. F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut. 2021;70(11):2123–37.
Sun J, et al. F. nucleatum facilitates oral squamous cell carcinoma progression via GLUT1-driven lactate production. EBioMedicine. 2023;88:104444.
Yamamura K, et al. Intratumoral Fusobacterium nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin Cancer Res. 2019;25(20):6170–9.
Huang ST, et al. Intratumoral levels and prognostic significance of Fusobacterium nucleatum in cervical carcinoma. Aging (Albany NY). 2020;12(22):23337–50.
Mima K, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973–80.
Mouradov D, et al. Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology. 2023;165(1):104–20.
Zhang Y, et al. Pan-cancer analyses reveal the stratification of patient prognosis by viral composition in tumor tissues. Comput Biol Med. 2023;167: 107586.
Huang X, et al. Metagenomic analysis of intratumoral microbiome linking to response to neoadjuvant chemoradiotherapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2023;117(5):1255–69.
Colbert LE, et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell. 2023. https://doi.org/10.1016/j.ccell.2023.09.012.
Wang N, et al. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes. 2024;16(1):2333790.
Li B, et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free Radic Biol Med. 2024. https://doi.org/10.1016/j.freeradbiomed.2024.04.226.
LaCourse KD, et al. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota. Cell Rep. 2022;41(7): 111625.
Li G, et al. Desulfovibrio desulfuricans and its derived metabolites confer resistance to FOLFOX through METTL3. EBioMedicine. 2024;102: 105041.
Dalmasso G, et al. Colibactin-producing Escherichia coli enhance resistance to chemotherapeutic drugs by promoting epithelial to mesenchymal transition and cancer stem cell emergence. Gut Microbes. 2024;16(1):2310215.
Guillot N, et al. Manipulating the gut and tumor microbiota for immune checkpoint inhibitor therapy: from dream to reality. Trends Mol Med. 2023;29(11):897–911.
Bender MJ, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186(9):1846-1862.e26.
Dana H, et al. CAR-T cells: early successes in blood cancer and challenges in solid tumors. Acta Pharm Sin B. 2021;11(5):1129–47.
Wang G, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134–53.
Siegel RL, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Shi Z, et al. Emerging roles of the gut microbiota in cancer immunotherapy. Front Immunol. 2023;14:1139821.
Wang M, et al. Killing tumor-associated bacteria with a liposomal antibiotic generates neoantigens that induce anti-tumor immune responses. Nat Biotechnol. 2023. https://doi.org/10.1038/s41587-023-01957-8.
Gao C, et al. Synergistic target of intratumoral microbiome and tumor by metronidazole-fluorouridine nanoparticles. ACS Nano. 2023;17(8):7335–51.
Caproni E, et al. Anti-tumor efficacy of in situ vaccination using bacterial outer membrane vesicles. Cancers (Basel). 2023;15(13):3328.
Peng S, et al. PD-1 blockade synergizes with intratumoral vaccination of a therapeutic HPV protein vaccine and elicits regression of tumor in a preclinical model. Cancer Immunol Immunother. 2021;70(4):1049–62.
Zalatan JG, Petrini L, Geiger R. Engineering bacteria for cancer immunotherapy. Curr Opin Biotechnol. 2024;85: 103061.
Suh S, et al. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci (Weinh). 2019;6(3):1801309.
Gurbatri CR, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med. 2020;12(530):eaax0876.
Savage TM, et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Sci Adv. 2023;9(10):eadc9436.
Luke JJ, et al. Phase I study of SYNB1891, an engineered E. coli Nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin Cancer Res. 2023;29(13):2435–44.
Zhou S, et al. Salmonella-mediated methionine deprivation drives immune activation and enhances immune checkpoint blockade therapy in melanoma. J Immunother Cancer. 2024;12(2): e008238.
Guo L, Ding J, Zhou W. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm Sin B. 2023;13(12):5074–90.
Zhu J, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy. Nat Commun. 2022;13(1):7466.
Tian Y, Xie D, Yang L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct Target Ther. 2022;7(1):117.
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–9.
Abd-Aziz N, Poh CL. Development of oncolytic viruses for cancer therapy. Transl Res. 2021;237:98–123.
Wang S, et al. An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy. J Immunother Cancer. 2024;12(3):e008431.
Ban W, et al. Emerging systemic delivery strategies of oncolytic viruses: a key step toward cancer immunotherapy. Nano Res. 2022;15(5):4137–53.
Lou J, et al. Remodeling of the tumor microenvironment using an engineered oncolytic vaccinia virus improves PD-L1 inhibition outcomes. Biosci Rep. 2021;41(6):BSR20204186.
Kajiwara Y, et al. Oncolytic virus-mediated reducing of myeloid-derived suppressor cells enhances the efficacy of PD-L1 blockade in gemcitabine-resistant pancreatic cancer. Cancer Immunol Immunother. 2023;72(5):1285–300.
Chen L, et al. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol Ther Oncolytics. 2022;24:522–34.
Toulmonde M, et al. Reshaping the tumor microenvironment of cold soft-tissue sarcomas with oncolytic viral therapy: a phase 2 trial of intratumoral JX-594 combined with avelumab and low-dose cyclophosphamide. Mol Cancer. 2024;23(1):38.
Panagioti E, et al. Immunostimulatory bacterial antigen-armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy. J Clin Invest. 2021;131(13): e141614.
Huang L, et al. Oncolytic adenovirus H101 ameliorate the efficacy of anti-PD-1 monotherapy in colorectal cancer. Cancer Med. 2022;11(23):4575–87.
Sun K, et al. A phase 2 trial of enhancing immune checkpoint blockade by stereotactic radiation and in situ virus gene therapy in metastatic triple-negative breast cancer. Clin Cancer Res. 2022;28(20):4392–401.
Rudin CM, et al. Phase 1, open-label, dose-escalation study on the safety, pharmacokinetics, and preliminary efficacy of intravenous Coxsackievirus A21 (V937), with or without pembrolizumab, in patients with advanced solid tumors. J Immunother Cancer. 2023;11(1): e005007.
Fakih M, et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: a phase I clinical trial (SPICE). J Immunother Cancer. 2023;11(4):e006561.
Nassiri F, et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial. Nat Med. 2023;29(6):1370–8.
Silk AW, et al. A phase 1b single-arm trial of intratumoral oncolytic virus V937 in combination with pembrolizumab in patients with advanced melanoma: results from the CAPRA study. Cancer Immunol Immunother. 2023;72(6):1405–15.
Gui M, et al. Swine pseudorabies virus attenuated vaccine reprograms the kidney cancer tumor microenvironment and synergizes with PD-1 blockade. J Med Virol. 2024;96(4): e29568.
Park JS, et al. PD-1 inhibitor plus oncolytic vaccinia virus is a safe and effective treatment option for metastatic renal cell carcinoma. Cancer Cell Int. 2024;24(1):50.
Yu H, et al. Combination immunotherapy of oncolytic flu-vectored virus and programmed cell death 1 blockade enhances antitumor activity in hepatocellular carcinoma. Hum Gene Ther. 2024;35(5–6):177–91.
Curti BD, et al. Intratumoral oncolytic virus V937 plus ipilimumab in patients with advanced melanoma: the phase 1b MITCI study. J Immunother Cancer. 2022;10(12): e005224.
Chesney JA, et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J Immunother Cancer. 2023;11(5): e006270.
Lutzky J, et al. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. J Cancer Res Clin Oncol. 2023;149(9):6059–66.
Zuo S, et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J Immunother Cancer. 2021;9(12): e002843.
Ju F, et al. Oncolytic virus expressing PD-1 inhibitors activates a collaborative intratumoral immune response to control tumor and synergizes with CTLA-4 or TIM-3 blockade. J Immunother Cancer. 2022;10(6): e004762.
Monge C, et al. Phase I/II study of PexaVec in combination with immune checkpoint inhibition in refractory metastatic colorectal cancer. J Immunother Cancer. 2023;11(2): e005640.
Rosewell Shaw A, et al. Oncolytic adeno-immunotherapy modulates the immune system enabling CAR T-cells to cure pancreatic tumors. Commun Biol. 2021;4(1):368.
Huang J, et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother. 2021;70(9):2453–65.
Wenthe J, et al. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol Immunother. 2021;70(10):2851–65.
Chalise L, et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Δ combination therapy against glioblastoma. Mol Ther Oncolytics. 2022;26:265–74.
Evgin L, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. 2022;14(640):eabn2231.
Sonzogni O, et al. T-SIGn tumor reengineering therapy and CAR T cells synergize in combination therapy to clear human lung tumor xenografts and lung metastases in NSG mice. Oncoimmunology. 2022;11(1):2029070.
Zhu G, et al. Enhancement of CD70-specific CAR T treatment by IFN-γ released from oHSV-1-infected glioblastoma. Cancer Immunol Immunother. 2022;71(10):2433–48.
Fang L, et al. Oncolytic adenovirus-mediated expression of CCL5 and IL12 facilitates CA9-targeting CAR-T therapy against renal cell carcinoma. Pharmacol Res. 2023;189: 106701.
Chen C, et al. Using oncolytic virus to retask CD19-chimeric antigen receptor T cells for treatment of pancreatic cancer: toward a universal chimeric antigen receptor T-cell strategy for solid tumor. J Am Coll Surg. 2024;238(4):436–47.
Ma R, et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 2021;81(13):3635–48.
Strecker MI, et al. AAV-mediated gene transfer of a checkpoint inhibitor in combination with HER2-targeted CAR-NK cells as experimental therapy for glioblastoma. Oncoimmunology. 2022;11(1):2127508.
Wang Y, et al. Bacteriolytic therapy with Clostridium ghonii for experimental solid tumors. Biochem Biophys Res Commun. 2022;634:114–21.
Goto Y, et al. Discovery of intratumoral oncolytic bacteria toward targeted anticancer theranostics. Adv Sci (Weinh). 2023;10(20): e2301679.
Chintalapati S, et al. Tumor-isolated cutibacterium acnes as an effective tumor suppressive living drug. Biomed Pharmacother. 2024;170: 116041.
Janku F, et al. Intratumoral injection of Clostridium novyi-NT spores in patients with treatment-refractory advanced solid tumors. Clin Cancer Res. 2021;27(1):96–106.
Tito RY, et al. Microbiome confounders and quantitative profiling challenge predicted microbial targets in colorectal cancer development. Nat Med. 2024. https://doi.org/10.1038/s41591-024-02963-2.
Acknowledgements
The authors have no acknowledgement to make.
Funding
This work was supported by the Natural Science Foundation of Henan (242300421019), Henan Province Youth Health Science and Technology Innovation Project (LJRC2023014), Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023012), Joint Construction Project of Medical Science and Technology of Henan Province, China (LHGJ20220386), and National Natural Science Foundation of China (82070209, 82170183, 81970184, U1904139).
Author information
Authors and Affiliations
Contributions
MZ, ZL and ZS conceived the study. ZS and ZL collected the data. ZS and ZL drafted the manuscript and prepared the figures. MZ and ZS revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declare no potential competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Z., Li, Z. & Zhang, M. Emerging roles of intratumor microbiota in cancer: tumorigenesis and management strategies. J Transl Med 22, 837 (2024). https://doi.org/10.1186/s12967-024-05640-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05640-7