Abstract
Understanding the types of haemocytes involved in the immune response in insects, and the mechanism involved in determining that response, can provide a scientific reference for developing effective microbial insecticides. Therefore, the current study examined haemocytes from Agrotis ipsilon (Hufnagel) larvae infected with Escherichia coli in terms of their morphology, total counts, and relative proportions at different time points post-infection by optical microscopy technology. The results revealed six types of haemocytes (prohemocytes, plasmatocytes, granulocytes, spherule cells, oenocytoids, and cystocytes) in the haemolymph of sixth-instar larvae. Haemocyte deformation, disruption, nuclear changes, and vacuoles were recorded after infection with different dosages of E. coli. At each time period post-infection, the total haemocyte count was significantly higher than in the controls, peaking at 24 h post-infection and then decreasing by 48 h post-infection. The proportion of prohemocytes decreased significantly until 24 h post-infection, and then began to increase. By contrast, the proportions of plasmatocytes, granulocytes, spherule cells, oenocytoids, and cystocytes relatively increased, and peaked by 24 h post-infection, and then decreased. This revealed that strong immune response was stimulated in larva of A. ipsilon in a short time after infection with E.coli, and the results shed addition light on the cellular immune response of insects to pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects have a complex immune system, including both cellular immunity and humoral immunity, enabling them to defend against pathogenic infections (Ardia et al. 2012; Berger and Jurčová 2012; Zdybicka-Barabas and Cytryńska 2013; Zhang and Zhang 2019). Haemocytes mainly perform the cellular immune functions, with an important role in defending against pathogen invasion. Previous studies have shown that haemocytes in the insect haemolymph can be categorized into several types, namely prohemocytes, plasmatocytes, granulocytes, cystocytes, adipohemocytes, spherule cells, and oenocytoids (Jones 1977). However, these types of haemocytes differ both among insect species and between developmental and physiological stages within the same insect species (Gillespie et al. 2000; Giannoulis et al. 2005; Beetz et al. 2008; Ruchita and Krishna 2014). Morphological studies of haemocytes can be used to investigate the cellular immune response. More than 100 species of insects have been studied in terms of their haemocytes (Ribeiro and Brehélin 2006; Strand 2008; Wu et al. 2016; Mahmood et al. 2018; Boguś et al. 2018). The primary functions of haemocytes are coagulation, phagocytosis, encapsulation, detoxification, and the storage and distribution of nutritive materials (Siddiqui and Al-Khalifa 2014). Haemocytes come into contact with pathogens via the haemolymph, whereupon they engulf the pathogen, forming nodules that are then destroyed by the immune system (Hillyer and Christensen 2002; Hillyer et al. 2003; Siddiqui and Al-Khalifa 2014). However, during the immune response, pathogens can produce toxins that damage the haemocytes, resulting in variation in the morphology, quantity and proportion of the different types of haemocytes (Wang et al. 1990; Mazet et al. 1994; Vilcinskas et al. 1997; Griesch and Vilcinskas 1998; Perveen and Ahmad 2017; Mahmood et al. 2018; Boguś et al. 2018). Such interactions can affect the efficacy of biological control approaches against pests. Therefore, studying the immune function of haemocytes, and the mechanism involved, will provide a theoretical reference for biological control of the pests. However, because the haemocytes and mechanism involved can differ among insect species (Wang et al. 1990; Feng et al. 2011; Ruchita and Krishna 2014), further studies on the cellular immune responses of additional insects species are necessary.
The black cutworm moth, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae) is a pest of a variety of crops in many areas of the world (Gesraha and Ebeid 2021), and has become one of the most important pests on vegetables and crops throughout China (Ding et al. 2018). Several studies have investigated the biological characteristics of the pest and its control measures (Gemeno and Haynes 2000; Amin et al. 2019; Sobhy et al. 2020; Gesraha and Ebeid 2021). In the previous study, five types of haemocytes in fourth-larval instars of A. ipsilon were reported, changes in the haemocytes and a reduction in the total haemocyte count of A. ipsilon larvae after infection with insecticide dimilin and Bacillus thuringiensis was also observed (El-Aziz and Awad 2010). However, given that haemocyte types can vary among developmental stages of the same insect species, and under different ecological and physiological conditions (Gillespie et al. 2000), as well as in terms of infection with different pathogens (Wang et al. 1990; Feng et al. 2011), there is a need to understand the effects of pathogens on the morphology of haemocytes across the developmental stages of the Chinese population of A. ipsilon.
In this study, we investigated the haemocytes of A. ipsilon in terms of their morphology, total number and relative proportion of count numbers in A. ipsilon larvae at different time points following infection with Escherichia coli. The results will be useful to further understanding of the types of haemocytes involved in the immune response and the mechanisms involved in their immune functions, providing a scientific reference for the development of more effective microbial insecticides.
Materials and methods
Insects
Larvae of A. ipsilon were collected from Leshan tobacco-planting areas around Zunyi in Guizhou province, China in March 2015. The larvae were reared in the laboratory at 25 ± 1℃ and 70 ± 7 relative humidity (RH) under a 14:10 (L:D) hours photoperiod until the third instar stage. Thereafter, they were reared individually to avoid cannibalism. Larvae were fed on Chinese cabbage leaves, Brassica pekinensis. Four generations of larvae were reared, and the sixth-instar larvae from each generation were used in the study.
Preparation of bacterial suspension
Escherichia coli was purchased from Shanghai Luwei Technology company, China, and cultivated on Luria–Bertani (LB) medium at 37 °C. After centrifugation at 2, 000 rpm for 10 min, the bacteria were collected and diluted with normal saline to give six concentrations: 1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 cells/mL. Concentrations of bacteria were determined using a hemocytometer under the optical microscope. First, the number of bacteria in each small square of hemocytometer was measured, and then converted into the number of bacteria in each milliliter of bacterial solution. So, the bacteria number in 1 mL of bacterial suspension = the average number of bacteria in each square (n) × coefficient (k) × dilution ratio of bacterial suspension (d).
Morphological observations on haemocytes of larvae
Ten sixth-instar larvae of A. ipsilon were collected and washed with sterile water. The abdomen of each larva was punctured with a dissecting needle, and a small amount of haemolymph was extracted and placed on a microscope slide with a pipette, forming a blood film. Three drops of Wright’s dye was added to the blood film, followed, 2 min later, by three drops of Giemsa stain and a phosphate buffered solution (PBS, pH7.2). The slides were left for 30 min, washed with tap water, and allowed to dry at room temperature. Each slide was then observed under a light microscope. The haemocytes observed were classified according to the classification standard suggested by Jones, who identified the commonest seven types of haemocytes in insects haemolymph, namely prohemocytes, plasmatocytes, granulocytes, cystocytes, adipohemocytes, spherule cells, and oenocytoids (1977).
Variations in morphology and counts of haemocytes infected by E. coli
To study the variation in morphology and counts of haemocytes from larvae infected by E. coli, 3 μL of a bacterial suspension was injected into the abdomen of larvae using a microinjector. The larvae were then transferred to an artificial climate box and fed on Chinese cabbage leaves. Haemolymph of larvae was collected at 6, 12, 24, 48 h post-injection. The total haemocyte counts (THCs) in each sample of haemolymph was determined using a hemocytometer filled with anticoagulant buffer (Leonard et al. 1985). The haemolymph samples were each placed on a microscope slide, and dyed using three drops of Wright’s-Giemsa stain. Each slide was then examined under a light microscope(640 ×) and haemocyte counts and variation in haemocyte morphology were recorded. Photographs of haemocytes were captured with a digital camera (Canon, EOS-200D). To accurately measure the counts of haemocytes, the haemolymph was thoroughly mixed with the anticoagulant buffer to disperse the haemocytes. For each treatment,45 larvae / time point / dosage were used, and three replicates were conducted. A control experiment was also run using larvae treated with normal saline.
Statistical analysis
All data were analyzed by SPSS version 11.5 software (SPSS Inc., Chicago, IL, USA). Comparisons between the mean of groups at different treatment times and bacterial dosages were analyzed using one-way analysis of variance (ANOVA) and two-way analysis of variance (ANOVA), where the differences among means were compared with the Tukey’s multiple comparison method at P < 0.05 level that the test results are false, reject.
Results
Morphology of larval haemocytes
Six types of haemocytes were found in the haemolymph of A. ipsilon sixth-instar larvae: prohemocytes (PRs), plasmatocytes (PLs), granulocytes (GRs), spherule cells (SPs), oenocytoids (OEs), and cystocytes (CYs). The total haemocyte count was 18,897.33 indiv./mL in the haemolymph, prohemocytes were the most abundant haemocytes with the count of 8,999.45 indiv./mL, followed by plasmatocytes (2,758.66 indiv./mL), granulocytes (2,346.71indiv./mL), spherule cells (2,122.05 indiv./mL) and cystocytes (1,514.26 indiv./mL), oenocytoids were the least abundant (1,156.20 indiv./mL) (Fig. 1A), the difference among them reached significant level (df1 = 6, df2 =13, F = 1703.82, P < 0.0001). Each cell type in the total haemocyte count accounted for 47.62%, 14.60%, 12.42%, 11.23%, 8.01% and 6.12% respectively (Fig. 1B). Healthy haemocytes had well-developed cell membranes and nuclei.
Haemocyte counts and percentages of A. ipsilon larva. A:Haemocyte counts; B:Haemocyte percentages. The letters on the column are the results of Tukey’s multi comparison, the different lowercase letters(a,b,c,d,e,f) represent statistically significant differences in cell counts or percentage among different haemocyte type at P < 0.05 level. The error bars represent the standard error (SE)
Prohemocytes
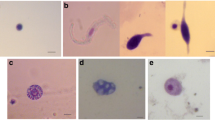
Prophemocytes were identified as small haemocytes (10.25 ~ 12.13 μm × 7.34 ~ 8.81 μm) in the haemolymph of A. ipsilon sixth-instar larva. They were circular or ovate in shape with a clear outline, a nucleus in the center of cell, and a high nuclear:cytoplasmic ratio. The cytoplasm was relatively homogeneous, without any obvious granular materials (Fig. 2A).
Haemocytes isolated from the haemolymph of sixth-instars of A. ipsilon larva. A: Prophemocytes (640 ×); B: Plasmatocyte (640 ×); C: Granulocytes (640 ×); D: Spherule cells (640 ×); E: Oenocytoids (640 ×); F: Cystocytes (640 ×). N, nucleus; LLM, lysosome-like materials; PLI, pearl-like inclusions; RG,refractive granules
Plasmatocytes
Plasmatocyte, also called protogonocytes, occurred in different shapes and sizes (16.13~17.65μm × 8.87~10.29μm). There was a single nucleus in the center of the cell, accounting for half of the overall volume of the cell; the cytoplasm was relatively homogeneous, without any granules (Fig. 2B).
Granulocytes
Granulocytes were common in the haemolymph of A. ipsilon sixth-instar larva, and were circular, ovate, or irregular in shape with different sizes (19.23 ~ 29.41 μm × 8.85 ~ 14.71 μm) (Fig. 2C). The nucleus was circular or ovate, and centrally located. There were heterogeneous lysosome-like materials present in the cytoplasm, enabling these cells to be distinguished from plasmatocytes.
Spherule cells
Spherule cells were circular haemocytes of medium-large size, in the range of 13.53 ~ 16.67 μm × 9.86 ~ 14.17 μm, and contained many pearl-like inclusions forming a circle within the cells (Fig. 2D). It was difficult to locate the nucleus.
Oenocytoids
Oenocytoids were irregular in shape, with a small, circular nucleus. The cell sizes were 17.68 ~ 20.39 μm × 12.92 ~ 15.83 μm. The cytoplasm was dense and homogeneous (Fig. 2E).
Cystocytes
Cystocytes were usually medium-sized haemocyte (14.32 ~ 25.11 μm × 13.33 ~ 23.53 μm), with a circular or oval shape. The outline was relative smooth, and the cytoplasm contained some refractive granules of different sizes (Fig. 2F).
Morphological variations in haemocytes from A. ipsilon larvae infected by E. coli
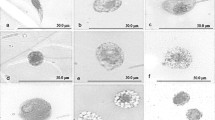
The infection of A. ipsilon by E.coli induced haemocytes to adhere to each other to form aggregates (Fig. 3A), of which most of the haemocytes were plasmatocytes and granulocytes. Six hours after infection, some haemocytes showed considerable structural changes. For example, plasmatocytes lost their smooth outline and complete profile, developing a pleated or distorted outer membrane. The nucleus appeared displaced to one side of the cells with presence of vacuoles of various sizes (Fig. 3B). The granulocytes appeared deformed with many vacuoles in the cytoplasm (Fig. 3C). The membranes of cystocytes became uneven, and ruptured in some cells, which resulted in the out-flowing of cytoplasm content including nucleus (Fig. 3D).
Variation in total haemocytes counts in A. ipsilon larva infected by E. coli
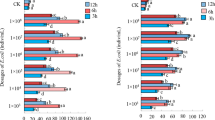
Infection of sixth-instar larva of A. ipsilon with different dosages of E. coli led to significant increases in the number of THCs after 6, 12, 24, and 48 h post-infection (Fig. 4), with the number of THCs increasing significantly with increasing bacterial dosage (df1 = 6, df2 = 21, F = 34.945, P < 0.0001). The number of THCs in each bacterial dosage group peaked at 24 h post-infection (Fig. 4). Overall, infection of A. ipsilon with different dosages of E. coli (1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 cells/mL.) increased the number of THCs by 82.46%, 97.64%, 111.29%, 126.35%, 139.27%, and 155.39% as compared to the control after 24 h post-infection, respectively. After 48 h post-infection, the number of THCs declined, although the differences among the different treatment time remained significant (df1 = 3, df2 = 20, F = 2.498, P < 0.0001). Two-way analysis of variance showed that the interaction of dosages of E. coli and treatment time had a significant influence on the number of THCs in sixth-instar larva of A. ipsilon (df = 15, F = 7.835, P < 0.0001).
Total haemocyte counts of A. ipsilon larva infected by E. coli. The letters on the column are the results of Tukey’s multi comparison between different time points at the same dosage, the different lowercase letters(a,b,c,d) represent statistically significant differences in total cell counts among different time points post-infection at P < 0.05 level. CK is the control treatment, the error bars represent the standard error (SE)
Proportions of haemocyte types of A. ipsilon larva infected by E. coli
Variations in the relative proportion of the six haemocyte types in A. ipsilon sixth instar larva were observed at different time points post-infection with E. coli (Fig. 5). The percentage in number of prohemocyte decreased significantly relative to the control with increasing bacterial dosage (df1 = 6, df2 = 21, F = 7.586, P < 0.0001) and time post infection up until 24 h post-infection (df1 = 3, df2 = 20, F = 14.317, P < 0.0001) (Fig. 5A), which decreased 22.78%, 26.98%, 29.10%, 31.06%, 33.15%, and 35.10% than control, separately. Whereupon it began to increase.
Percentages of count number of haemocytes of A. ipsilon larva infected by E. coli. A. Percentages of prohemocyt; B. Percentages of plasmatocyte; C. Percentages of granulocyte; D. Percentages of spherule cell; E. Percentages of oenocytoid; F. Percentages of cystocyte.The letters on the column are the results of Tukey’s multi comparison between different time points at the same dosage, the different lowercase letters(a,b,c,c,d) represent statistically significant differences in percentages of count number of haemocytes among different time points post-infection at P < 0.05 level. CK is the control treatment, the error bars represent the standard error (SE)
The percentages in number of plasmatocyte (df1 = 6, df2 = 14, F = 4.80, P = 0.007), granulocyte (df1 = 6, df2 = 14, F = 4.162, P = 0.013), spherule cell (df1 = 6, df2 = 14, F = 2.518, P = 0.072), oenocytoid (df1 = 6, df2 = 14, F = 3.242, P = 0.033), and cystocyte (df1 = 6, df2 = 14, F = 5.343, P = 0.005) all increased relative to the control at 6 h post-infection, and with increasing bacterial dosages, peaking at 24 h post-infection (Fig. 5B−F). For each dosage, the percentage in number of plasmatocyte increased 5.39%, 6.21%, 6.85%, 7.44%, 8.05%, and 8.44%, separately, granulocyte increased 4.70%, 5.52%, 6.11%, 6.83%, 7.49%, and 7.87%, spherule cell increased 3.90%, 4.72%, 5.04%, 5.20%, 5.51%, and 5.65%, oenocytoid increased 4.01%, 4.56%, 5.04%, 5.18%, 5.50%, and 5.64%, and cystocyte increased 4.78%, 5.97%, 6.06%, 6.41%, 6.60%, and 7.50%. The difference between different treatment times were not significant in plasmatocyte (df1 = 3, df2 = 20, F = 12.987, P = 0.955), granulocyte (df1 = 3, df2 = 20, F = 12.35, P = 0.995), spherule cell (df1 = 3, df2 = 20, F = 13.926, P = 0.509), oenocytoid (df1 = 3, df2 = 20, F = 14.159, P = 0.306), and cystocyte (df1 = 3, df2 = 20, F = 17.978, P = 0.59). Thereafter, the percentages in number of the five haemocytes had begun to decrease by 48 h post-infection.
Two-way analysis of variance showed that the interaction of dosages of E. coli and treatment time had no significant influence on the percentage in number of prohemocyte (df = 15, F = 1.048, P = 0.427), plasmatocyte (df = 15, F = 0.014, P = 1.00), granulocyte (df = 15, F = 0.012, P = 1.00), spherule cell (df = 15, F = 0.087, P = 1.00), oenocytoid (df = 15, F = 0.112, P = 1.00), and cystocyte (df = 15, F = 0.149, P = 1.00) in sixth-instar larva of A. ipsilon.
Discussion
Haemocytes are the main cells involved in vital physiological activities in insects, for example, prohemocytes are putative stem cells that involved in division of haemocytes; plasmatocytes are the main capsule formation cells that involved in forming of capsule (Jones 1977; Schmidt et al. 2001; Lavine and Strand 2002; Hillyer et al. 2003; Castillo et al. 2006). Studies shows that granulocytes are the professional phagocytes that involved in phagocytizing the pathogens, and they also participated in nodule formation and envelopment (Jones 1977; Schmidt et al. 2001; Lavine and Strand 2002; Hillyer et al. 2003; Castillo et al. 2006). In addition, spherule cells are potentially a source of cuticular components that involved in secretions and storage; oenocytoids are a source of phenoloxidases, and coagulocytes are involved in clotting (Jones 1977; Schmidt et al. 2001; Lavine and Strand 2002; Hillyer et al. 2003; Castillo et al. 2006). However, the proportions and types of haemocytes vary among insect species. Our results revealed six types of haemocytes in the haemolymph of sixth instar larva of A. ipsilon (prohemocytes, plasmatocytes, granulocytes, spherule cells, oenocytoids, and cystocytes). Prohemocytes are by far the most abundant cell type, followed by plasmatocytes and granulocytes, and oenocytoids comprise the least proportion of total haemocyte population. These haemocyte types have already been described in diverse species, including Lepidoptera, Orthoptera, Diptera, Blattaria, Coleoptera, Hymenoptera, Hemiptera, and Collembola (Jones 1977; Lavine and Strand 2002; Hillyer et al. 2003; Ribeiro and Brehélin 2006; Castillo et al. 2006; Boguś et al. 2018). However, five types of haemocytes were found in the haemolymph of fourth-instar larvae of A. ipsilon (prohemocytes, plasmatocytes, granulocytes, spherule cells, and adipohemocytes) (El-Aziz and Awad 2010). These differences further demonstrated that haemocytes differ among developmental stages of the same insect species (Gillespie et al. 2000; Beetz et al. 2008). Furthermore, different foods eaten by the insect and different collection methods of haemocytes may also greatly affect the number and types of haemocytes obtained from the haemolymph (Castillo et al. 2006).
Cellular immunity depends on the phagocytosis, aggregation and encapsulation of haemocytes to pathogens. Plasmatocytes, granulocytes, and oenocytoids are the main haemocytes that involved in the procedure of cellular immunity (Hillyer et al. 2003). Our study shows that plasmatocytes and granulocytes of A. ipsilon adhere to each other to form aggregations after infection with E.coli. These unstructured aggregations may be encapsulated by other haemocytes. Given the role of haemocytes in the cellular immune response against pathogens, it can be hypothesized that pathogen invasion is enhanced by changes in morphology and quantity of the haemocytes in affected insects. In our study, haemocytes from sixth-instar larva of A. ipsilon infected by E. coli underwent considerable structural changes, including deformation, membrane disruption, changes in the position of the nucleus, and occurrence of vacuoles. This showed that larva of A. ipsilon rapidly elicited strong immune responses against inoculated bacteria. These results are in concordance with those of El-Aziz and Awad, who reported that the infection of A. ipsilon with B. thuringiensis induced several pathological detribitons in haemocytes, the contents of the granules seem to swell giving the cells an extremely vacuolated appearance (El-Aziz and Awad 2010). And in other species, such as Plodia interpunctella and Musca domestica, many haemocytes also showed considerable structural changes after infection with bacteria, including occurrence of vacuoles (Wang et al. 1990; Yan et al. 2009; Boguś et al. 2018).
Bacterial infection has also caused a significant increase in number of THCs of sixth-instar larva of A. ipsilon relative to the control (P < 0.05), at 6, 12, and 24 h post-infection. The number of THCs increased with the treatment time extended, and reached highest at 24 h, then it began to decrease after 48 h post-infection. This revealed that strong immune response was stimulated in larva of A. ipsilon in a short time after infection with E.coli, and large numbers of haemocytes were produced and rapidly released into the haemolymph to phagocytose and encapsulate the bacteria. Similar results were obtained in other species, for example, the number of THCs in Manduca sexta larvae showed a marked increase after injected with Pseudomonas aeruginosa (Horohov and Dunn 1982). In another study, injection of Enterobacter cloacae also caused a sharp increase in the number of THCs of Rhodnius prolixus up to 7 days post-infection, followed by a decline in the number of THCs after this time (De Azambuja et al. 1991). Previous studies have showed that the development of hematopoietic organs, the proliferation of haemocytes, and the release of immobilized haemocytes all cause an increase in the number of THCs of insects (Feng et al. 2011). However, the speed of hematopoietic organs development and the proliferation of haemocytes were relatively slow, making it difficult to produce large numbers of haemocytes over a short period of time (Feng et al. 2011). Thus, we hypothesize that the immediate increase in the number of THCs in A. ipsilon larvae after infection with E. coli resulted from the rapid release of immobilized haemocytes into the haemolymph after invasion, and that the increase in the number of THCs seen at later times during the post-infection period could result from the development of hematopoietic organs and the proliferation of haemocytes. With increasing time post-infection, the number of haemocytes that be destroyed also increased, resulting in a decrease in the number of THCs (Feng et al. 2011). However, our results were in contrast to the findings of El-Aziz and Awad, who reported a significant decrease in the number of THCs of forth instar larva of A. ipsilon relative to the controls at 12, 24, and 48 h post-infection with B. thuringiensis (El-Aziz and Awad 2010). In addition, a reduction in haemocyte counts of Trichoplusia ni was found after exposure to B.thuringiensis subsp.kurstaki (Btk) and after injection with E.coli (Ericsson et al. 2009). Infection with Conidiobolus coronatus also caused a significant drop in the number of haemocyte types in Galleria mellonella (Boguś et al. 2018). These variations in the number of THCs might result, in part, from the unusual resistance of different insects to different bacterial pathogens.
After infection with E.coli, the relative proportion of the six haemocyte types all changed. The percentage in number of prohemocyte decreased until 24 h post-infection, and then began to increase. However, the percentages in number of plasmatocyte, granulocyte, spherule cell, oenocytoid, and cystocyte all increased, peaking at 24 h post-infection, and then decreased. The reasons may be that the prohemocyte was activated and differentiated into other haemocyte types during early post-infection periods, caused the percentage in number of prohemocyte to decrease and the percentages in number of plasmatocyte, granulocyte, spherule cell, oenocytoid, and cystocyte to increase. At later post-infection period, the plasmatocyte, granulocyte, spherule cell, oenocytoid, and cystocyte were destroyed, and the differentiation capacity of prohemocyte declined, caused the percentage in number of prohemocyte to increase and the percentages in number of other haemocyte types to decrease in the THCs. However, these results are in disagreement with those of Musca domestica, the percentages in number of plasmatocytes and granulocytes in the larva of M. domestica increased significantly at 4, 6, 8 h post-infection with E.coli, the percentage in number of spherule cells decreased, and the percentages in number of prohemocytes and oenocytoids did not change significantly (Yan et al. 2009). Also, bacterial injection into M. sexta larvae caused a significant increase in percentage in number of spherule cells and significant decrease in percentages in number of granulocytes and plasmatocytes (Horohov and Dunn 1982). No significant changes in the number of oenocytoids were detected. In addition, the infection of Parasarcophaga surcoufi third-instar larvae with nematode decreased the percentages in number of plasmatocytes and granulocytes at 40 h of injection (Ayaad et al. 2001). These discrepancies further highlighted that the types and counts of haemocytes revolved in the immune response differed among insect species when infected with different microorganisms.
In conclusion, after infection with E.coli, the morphology, quantity and proportion of the haemocytes in sixth-instar larva of A. ipsilon all varied, revealing a particular pattern of cellular immune response in these larvae. In the immune response procedure, plasmatocyte, granulocyte, and cystocyte showed significant morphological variation, and their percentage variations was higher than other haemocytes in haemolymph. These indicate that plasmatocyte, granulocyte, and cystocyte are the main participants in immune response of sixth-instar larva of A. ipsilon. The results shed addition light on the cellular immune response of insects to pathogens. Haemocytes are not only responsible for cellular immune response, they also provide humoral immunity factors, e.g. they often release a variety of hydrolases and antioxidase by degranulation during the phagocytosis, which are involved in clearing the pathogen. Further studies on the relationship between haemocytes and humoral factors in immune response are needed.
References
Amin ARH, Bayoumi AED, Dimetry NZ, Youssef DA (2019) Efficiency of nano-formulations of neem and peppermint oils on the bionomics and enzymatic Activities of Agrotis ipsilon larvae (Lepidoptera: Noctuidae). International Journal of Natural Resource Ecology and Management 4(5):102–111
Ardia DR, Gantz JE, Strebel S (2012) Costs of immunity in insects: an induced immune response increases metabolic rate and decreases antimicrobial activity. Funct Ecol 26(3):732–739
Ayaad TH, Dorrah MA, Shaurub EH, El-Sadawy HA (2001) Effects of the entomopathogenic nematode, Heterorhabditis bacteriophora HP88 and azadirachtin on the immune defense response and prophenoloxidase of Parasarcophaga surcoufi larvae (Diptera: Sarcophagidae). J Egypt Soc Parasitol 31(1):295–325
Beetz S, Holthusen TK, Koolman J, Trenczek T (2008) Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol 67:63–75
Berger J, Jurčová M (2012) Phagocytosis of insect haemocytes as a new alternative model. J Appl Biomed 10(1):35–44
Boguś MI, Ligęza-Żuberc M, Polańska MA, Mosiewicz M, Włóka E, Sobocińska M (2018) Fungal infection causes changes in the number, morphology and spreading ability of Galleria mellonella haemocytes. Physiol Entomol 43(3):214–226
Castillo JC, Robertson AE, Strand MR (2006) Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem Mol Biol 36:891–903
De Azambuja P, Garcia ES, Ratcliffe NA, Warthen JRJD (1991) Immune-depression in Rhodnius prolixus induced by the growth inhibitor, azadirachtin. J Insect Physiol 37(10):771–777
Ding JF, Zhao YH, Zhang ZQ, Xu CM, Mu W (2018) Sublethal and hormesis effects of clothianidin on the Black Cutworm (Lepidoptera: Noctuidae). J Econ Entomol 111(6):2809–2816
El-Aziz NMA, Awad HH (2010) Changes in the haemocytes of Agrotis ipsilon larvae (Lepidoptera: Noctuidae) in relation to dimilin and Bacillus thuringiensis infections. Micron 41:203–209
Ericsson JD, Janmaat AF, Lowenberger C, Myers JH (2009) Is decreased generalized immunity a cost of Bt resistance in cabbage loopers Trichoplusia ni. J Invertebr Pathol 100(2):61–67
Feng CJ, Dong QA, Zhai HF, Chen GB, Yang JM, Miao JL (2011) Immunological and stress response of the hemolymph of Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae) larvae to the injection of Escherichia coli. Acta Entomol Sin 54(2):117–126. (in Chinese with English abstract)
Gemeno C, Haynes KF (2000) Periodical and age-related variation in chemical communication system of black cutworm moth, Agrotis ipsilon. J Chem Ecol 26:329–342
Gesraha MA, Ebeid AR (2021) Evaluate the effects of potential botanical and conventional insecticides on the reproductive and developmental aspects of the pest Agrotis Ipsilion (Lepidoptera: Noctuidae). Asian J Biol 10(4):83–91
Giannoulis P, Brooks CL, Gulii V, Dunphy GB (2005) Haemocytes of larval Malacosoma disstria (Lepidoptera: Lasiocampidae) and factors affecting their adhesion to glass slides. Physiol Entomol 30(3):278–286
Gillespie JP, Burnett R, Chanley KJ (2000) Changes in differential hemocyte count and in vitro behaviour of plasmatocytes from host. J Insect Physiol 46(4):429–437
Griesch J, Vilcinskas A (1998) Proteases released by entomopathogenic fungi impair phagocytic activity and spreading of plasmatocytes isolated from haemolymph of the greather wax moth Galleria mellonella. Biocontrol Sci Tech 8:517–531
Hillyer JF, Christensen BM (2002) Characterization of hemocytes from the yellow fever mosquito, Aedes Aegypti. Histochem Cell Biol 117(5):431–440
Hillyer JF, Schmidt SL, Christensen BM (2003) Hemocyte mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res 313(1):117–127
Horohov DW, Dunn PE (1982) Changes in the circulating haemocyte population on Manduca sexta larvae following injection of bacteria. J Invertebr Pathol 40:327–339
Jones JC (1977) The circulatory system of insects. Thomas Press, Springfield, pp 144–170
Lavine MD, Strand MR (2002) Insect hemocytes and their role in cellular immune responses. Insect Biochem Molecul Biol 32:1237–1242
Leonard C, Soderhall K, Ratcliffe NA (1985) Studies on prophenoloxidase and protease activity of Balbifer cranifer haemocytes. Insect Biochem 15:803–810
Mahmood SZ, Ijaz M, Altaf SM, HasnainY MM (2018) Effects of selected synthetic insecticides on the total and differential populations of circulating haemocytes in adults of the red cotton stainer bug Dysdercus koenigii (Fabricius) (Hemiptera: Pyrrhocoridae). Environ Sci Pollut Res 25(17):17033–17037
Mazet I, Hung SY, Boucias DG (1994) Detection of toxic metabolites in the hemolymph of Beauveria bassiana infected Spodoptera exigua larvae. Experentia 50:142–147
Perveen N, Ahmad M (2017) Toxicity of some insecticides to the haemocytes of giant honeybee, Apis dorsata F. under laboratory conditions. Saudi J Biol Sci 24(5):1016–1022
Ribeiro C, Brehélin M (2006) Insect haemocytes: what type of cell is that? J Insect Physiol 52:417–429
Ruchita P, Krishna K (2014) A comparative study of haemocytes in three cyclorrhaphous dipteran flies. Int J Trop Insect Sci 34(3):207–216
Schmidt O, Theopold U, Strand M (2001) Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bio Essay 23:344–351
Siddiqui MI, Al-Khalifa MS (2014) Review of haemocyte count, response to chemicals, phagocytosis, encapsulation and metamorphosis in insects. Ital J Zool 81(1):2–15
Sobhy HM, Abdel-Bary NA, Harras FA, Faragalla FH, Husseinen HI (2020) Efficacy of entomopathogenic nematodes against Spodoptera littoralis (Boisd.) and Agrotis ipsilon (H.) (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 30(2):265–267
Strand MR (2008) The insect cellular immune response. Insect Science 15:1–14
Vilcinskas A, Matha V, Götz P (1997) Inhibition of fagocytic activity of plasmatocytes isolated from Galleria mellonella by entomogenous fungi and their secondary metabolites. J Insect Physiol 43:475–483
Wang YZ, Zhang LH, Huang CY (1990) Pathogenicity changes in the hemocytes of Indian meal moth after being infected by Bacillus thuringiensis. Chin Bull Entomol 27(1):61–62. (in Chinese)
Wu G, Liu Y, Ding Y, Yi Y (2016) Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva:cell types and their role in the innate immunity. Tissue Cell 48:297–304
Yan R, Liu H, He LF, Liu L, Wan QH (2009) Observation on cell immunity of the Musca domestica larva infected by Escherichia coli. Sichuan J Zool 28(6):827–830. (in Chinese with English abstract)
Zdybicka-Barabas A, Cytryńska M (2013) Apolipophorins and insects immune response. Invertebr Surviv J 10:58–68
Zhang XM, Zhang KS (2019) Cellular response to bacterial infection in the grasshopper Oxya chinensis. Biol Open 8(10):1–10
Acknowledgements
We thank Prof. Jian feng Liu, Guizhou University, for a critical review of an earlier version of the manuscript.
Funding
This study was funded by the Open Project of State Key Laboratory of Crop Stress Biology for Arid Areas (CSBAA2015011), the Academic Funding Project for the Top-Notch Personnel of College Subject Specialty (Grant No. gxbjZD2020089), and the Scientific Research Foundation of Chuzhou University (Grant No.2019qd02), China.
Author information
Authors and Affiliations
Contributions
Yu-yong Xiang contributed with the experimental design, data analysis, manuscript writing, and manuscript review. Ming Ni contributed with the experimental study. Pei-feng Yin contributed with the experimental design. Yuan-chang Zhang contributed with the data analysis.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiang, Yy., Ni, M., Yin, Pf. et al. Morphological observations of haemocytes from Agrotis ipsilon (Lepidoptera: Noctuidae) larvae infected by Escherichia coli. Int J Trop Insect Sci 42, 2683–2691 (2022). https://doi.org/10.1007/s42690-022-00797-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00797-4