Abstract
Red cotton bug, Dysdercus koenigii (Hemiptera: Pyrrhocoridae), has become the major insect pest of various crops, including cotton, and thereby reducing the yield qualitatively and quantitatively and synthetic insecticides belonging to different groups are the major control agents for such insect pests. A laboratory experiment was carried out to evaluate the effect of different conventional insecticides, i.e., imidacloprid, deltamethrin, lambda cyhalothrin, gamma cyhalothrin and cyfluthirn on haemocytes of D. koenigii. The individuals were exposed to insecticides separately and data was recorded after 30 and 60 min of the exposure. The findings of current study depicted chlorpyrifos to be more effective and significant alterations in total haemocyte counts and differential haemocyte counts were observed in the cyfluthirn treated D. koenigii. In addition to this, cell structure was also disrupted as an immune response. Similar studies would also be helpful to understand the defence mechanisms of insects against the xenobiotics which will help to device efficient management tools for D. koenigii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Red cotton bug, Dysdercus koenigii F., has become the major insect pest of cotton worldwide from the last few years (Shah 2014). Owing to its polyphagous nature it is found on number of crops but cotton is the most preferred host. More damage is done to the cotton seeds by reducing quality and oil content (Sweet 2000). Adults and nymphs damage the lint quality by staining thus indirectly reducing the yield (Khan and Qamar 2012; Jaleel et al. 2014). Feeding by large populations of the red cotton bug causes a significant decrease in cotton seed weight (up to 15%). The germinating ability of seeds is also significantly reduced, potentially as much as 88% (Henry 1983). In particular, the cotton stainer, Dysdercus spp., has always been a source of severe damage to the crop by feeding on developing cotton bolls and mature cotton seeds (Ahmad and Khan 1980; Ahmad and Schaefer 1987; Yasuda 1992). Alternate hosts of D. koenigii include hollyhock (Kamble 1971) okra, ground nut, yellow corn, castor (Jamal 2014) and plants of family Bombacaceae (Kohno and Ngan 2004). However, this boll-feeding insect now becomes a more common pest of cotton. It is thought that major source of cotton staining is D. koenigii (Shah 2014). Cotton stainer especially D. koenigii (Hemiptera: Pyrrhocoridae) has been found the most destructive pests of cotton in the cotton zones of Pakistan (Jaleel et al. 2013). Haematological studies are of greater importance in physiology, which help to identify the exact line of defence and immune responses to the xenobiotics. Haemolymph of the insects contain different types of cells including the haemocytes, which are responsible for different functions such as phagocytosis, transfer of nutrients, storage of food and formation of connective tissues (Wigglesworth 1959). In addition to this, haemocytes are also, responsible for cellular defence mechanism and immune system of insects (Ratcliffe and Rowley 1979; Șapcaliu et al. 2009).
Insecticides are the most important tools for the management of various insect pests (Afzal and Shad 2015), and in Pakistan, insect pests of horticultural and field crops including cotton are managed by insecticides, owing to the reason that these have quick knock down and are relatively cheaper in price. The insecticides, in addition to mortality, also affect many physiological functions in different systems of insect pests. Exposure of different insecticides affects the normal functioning such as phagocytosis, encapsulation, nodule formation and coagulation of the haemocytes. These haematological studies help to understand the physiological mechanisms of insect body that would help to develop a successful pest management program (Richards and Davies 1977). Keeping in view the importance of D. koenigii and the problems associated with application of insecticides after its management, the present study was conducted aiming to evaluate the toxicological effects of selected insecticides on the total and differential haemocyte counts in D. koenigii adults.
Materials and methods
Collection of D. koenigii
Adults of D. koenigii were collected from the cotton fields of research farms of the University of Agriculture, Faisalabad, Pakistan, in the month of July to August, 2016, and reared in rearing cages on fresh cotton leaves under laboratory conditions of 28 ± 1 °C, 60–70% RH and 10 L:14 D-hour photoperiod (Khan et al. 2014).
Insecticides tested against D. koenigii
Five insecticides, namely, imidacloprid (250 ml/acre), deltamethrin (250 ml/acre), lambda cyhalothrin (330 ml/acre), gamma cyhalothrin (100 ml/acre) and cyfluthirn (800 ml/acre) at recommended field doses, were checked for their effect on haemocytes of D. koenigii.
Insecticide application
The insecticides were applied individually on the insect’s thorax with the help of a micro-applicator (Pro-Shot 50 cc Pistol Grip Syringe) at the rate of 8 μl/insect. Insects were anesthetized by keeping in the freezer for 5–7 s. Insects were then placed on a tissue paper to absorb the extra moisture. Effects of the tested insecticides were observed immediately after 30 and 60 min of exposure (Perveen and Ahmad 2017).
Statistical analysis
The experiment was repeated four times using completely randomized design (CRD) and data were subjected to analysis of variance (ANOVA) (Perveen and Ahmad 2017).
Preparation of permanent slides
For haematological investigation, following procedure was followed; the haemolymph of fully grown adults (Rosenberger and Jones 1960) of red cotton bug was taken on a clean glass slide by removing one or two abdominal legs using fine scissors and needle (Ghoneim et al. 2015; Jones 1962). Gentle pressure was applied on the insect abdomen for getting more quantity of haemolymph (Barkat et al. 2002). Smear was prepared in conventional manner by drawing a second slide across the first slide at an angle of 45°. The thin blood films were treated with methyl alcohol for 5–7 min and air dried. Dried blood films were immersed in the Giemsa solution (Conn et al. 1962) for 15 min and washed with distilled water for removing excess stain. Later on, permanent slides were made by Canada balsam under Motic BA310 compound microscope to calculate the number and types of the haemocytes.
Total haemocyte count
The total haemocyte count of adult was calculated before and after the application of insecticides by using Neubauer haemocytometer. For standard sampling of the haemolymph, Thoma white cell pipette was used throughout the studies. Haemolymph was collected on a glass slide and quickly sucked up into Thoma white cell pipette up to the mark 0.5. The end of the pipette was wiped and diluting fluid (glacial acetic acid, 1 ml; distilled water, 100 ml; gentian violet, 0.3%) was drawn up to the mark 11, for producing a dilution of 1/20, (1 part of blood: 20 parts of WBC. fluid). The contents of the pipette were properly mixed and first three drops were discarded (Jones 1962) and Neubauer’s chamber was charged with the dilutant. The haemocytes were counted from the four corner squares (white cell squares) in each of the two chambers.
Differential haemocyte counts
Differential haemocyte count (DHC) from the stained slides was carried out with help of a telecounter by following the battlement method (Perveen and Ahmad 2017). The film was examined systematically by observing traversed three fields along the edge and two fields down starting at the thin end of the smear. This sequence was continued until minimum of 200 cells were enumerated (Jones 1967) and percentage of various classes of haemocytes was also determined (Mahmood and Yousaf 1985).
Results and discussion
Total haemocyte count for normal adults
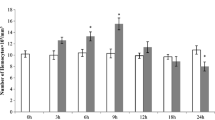
During the present studies, the total haemocyte count (THC) was calculated from the haemolymph of red cotton bug adults (Table 1). Under normal conditions without the exposure of insecticides on an average, 8450 blood cells/mm3 were present in blood of untreated red cotton bug adults. These results disagreed with the findings of Haq et al. (2005) in which an average of 17,000 cells/mm3 were recorded in the haemolymph. This may be due to two reasons, i.e., firstly, due to the difference in the test insects, while secondly, it may be due to the difference in the method of studying the haemocytes.
Differential haemocyte count for normal adults
The percentage of different types of haemocytes in haemolymph of red cotton bug was carried out by the DHC under normal conditions with the help of microscope. The prohaemocytes were recorded the highest (44.25%), followed by granulocytes (29.50%), plasmatocytes (19.0%), oenocytes (5.0%) and spherulocytes (2.25%), respectively (Table 2).
Total haemocyte count for treated adults
The results showed that the THC decreased significantly (5650 cells/mm3) just after the application of imidacloprid 20SL, increased (8000 cells/mm3) after 30 min of application and again decreased remarkably (6000 cells/mm3) after 1 h of application which is much lesser than the normal count (8450 cells/mm3) (Table 2). These findings differ from the result of Sabri and Tariq (2004), Haq et al. (2005), Al-Hariri and Suhail (2001) and Fatima et al. (2016) who reported increased THC after the application of insecticides. Effect of deltamethrin showed decreased THC (5,675cells/mm3) than the normal count just after the application of deltamethrin, increased slightly (8725 cells/mm3) after 30 min and increased (9,200cells/mm3) after 1 h of application (Table 2). These results differ from those of Iqbal et al. (2002), Haq et al. (2005) and Pugazhvendan and Soundararajan (2009) who noticed that THC increased firstly and then decreased after the application of insecticides.
In case of the application of lambda cyhalothrin, it was observed that the THC decreased remarkably (5875 cells/mm3) than the normal count subsequent to insecticide application; then, it increased to a smaller extent (6325 cells/mm3) after half an hour of application and then further increased (7050 cells/mm3) but still remained lower than the normal count. These results differ from those of Iqbal et al. (2002), Haq et al. (2005) and Pugazhvendan and Soundararajan (2009) who noticed that the THC increased firstly and then decreased after the application of insecticides. The application of gamma cyhalothrin depicted that the THC decreased (5525 cells/mm3) simply after the application of insecticide, but it increased (7700 cells/mm3) after half hour of application and remained lesser than the normal and then increased (9100 cells/mm3) after 1 h. These research findings differ from the studies of Iqbal et al. (2002), Haq et al. (2005) and Pugazhvendan and Soundararajan (2009). The results of application of cyfluthirn showed that the THC decreased drastically (4975 cells/mm3) subsequent to the application and increased (7300 cells/mm3) after half hour of application but still remained lower than the normal value and increased further (8,825cells/mm3) after 1 h. The results are in contradiction to those of Iqbal et al. (2002), Haq et al. (2005) and Pugazhvendan and Soundararajan (2009) where it was reported that the THC increased firstly and then decreased after the application of insecticides.
Effect of insecticides on differential haemocyte counts in adults
The percentage of various classes of haemocytes of red cotton bug obtained after the application of imidacloprid showed that the percentage of plasmatocytes, granulocytes and spherulocytes increased from the normal, i.e., 19.0, 29.0 and 2.25% to 31.5, 32.0 and 4.75%, respectively. Whereas the percentage of prohaemocytes and oenocytes was decreased from the normal, i.e., 44.25 and 5.0% to 27.0 and 3.5%, respectively (Table 2). These investigations differ from those of Haq et al. (2005) and Teleb (2011) where it was noticed that the percentage of prohaemocytes and oenocytoids increased after the application of insecticides, while plasmatocytes, spherulocytes and granulocytes were decreased.
The percentage of plasmatocytes and spherulocytes increased up to 30.75 and 7.75% then the normal value, respectively after the application of deltamethrin. Whereas the percentage of prohaemocytes, granulocytes and oenocytes decreased to 31.75, 25.25 and 4.5%, respectively. These results slightly contradict with those of Al-Hariri and Suhail (2001), Iqbal et al. (2002), Suhail et al. (2007), Teleb (2011) and Fatima et al. (2016) who noticed increased in plasmatocytes, while prohaemocytes and granulocytes were decreased after the application of insecticides. The percentage of various classes of haemocytes of red cotton bug obtained after the application of lambda cyhalothrin exhibited that the percentage of plasmatocytes, spherulocytes and oenocytes increased of 30, 11 and 6.5%, then the average value, respectively, whereas the percentage of prohaemocytes and granulocytes was decreased to the percentage of 31.5 and 21.5%, respectively. Results differ from Haq et al. (2005) who reported that the percentage of plasmatocytes, spherulocytes and oenocytoids decreased, while the percentage of prohaemocytes and granulocytes increased after the application of insecticides. The application of gamma cyhalothrin increased the percentage of plasmatocytes and spherulocytes to 33.0 and 5.0%, respectively, whereas the percentage of prohaemocytes and oenocytes was decreased to 31.75 and 0.75%, respectively, while the percentage of granulocytes remained constant, i.e., 29.5. These results reported here in differ from those of Teleb (2011), where it states that the percentage of plasmatocytes and spherulcytes decreased, while oenocytoids and prohaemocytes increased after the application of insecticides.
Calculation of the percentage of plasmatocytes, and spherulocytes for lambda cyhalothrin increased from 26.25 and 2.75%, then the average values, respectively, whereas the percentage of prohaemocytes, oenocytes and granulocytes was decreased to 38.75, 4.75 and 24.75%, respectively. These findings are not in accordance with the results of Haq et al. (2005) and Teleb (2011) where it was observed that the percentage of prohaemocytes and granulocytes increased, while plasmatocytes and spherulocytes decreased after the application of insecticides (Table 2).
Conclusion
The findings of the current study clearly state that the xenobiotics have certain role in life history of insects. As the tested insecticides have vital effect on the number of haemocytes in red cotton bug. Further study is required to check the effect of these insecticides on the cells that reduce immunity in red cotton bug.
References
Afzal MBS, Shad SA (2015) Resistance inheritance and mechanism to emamectin benzoate in Phenacoccus solenopsis (Homoptera: Pseudococcidae). Crop Prot 71:60–65
Ahmad I, Khan NH (1980) Effects of starvation on the longevity and fecundity of red cotton bug, Dysdercus cingulatus (Hemiptera: Pyrrhocoridae) in successive selected generations. Appl Entomol Zool 15:182–183
Ahmad I, Schaefer CW (1987) Food plant and feeding biology of the Pyrrhocoroidea (Hemiptera). Phytophaga 1:75–92
Al-Hariri MK, Suhail A (2001) Effect of lambdacyhalothrin and deltamethrin on the haemocytes of desert locust, Schistocerca gregaria Forsk. Int J Agric Biol 3:81–84
Barkat EMS, Meshrif WS, Shehata MG (2002) Changes in the haemolymph of the desert locust, Schistocerca gregaria after injection with Bacillus thuringiensis. J Egypt Acad Soc Environ Develop 2:95–115
Conn HJM, Darrow A, Emmel VM (1962) Staining procedures, 2nd edn. The Williams and Wilkins Co., Baltimore, pp 136–137
Fatima M, Tariq M, Tariq K, Naz G, Anwar Z, Gulzar A, Ali A, Khan AA, Khursheed I, Gulzar A, Ali K, Nadeem M (2016) Effect of thiacloprid and imidacloprid on the haemocytes of American bollworm, Helicoverpa armigera. Amer. Eurasian. J Toxicol Sci 8:139–143
Ghoneim K, Tanani M, Hamadah K, Basiouny A, Waheeb H (2015) Effects of novaluron and cyromazine, chitin synthesis inhibitors, on the larval haemogram of Spodoptera littoralis (Boisd. ) (Lepidoptera: Noctuidae). Int J Adv Res 3:554–557
Haq MR, Sabri MA, Rashid A (2005) Toxicity of nicotinyl insecticides on the haemocytes of red cotton bug, Dysdercus koengii (Fb.) (Pyrrhocoridae: Hemiptera). J Agric Social Sci 3:239–241
Henry TJ (1983) Pests not known to occur in the United States or of limited distribution, no. 38: cottonseed bug. USDA–APHIS–PPQ, pp 6
Iqbal J, Sohail A, Abdin Z, Anjum AH (2002) Toxicity of tracer 240 SC on haemocytes of last larval instar of brinjal fruit borer Leucinodes orbonalis (Geun.). Pak Entomol 24:115–119
Jaleel W, Saeed S, Naqqash MN (2013) Biology and bionomic of Dysdercus koenigii F. (Hemiptera: Pyrrhocoridae) under laboratory conditions. Pak J Agric Sci 50:373–378
Jaleel W, Saeed S, Naqqash MN, Zaka SM (2014) Survey of Bt cotton in Punjab Pakistan related to the knowledge, perception and practices of farmers regarding insect pests. Int J Agric Crop Sci 7:10–20
Jamal K (2014) Effect of host plant on survivability, development and reproductive potential of cotton stainer bug Dysdercus koenigii (Hemiptera: Pyrrhocoridae). J Funct Environ Bot 7:100–105
Jones JC (1962) Current concepts concerning insect hemocytes. Am Zool 2:209–246
Jones JC (1967) Change in the haemocyte picture of Galleria mellonella L. Biol Bull (Woods Hole) 132:211–221
Kamble ST (1971) Bionomic of Dysdercus koenigii Fab. J N Y Entomol Soc 79:154–157
Khan I, Qamar A (2012) Andalin, an insect growth regulator, as reproductive inhibitor for the red cotton stainer, Dysdercus koenigii (F.) (Hemiptera: Pyrrhocoridae). Academic J Entomol 5:113–121
Khan BA, Freed S, Zafar J, Farooq M (2014) Evaluation of three different insect pathogenic fungi for the control of Dysdercus koenigii and Oxycarenus hyalinipennis. Pakistan J Zool 46:1759–1766
Kohno k, Ngan BT (2004) Effect of host plant on the development of Dysdercus cingulatus (Heteroptera: Pyrrhocoridae). Appl Entomol Zool 39:183–187
Mahmood A, Yousaf N (1985) Effect of insecticides on the haemocytes of Gryllus bimaculatus. Pak J Zool 17:71–84
Perveen N, Ahmad M (2017) Toxicity of some insecticides to the haemocytes of giant honeybee, Apis dorsata F. under laboratory conditions. Saudi J Biol Sci 24:1016–1022
Pugazhvendan SR, Soundararajan M (2009) Effect of penfluron on total haemocyte count of Chrysocoris purpureus. Middle-East J Sci Res 4:338–340
Ratcliffe NA, Rowley AF (1979) Role of haemocytes in defense against biological agents. In: Insect haemocytes. Cambridge University Press, Cambridge
Richards OW, Davies RG (1977) Imm’s general textbook of entomology, 10th edn. Chapman and Hall, London, pp 238–242
Rosenberger CR, Jones JC (1960) Studies on total blood cell counts of southern armyworm larvae, Prodenia eridania (Lepidoptera). Ann Entomol Soc Am 53:351–355
Sabri MA, Tariq B (2004) Toxicity of some insecticides on the haemocytes of red pumpkin beetle, Aulacophora foveicollis Lucas. Pak Entomol 26:109–114
Șapcaliu A, Rădoi I, Pavel C, Tudor N, Căuia E, Siceanu A, Meiu F (2009) Research regarding haemocyte profile from Apis mellifera carpatica bee haemolymph originated in the south of Romania. Lucrari Stiintifice-Universitatea de Stiinte Agricole a Banatului Timisoara, Medicina Veterinara 42:393–7
Shah SI (2014) The cotton stainer (Dysdercus koenigii): an emerging serious threat for cotton crop in Pakistan. Pak J Zool 46:329–335
Suhail A, Gogi MD, Arif MJ, Arshad M, Rana M, Sarfraz M (2007) Effect of various treatments of azadirachtin, spinosad and abamectin on the haemogram of Coccinella septempunctata L. (Coleoptera: Coccinellidae). Pak Entomol 29:151–164
Sweet MH (2000) Seed and chinch bugs. In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC, Boca Raton, pp 143–264
Teleb SS (2011) Effect of Nomolt on differential and total haemocytes in the desert locust Schistocerca gregaria Forskal (Orthoptera: Acrididae). J Am Sci 7:479–484
Wigglesworth VB (1959) Insect blood cells. Annu Rev Entomol 4:1–16
Yasuda K (1992) Cotton bug. In: Hidaka T (ed) Insect pests of vegetables in the tropics. Association for International Cooperation of Agriculture and Forestry, Tokyo, pp 22–23
Acknowledgements
We are highly thankful to the anonymous reviewers and editor for their valuable comments for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sarwar, Z.M., Ijaz, M., Sabri, M.A. et al. Effects of selected synthetic insecticides on the total and differential populations of circulating haemocytes in adults of the red cotton stainer bug Dysdercus koenigii (Fabricius) (Hemiptera: Pyrrhocoridae). Environ Sci Pollut Res 25, 17033–17037 (2018). https://doi.org/10.1007/s11356-018-1898-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1898-1