Abstract
Understanding the variations in activity of enzymes involved in the immune response of insects is important for developing effective microbial insecticides against pest species, the determination of bacteria dosage that caused variation of enzyme activities would assist us to prepare effective concentrations of microbial insecticides. In this study, we measured the activities of acid phosphatase (ACP), phenoloxidase (PO), peroxidase (POD), glutathione peroxidase (GSH-px), glutathione reductase (GR), and glutathione S-transferase (GST) in haemocytes and serum of larvae of the pest Heterolocha jinyinhuaphaga Chu (Lepidoptera: Geometridae) infected with Escherichia coli at different time points post-infection. Infection of fifth-instar larvae with different dosages of E. coli led to significant increases in the activities of these enzymes in haemocytes and serum at 3, 6, 12, and 24 h post-infection. Enzyme activities in haemocytes peaked at 6 h post-infection, but their activities began to decrease at 12 h post-infection. Enzyme activities in serum peaked at 12 h post-infection, and they began to decrease at 24 h post-infection. Additionally, enzyme activities increased with increasing dosages of E. coli. The activities of ACP, PO, POD, and GSH-px peaked at 1 × 107 indiv/mL, and the activities of GR and GST peaked at 1 × 106 indiv/mL, activities decreased thereafter. Two - way analysis of variance showed that the interaction of infection time and dosages of E. coli had no significant effect on the enzyme activities in haemocytes and serum of H. jinyinhuaphaga larvae (P > 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nature, species resist the invasion of pathogens via their immune system. When insects are invaded by pathogens such as bacteria, their cellular and humoral immune defense responses are activated (Hoffmann et al. 1999). Haemocytes are the main enforcers of cellular immunity, and the humoral immune response is activated by haemolymph (Beck et al. 2000). During the immune response, haemocytes kill pathogens through phagocytosis, nodule formation, or encapsulation. Previous studies showed that most pathogens that invade insects are removed by phagocytosis of granulocytes and plasmatocytes (Hillyer et al. 2003; Castillo et al. 2006; Williams 2007; Marmaras and Lampropoulou 2009). In addition, a variety of hydrolases, oxidase and antioxidant enzymes were released in form of degranulation by haemocytes (Giannoulis et al. 2007; Feng at al. 2011).
A number of enzymes play important roles in the killing and removal of pathogens. For example, phenoloxidase (PO) catalyzes the formation of melanin from tyrosine, and melanin deposits around the pathogens participate in their encapsulation (Söderhäll 1982). During the encapsulation process, melanin produces reactive oxygen radicals, such as superoxide anion free radical (O–2) and hydroxyl free radicals (·OH) (Nappi 1995), which may help to kill pathogens. However, too many reactive oxygen radicals can damage the organism (Packer 1984). Catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione peroxidase (GSH-px), glutathione S-transferase (GST), and glutathione reductase (GR) help to remove excess reactive oxygen radicals and block the chain reaction of lipid peroxidation, thereby protecting the structural and functional integrity of the cell membrane (Sheehan et al. 2001; Freitas et al. 2007). Acid phosphatase (ACP) changes the surface structure of pathogens to expedite their recognition, phagocytosis and removal (Cheng 1978; Yan et al. 2008).
Previous studies have shown that the activities of enzymes involved in the immune response change significantly within a short period of time when insects are invaded by pathogens. Therefore, their activity levels indicate the immune status of insects. For example, activation of prophenoloxidase (PPO) in Spodoptera exigua was inhibited after specimens were injected with Xenorhabdus nematophila (Park and Kim 2003), and PO activity in Pieris rapae decreased after it was parasitized by Cotesia rubecula (Asgari et al. 2003). In Brontispa longissima, PO activity in pupae increased significantly at 0.5, 1, and 4 day post-parasitization by Tetrastichus brontispae (Liu et al. 2008), and PO activity increased in Chilo suppressalis larvae hemolymph at 0.5, 1, 2, and 3 day post-parasitization by Cotesia chilonis (Li et al. 2011). The activities of ACP, CAT, and diphenol oxidase (DPO) in the serum of Musca domestica larvae all increased after infection with Escherichia coli (Yan et al. 2008, 2011), and the activities of GSH-px, GST, and GR in the serum of Ostrinia furnacalis also increased after infection with E. coli (Feng et al. 2011). These findings show that variations in activity of enzymes involved in the immune response differ among host species when they are invaded by pathogens. Studying the activities of immune-related enzymes will improve our understanding of the immune functions and mechanisms of insects.
The honeysuckle geometrid, Heterolocha jinyinhuaphaga Chu (Lepidoptera: Geometridae), is a major pest of the honeysuckle plant (Lonicera japonica Thunb). This insect appears to have three generations per year in China (Xiang et al. 2010), and the life cycle consists of egg, larva, pupa, and adult stages. This moth can mate on the first night after emergence, and females oviposit during 6–8 h after mating. After hatching, the larvae feed on honeysuckle leaves and often cause extensive damage to the plant, which results in great economic losses in many regions of China (e.g., Shandong, Henan, and Anhui provinces) (Xiang et al. 2010). The larval amount of the first generation peaks in mid-May, following the second generation peaks in mid-late July, and the third generation peaks in late September. Overwintering of pupae begins in early October.
Several studies have focused on the biological characteristics and control methods of H. jinyinhuaphaga (Ni et al. 2006; Xiang et al. 2010, 2016a, 2018, 2019), but few studies have investigated its immune system. Escherichia coli is a pathogenic bacteria which may activate immune responses in insects (Liang et al. 1991 Whitten and Ratcliffe 1999; Yan et al. 2011; Feng et al. 2011; Xiang et al. 2016b). In this study, we measured the enzyme activities of ACP, PO, POD, GSH-px, GR, and GST in haemocytes and serum of H. jinyinhuaphaga larvae infected with E. coli at different time points post-infection. The results of this study will increase our understanding of the immune response and the mechanisms involved in immunity of H. jinyinhuaphaga (Beck et al. 2000) and provide a scientific reference for the development of more effective microbial insecticides against this pest. The determination of bacteria dosage that caused variation of enzyme activities would assist us to prepare effective concentrations of microbial insecticides.
Materials and methods
Insects
In May of 2018, larvae of H. jinyinhuaphaga were collected from honeysuckle plantations growing the Jiufeng 1 variety of the plant, which are located in Chuzhou City, Anhui Province, China. Larvae were reared with honeysuckle leaves (Jiufeng 1) in the laboratory at 25 ± 1 °C and 70 ± 7% relative humidity under a 14:10 h (light: dark) photoperiod. Larvae were reared together before the third instar stage and individually thereafter to avoid cannibalism. Larval instar stage were identified according to their head capsule widths. Four generations of larvae were reared, and the fifth-instar larvae from each generation were used in the study.
Preparation of bacterial suspension
Escherichia coli (ATCC25922) was purchased from Shanghai Luwei Technology Company, China, and cultivated on Luria-Bertani medium at 37 °C. After centrifugation at 2 000 rpm for 10 min, the bacteria were collected and diluted with normal saline (0.65% NaCl, 0.025% KCl, 0.03% CaCl2, 0.025% NaHCO3) to generate six concentrations: 1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 ind/mL. The bacteria concentrations were chosen according to the previous study which could cause hemocytes immune response in H. jinyinhuaphaga, and result in morphological and quantitave variations in hemocytes (Xiang et al. 2016b). The bacteria concentration was measured using a hemocytometer. First, the number of bacteria in each small square of hemocytometer was measured, and then converted into the number of bacteria in each milliliter of bacterial solution. So, the bacteria number in 1 mL of bacterial suspension = the average number of bacteria in each square (n) × coefficient (k) × dilution ratio of bacterial suspension (d).
Preparation of serum and haemocytes of H. jinyinhuaphaga larvae
Three microlitres of a bacterial suspension were injected into the abdomen of fifth-instar larvae using a microinjector. The larvae were then transferred to an artificial climate box and fed on honeysuckle leaves (Jiufeng 1). For each bacterial suspension treatment, 15 larvae / time point / dosage were used, and three replicates were conducted. A control experiment (CK) was also run using larvae treated with normal saline.
Larvae haemolymph was collected at 3, 6, 12, and 24 h post-injection, the larval prolegs were excised with a pair of fine scissors, and the body was pressured gently. An amount of haemolymph was extracted and placed in a conical centrifuge tube with a pipetting gun, and centrifuged for 10 min (4 °C, 5000 r/min) (Yan et al. 2011), and the supernatant was taken as the serum. The precipitated haemocytes were put into insect protection liquid (10% glycerin) with the equal volume to the serum. The haemocytes were treated with a cell ultrasonic pulverizer for 30 s to break them. The homogenate was centrifuged for 10 min (4 °C, 5000 r/min), and the haemocytes extract was obtained after removing the precipitate. For each analysis described below, the experiment was repeated three times.
Activity of acid phosphatase (ACP)
Fifty microlitres of serum or haemocyte extract from the fifth-instar larvae were used to measure ACP activity. The method was conducted according to the kit instructions (Suzhou Comin Biotechnology Com. LTD, China ). The enzyme activity unit (U) was defined as 1µmol of phenol production catalyzed by 1 mg of protein per minute at 37 °C. The result was expressed as U/mg.
Activity of phenoloxidase (PO)
The PO activity was measured following Jiao et al. (2010). Two hundred microlitres of serum or haemocyte extract, 2 mL of phosphate buffer (pH 6.5, 0.1 mol/L), and 0.8 mL of catechol (37.5 mmol /L) were mixed in a test tube, which was then placed in a water bath (30 °C). The absorbance value (optical density at 420 nm, OD420) of the reaction solution was measured every 20 s using an ultraviolet spectrophotometer, and the changes in absorbance value were recorded for two consecutive minutes. Based on the OD420 value, a straight line was obtained and the enzyme activity was calculated from the slope of the line. A 0.01 increase in the catalytic substrate’s oxidative absorbance per minute was defined as 1 U.
Activity of peroxidase (POD)
The POD activity was measured by Guaiacol colorimetry (Zhang et al. 2013). After putting 0.5 mL of phosphate buffer (pH = 6.0, 0.2 mol/L), 28 µL of 30% hydrogen peroxide, and 19 µL of guaiacol together in a test tube, 50 µL of serum or haemocyte extract were added and the solution was fully mixed. The OD value of the reaction solution was determined at 470 nm after being allowed to react for 10 min. The enzyme activity was calculated according to following formula: (Zhong et al. 2011)
POD activity = OD470 / [ε × t×W×(V0/V)].
Where OD470 represents the changes of the absorbance value during the reaction time, t is the reaction time (min), V is the total liquid volume (mL), V0 is the measured sample volume (mL), W is the sample quality (mg), and ε is the extinction coefficient (26.6 mmol·L–1·cm–1).
Activity of glutathione peroxidase (GSH-px)
The GSH-px activity was measured following Feng et al. (2001). The standard curve was generated by reacting different dosages of GSH with the saturated amount of 5, 5-disulfide dinitrobenzoic acid (DTNB). In the sample tube, 2.0 mL of phosphate buffer (pH = 7.0, 0.2 mol/L), 0.2 mL of NaN3 (0.01 mol/L), 0.3 mL of GSH (0.002 mol/L), 0.4 mL of DTNB (0.04%), and 200 µL of enzyme liquid were added. In the blank tube, 2.0 mL of phosphate buffer (pH = 7.0, 0.2 mol/L), 0.2 mL of NaN3 (0.01 mol/L), 0.4 mL of DTNB (0.04%), and 0.6 mL of H2O were added. In the control tube, 2.0 mL of phosphate buffer (pH = 7.0, 0.2 mol/L), 0.2 mL of NaN3 (0.01 mol/L), 0.3 mL of GSH (0.002 mol/L), 0.4 mL of DTNB (0.04%), and 0.2 mL of H2O were added. After heating the tubes in a water bath (30 °C) for 5 min, 0.1mL of preheated H2O2 (1.25mmol/L) was added to the sample tube and control tube. Two minutes later, the OD value of each tube was measured at 412 nm. The log (GSH) drop value per minute was obtained by deducting the non-enzymatic reaction (sample OD - non-enzymatic OD). According to the Hafeman method, a log (GSH) decrease by 0.001 per minute is defined as one unit of GSH-px activity, and the GSH-px activity was expressed as U/mg.
Activity of glutathione reductase (GR)
The GR activity was measured following Yang et al. (1988). First, 2.0 mL of phosphate buffer (pH = 7.2, 0.1 mol/L), 0.05 mL of ethylenediaminetetraacetic acid (EDTA, 80 mmol/L), 0.1mL of glutathione disulfide (GSSG, 50 mmol/L), and 0.1mL of flavin adenine dinucleotide (FAD, 0.25 mmol/L) were placed in the sample tube. For the control, 2.35 mL of phosphate buffer (pH = 7.2, 0.1 mol/L) were placed in the tube. Before being heated in a water bath (37 °C), 0.1mL of serum or haemocyte extract was added to the two tubes separately. Thirty minutes later, 0.1 mL of reduced nicotinamide adenine dinucleotide phosphoric acid (NADPH, 4 mmol/L) was added to the sample tube, the OD value of the sample was measured every 30s at 340 nm using the control tube as zero calibration, and the reduction in OD340 value was recorded for 0.5 to 5 min. At 37 °C, the required amount of enzyme that catalyzes the oxidation of 1 µmol of NADPH per minute was defined as one GR activity unit. The GR activity was calculated according to the following formula:
GR activity (U/mg) = △OD340/5min×78.78.
Activity of glutathione S-transferase (GST)
The GST activity was measured following Liu and Wang (1992). The matrix mixture contained 50 mL of phosphate buffer (pH = 6.5, 0.2 mol/L), 5 mL of 1-chloro-2, 4-dinitrobenzene (CDNB, 20 mmol/L), and 10 mL of reduced GSH (10 mmol/L). The mixture was diluted to 100 mL by adding distilled water. Next, 0.5 mL of matrix mixture and 30 µL of serum or haemocyte extract were added to the colorimetric cup, and the OD value was measured every 15 s for 5 min at 340 nm. Distilled water served as the blank control. At 25 °C and pH 6.5, when the final dosage of CDNB and GSH was 1 mmol/L, the amount of enzyme required to catalyze the combination of 1µmol of CDNB and GSH was defined as one GST activity unit. GST activity was calculated according to following formula:
GST activity (U/mg) = OD340/min×1840.
Where OD340/min represents the net increase of absorbance per minute at the wavelength of 340 nm.
Measurement of protein content
The protein content was measured using the Coomassie brilliant blue (G-250) method (Bradford 1976), and bovine serum albumin was used as the standard protein.
Statistical analysis
All data were analyzed using SPSS version 11.5 software (SPSS Inc., Chicago, IL, USA). Comparisons among the means of groups at different treatment times, or comparisons among the means of groups at different bacterial dosages were analyzed using one-way analysis of variance (ANOVA). The interaction of infection time and dosages of E. coli on means of groups were analyzed using two-way analysis of variance (ANOVA). Mean values among groups at different treatment times and bacterial dosages were separated using Tukey’s multiple comparison method at P < 0.05.
Results
Activity of ACP
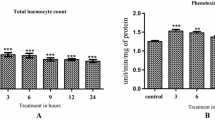
Compared to the control, infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in ACP activity in haemocytes (F = 3.435, df1 = 6, df2 = 21, P = 0.016) (Fig. 1-A) and serum (F = 11.983, df1 = 6, df2 = 21, P < 0.0001) (Fig. 1-B) at 3, 6, 12, and 24 h post-infection. The ACP activity in haemocytes peaked at 6 h post-infection, and activities for the different doses were 92.70 U/mg, 108.35 U/mg, 121.24 U/mg, 140.31 U/mg, 148.65 U/mg, and 142.33 U/mg, resulted in activity increases of 703.29%, 838.91%, 950.61%, 1115.86%, 1188.13%, and 1133.36% compared to the control, respectively. At 12 h post-infection, ACP activity in haemocytes began to decrease. The ACP activity in serum peaked at 12 h post-infection, and activities for the different doses were 53.36 U/mg, 62.55 U/mg, 70.13 U/mg, 80.79 U/mg, 94.42 U/mg, and 87.81 U/mg, resulted in activity increases of 385.97%, 469.67%, 538.71%, 635.79%, 759.93%, and 699.73% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of ACP in serum.
At the same time point after infection, the ACP activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 107 ind/mL. Two-way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the ACP activity in haemocytes (F = 1.342, df1 = 3, df2 = 5, P = 0.215) or serum (F = 0.697, df1 = 3, df2 = 5, P = 0.775).
Activity of PO
Infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in PO activity in haemocytes (F = 2.863, df1 = 6, df2 = 21, P = 0.034) (Fig. 2-A) and serum (F = 2.114, df1 = 6, df2 = 21, P = 0.01) (Fig. 2-B) at 3, 6, 12, and 24 h post-infection. The PO activity in haemocytes peaked at 6 h post-infection, and activities for the different doses were 86.90 U/mg, 97.53 U/mg, 108.37 U/mg, 122.57 U/mg, 132.49 U/mg, 125.46 U/mg, resulted in activity increases of 797.73%, 907.54%, 1019.52%, 1166.22%, 1268.69%, and 1196.07% compared to the control, respectively. At 12 h post-infection, the PO activity in haemocytes began to decrease. The PO activity in serum peaked at 12 h post-infection, and activities for the different doses were 80.26 U/mg, 88.95 U/mg, 109.37 U/mg, 117.67 U/mg, 127.83 U/mg, and 120.33 U/mg, resulted in activity increases of 986.06%, 1103.65%, 1379.98%, 1492.29%, 1629.77%, and 1528.28% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of PO in serum.
At the same time point after infection, the PO activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 107 ind/mL. Two - way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the PO activity in haemocytes (F = 0.82, df1 = 3, df2 = 5, P = 0.651) or serum (F = 1.068, df1 = 3, df2 = 5, P = 0.409).
Activity of POD
Infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in POD activity in haemocytes (F = 6.229, df1 = 6, df2 = 21, P = 0.001)(Fig. 3-A) and serum (F = 8.09, df1 = 6, df2 = 21, P < 0.0001) (Fig. 3-B) at 3, 6, 12, and 24 h post-infection. The POD activity in haemocytes peaked at 6 h post-infection, and activities for the different doses were 83.32 U/mg, 90.71 U/mg, 101.48 U/mg, 114.25 U/mg, 123.46 U/mg, and 112.73 U/mg, resulted in activity increases of 1070.22%, 1174.02%, 1325.28%, 1504.63%, 1633.99%, and 1483.29% compared to the control, respectively. At 12 h post-infection, the POD activity in haemocytes began to decrease. The POD activity in serum peaked at 12 h post-infection, and activities for the different doses were 70.82 U/mg, 81.33 U/mg, 90.36 U/mg, 101.23 U/mg, 111.55 U/mg, and 97.78 U/mg, resulted in activity increases of 1470.29%, 1703.33%, 1903.54%, 2144.57%, 2373.29%, and 2068.07% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of POD in serum.
At the same time point after infection, the POD activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 107 ind/mL. Two-way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the POD activity in haemocytes (F = 0.503, df1 = 3, df2 = 5, P = 0.927) or serum (F = 1.036, df1 = 3, df2 = 5, P = 0.438).
Activity of GSH-px
Infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in GSH-px activity in haemocytes (F = 5.525, df1 = 6, df2 = 21, P = 0.001) (Fig. 4-A) and serum (F = 15.826, df1 = 6, df2 = 21, P < 0.0001) (Fig. 4-B) at 3, 6, 12, and 24 h post-infection. The GSH-px activity in haemocytes peaked at 6 h post-infection, and activities for the different doses were 117.35 U/mg, 127.57 U/mg, 139.76 U/mg, 150.11 U/mg, 164.25 U/mg, and 147.34 U/mg, resulted in activity increases of 470.21%, 519.87%, 579.11%, 629.39%, 698.10%, and 615.93% compared to the control, respectively. At 12 h post-infection, the GSH-px activity in haemocytes began to decrease. The GSH-px activity in serum peaked at 12 h post-infection, and activities for the different doses (1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 ind/mL) were 71.51 U/mg, 82.36 U/mg, 95.77 U/mg, 109.95 U/mg, 124.33 U/mg, and 112.21 U/mg, resulted in activity increases of 235.57%, 286.49%, 349.41%, 415.95%, 483.44%, and 426.56% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of GSH-px in serum.
At the same time point after infection, the GSH-px activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 107 ind/mL. Two -way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the GSH-px activity in haemocytes (F = 0.909, df1 = 3, df2 = 5, P = 0.56) or serum (F = 1.551, df1 = 3, df2 = 5, P = 0.125).
Activity of GR
Infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in GR activity in haemocytes (F = 15.519, df1 = 6, df2 = 21, P < 0.0001) (Fig. 5-A) and serum (F = 22.163, df1 = 6, df2 = 21, P < 0.0001) (Fig. 5-B) at 3, 6, 12, and 24 h post-infection. The GR activity in haemocytes peaked at 6 h post-infection, and activities for the different doses (1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 ind/mL) were 28.74 U/mg, 35.86 U/mg, 43.35 U/mg, 54.44 U/mg, 45.23 U/mg, and 34.69 U/mg, resulted in activity increases of 428.31%, 559.19%, 696.88%, 900.74%, 731.43%, and 537.68% compared to the control, respectively. At 12 h post-infection, the GR activity in haemocytes began to decrease. The GR activity in serum peaked at 12 h post-infection, and activities for the different doses were 18.85 U/mg, 25.93 U/mg, 31.44 U/mg, 42.52 U/mg, 35.25 U/mg, and 29.43 U/mg, resulted in activity increases of 373.62%, 551.51%, 689.95%, 968.34%, 785.68%, and 639.44% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of GR in serum.
At the same time point after infection, the GR activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 106 ind/mL. Two -way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the GR activity in haemocytes (F = 0.856, df1 = 3, df2 = 5, P = 0.614) or serum (F = 0.241, df1 = 3, df2 = 5, P = 0.998).
Activity of GST
Infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in GST activity in haemocytes (F = 17.114, df1 = 6, df2 = 21, P < 0.0001) (Fig. 6-A) and serum (F = 28.647, df1 = 6, df2 = 21, P < 0.0001)(Fig. 6-B) at 3, 6, 12, and 24 h post-infection. The GST activity in haemocytes peaked at 6 h post-infection, and activities for the different doses were 35.39 U/mg, 45.92 U/mg, 53.37 U/mg, 64.22 U/mg, 56.47 U/mg, and 47.25 U/mg, resulted in activity increases of 453.79%, 670.47%, 795.47%, 977.52%, 847.48%, and 692.79% compared to the control, respectively. At 12 h post-infection, the GST activity in haemocytes began to decrease. The GST activity in serum peaked at 12 h post-infection, and activities for the different doses were 26.71 U/mg, 35.56 U/mg, 45.43 U/mg, 53.63 U/mg, 46.41 U/mg, and 37.67 U/mg, resulted in activity increases of 436.35%, 614.06%, 812.25%, 976.91%, 831.93%, and 656.43% compared to the control, respectively. At 24 h post-infection, there was a decrease in the activity of GST in serum.
At the same time point after infection, the GST activity in haemocytes and serum increased with increasing dosages of E. coli, with the peak at 1 × 106 ind/mL. Two-way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the GST activity in haemocytes (F = 0.189, df1 = 3, df2 = 5, P = 0.999) or serum (F = 0.716, df1 = 3, df2 = 5, P = 0.756).
Discussion
The immune defense system of insects is very sophisticated and complicated, and it allows insects to specifically recognize and kill the invading bacteria, fungi and other pathogens. However, during the immune response, pathogens produce toxins that damage the immune defense system and cause significant changes in activities of immunity-related enzymes in a short period of time (Ashida and Brey 1998). In our study, infection of fifth-instar larvae of H. jinyinhuaphaga with different dosages of E. coli led to significant increases in activities of ACP, PO, POD, GSH-px, GR, and GST in haemocytes and serum at 3, 6, 12, and 24 h post-infection. In haemocytes, activities of all six enzymes peaked at 6 h post-infection and decreased thereafter. In serum, the enzyme activities all peaked at 12 h post-infection, but by 24 h post-infection, their activities had decreased. These results show that significant immune responses were induced in the fifth-instar larvae of H. jinyinhuaphaga during the early post-infection period, resulting in the increased activity of these enzymes in vivo to adapt to the invasion of foreign pathogens. Similar results were obtained for M. domestica larvae, in which ACP and POD activities in serum increased at 4, 8, 16, and 24 h post-infection with E. coli (Yan et al. 2011). Additionally, ACP peaked at 8 h post-infection and POD activity peaked at 12 h post-infection. One possible explanation for these results is that the haemocytes synthesized these enzymes soon after stimulation by pathogens, and they then are released into the serum to participate in the immune response. As pathogens are removed and haemocytes are consumed, enzymes synthesis and activity decrease.
Other insect species exhibit a variety of trends in enzyme activity changes. For example, the PO activity of fifth-instar larvae of Spodoptera litura increased significantly over time after treatment with hexaflumuron (Jiao et al. 2010). The PO activity in hemolymph of C. suppressalis larvae increased significantly after they were parasitized by C. chilonis, but it showed a decreasing tendency over time, and reached a level lower than that of the control at 4 d post-parasitization (Li et al. 2011). Feng et al. (2011) reported that, after O. furnacalis were infected with E. coli, the activities of PO, GSH-px, GST, and GR in serum all increased. After infection with lower dosages of E. coli, the activities of PO and GSH-px increased as the duration of the treatment increased, whereas treatment with higher dosages resulted in their activities peaking at 12 h post-infection. They found that the GST activity under the lower dosages of E. coli decreased over treatment time, but higher dosages resulted in peak activities at 12 h post-infection. Additionally, they reported no significant variation of GR activity compared to the control under lower dosages of E. coli, but higher dosages of E. coli resulted in a peak at 36 h post-infection. These differences among studies show that variations in activities of enzymes involved in the immune response differ among host and pathogen species.
At the same time point after infection, we found that the activities of the six enzymes in haemocytes and serum of fifth-instar larvae of H. jinyinhuaphaga all increased with increasing dosages of E. coli. The activities of ACP, PO, POD, and GSH-px peaked at 1 × 107 ind/mL, and the activities of GR and GST peaked at 1 × 106 ind/mL. Increased dosages of E. coli could induce haemocytes to produce more enzymes and enhance their activities to remove pathogens, but beyond a dosage threshold, too much E. coli could damage the haemocytes and inhibit enzymes activity, thereby reducing the immune function of H. jinyinhuaphaga. Two -way ANOVA showed that the interaction of infection time and dosages of E. coli had no significant effect on the activities of ACP, PO, POD, GSH-px, GR, and GST in haemocytes and serum of H. jinyinhuaphaga larvae (P>0.05). This result indicates that the infection time and dosages of E. coli acted alone on the enzymes activities.
In summary, this result indicates that ACP, PO, POD, GSH-px, GR, and GST constitute the protective enzyme system in H. jinyinhuaphaga larvae, they participated the immune response after infection with E. coli. Infection of E. coli may cause H. jinyinhuaphaga larvae to produce many reactive oxygen radicals in a short time (Whitten et al. 1999; Pereira et al. 2001; Feng et al. 2011), the reactive oxygen radicals induced the increase of these enzymes activity, so to prevent the poisoning of the reactive oxygen radicals, and adapt to the infection of bacteria. These findings are important for guiding the development of microbial insecticides, the concentration of microbial insecticides must exceed 1 × 107 ind/mL, so as to damage the activity of protective enzymes in H. jinyinhuaphaga and cause them to die. However, the insecticidal effect of microbial insecticides is affected by temperature, humidity, light and other environmental factors. So comprehensive effect of these factors on the microbial insecticides should be studied in future, and thus improve programs of integrated pest management.
References
Asgari S, Zhang GM, Zareie R, Schmidt O (2003) A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem Mol Biol 33(10):1017–1024
Ashida M, Brey PT (1998) Recent advances on the research of the insect prophenoloxidase cascade. In: Brey PT, Hultmark D (eds) Molecular mechanisms of Immune responses in insects. Chapman & Hall, London, pp 135–172
Beck M, Theopold U, Schmidt O (2000) Evidence for serine protease inhibitor activity in the ovarian calyx fluid of the endoparasitoid Ventrria canescens. J Insect Physiol 46(1):275–1283
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytic Biochem 72:248–254
Castillo JC, Robertson AE, Strand MR (2006) Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedesaegypti. Insect Biochem Mol Biol 36(12):891–903
Cheng TC (1978) The role of lysosimales in molluscan cellular response to immunologic challenge.Comp. Pathobiol 4:59–71
Feng CJ, Dai HG, Wu SW (2001) Stress response of Nilaparvata lugens at high temperature and activities of its protective enzyme systems. Chin J Appl Ecol 12(3):409–413 (in Chinese with English abstract)
Feng CJ, Dong QA, Zhai HF, Chen GB, Yang JM, Miao JL (2011) Immunological and stress response of the hemolymph of Ostrinia Furnacalis Guenée (Lepidoptera: Pyralidae) larvae to the injection of Escherichia coli. Acta Entomol Sin 54(2):117–126 (in Chinese with English abstract)
Freitas DRJ, Rosa RM, Moraes J, Campos E, Logullo C, SilvaVaz I, Da, Masuda A (2007) Relationship between glutathione S-transferase, catalase, oxygen consumption, lipid peroxidation and oxidative stress in eggs and larvae of Boophilus microplus (Acarina: Ixodidae).Comp. Biochem. Phys., PartA, Mole & integrative physiol 146:688–694
Giannoulis P, Brooks CL, Dunphy GB, Mandato CA, Niven DF, Zakarian RJ (2007) Interaction of the bacteria Xenorhabdus nematophila (Enterobactericeae) and Bacillus subtilis (Bacillaceae) with the hemocytes of larval Malacosoma disstria (Insecta: Lepidoptera: Lasiocampidae). J Invertebr Pathol 94:20–30
Hillyer JF, Schmidt SL, Christensen BM (2003) Hemocyte-mediated hagocytosis and melanization in the mosquito Armigeres Subalbatus following immune challenge by bacteria. Cell Tissue Res 313(1):117–127
Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science 284:1313–1318
Jiao YY, LiuYJ, Qiu XC, Liu H (2010) Effects of hexaflumuron on phenoloxidase activity in Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Acta Entomol Sin 53(5):517–524 (in Chinese with English abstract)
Li XH, Yao HW, Ye GY (2011) Effects of parasitization by Cotesia chilonis (Hymenoptera: Braconidae) on larval immune responses of Chilo Suppressalis (Lepidoptera: Pyralidae). Acta Phytophy Sin 38(4):313–319 (in Chinese with English abstract)
Liang ZC, Cheng ZH (1991) Cellular immune responses of Asian cornborer larvae,Ostrinia Furnacalis. Acta Entomol Sin 34(2):141–145 (in Chinese with English abstract)
Liu WH, Wang SJ (1992) Dynamic force measurement method and preliminary application of glutathione S-transferase in serum. J Clin Lab Sci 10(1):2–4 (in Chinese)
Liu K, Lin JR, FuYG, Peng ZQ, Jin QA (2008) Effects of parasitization by Tetrastichus brontispae (Hymenoptera: Eulophidae) on immunoreaction of the coconut hispine beetle, Brontispa longissima (Coleoptera:Chrysomelidae). Acta Entomol Sin 51(10):1011–1016 (in Chinese with English abstract)
Marmaras VJ, Lampropoulou M (2009) Regulators and signalling in insect haemocyte immunity. Cell Signal 21(2):186–195
Nappi AJ, Vass E, Frey F, Carton Y (1995) Superoxid Eanion generation in Drosophila during melanotic encapsulation of parasites. Eur J Cell Biol 68:450–456
Ni YX, Liu XT, Liu YX, Liu HY (2006) Pesticide control to Heterolocha Jinyinhuaphaga Chu. J Henan Agri Sci 12:78–79.(in Chinese)
Packer L (1984) Oxygen radicals in biological systems. Harcourt BJed. Methods in Enzymology.INC.Vol.105. Academic, London, pp 273–280
Park Y, Kim Y (2003) Xenorhabdus Nematophilus inhibits p-bromophenacyl bromide (BPB)-sensitive PLA2 of Spodoptera exigua. Arch Insect Biochem Physiol 54:134–142
Pereira LS, Oliveira PL, Baria-Fidalgo C, Daffre S (2001) Production of reactive oxygen species by hemocytes from the cattle tick, Boophilus microplus. Exp Parasitol 99:66–72
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360(1):1–16
Söderhäll K (1982) Prophenoloxidase activating system and melanization - a recognition mechanism of arthropods? A review. Dev Comp Immunol 6(4):601–611
Whitten MMA, Ratcliffe NA (1999) In vitro superoxide activity in the haemolymph of the west Indian leaf cockroach, Blaberus discoidalis. J Insect Physiol 45:667–675
Williams MJ (2007) Drosophila hemopoiesis and cellular immunity. J Immunol 178(8):4711–4716
Xiang YY, Yin PF, Wang MY, Luo X, Zhang YC (2010) Developmental threshold temperature and effective accumulative temperature of Heterolocha Jinyinhuaphaga. Chin J Appl Entomol 48(1):152–155 (in Chinese with English abstract)
Xiang YY, Chen Y, Yin PF (2016a) Feeding preference of honeysuckle geometrid, Heterolocha Jinyinhuaphaga Chu to different varieties of honeysuckle. J Plant Prot 43(5):745–751 (in Chinese with English abstract)
Xiang YY, Zhang Y, Yin PF, Zhu P (2016b) Variation of hemocytes of Heterolocha Jinyinhuaphaga Chu larva infected by Escherichia coli. J Zhejiang Uni (Agric Life Sci) 42(2):163–168 (in Chinese with English abstract)
Xiang YY, Zhang XW, Xu GM (2018) The timing of calling and mating in Heterolocha Jinyinhuaphaga and the influence of environmental determinants. J Insect Behav 31(3):334–346
Xiang YY, Dong SL, Liu C, Wang ZW (2019) Physical and chemical traits affecting the oviposition preference of honeysuckle geometrid, Heterolocha Jinyinhuaphaga Chu among honeysuckle varieties. Arthropod-Plant Int 13:905–913
Yan R, Liu H, He LF, Wan QH (2008) Activity change of three enzymes in serum of the Musca domestica larva infected by Escherichia coli. Chin J Vector Bio &Control 19(5):426–428 (in Chinese with English abstract)
Yan R, Liu L, Liu H, He LF, Gao L (2011) The influence of Escherichia coli infection on the activity of ACP and POD in Musca domestica Larvae. Hubei Agri Sci 50(21):4396–4398 (in Chinese with English abstract)
Yang WZ, Yin YJ, Seng FN, Xu ZJ, Wang HL (1988) Determination of the glutathione reductase activity in red blood cells. J Clin Lab Sci 6(1):11–12 (in Chinese)
Zhang LL, Li YH, Wu JX (2013) Effects of various host plants on the development and protective enzyme activity of the black cutworm, Agrotis Ypsilon. Chin J Appl Entomol 50(4):1049–1054 (in Chinese with English abstract)
Zhong L, LÜ C, Yang WL, Liang P, Shi XY, Gao XW (2011) Effects of imidacloprid and omethoate on the activity of peroxidase, glutathione reductase and catalase in wheat seedlings. Chin J Pesti Sci 13(3):276–280 (in Chinese with English abstract)
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This study was supported by the Academic Funding Project for the Top-Notch Personnel of College Subject Specialty (Grant No. gxbjZD2020089), China, and Scientific Research Foundation of Chuzhou University (Grant No.2019qd02), China.
Author information
Authors and Affiliations
Contributions
Yu-yong Xiang contributed with the experimental design, data analysis, manuscript writing, and manuscript review. Xiu-jie Mu contributed with the experimental study. Yan Zhao contributed with the experimental design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, Yy., Mu, Xj. & Zhao, Y. Variation of enzymes activities in haemocytes and serum of honeysuckle geometrid, Heterolocha jinyinhuaphaga Chu (Lepidoptera: Geometridae) larvae infected by Escherichia coli. Int J Trop Insect Sci 44, 1703–1712 (2024). https://doi.org/10.1007/s42690-024-01270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-024-01270-0