Abstract
The humoral and cellular defenses are crtitical in insects’ immunity. In the present study we focused on individual haemocyt types of mulberry pyralid, Glyphodes pyloalis Walker (Lep.; Pyralidae) and examined them for the first time by light, differential interference contrast, scanning and transmission electron microscopy. Like many lepidopterous insects five types of haemocytes were identified; prohaemocytes, plasmatocytes, granulocytes, oenocytoids and spherulocytes. The small, rounded to ovoid cells with homogenous cytoplasm and large nuclei compared to cytoplasm were designated as prohaemocyts. The plasmatocytes were polymorphic and variable in sizes. The oval to spherical cells, with plenty of rough endoplasmic reticula, mitochondria, and microtubules in the cytoplasm were designated as the granulocytes. The oenocytoids were round or spherical with elongated eccentric nucleus and cytoplasm with small mitochondria and few rough endoplasmic reticula. Total haemocyte counts in different larval instars showed that THC increases as the larval age increase. Differential counts of haemocytes revealed the plasmatocytes and granulocytes as the most abundant haemocyte types in comparison with other types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect haemocytes—as the major components of innate immune system—are not only responsible for cellular immunity, but participate in the humoral defenses by a typical factor such as antimicrobial peptides (AMPs), lectin and lysozyme, etc. as well ultimately killing pathogens through lysis (Lavine and Strand 2002; Nappi et al. 2004; Schmid-Hempel 2005; Eleftherianos and Revenis 2011; Hillyer 2016; Li et al. 2019; Garcia-Robles et al. 2020; Manjula et al. 2020). The main functions start with recognition of non-self and then initiating appropriate responses in association with humoral system (Strand 2008; Silva et al. 2002). Haemocytes in insects comprise various types with obvious morphological and functional differences (Ling et al. 2005).
Hemocytes are classified into distinct types based on morphological and functional characteristics and have different names in different insect orders (Lavine and Strand 2002; Lamprou et al. 2007; Kanost et al. 2004; Becchimanzi et al. 2020) Four haemocyte types have been described in lepidopteran larvae based on morphology and functions (Ribeiro and Brehélin 2006; Strand 2008). The plasmatocytes and granulocytes are involved in phagocytosis, nodulation, and capsule formation; oenocytoids produce enzymes (e.g., phenoloxidase) involved in the melanization cascade and spherule cells whose immune function remains unclear (Lavine and Strand 2002 ; Strand 2008; (Pandey and Tiwari 2012; Becchimanzi et al. 2020).
The development of haemocytes in insects is initiated during embryogenesis from head or dorsal mesoderm. Later, the next population comes from the mesodermal haematopoietic organs in juvenile stages and also from circulating prohaemocytes (Yamashita and Iwabuchi 2001; Strand 2008; Grigorian and Hartenstein 2013; Öztürk et al. 2018). Ling et al. (2005) however, believes that in H. armigera the haematopoietic organs are important contributors to plasmatocyte production/ maturation and not the prohemocytes. On the other hand a positive correlation between prohemocytes with granulocytes and oenocytoids are indicative of their origin from circulating prohemocytes (Istkhar and Chaubey 2018). Nakahara et al. (2009 and 2010) in B. mori proposed two major lineages in haemocytes origin, a granulocyte lineage and a plasmatocyte-oenocytoid lineage. The origins of the spherulocytes could not be determined in their studies. Subsequently the number of haemocytes change during development (Gardiner and Strand 2000; Okazaki et al. 2006) or when stressed by wounding, infection or even the number varies with varied food regimes (Ratcliffe et al. 1985; Vogelweith et al. 2016). The populations of haemocytes are usually maintained by mitotic division of circulating cells (Saito and Iwabuchi 2003) or through production and release of haemocytes in the hematopoietic organs (Gardiner and Strand 2000). The number of cells has been proved to fluctuate during molting cycle (Kiuchi et al. 2008).
The Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) is a major pest of mulberry plantations in silk growing countries including India, China, Korea, Japan, Malaysia, Pakistan, Uzbekistan and Burma (Madyarov et al. 2006; Moallem et al. 2017; Shao et al. 2020). This pest has also turned into a major pest of mulberry in northern Iran and has become a serious threat to silk industry (Khosravi and Jalali Sendi 2010 ; Mahadeva 2018; Hosamani et al. 2020). The larvae fold the leaves on the outer part and then feed underside mesenchyme of leaves, thus retaining only a network of epidermis. If the infested leaves are provided to silkworms, they are unable to defecate due to constipation (Oftadeh et al. 2014). The G. pyloalis larvae are also regarded as the secondary hosts for Bombyx densoviruses and picornaviruses (Watanabe et al. 1988; Shao et al. 2020).
The first step to study the immune responses in any insect pests, could be the characterization of the haemocytic form and population and changes in haemocytic profile in the haemolymph during developmental stages (3rd instar larvae to pupal stage).Again an additional morphological information on haemocyte types of mulberry pyralid, Glyphodes pyloalis Walker in the 5th larval stage was performed using light, differential interference contrast and also scanning and transmission electron microscopy from mulberry plantations in Rasht, Iran.
Materials and methods
Insect rearing
The G. pyloalis larvae of various stages were handpicked from mulberry plantations in Rasht city (37°17′ N, 49°35′ E) north of Iran. The larvae were provided with fresh mulberry leaves (kenmouchi variety) in an insect culture room (Department of plant Protection, University of Guilan, Rasht, Iran) set at 24 ± 1 °C, 75 ± 5% RH and 16:8 (L: D) h photoperiod in transparent plastic boxes (18 × 15 × 7 cm) covered with muslin cloth to provid sufficient air ventilation. The emerging adults were also placed in similar transparent plastic boxes in pairs and were provided with cotton wool soaked in 10% honey solution and tender mulberry leaves for feeding and oviposition respectively. The resulting larvae of this culture were used to perform the experiments (Khosravi and Jalali Sendi 2010).
Light microscopy
The haemolymph (5 µL) from each 5th instar larva (a total of 10 larvae were used) was directly bled onto a clean slide and thin smears were made with the help of another slide and then let to settle for 10–20 min at room temperature. After collection of the blood from larvae they were discarded (Khosravi et al. 2016).
The haemocytes were stained with Giemsa (diluted 1:9 in distilled water) for 15 min, then washed quickly in distilled water (Khosravi et al. 2016). They were observed under an Olympus (BX51)microscope (Japan) in Insect Physiology laboratory, University of Guilan, Rasht, Iran. The identification clues for cell types were made by keys provided in pioneering work of Jones (1962), Gupta (1979), and later scientists including Strand (2008) and Huang et al. (2010).
Measurements of each cell width were already reported by us using a digital objective lens of light microscope (Olympus BX51) (see also Zibaee and Jalali Sendi 2011). A minimum of 30 cells of each morphotype were measured.The differential haemocyte counts were evaluated after counting 100 stained cells.
Differential interference contrast (DIC) microscopy
The haemolymph of another set of ten 5th instar larvae was considered for differential interference contrast (DIC). The haemolymph from each larva was mixed with anticoagulant solution (0.186 M NaCl, 0.098 M NaOH, 0.041 M citric acid, and 0.017 M EDTA, pH 4.5). A few drops were placed on slide and then were covered by a cover glass producing thin haemolymph films, the larvae after collection haemolymph were discarded. These wet smears highly facilitated haemocyte observation and identification. The cells were identified by DIC microscope, and images were taken with a camera (Dino Capture 2.0) in the Insect Physiology laboratory, University of Guilan.Rasht, Iran.
Scanning electron microscopy
The haemolymph from ten fifth instars of G. pyloalis was directly dropped on a slide and was then mixed with anticoagulant solution (30% v/v). The haemocyte monolayers were fixed in phosphate buffer (0.1 M, pH 7.3) with glutaraldehyde solution (2.5%), post-fixed in osmium tetroxide (1%), dehydrated in graded ethanol (Merck) series, 15 min each in 30%, 50%, 70%, 80%, 90%, and 100% (v/v) (Merck, Germany), and then dried with hexamethyldisilazane (HMDS) (Sigma grade) in Insect Physiology lab.University of Guilan. The preparations were mounted on stubs, gold coated in a sputter coater, and later observed under the electron microscope (VEGA) (Manachini et al. 2011) in Razi Metallorgy Research Center, Tehran, Iran.
Transmission electron microscopy (TEM)
Haemolymph from ten fifth instar larva of G. pyloalis was collected and was immediately mixed with an equal volume of 5% glutaraldehyde (5% glutaraldehyde in 0.1 M cacodylate buffer; pH 7.3,) and centrifuged at 800 × g for 10 min at 4 °C. The supernatant was removed and the cell pellets were again prefixed with 2.5% glutaraldehyde solution for 1.5 h at 4 °C. The pellets were then rinsed few times with 0.2 M cold cacodylate buffer at pH 7.2, post fixation was done in osmium tetroxide (1%) in 0.1 M cacodylate buffer for 2 h. The sample was rinsed with the same buffer thrice. They were then dehydrated in ethanol series (15 min each in 30%, 50%, 70%, 80%, 90%, and 100% (v/v)) (Hypša and Grubhoffer 1997) and was then embedded in Epon 812. The samples were preserved at 30 °C for 24 h and then at 60 °C for 48 h.The preparations of the samples were performed in Insect Physiology Lab. University of Guilan, Rasht, Iran. Then, the samples were posted to Aggeu Magalhaes Research Center, Brazil. Ultrathin sections were made by Leica EM UC6 Ultra microtome. The double contrast method of ultrathin sections with uranyl acetate (UA) and lead citrate was used as the standard contrasting technique. Finally, these sections were observed with a Tecnai™ G2 Spirit electron microscope.
Total haemocyte counts (THC)
Total haemocyts (cells/mL) at various life stages (based on head capsule size), second, third, fourth, and fifth instar larvae at 48 h after molt (h.a.m.), prepupae (24 and 48 h.a.m.), and pupae (48 h.a.m.) of G. pyloalis was determined from 5 µL of fresh haemolymph was diluted in 15 µL anticoagulant with the help of Neubauer haemocytometer (Khosravi et al. 2016).The count was estimated from ten larvae in each stage.
Differential haemocyte counts (DHC)
Giemsa-stained smears from ten different larvae in each developmental stage were used. Differential haemocyte counts were determined upon counting the haemocyte cell types and calculating their relative percentages. The haemolymph was bled directly on glass slide, mixed 1:1 with phosphate buffered saline (PBS), and allowed to dry at room temperature. Then haemocyte slides were stained with Giemsa (diluted 1:9 in distilled water) for 15 min, washed with distilled water. After air drying, the slides were covered with cover slips with a drop of Canada balsam and observed with light microscopy. From each larva 100 cells were randomly counted in bright-field microscopy at × 40 magnification.
Statistical analysis
Statistical analysis was done in haemocyte count changes in different larval and pupal stages (from 1st to 5th larva and prepupal, pupal stages of silkworm with means were considered significant at P ≤ 0.05.
Results and discussion
Haemocyte morphological characterization
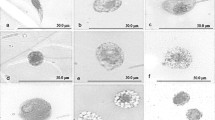
Using light microscopy, five main types of haemocytes including. prohaemocytes, plasmatocytes, granulocytes, oenocytoids and spherulocytes were identified:
-
Prohaemocytes
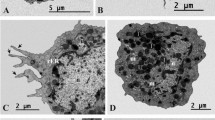
The prohaemocyts were round or oval cells, they are among the smallest blood in the insect. Our previous report show the cells measuring 4.0 ± 0.61 µm in G. pyloalis (Zibaee and Jalali Sendi 2011). Their nucleus occupies a major portion of cells (Figs. 1 and 2A) thus the cytoplasm is in the periphery forming just a thin layer around the nucleus. Observation of TEM of these cells are indicative of rough endoplasmic reticulum (RER), lysozyme and mitochondria. The cell membrane is smooth (Figs. 3-A and 4-A). Prohaemocyts are the cells considered to differentiate to other haemocyte types Silva et al.(2002).
Our naming of these cells were based on their small size, agranular structure, and the absence of granules which is their unique feature. However, some authors believe that prohaemocytes as well as plasmatocytes originate from haematopiotic organs. Hence, prohaemocytes are the cells forming the rest of haemocytes including granulocytes, oenocytoids and spherolucytes (Ling et al. 2005; Istkhar Chaubey 2018).
Plasmatocytes
Plasmatocytes are considered the pleomorphic cells and make the largest number in circulating cells in the larvae of the lesser mulberry pyralid measuring 8.5 ± 0.61 µm in width. The nucleus is more or less circular with a distinct central or pericentral position (Figs. 1, 2 and 3b)The chromatin is generally sprinkled. The cytoplasm includes low electron density vesicles; the mitochondria are either round or stretched with amounts of rough endoplasmic reticulum and developed cisternae. The presence of many vesicles that are attached to a very nonhomogeneous cytoplasm are considered as the main feature in these cells. The plasma membrane is folded and possess transparent electrolytic vesicles with irregular or rounded shape (Fig. 4A, B and C) Akai and Sato (1971) believe that the enhanced ecdysteroids level in hemolymph of Bombyx mori lead to excessive release of hemocytes from the haematopoietic organs, and Nakahara et al. (2003) showed these haemocytes are mainly prohemocytes and plasmatocytes. In Pseudoplusia includens and Spodoptera frugiperda (Gardiner and Strand 2000), and Manduca sexta (Nardi et al. 2003) this theory was also verified. Strand (2008) stated that haematopoietic organs in Lepidoptera are an important source of plasmatocytes.
The spreading potential of cytoplasm in plasmatocytes is indicative of its phagocytosis behaviour. Thus, vermicytes are likely formed by cytoplasm elongation on both sides of the spherical plasmatocytes (Gupta 1985). These types of cells are seen in G. pyloalis haemolymph as in other lepidopterous insects. The plasmatocytes are considered immunocytes since they take part in phagocytosis, encapsulation and nodulation. However, the controversies does exist, while a good number of researchers are sure on the phagocytosis role of plasmatocytes (Tojo et al. 2000) but others have questioned this role (Akai and Sato 1973; Neuwirth 1974; Beaulaton 1979; Gongqing et al. 2016; Wu et al. 2016). This difference is probably due to variations in experiments thus altering the amounts being ingested by these cells.
Granulocytes
A circular or oval cell that is variable in size, larger than prohaemocytes but smaller than plasmatocytes measuring 6.5 ± 0.5 µm in G. polyalis. The nucleus of granulocyte is different in size, more polymorphic and the nucleus being central with dispersed chromatin (Figs. 1 and 2 C). The granules are plenty in the granulocyte cytoplasm, which provides an irregular surface to the cell and remains as the main character of these cells. There exists a very profound rough endoplasmic reticulum, Golgi apparatus, small circular mitochondria, ribosomes and polysomes. The granulocytes enjoy the presence of transparent electrons, and membrane- bound granules (Fig. 4E). Granulocytes of G. pyloalis are easily recognizable by Giemsa staining due to their basophilic cytoplasmic granules. Granulocytes can be differentiated into types because of their granule numbers. This variation is of prime importance at the time of their phagocytosis as in case of Rhodnius prolixus reported by Borges et al. (2008). These cells are considered more phagocytic than other cell types, involved in encapsulation and nodule process, which they do so by contacting the foreign body and releasing their granular content Clark and Strand 2013; Ghosh and Venkatesan 2019). Chain et al. (1992) believed that granulocytes are formed by plasmatocytes in cockroach Periplaneta americana (L.), but Yamashita and Iwabuch (2001) verified that both plasmatocytes or granulocytes are differenciated from prohemocytes in B. mori. The granulocytes are strongly adhering and phagocytic cells and showing deep phenoloxidase activity following immune challenges (Beckage 2008). The ultrastructure picture of granulocyte shows the formation of filopodia. The present result is in agreement with observations already reported for granulocytes in Blatella germinica (Chiang et al. 1988) and in Melipona scutellaris (Amaral et al. 2010).
Oenocytoids
The oenocytoids are round or spherical in shape. They possess a large and homogenous cytoplasm. Giemsa staining depicted a basophilic cytoplasm in oenocytoids. The nucleus is pushed to the periphery of cytoplasm (Figs. 1, 2E and 3D). Diffused chromatin and sometimes a clear nucleus are seen in TEM sections. The oenocytoids of the mulberry pyralid are smaller measuring 8.5 ± 0.61 than those reported for other insects examples are ; Anastrepha oblique (Silva et al. 2002) in Hyphantria cunea (Zibaee and Jalali Sendi 2011) and also in Arge ochropus (Khosravi et al. 2016 ) (Fig. 4F). A homogeneous with no apparent organelles is the main features of oenocytoids in this insect. The mitochondria are few with electron free -vacuoles which is contrary to the reports for Diatraea saccharalis where dense mitochondria, generally ring-shaped was observed (Falleiros et al. 2003). However, similar to the reports of Huang et al. (2010) in Plutella xylostella who reported fewer mitochondria. The reports of Huang et al. (2010) in Plutella xylostella is also indicative of the presence of numerous polysomes but not seen in our preparations.
Spherule cells
Spherulocytes are small or large cells measuring 5.00 ± 0.0 µm with several spherules in the cytoplasm. Huang et al. (2010) reported these cells to measure 10.15 ± 0.20 µm.The differences in sizes are certainly related to the state of spherules at the time of preperations. In Spherulocytes, the cytoplasm is filled with large granules round to oval (Figs. 1 and 2D). The rough endoplasmic reticulum is clearly visible in this cell. In Diatraea saccharalis it is reported that the cytoplasm contained few organelles around the nucleus, such as ribosome, Golgi complex, RER and few mitochondria (Falleiros et al. 2003). Spherulocytes contain little number of large spherules that infer irregular shape to the cells. Spherulocytes show no certain nucleus Falleiros et al. (2003) in D. saccharalis reported that the nucleus was small, centrally located or eccentric, mostly deformed by the spherules. These cells are completely different from granulocytes containing phagocytes. The spherulocytes in the G. pyloalis, possess golgi apparatus that is filled with mature granules. The micro tubular bundle forming a micro tubular ring beneath the plasma membrane is evident in this haemocyte type (Fig. 4G).
Spherule cells or spherulocytes are possible sources of cuticle constituents (Strand 2008).
Haemocyte counts
Because haemocytes are expected to vary in numbers and proportion upon exogenous pressures (Vogelweith et al. 2016), we thus quantified their concentration in G. pyloalis in different larval stages until pupation.
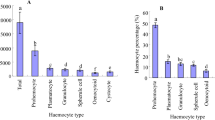
The total haemocyte count (THC) is the number of blood cells in the haemolymph. The THC count showed that the number of haemocytes per mL of haemolymph increased with development (Fig. 5). As it can be depicted from Fig. 5 the lowest number of haemocytes per mL is seen in the 3rd instar larvae (18 cells/mL) and then higher in 4th instar (37/mL). A slight decline in 5th instar (34/mL) but the highest number is observed in the beginning of prepupal stage (63 /mL), after which it declines sharply in late prepupa (31cells/mL) and then in pupal stage (27 cells/mL) (Fig. 5). During growth and development, the THCs of P. americana increased progressively from the 1st instars to adults. The population of haemocytes were as low as 3515/mm3 initially in the 1st instar, and as high as 12,620/mm3 in the 120 h old adults, a 3.59 - fold increase (Mudoi et al. 2020). The changes in haemocyte number in a hemimetabolous insect like P. americana is observed in an increasing trend from 1st instar nymph but reaching its maximal number in adults is acceptable. Because, as the insect grows, the need for intermediary metabolism will also increase. Hence, the services of many cells are required and these services are even required in active adult stage because the haemocytes continue their involvement in metabolism but this time in favour of future generation. However, in a holometabolous insect like Papilio demoleus the increasing trend is limited up to prepupal stage and then decreasing trend is initiated (Jalali and Salehi, 2008) since the adults in holometabolous insects seldom need the feeding for investment on future generation, this process has been completed in most cases by larval stages ( Chapman 2013). Hence the differences in two named insects can be referred to their differences in the type of development.
Changes in total hemocyte count (THC) during larval instars and pupa of Glyphodes pyloalis. Each data point is the mean ± SE of at least ten individual analyses. Different letters indicate significant differences between groups (Tukey test, p < 0.05). L3 – L5 are the larval instars, Pp1 is the beginning of prepupa, Pp2 is the end of prepupa
The study of differential haemocyte count (DHC) showed a significant difference in the abundance of haemocyte types in different stages of larvae. The number of prohomocytes in the early larval instars was higher than the other larval instars, but in the later stages of growth, their density decreased (Fig. 6). The amount of prohaemocytes in our study in the early instar larvae is acceptable as these cells are originating from haematopiotic organ and are released into haemolymph. They are the stem cells giving rise to other cell types (Ling et al. 2005; Strand 2008; Istkhar Chaubey 2018). Conversely, the plasmatocytes and granulocytes’ number would increase in later instars since they are formed by previously abundant cells. They are the so called functional cells and take the burden of many physiological processes, including immunity. Plasmatocytes at all stages of development have the highest frequency and then are the granulocytes. The frequency of plasmatocytes increased gradually with increasing age of larvae because the activity, movement, feeding and facing challenges against aliens are also increased (Khosravi et al. 2016; Ghosh and Venkatesan 2019).
Changes in percentage of haemocyte types during larval instars and pupa of Glyphodes pyloalis. Each data point is the mean ± SE of at least ten individual analyses. Different letters indicate significant differences between groups (Tukey test, p < 0.05). L3 – L5 are the larval instars, Pp1 is the beginning of prepupa, P is the pupa
Gardiner and Strand (2000) showed that in Pseudoplusia includens and Spodoptera frugiperda, the occurrence of plasmatocytes was higher during the early larval stages, while granulocytes were most prevalent in the late larval stages. However, in the present study the plasmatocytes in last larval stage was more than the granulocyte population. Beetz et al. (2008) reported that the population of plasmatocytes was larger than the population of granulocytes in the 5th larval stage of Manduca sexta (Lep.: Sphingidae). By counting only the free haemocytes in the haemolymph, haemocytes bound to the tissue are not evaluated in the DHC. In fact, the separation of tissues from pupae showed that a large number of granulocytes are attached to muscle fibers and lobes of fat bodies. During metamorphosis, haemocytes bind to tissues that are decomposed and rebuilt (Kiger et al. 2001).
We conclude that the present work has contributed to further our knowledge in characterization of Glyphodes pyloalis haemocytes using various methods. However the behavior of cells in responding to parasitoids or pathogenic attack in vivo is of immense importance which requires further research.
References
Akai H, Sato S (1971) An ultrastructural study of the haemopoietic organs of the silkworm, Bombyx mori. J Insect Physiol 17:1665–1676
Akai H, Sato S (1973) Ultrastructure of the larval hemocytes of the silkworm Bombyx mori L. (Lepidoptera: Bombycidae). Int J Insect Morph Embryol 2:207–231
Amaral IMR, Neto JFM, Pereira GB, Franco MB, Beletti ME, Kerr WE, Bonetti AM, Ueira-Vieira C (2010) Circulating hemocytes from larvae of Melipona scutellaris (Hymenoptera, Apidae, Meliponini): cell types and their role in phagocytosis. Micron 41:123–129
Beaulaton J (1979) Haemocyts and hemocytopoiesis in silkworms. Biochem Mol Biol 61:157–164
Beetz S, Holthusen TK, Koolman J, Trenczek T (2008) Correlation of hemocyte counts with different developmental parameters during the last larval instar of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol 67:63–75
Becchimanzi A, Avolio M, Bostan H, Colantuono C, Cozzolino F, Mancini D, Chiusano ML, Pucci P, Caccia S, Pennacchio F (2020) Venomics of the ectoparasitoid wasp Bracon nigricans. BMC Genom. https://doi.org/10.1186/s12864-019-6396-4
Borges AR, Santos PN, Furtado AF, Figueiredo RCBQ (2008) Phagocytosis of latex beads and bacteria by hemocytes of the triatomine bug Rhodnius prolixus (Hemiptera: Reduvidae). Micron 39:486–494
Beckage NE (2008) Insect immunology, 348 pp. Academic Press, New York
Chain BM, Leyshon-Soland K, Siva-Jothy MT (1992) Haemocyte heterogeneity in the cockroach Periplaneta americana analyzed using monoclonal antibodies. J Cell Sci 103:1261–1267
Chapman RF (2013) The insects structure and function. Edited by S. T. Simpson and A. E. Douglas, Cambridge University Press. pp 88–93
Chiang AS, Gupta AP, Han SS (1988) Arthropod immune system: I. Comparative light and electron microscopy accounts of immunocytes and other hemocytesof Blattella germanica (Dictyoptera: Blattellidae). J Morphol 198:257–267
Clark KD, Strand MR (2013) Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J Biol Chem 288:14476–14487
Eleftherianos I, Revenis C (2011) Role and importance of phenoloxidase in insect hemostasis. J Innate Immun 3:28–33
Falleiros AMF, Bombonato MTS, Gregório EA (2003) Ultrastructural and quantitative studies of hemocytes in the sugarcane borer, Diatraea saccharalis (Lepidoptera: Pyralidae). Braz Arch Biol Technol 46:287–294
Gardiner EMM, Strand MR (2000) Hematopoiesis in larval Pseudoplusia includens and Spodoptera frugiperda. Arch Insect Biochem Physiol 43:147–164
García-Robles I, De Loma J, Capilla M, Roger I, Boix-Montesinos P, Carrió P, Vicente M, López-Galiano J, DoloresReal M, Rausell C (2020) Proteomic insights into the immune response of the Colorado potato beetle larvae challenged with Bacillus thuringiensis. Dev Comp Immunol 104:103525
Gupta AP (1979) Hemocyte types: their structures,synonymies, interrelationships, and taxonomic significance. In: Gupta AP (ed) Insect Hemocytes. Cambridge University Press,Cambridge, p 85–127
Gupta AP (1985) Cellular elements in the hemolymph. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology biochemistry and pharmacology. Pergamon Press, Oxford, pp 402–444
Ghosh E, Venkatesan R (2019) Plant Volatiles Modulate Immune Responses of Spodoptera litura. J Chem Ecol 45:715–724
Gongqing W, Yi L, Ying D, Yunhong Y (2016) Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 48(4). https://doi.org/10.1016/j.tice.2016.06.007
Grigorian M, Hartenstein V (2013) Hematopoiesis and hematopoietic organs in arthropods. Dev Genes Evol 223:103–115
Hosamani V, Yalagi M, Sasvihalli P, Hosamani V, Nair KS, Harlapur VK, Hegde CR, Mishra RK (2020) Sucking pest and their management in mulberry (Morus alba): a review. Int J Chem Stud 8:1065–1070
Hillyer JF (2016) Insect immunology and hematopoiesis. Dev Comp Immunol 58:102–118
Huang F, Yang YY, Shi M, Li JY, Chen ZQ, Chen FS, Chen XX (2010) Ultrastructural and functional characterization of circulating hemocyte from Plutella xylostella larva: cell types and their role in phagocytosis. Tissue Cell 42(6):360–364
Hypša V, Grubhoffer L (1997) Two haemocyt populations in Triatoma infestans: ultrastructural and lectin-binding characterization. Folia Parasitol 44:62–70
Istkhar, Chaubey AK (2018) Challenging the larvae of Helicoverpa armigera and assessing the immune responses to nematode-bacterium complex. Phytopara 46:75–87
Jalali J, Salehi R (2008) The hemocyte types, differential and total count in Papilio demoleus L. (Lepidoptera: Papilionidae) during post-embryonic development. Mun Ent Zool 3:199–216
Jones JC (1962) Current concepts concerning insect haemocytes. Am Zool 2:209–246
Kanost MR, Jiang H, Yu XQ (2004) Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev 198:97–105
Khosravi R, Jalali Sendi J (2010) Biology and demography of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) on mulberry. J Asia Pac Entomol 13:273–276
Khosravi R, Sendi JJ, Brayner FA, Alves LC, Feitosa APS (2016) Hemocytes of the Rose Sawfly Arge ochropus (Gmelin) (Hymenoptera: Argidae). Neotrop Entomol 45:58–65
Kiuchi T, Aoki F, Nagata M (2008) Effects of high temperature on the hemocyte cell cycle in silkworm larvae. J Insect Physiol 54:454–461
Kiger JA, Natzle JE, Green MM (2001) Hemocytes are essential for wing maturation in Drosophila melanogaster. Proc Natl Acad Sci U S A 98:10190–10195
Lamprou I, Mamali I, Dallas K, Fertakis V, Lampropoulou M, Marmaras VJ (2007) Distinct signaling pathways promote phagocytosis of bacteria, latex beads and lipopolysaccharide in medfly hemocytes. Immunology 121:314–327
Lavine MD, Strand MR (2002) Insect haemocyts and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Li T, Yan D, Wang X, Zhang L, Chen P (2019) Hemocyte changes during immune melanization in Bombyx mori infected with Escherichia coli. Insects 10:301
Ling E, Shirai K, Kanekatsu R, Kiguchi K (2005) Haemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: Prohaemocyts have the function of phagocytosis. Cell Tissue Res 320:535–543
Madyarov SR, Khamraev AS, Otarbaev DO, Kamita SG, Hammock BD (2006) Comparative effects of wild and recombinant baculoviral insecticides on Glyphodes pyloalis and mulberry silkworm Bombyx mori, pp. 230–231. In International Workshop on Silk Handcrafts Cottage Industries and Silk Enterprises Development in Africa, Europe, Central Asia and the Near East, & Second Executive Meeting of Black, Caspian seas and Central Asia Silk Association (BACSA), 6–10 March, Bursa, Turkey
Mahadeva A (2018) Insect pest infestation, an obstacle in quality mulberry leaves production. Asian J Biol Sci 11:41–52
Manachini B, Arizza V, Parrinello D, Parrinello N (2011) Haemocyts of Rhynchophoru ferrugineus (Olivier) (Coleoptera: Curculionidae) and their response to Saccharomyces cerevisiae and Bacillus thuringiensis. J Invertebr Pathol 106:360–365
Manjula P, Lalitha K, Vengateswari G, Patil J, Senthil Nathan S, Shivakumar MS (2020) Effect of Manihot esculenta (Crantz) leaf extracts on antioxidant and immune system of Spodoptera litura (Lepidoptera: Noctuidae). Biocatal Agri Biotech https://doi.org/10.1016/j.bcab.2019.101476
Moallem Z, Karimi-Malati A, Sahragard A, Zibaee A (2017) Modeling temperature-dependent development of Glyphodes pyloalis (Lepidoptera: Pyralidae). J Insect Sci 37:1–8
Mudoi A, Das P, Hazarika LK (2020) Hemocytes of Periplaneta americana (Blattodea: Blattidae). J Entomol Res. https://doi.org/10.5958/0974-4576.2019.00070.7
Nakahara Y, Kanamori Y, Kiuchi M, Kamimura M (2003) Effects of silkworm paralytic peptide on in vitro hematopoiesis and plasmatocyte spreading. Arch Insect Biochem Physiol 52:163–174
Nakahara Y, Shimura S, Ueno C, Kanamori Y, Mita K (2009) Purification and characterization of silkworm hemocytes by flow cytometry. Dev Comp Immunol 33:439–448
Nakahara Y, Kanamori Y, Kiuchi M, Kamimura M (2010) Two hemocyte lineages exist in silkworm larval hematopoietic organ. PLoS ONE 8:e11816
Nappi AJ, Kohler L, Mastore M (2004) Signalling pathways implicated in the cellular innate immune responses of Drosophila. Invert Surviv J 1:5–33
Nardi JB, Pilas B, Ujhelyi E, Garsha K, Kanost MR (2003) Hematopoietic organs of Manduca sexta and hemocyte lineages. Dev Genes Evol 213:477–491
Neuwirth M (1974) Granular hemocytes, the main phagocytic blood cells in Calpodes ethlius (Lepidoptera, Hesperiidae). Can J Zool 52:783–784
Oftadeh M, Sendi JJ, Zibaee A, Valizadeh B (2014) Effect of four varieties of mulberry on biochemistry and nutritional physiology of mulberry pyralid, Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). J EntomolAcarol Res 46:42–49
Okazaki T, Okudaira N, Iwabuchi K, Fugo H, Nagai T (2006) Apoptosis and adhesion of haemocyts during molting stage of silkworm, Bombyx mori. Zool Sci 23:299–304
Öztürk G, Çakici O, Arikan H (2018) Morphological characterization of hemocyte types in some species belonging to Tettigoniidae and Pamphagidae (Insecta: Orthoptera). Turk J Zool 42:340–345
Pandey JP, Tiwari RK (2012) An overview of insect haemocytes science and its future application in applied and biomedical fields. Am J Biochem Mol Biol 2:82–105
Ratcliffe NA, Rowley AF, Fitzgerald SW, Rhodes CP (1985) Invertebrate immunity: basic concepts and recent advances. Int Rev Cytol 97:186–350
Ribeiro C, Brehelin M (2006) Insect haemocytes: what type of cell is that? J Insect Physiol 52:417–429
Saito T, Iwabuchi K (2003) Effect of bombyxin-II, an insulin-related peptide of insects, on Bombyx mori haemocyt division in single-cell culture. Appl Entomol Zool 39:583–588
Schmid-Hempel P (2005) Evolutionary ecology of insect immune defenses. Annu Rev Entomol 50:529–551
Shao Z, Li Y, Zhang X, Ch J, Ma J, Liu Z, Wang J, Sheng S, Wu F (2020) Identification and functional study of chitin metabolism and detoxification-related genes in Glyphodes pyloalis walker (Lepidoptera: Pyralidae) based on transcriptome analysis. Int J Mol Sci 21:1904
Shao Z et al (2020) Identification and functional study of chitin metabolism and detoxification-related genes in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) based on transcriptome analysis. Int J Mol Sci 21(5) https://doi.org/10.3390/ijms21051904
Silva JEB, BoleliI C, Simões ZLP (2002) Hemocyte types and total and differential counts in unparasitized and parasitized Anastrepha obliqua (Diptera, Tephritidae) larvae. Braz J Biol 62:689–699
Strand MR (2008) The insect cellular immune response. Insect Sci 15:1–14
Tojo S, Naganuma F, Arakawa FK, Yokoo S (2000) Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol 46:1129–1135
Vogelweith F, Moret Y, Monceau K, Thiéry D, Moreau J (2016) The relative abundance of hemocyte types in a polyphagous moth larva depends on diet. J Insect Physiol 88:33–39
Watanabe H, Kurihara Y, Wang YX, Shimizu T (1988) Mulberry pyralid, Glyphodes pyloalis: Habitual host of non -occluded viruses pathogenic to the silkworm, Bombyx mori. J Inverteb Pathol 52:401–408
Wu G, Liu Y, Ding Y, Yunhong Y (2016) Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 48:297–304
Yamashita M, Iwabuch K (2001) Bombyx mori prohaemocyt division and differentiation in individual microcultures. J Insect Physiol 47:325–331
Zibaee I, Jalali Sendi J (2011) Identification, differential and total count on haemocytes of Hyphantria cunea (Lep.: Arctiidae) and Glyphodes pyloalis (Lep.: Crambidae), and investigation on the effect of juvenile hormone I on these cells. J Entomol Soc Iran 30: 47–67
Acknowledgements
Our sincere thanks are due to Núcleo de Plataformas Tecnológicas-CPqAM/FIOCRUZ for transmission electron microscopy.
Funding
This study was funded by Iran National Science Foundation (INSF) (Grant number 91003789).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khosravi, R., Sendi, J.J., Valizadeh, B. et al. Mulberry pyralid haemocyts, a structural and functional study. Int J Trop Insect Sci 41, 75–84 (2021). https://doi.org/10.1007/s42690-020-00177-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00177-w