Abstract
Purpose of Review
After several decades of research on edge effects in marine habitats, we still have little understanding of how organisms respond to marine ecotones, and methodological gaps appear to be limiting our progress. Using recent literature (2010–2018), we synthesized responses and processes of organisms across several marine habitats. Specifically, we examined the uniformity of studies across biogenic habitats, the scales selected for exploring edge effects, the experimental approaches used, and the confounding influences that muddle our interpretation of results.
Recent Findings
The majority of edge effect studies are still conducted in seagrass systems and focused on response patterns. We found that the majority of studies were equally likely to report an increase, decrease, neutral, or equivocal effect depending on the context of the organism or habitat. Additionally, only a single measure, or a few related responses, is assessed and causal mechanisms are rarely tested. We note that most studies quantitatively defined an edge habitat as a linear distance from a habitat boundary (e.g., < 1 m, < 5 m), but the distances were not usually scaled to the size, trophic level, or mobility of focal organisms.
Summary
We provide a conceptual diagram as a roadmap for researchers for navigating the myriad influences that affect floral and faunal responses to marine habitat edges. Future efforts should seek to move beyond mensurative searches, explicitly incorporate potentially confounding variables, and more consistently test putative causal factors when known or hypothesized. Additionally, we advise expanding research on habitat types other than seagrasses (e.g., mangroves, shellfish, corals) and adjusting observational scales to more appropriately match mechanisms. Ultimately, we should move beyond pattern description, repeated in a limited subset of nearshore habitats, and toward a quantitative understanding of the processes acting in these unique and potentially impactful marine ecotones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biogenic habitats are critical to marine and coastal productivity and exist as a mosaic of patches that vary in size, shape, and degree of isolation due to a combination of anthropogenic and natural dynamics, including eutrophication, destructive fishing practices, navigational dredging, wind and wave activity, currents, and a suite of ecological processes. For the past 30 years, marine ecologists have largely applied terrestrial landscape-scale principles while investigating how watershed modification, habitat loss, and fragmentation affect the management and conservation of important marine species and foundational habitats. Dubbed ‘seascape ecology,’ the still nascent field focuses on the ways in which complex spatial patterning in marine habitats modifies interactions between species and the environment, controlling the abundance and distribution of marine organisms. One of the most pronounced features in seascapes are habitat edges or ecotones, typically defined as transitions between habitats of different structural complexity. Associated with these transition zones are changes in abiotic conditions (i.e., flow [1•, 2]), as well as changes in community and habitat structure relative to the interior of either habitat [3]. Edge effects have received considerable attention in the literature due to both observed and predicted responses of organisms to habitat boundaries.
Edge habitats are thought to have different environmental conditions than ‘core’ or interior habitats, which should influence ecological processes and subsequently community structure. Edges can differentially influence the movement of organisms, alter mortality rates, facilitate cross-boundary subsidies, and create opportunities for novel species interactions [4]. During the past 15 years, researchers have used empirical and theoretical models to predict species responses to edges in terrestrial [5] and marine [3] habitats. Edge zones that offer greater access to resources and/or complementary resources between two habitats may enhance abundance and diversity at patch edges [3], resulting in what is often termed a ‘positive edge response’ or one in which the estimated variable increases from core to edge. Conversely, negative edge responses may result from higher predation rates [6] and/or invasions [7] along edges. Most often, however, species do not appear to respond to edges at all, with neither increased or decreased abundance and diversity between core and edge areas, resulting in a ‘neutral edge response’ (sensu Boström et al [8]). The study of edge effects has achieved prominence in seascape ecology because many anthropogenic activities result in habitat loss and fragmentation, which often generate edge habitat. Thus, it is critical to develop a clear understanding of how edges modify organismal biology and ecosystem function if urbanized estuaries and coasts are to be managed successfully.
Tenets of landscape ecology, such as the effects of habitat fragmentation and landscape composition and configuration on ecosystem function, have been applied with little modification to the studies of edge effects in marine systems [9]. Terrestrial studies have documented how the vegetative structure of ecotones can result from even subtle gradients in environmental conditions (e.g., light [10], microclimate [11, 12]) compared to the core habitats. Organism distribution and net species interactions often respond accordingly [13, 14], resulting in edges with accumulations of organisms and resources. However, despite environmental gradients, Ries et al. [5] found that this is by no means a universal pattern, with a large number of terrestrial studies, involving many taxa, reporting neutral responses to patch edges. Similarly, in marine systems, we routinely observe edge-related patterns that vary by species, system, and sampling method, in part because the field is still developing [8]. Inconsistent edge effects in marine systems might be attributable to a still emerging field; however, we argue that major differences in the physics of the constituent medium (i.e., water vs. air) lead to important distinctions in density, heat capacity, light attenuation, oxygen concentration, etc. between marine and terrestrial habitats, creating a number of challenges to the translation of terrestrial concepts and experimental approaches to marine systems [15••]. Thus, our expectations of the factors driving edge responses and the assumed ubiquity of edge effects may be misplaced. By identifying which physical and biological processes are edge-responsive, we can better predict where and when we might see edge effects.
Despite the ambiguity of edge effects, they remain critical for management purposes. There is a gap in our understanding of the ecological consequences of changing seascape spatial arrangement, and habitat boundaries, and the edge habitats they create, remain important structural attributes of seascapes. The predictive framework of faunal responses to habitat edge is dependent upon resource distribution between the two adjacent habitats [3, 10]. However, shape, magnitude, and direction of the response across edges vary with habitat complexity, resource distribution, and the causal process or processes at work [3, 16, 17]. While a majority of previous studies showed no edge effects on abundance, richness, or diversity, we commonly see effects on recruitment [18••], predation [19], and animal movement [20], suggesting that the snapshot nature of many studies may not be sufficient for uncovering edge effect patterns.
In a broad review of seascape ecology, Boström et al. [8] discussed edge effects from the literature within the context of three biogenic habitats—seagrasses, salt marshes, and coral reefs. The spatiotemporal scales varied within and across habitats, and some of the studies used the amount of edge habitat or differing perimeter to area ratios to determine an edge response, rather than a direct comparison of edge to core locations. Although there were some taxon-specific responses to patch edges, the majority of the studies yielded equivocal results [8]. The authors concluded that future edge studies should seek to test the mechanisms underlying spatial patterns, where and when they occur. Additionally, they suggested expanding work in other biogenic habitats, across multiple life history stages, and on animal movement and behavior within edge habitats. To expand upon their synthetic critique, we reviewed the literature on marine biogenic ecotones published since the Boström et al. [8] review. By examining edge responses of fauna across six biogenic habitats (seagrass, salt marsh, mangrove, shellfish reef/bed, coral reef, and macroalgae), we answered the following questions: (1) How common are edge studies across biogenic habitats in general? (2) Are the spatial scales typically used appropriate for evaluating edge effects? (3) Has the experimental approach to edge effects in marine habitats matured over time? and (4) What variables confound the interpretation of edge effects, and are they being directly measured or implied by literature post hoc? Our goals were to audit the investigation of edge effects in coastal seascapes, assess the commonalities in questions and results across habitats, identify conceptual pitfalls or roadblocks, assess experimental rigor, locate missed opportunities for discovery, reassess starting assumptions and identify circular reasoning, and make recommendations on future research directions to improve our understanding of edge effects in marine systems.
Methods
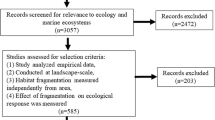
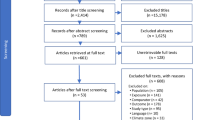
The aforementioned review of seascape ecology by Boström et al. [8] evaluated literature for the years preceding 2010; therefore, we reviewed the literature appearing after 2010 on edge effects in biogenic marine habitats. Studies were amassed through the use of personal libraries as well as database searches. The database ISI Web of Knowledge (www.webofknowledge.com) was used, and searches were bounded from studies published from July 2010 through October 2018; wildcards (e.g., seagrass*) were combined with the following key terms: edge, edge effect, and ecotone, to search the title, abstract, and keywords. Our main criterion was explicit comparisons of a process or pattern between the edge and interior of the focal biogenic habitat. We included seagrasses, salt marshes, mangroves, coral reefs, shellfish beds (oysters and mussels), and macroalgae as focal habitats. In addition, we included mesocosm studies that made direct comparison between the edge and interior of a focal habitat.
Once qualifying studies were compiled, we identified the habitat, the focal organisms, the matrix habitat, the spatial scale of the edge, period of observation, the process or response measured, and the overall edge effect result (i.e., increase, decrease, neutral, or equivocal). We defined an increase in process or pattern as a positive response, a decrease as a negative, and no change (as defined by the authors) as neutral. Importantly, we used the manuscripts’ focal questions to determine the response (i.e., if a manuscript was examining survival of a prey species and showed a decrease near the edge, we considered that a negative response, whereas if a manuscript was examining foraging of a predator species, which was higher at the patch edge, we considered that a positive response). In addition, we classified studies that displayed multiple but inconsistent responses within and among species, or across time, as having equivocal results. We considered both patterns (e.g., biomass, density, diversity, richness; Table 1) as well as ecological processes (i.e., events or actions resulting in the measured responses, such as predation or water/resource flow; Table 2). We noted the author definitions of edge distance, when available, to determine if authors were arbitrarily determining the edge distance or if they were designing experiments sensitive to the organism size, trophic level, or mobility. One paper was excluded because it selected an anomalously large edge distance (150 m) for a sessile organism (mussels) without sufficient justification.
Results
Our search terms identified 105 potential papers using the terms ‘edge’, ‘edge effects’, or ‘ecotones’ within the title, abstract, or as keywords. From these, we eliminated 33 papers from consideration that referred to species range edges rather than habitat edges, did not make direct comparisons between an edge and core habitat, or were reviews or meta-analyses, leaving us with 71 publications for analysis. Almost half of the papers examined edge effects in seagrass habitat (n = 33), with relatively few studies focused on mangroves (n = 12), shellfish beds/reefs (n = 12), salt marshes (n = 10), macroalgal habitats (n = 3), and coral reefs (n = 1) (Fig. 1a). Study organisms ranged from bacteria to fishes, although 20% of studies examined broader taxonomic categories or functional groups (e.g., ‘invertebrates,’ ‘macrofauna,’ ‘epifauna’ etc.). Observational time scales varied considerably, with almost as many studies using a single sampling event (32%, n = 23) as those replicated temporally over a few months (30%, n = 22). A few studies were repeated seasonally (8%, n = 6), or across multiple years (12%, n = 8). The rest of the studies lasted less than a month, with a number of experiments lasting only a few hours to days (13%, n = 9).
The majority of studies examined edge effects across boundaries between structured focal habitat patches and unstructured matrix habitat, typically soft sediments, although 15 studies compared two structured habitats. Of the 71 studies, 22 (34%) adopted a continuous sampling design from the habitat transition toward the interior of the focal habitat, and thus did not explicitly delineate habitats as edge or interior/core. The remaining studies (n = 49) examined categorical edge and interior habitats where 43 explicitly defined an edge (e.g., < 1 m, < 5 m). The remaining six categorical studies compared edge to interior but did not clearly define the width of the edge habitat. Of the studies that defined an edge, whether using a categorical or continuous scheme, the range was 0.03–20 m, although the majority defined the edge habitat as less than 5 m (n = 40; 56%; Fig. 1b). The mean edge distance was 3.0 m ± 4.7 m (mean ± SD), and grouping the study organisms into categories defining their mobility (sessile, mobile, plankton), trophic level (primary producer, primary consumer, predator), or individual body size did not show any clear pattern related to corresponding edge size (Supplemental Fig. S1).
Overall, the papers we examined measured more patterns (65%) than processes (35%; Supplemental Table S1) Out of the 46 studies that measured patterns in relation to patch edges, the majority measured organismal density (52%, n = 24), but species diversity was also common (28%, n = 13) (Table 1). Focal taxa were variable, with the highest number of studies examining ‘fauna’ (17%, n = 8) and bivalves (15%, n = 7). These patterns showed a decreased response 32% of the time; it was more common to find a negative response on species density, diversity, and survival at patch edges while equivocal results also common (27%) due to differences across multiple species of fauna, at different edge locations, or in metric used (i.e., diversity vs. richness) (Table 1).
We found 24 papers that examined edge effects on processes with the majority conducted in seagrass (50%, n = 12), followed by mussel beds (21%, n = 5), and saltmarsh (17%, n = 4) (Table 2). Of these studies, the most common processes examined were settlement/recruitment (n = 8) and predation (which we included foraging and grazing; n = 9). Additionally, nine studies examined the structural complexity/density of the habitat as a proxy for the mechanism of interest. As above, the effects of habitat edges on processes were variable across taxa and habitats (Table 2). Settlement and recruitment were often higher at habitat edges (n = 4), although one study demonstrated a decreased response and two studies reported no effect. Similarly, when predation, grazing, and foraging were considered together, four studies showed an increase at patch edges, while decreased and neutral responses were found in two studies each. In total, most of the studies (n = 50) were strictly mensurative (observing responses only); many fewer (n = 13) actually measured or experimentally assessed the mechanism that was thought to be driving the observed edge effect, while the rest of the papers invoked mechanisms from the literature.
Discussion
The distribution of marine species is driven by both the surrounding physical environment and ecological interactions and processes, both of which can abruptly change at habitat edges. As such, these transition zones are recognized as important features in seascape ecology, and organismal and ecological processes at edges continue to be major research foci. Similar to previous assessments of the edge effect literature, we report inconsistent or equivocal edge effects on species responses across major marine habitats. Further, we found that (1) most studies were conducted in seagrass habitats, (2) most researchers took a mensurative approach to exploring responses with few examples of manipulative work, (3) studies were mostly conducted along non-structured matrix habitats, and at relatively fine spatiotemporal scales. We have identified a number of potential issues pertaining to the study of marine ecotones that may have stymied progress in this nascent subdiscipline.
- (1)
How common are edge studies across biogenic habitats?
Boström et al. [8] found that the majority of the research on edge effects remains within seagrass habitats, predominantly within a binary (habitat vs. no habitat) seascape context. Although our review shows that edge effect studies have increased in certain habitats (i.e., shellfish, mangroves), seagrasses still represent the preponderance of effort. There is likely a historical component favoring seagrasses, as these were the first marine habitats in which landscape ecological principles were applied [21] and seagrasses often exist in patchy mosaics with distinct boundaries, making edge delineation relatively straightforward. In addition, seagrass patches are easy to replicate (i.e., using artificial seagrass units), which can allow researchers to perform more controlled, manipulative studies. Experiments using natural and artificial seagrasses typically explore questions within a binary landscape (seagrass vs. sand) with well-defined habitat edges, rather than transitions between two complex habitats where pattern interpretation is more difficult.
Marine habitats other than seagrasses are less represented in the literature and may be fundamentally more difficult to work within. Edges may be less distinct and therefore more difficult to delineate in situ. They may be ephemeral due to stochastic species occurrence, as is the case for some macroalgal canopies, or result from canopy movement, common in kelp systems, where wave activity shifts edges over very short timescales. Similarly, shellfish beds and coral reefs may not have a distinct edge, particularly in natural reefs/beds where edges may be surrounded by shell debris fields or intermittently covered by shifting sediments. Non-seagrass biogenic habitats are often tidally exposed and bounded by other complex habitats (i.e., marshes and oysters), which could complicate the interpretation of edge-related patterns. In addition, the structural complexity of some of these habitats can potentially confound results; while seagrasses extend upwards into the water column, the structure and rugosity of oyster reefs are much more complex and difficult to standardize when comparing among or between study sites. Regardless of the underlying motivation, it is clear that seagrass habitats are preferentially selected by investigators and so dominate the seascape and edge effects literature. To address this issue, we recommend expanded focus on other biogenic habitats and a clearer examination of edge effects related to both the habitat forming species themselves and their associated communities.
- (2)
Are the spatial scales relevant?
Appropriate scaling remains one of the central issues in ecological studies [22]. Our review indicates that, for most studies, edge sizes have not been selected with deference to the size, trophic level, or level of mobility of focal organisms. For example, a 1 m distance has been used to describe the edge habitat for studies of organisms ranging from bacteria [23] to seagrass and mangrove propagules [24•, 25•] to low-mobility invertebrates such as scallops [6] or to high-mobility animals such as fishes [26••]. Meanwhile, within groups of similar size, mobility, and trophic level, such as gastropods, the distance of the edge habitat can range from 0.1 to 15 m [27,28,29]. In general, edge distances used were relatively small (< 2 m) which may be appropriate for bacteria and sessile organisms that experience differences in flow, dissolved oxygen, predation, etc., at those scales, but may not be relevant for larger organisms such as crustaceans or fish. Mobility, paired with organism size, is therefore a key factor in defining an edge that is appropriate from an ecological and biological standpoint.
Beyond scaling to organism size, investigators should carefully consider the causal process being studied and attempt to scale their study appropriately. There are few examples within the literature where studies have established an edge boundary by first measuring environmental gradients (flow, temperature, turbidity etc.) to dictate the definition of edge size. For example, Jurgens and Gaylord [30••] identified patterns in mussel distribution, while Nicastro et al. [31] examined mussel behavior across temperature stress gradients which occurred at a very short spatial scale (12 cm and 15 cm, respectively). Mussel responses were observed over discernable gradients in temperature, indicating that the studies used the appropriate, albeit small, scale of the response. Predation on mussels in oyster habitat may also vary over extremely small spatial scales (3–12.5 cm) in mesocosm studies [19]. Alternatively, on oyster reefs in the field, differences in mussel abundance between the edge and interior were not found until sampling beyond 5 m [32•]. Other responses and processes are likely occurring at either smaller or larger scales than those arbitrarily chosen (1–2 m) in most edge effect studies. Frequent discrepancy between the scales of action (mechanism or focal organisms) and observation may contribute to the high number of studies finding equivocal edge effects. Lastly, the large number of studies that continuously sampled perpendicular to habitat boundaries represents a glaring missed opportunity to estimate the size of marine ecotones. Unfortunately, most of these studies merely sought an effect and made no attempt to quantify the width of edge habitats. Progress in our delineation of ecotonal habitat could be made by leveraging the data from regression-based sampling schemes.
At present, too many researchers select an arbitrary edge distance (i.e., 1 m) that may not be applicable to either the species or response being examined. In the future, when examining edge responses or processes, we make three recommendations. First, select the scale relevant to the organism, which includes considerations for organism size, life stage, mobility, and behavior. Second, select the scale relevant to the process/response, which would require examining changes in both physical environment and ecological interactions across edge-interior gradients. Third, we suggest that future studies clearly define the edge and interior habitats based on actual measurements of variables between the two areas (flow, temperature, resources, etc.), rather than differences implied from the literature. In this way, we should take advantage of research that continuously samples transects from the habitat boundary to help distinguish the ‘edge’ from the ‘core.’
- (3)
Has the experimental approach to marine edge effects studies matured over time?
The cause of edge effects across biogenic habitats is driven by several processes that are complex and interactive, and yet, very few studies directly measure the variables or examine the processes thought to be associated with the edge effect patterns. Predation is often invoked as an explanation for many observed or expected edge responses (n = 23 studies); yet, predation was only quantified in nine studies. Similarly, larval settlement/recruitment and food availability are cited as potential mechanisms for edge responses about twice as often as they are experimentally assessed in the literature. Published studies continue to be dominated by survey data and rarely test causal hypotheses. Thus, many studies are still exploring whether edge effects occur (responses) and not why they occur (processes).
There are few examples in the literature that take a more holistic approach to understanding edge effects. In a seagrass edge effect study which examined both responses and processes, Carroll et al. [16] were able to demonstrate that the ‘neutral’ edge effect on scallop abundance was actually driven by two component processes—larval recruitment and post-settlement survival—which operate in opposite directions across a patch edge, effectively counteracting each other. Likewise, Tuya et al. [28] made similar observations on gastropods, with species-specific patterns in predation and recruitment at the edge driving snail abundance patterns. A few other studies have actively tried to disentangle habitat complexity from edge effects using a manipulative approach [17, 26••]. Macreadie et al. [19] were able to demonstrate that edge effects on prey survival in mesocosm experiments were dependent upon both patch configuration and the presence of top predators. In perhaps the most complete study of marine edge effects, Macreadie et al. [33] used a hierarchical approach to demonstrate that pipefish positively respond to seagrass patch edges and this response was driven by food (zooplankton) abundance. While this type of explicit, hypothesis-testing approach should be commonplace in edge effects literature, it remains the exception. Importantly, this study, too, began with a blind survey of potential edge responsive species, ultimately selecting one with strong spatial patterns for follow-up hypothesis testing. As a microcosm of the subdiscipline, we argue that this sequence could offer a pathway for progress by future investigators. Therefore, we echo Boström et al. [8] that more explicit hypothesis-driven research should be conducted along habitat edges which combine both mensurative and manipulative approaches.
- (4)
What variables confound our ability to detect edge effects?
The detection of marine edge effects has been largely equivocal, with responses varying across studies, habitats, and species. We conclude that much of this variability results from complex factors overlooked by researchers. For example, we identified over a dozen variables that are capable of obscuring edge effects, which fall into four broad categories—(1) temporal, (2) patch, (3) landscape, and (4) organismal attributes (Fig. 2).
Conceptual diagram showing the different variables that may influence our ability to observe edge effects, broken into four main types of variables: temporal, patch, seascape and organismsal attributes. Number (n) in each box is the number of studies that at least measured/examined this variable, even if it was not used in subsequent analysis
First, and perhaps most importantly, temporal scales of observation vary considerably across studies, contributing to variable findings regarding edge effects. Response patterns have been shown to vary over relatively short (diel [34, 35]; tidal [36•]) to long (season [37]; years [6]) time scales. However, half of the studies we reviewed addressed their responses using a single synoptic sampling of no more than a few days [29, 38•, 39], which may not be sufficient to fully examine edge responses. For example, sampling season alters responses in fish [40] and crab [37] abundance along mangrove edges. The pattern of increased scallop growth at seagrass patch edges disappears in low growth years, while resource distribution (phytoplankton) varies across months within a growing season [6]. Long-term (6-month) patterns in probability of seagrass survival do not reflect short-term (6-day) patterns of grazing by fishes [41]. Therefore, selecting the wrong temporal scale could obscure the magnitude and direction of observed responses; temporal variability needs to be considered when designing edge effects experiments.
Second, numerous patch-level attributes may affect our ability to detect edge effects, such as patch size, shape, and within-patch variations in habitat complexity. For example, a number of studies scale the edge-core distances with patch size [18••, 20, 32•, 42, 43], or otherwise sample at a number of distances from the edge which inevitably increases with patch size [44,45,46,47]. High variability in distance between edge and core habitats can affect response detection. Habitat complexity (i.e., shoot density, % cover, shell volume) of the focal habitat might be the most important patch attribute, yet was only explicitly tested in 11% of the edge effect studies examined. Habitat complexity may covary with distance from patch edge (i.e., complexity declines with proximity to patch edges [1•, 17, 18••, 32•, 48•, 49, 50•]), which can confound patterns explored at patch edges. Faunal abundance across a sand-seagrass ecotone may be strongly impacted by seagrass morphology [44, 45], while the low complexity of oyster reef edges may actually increase diversity of mobile nekton relative to the interior [32•]. In some instances, when habitat complexity was considered as a variable, the overall effect was stronger than the edge effect [26••, 43], whereas others have demonstrated that edge effects were independent of structural complexity [17, 51]. Limited effort to control for complexity (i.e., artificial seagrass units [16]) may contribute to our poor understanding of the interactive effects of habitat complexity and edge effects.
Third, a number of landscape-level attributes likely hinder our identification of edge effects. Habitat patch composition and configuration can influence potential edge effects. Percent seagrass cover had a stronger effect on seed production than edge-interior differences [24•], whereas mussel survival at patch edges varied when oyster reef habitat was considered continuous or patchy [19]. Presumably, patchy habitats have more overall edge area in the surrounding seascape which can obscure potential responses observed at any given edge location within the seascape. In addition, edge location (i.e., which side of the focal habitat the edge is sampled) can affect whether faunal responses are observed [52], perhaps driven by differences in depth [43] or habitat complexity [25•, 53] at the edges on either side of a given patch. The matrix habitat surrounding the focal patch could also impact edge responses. However, the vast majority of studies we examined still treat edges in a binary context (habitat vs. no habitat, seagrass vs sand); only 15 studies compared edges between two complex habitats, and only 2 compared an edge between two high complexity habitats to an edge between a high and low complexity habitat. When two complex habitats make up the edge/ecotone, the responses are often stronger at the high complexity edge (i.e., seagrass-rocky reef; seagrass-mangrove) compared to the low complexity edge (e.g., seagrass-sand [27]; mangrove-sand [53]). Since a number of these complex biogenic habitats are found bordering each other in the same seascapes, more consideration should be given to examining transitions between complex focal and matrix habitats.
Fourth, there are numerous organism-level attributes that can obscure edge responses. Organism size is an important driver of their umwelt—that is smaller and/or sessile organisms may perceive their surrounding habitat as continuous at a different grain and over a smaller extent than larger and/or more mobile organisms. Despite this, the distance into patches considered the ‘edge habitat’ has remained relatively constant across studied organisms (see above). The size of the focal species can also play a role in whether edge responses are observed [54]. Organismal responses to habitat edges may also change with ontogeny, where different edge responses are observed between juveniles and adults [36•, 40]. Behavior and species interactions can also affect our ability to detect edge responses. Mussels can alter their behavior (gaping, byssus production) near patch edges which can increase their survival along environmental stress gradients (temperature, flow; [1•, 31]). When top predators are present, crab mesopredators may alter their behavior and reduce consumption along habitat patch edges [19]. Differences in behavior between blue crabs and pinfish can alter their susceptibility to predators in edge habitats [26••]. Furthermore, all fauna are a potential prey resource at some point in their life history, and their ability to respond to edge resources may be affected by the relative risk they experience in those areas [41, 55, 56]. Observing edge effects becomes more difficult at the community level. Multiple organismal attributes, such as life stages, recruitment patterns, mobility, behavior, and diet, both within and among species, should be considered and, where possible, controlled for in future edge effects studies.
In addition to the presence of potentially confounding variables, it is important to note that while we interpreted ‘neutral’ or ‘no effect’ based on the conclusions of each manuscript’s authors, an over-reliance on P-values within frequentist statistics could also be impacting our interpretations of edge effects [57]. While mining data for a meta-analysis was beyond the scope of our qualitative review, it is possible that if studies reported effect sizes [58], or emphasized the difference between statistical and ecological/biological significance [59•], it could impact our conclusions. We used the investigator interpretation to determine whether there was ‘no change’ or ‘no effect’ in each pattern or process explored; however, ‘not significant’ does not necessarily equate to ‘no effect,’ and likewise, ‘significant’ does not always equate to biologically or ecologically relevant or important [59•]. While we cannot say how widespread this issue might be in edge effects literature in seascapes, it is likely that if studies include more informative statistics (e.g., effect sizes, confidence intervals) coupled with traditional statistical significance, independent interpretation of response patterns (or lack thereof) to habitat edges would be improved.
In sum, the magnitude and direction of edge responses result from a confluence of interacting variables that may operate additively, synergistically and/or antagonistically, complicating our abilities to detect edge responses in seascapes. For future studies, researchers should use a more holistic research approach that measures not only the patterns but the processes, while considering the countervailing causal pathways. Studies should actually measure the variables thought to drive or explain potential edge effects rather than implying from the literature post hoc. Further, seasonal variation of biogenic habitat formers can alter complexity, patch size and configuration at various temporal scales [60, 61], and temporal patterns in other organisms can affect their responses to patch edges [62]. Beyond temporal considerations, habitat complexity, the overall amount of edge, and contrast between patch and matrix habitat can be quantified at multiple spatial scales across seascapes and can potentially influence edge responses. Future studies should focus not only on edge effects per se, but on the influence of patch and seascape metrics at appropriate timescales with suitable replication, and use hierarchical analytical approaches, such as structural equation modeling [63•] or regression tree analysis [64], as well as include information regarding effect sizes, which would allow researchers to evaluate the relative importance of these variables and the causal networks responsible for edge responses.
Conclusions
Human encroachment into coastal systems has led to dramatic declines across many valuable biogenic habitats, shrinking and fragmenting the seascape and increasing overall edge habitat [29, 65, 66], while reducing habitat complexity [67,68,69]. Appropriate management and restoration require a more complete understanding of how species and ecosystems respond to habitat edges and ecotones. Historically, all species are predicted to show positive, neutral, or negative responses to habitat edges, driven by assumptions that differences in abiotic factors and resource availability occur between edge and interior habitats, as well as between focal and matrix habitat types. However, gaps still exist in our understanding of the ecological processes operating across habitat edges, resulting in numerous equivocal, species-specific, and highly context-dependent results. The magnitude and direction of any observed edge effect is driven by the pattern/process being examined, the timing of the study, the organisms’ capacity to perceive and respond to the landscape, and a number of confounding variables. Further, clearing up inconsistent definitions (what defines the edge?), use of terms (core, interior, or center?), and verbiage (positive or negative response vs. increase or decrease in a metric) could help mitigate current ambiguity. Based on our synthesis, we provide the following conclusions:
Increased exploration of habitats other than seagrasses is still necessary
Studies need to be appropriately scaled to (1) actual rather than assumed environmental gradients (i.e., flow, resources); (2) the biology and umwelt of organism being studied (size, mobility, ontogeny); and/or (3) the causal process being explored
Studies should take a step-wise, hierarchical approach to both experimental design—identify patterns and then design experiments to test causal processes—and analysis (e.g., multivariate analysis, structural equation modeling, regression tree analysis) for a better mechanistic understanding of observed responses
Potential confounding variables should either be controlled, measured, or incorporated using appropriate analytical techniques to determine their relative importance to edge effects
We have begun to explore some of the poorly understood aspects of animal biology (movement, behavior), which has helped clarify some edge responses. However, our understanding of edge responses will likely remain uncertain until we move beyond seagrasses, stop over-interpreting isolated survey data, select suitable spatiotemporal scales, and consider, control, or incorporate confounding variables in truly manipulative experiments. By acknowledging issues pervasive in the marine edge effect literature, we can move toward a more complete understanding of these transition zones and begin to address their management implications.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Kangeri AKW, Jansen JM, Joppe DJ, Dankers N. In situ investigation of the effects of current velocity on sedimentary mussel bed stability. J Exp Mar Biol Ecol. 2016;485:65–72. https://doi.org/10.1016/j.jembe.2016.08.011A combination ofin situmeasurements and manipulations to examine how flow speed and sediment characteristics affect mussel byssus production and likelihood of being dislodged across a mussel bed. They found higher byssal production and courser sediment associated with higher flow at bed edge, the combination of which increased adhesive strength of mussels in these locations.
Peterson CH, Luettich RA, Micheli F, Skilleter GA. Attenuation of flow inside seagrass canopies of differing structure. Mar Ecol Prog Ser. 2004;268:81–92.
Macreadie PI, Connolly RM, Jenkins GP, Hindell JS, Keough MJ. Edge patterns in aquatic invertebrates explained by predictive models. Mar Freshw Res. 2010;61:214–8.
Fagan W, Cantrell R, Cosner C. How habitat edges change species interactions. Am Nat. 1999;153:165–82.
Ries L, Fletcher RJ, Battin J, Sisk TD. Ecological responses to habitat edges: mechanisms, models and variability explained. Annu Rev Ecol Evol Syst. 2004;35:491–522.
Carroll JM, Peterson BJ. Ecological trade-offs in seascape ecology: bay scallop survival and growth across a seagrass seascape. Landsc Ecol. 2013;28(7):1401–13. https://doi.org/10.1007/s10980-013-9893-x.
Ceccherelli G, Pinna S, Cusseddu V, Bulleri F. The role of disturbance in promoting the spread of the invasive seaweed Caulerpa racemosa in seagrass meadows. Biol Invasions. 2014;16(12):2737–45. https://doi.org/10.1007/s10530-014-0700-7.
Bostrom C, Pittman SJ, Simenstad C, Kneib RT. Seascape ecology of coastal biogenic habitats: advances, gaps, and challenges. Mar Ecol Prog Ser. 2011;427:191–217.
Hinchey E, Nicholson M, Zajac R, Irlandi EA. Preface: marine and coastal applications in landscape ecology. Landsc Ecol. 2008;23:1–5.
Didham RK, Lawton JH. Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropia. 1999;31:17–30.
Cadenasso ML, Traynor MM, Pickett STA. Functional location of forest edges: gradients of multiple physical factors. Can J For Res. 1997;27:774–82.
Meyer CL, Sisk TD, Covington WW. Microclimatic changes induced by ecological restoration of ponderosa pine forests in northern Arizona. Restor Ecol. 2001;9:443–52.
Watkins RZ, Chen J, Pickens J, Brosofske KD. Effects of forest roads on understory plants in a managed hardwood landscape. Conserv Biol. 2003;17:411–9.
Schlaepfer MA, Gavin TA. Edge effects on lizards and frogs in tropical forest fragments. Conserv Biol. 2001;15:1079–90.
•• Manderson JP. Seascapes are not landscapes: an analysis performed using Bernhard Reimann's rules. ICES J Mar Sci. 2016;73:1831–8 An important review paper because it describes the major differences in chemical and physical properties between air and water and why that might affect our ability to apply terrestrial landscape ecological principles to seascapes.
Carroll JM, Furman BT, Tettelbach ST, Peterson BJ. Balancing the edge effects budget: bay scallop settlement and loss along a seagrass edge. Ecology. 2012;93(7):1637–47. https://doi.org/10.1890/11-1904.1.
Moore EC, Hovel KA. Relative influence of habitat complexity and proximity to patch edges on seagrass epifaunal communities. Oikos. 2010;119(8):1299–311. https://doi.org/10.1111/j.1600-0706.2009.17909.x.
•• Hanke MH, Posey MH, Alphin TD. The influence of habitat characteristics on intertidal oyster Crassostrea virginica populations. Mar Ecol Prog Ser. 2017;571:121–38. https://doi.org/10.3354/meps12094One of just a few studies that investigated oyster reefs, this paper investigated within patch location and patch size effects on oyster demographic rates at both natural and constructed oyster reefs; they found mixed patterns. Oyster density increased with distance from the patch edge, although recruitment decreased over the same scale. Oyster condition was not affected.
• Macreadie PI, Geraldi NR, Peterson CH. Preference for feeding at habitat edges declines among juvenile blue crabs as oyster reef patchiness increases and predation risk grows. Mar Ecol Prog Ser. 2012;466:145–53. https://doi.org/10.3354/meps09986This paper investigated the role of predator behavior and feeding at habitat edges in artificially created oyster habitats in mesocosms. Even at a small spatial scale of operation, the authors demonstrated that small predators altered their edge habitat use, and thus their predation on prey resources, when their predators were present.
Sheaves M, Johnston R, Baker R. Use of mangroves by fish: new insights from in-forest videos. Mar Ecol Prog Ser. 2016;549:167–82. https://doi.org/10.3354/meps11690The authors investigated fish use of mangroves on incoming tides to determine whether and where they dispersed over time.
Robbins B, Bell S. Seagrass landscapes: a terrestrial approach to the marine subtidal environment. Trends Ecol Evol. 1994;9:301–4.
Levin SA. The problem of pattern and scale in ecology: the Robert H. MacArther Award Lecture. Ecology. 1992;73:1943–67.
Jiang XT, Peng X, Deng GH, Sheng HF, Wang Y, Zhou HW, et al. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb Ecol. 2013;66(1):96–104. https://doi.org/10.1007/s00248-013-0238-8.
• Stubler AD, Jackson LJ, Furman BT, Peterson BJ. Seed Production Patterns in Zostera marina: Effects of Patch Size and Landscape Configuration. Estuar Coasts. 2017;40(2):564–72. https://doi.org/10.1007/s12237-016-0165-2This paper investigated seed production inZostera marinabeds across multiple spatial scales, and found that within patch location did not have an effect on seed production, but rather, production was impacted by the total seagrass cover in the surrounding seascape.
• Langston AK, Kaplan DA, Angelini C. Predation restricts black mangrove (Avicennia germinans) colonization at its northern range limit along Florida's Gulf Coast. Hydrobiologia. 2017;803(1):317–31. https://doi.org/10.1007/s10750-017-3197-0A field survey and manipulative approach to asses location and predation effects on mangrove propagule survival. More soil disturbance and burrowing activity at creek edges, but almost 100% mortality of uncaged propagules regardless of location.
•• Mahoney RD, Kenworthy MD, Geyer JK, Hovel KA, Fodrie FJ. Distribution and relative predation risk of nekton reveal complex edge effects within temperate seagrass habitat. J Exp Mar Biol Ecol. 2018;503:52–9. https://doi.org/10.1016/j.jembe.2018.02.004A combination of mensurative and manipulative approaches were used to assess predation pressure on common mesopredators in seagrass landscapes. They found higher survival in seagrass edges, but no differences in their capture rates (i.e., spatial distribution) or the foraging patterns of an important higher order predator. Interactive effects of seagrass shoot density were also considered.
Ollivier QR, Bramwell NA, Hammill E, Foster-Thorpe C, Booth DJ. Are the effects of adjacent habitat type on seagrass gastropod communities being masked by previous focus on habitat dyads? Aust J Zool. 2015;63(5):357–63. https://doi.org/10.1071/zo15057.
Tuya F, Vanderklift MA, Hyndes GA, Wernberg T, Thomsen MS, Hanson C. Proximity to rocky reefs alters the balance between positive and negative effects on seagrass fauna. Mar Ecol Prog Ser. 2010;405:175–86. https://doi.org/10.3354/meps08516.
Amortegui-Torres V, Taborda-Marin A, Blanco JF. Effect of Neritina virginea (Neritimorpha, Neritinidae) population in a black mangrove stand (Magnoliopsida, Avicenniaceae: Avicennia germinans) in southern Caribbean. Pan-Am J Aquat Sci. 2013;8:68–78.
•• Jurgens LJ, Gaylord B. Edge effects reverse facilitation by a widespread foundation species. Sci Rep. 2016;6. https://doi.org/10.1038/srep37573The authors were able to demonstrate an edge effect on mussels across a small (~12cm) spatial scale but also were able to link this effect directly to a temperature stress gradient.
Nicastro KR, Zardi GI, McQuaid CD, Pearson GA, Serrao EA. Love Thy Neighbour: Group Properties of Gaping Behaviour in Mussel Aggregations. PLoS One. 2012;7(10). https://doi.org/10.1371/journal.pone.0047382.
• Hanke MH, Posey MH, Alphin TD. The effects of intertidal oyster reef habitat characteristics on faunal utilization. Mar Ecol Prog Ser. 2017;581:57–70. https://doi.org/10.3354/meps12261This paper investigated within patch location and patch size effects on oyster reef associated fauna on both natural and constructed reefs and found that spatial patterns varied across fauna, reef size and reef type.
Macreadie PI, Hindell JS, Keough MJ, Jenkins GP, Connolly RM. Resource distribution influences positive edge effects in a seagrass fish. Ecology. 2010;91(7):2013–21. https://doi.org/10.1890/08-1890.1.
Hitt S, Pittman SJ, Nemeth RS. Diel movements of fishes linked to benthic seascape structure in a Caribbean coral reef ecosystem. Mar Ecol Prog Ser. 2011;427:275–91. https://doi.org/10.3354/meps09093.
Hammerschlag N, Serafy JE. Nocturnal fish utilization of a subtropical mangrove-seagrass ecotone. Mar Ecol. 2010;31(2):364–74. https://doi.org/10.1111/j.1439-0485.2009.00337.x.
• Reis JA, Giarrizzo T, Barros F. Tidal migration and cross-habitat movements of fish assemblage within a mangrove ecotone. Mar Biol. 2016, 163(5). https://doi.org/10.1007/s00227-016-2885-zVideo analysis of fish use of mangroves. They found that the mangrove ecotone was used by the entire fish assemblage, but depending on tidal stage, fish migrated to a number of microhabitats.
Nobbs M, Blamires SJ. Spatiotemporal distribution and abundance of mangrove ecosystem engineers: burrowing crabs around canopy gaps. Ecosphere. 2015;6(5). https://doi.org/10.1890/es14-00498.1.
• Rietl AJ, Sorrentino MG, Roberts BJ. Spatial distribution and morphological responses to predation in the salt marsh periwinkle. Ecosphere. 2018;9(6). https://doi.org/10.1002/ecs2.2316A mensurative study of snail density and biomass along 50m transects from marsh edge to interior across multiple sites in Louisiana. They found that snail density tended to be highest around 10m from the edge, whereas biomass was highest 20-30m from the edge.
Coppa S, Guala I, DeLucia GA, Massaro G, Bressan M. Density and distribution patterns of the endangered species Pinna nobilis within a Posidonia oceanica meadow in the Gulf of Oristano (Italy). J Mar Biol Assoc UK. 2010;90:885–94.
Hammerschlag N, Ovando D, Serafy JE. Seasonal diet and feeding habits of juvenile fishes foraging along a subtropical marine ecotone. Aquat Biol. 2010;9(3):279–90. https://doi.org/10.3354/ab00251.
Statton J, Gustin-Craig S, Dixon KW, Kendrick GA. Edge Effects along a Seagrass Margin Result in an Increased Grazing Risk on Posidonia australis Transplants. PLoS One. 2015;10(10). https://doi.org/10.1371/journal.pone.0137778.
Kallen J, Muller H, Franken ML, Crisp A, Stroh C, Pillay D, et al. Seagrass-epifauna relationships in a temperate south African estuary: interplay between patch-size, within-patch location and algal fouling. Estuar Coast Shelf Sci. 2012;113:213–20. https://doi.org/10.1016/j.ecss.2012.08.006.
Murphy HM, Jenkins GP, Hindell JS, Connolly RM. Response of fauna in seagrass to habitat edges, patch attributes and hydrodynamics. Austral Ecol. 2010;35(5):535–43. https://doi.org/10.1111/j.1442-9993.2009.02062.x.
Tuya F, Vanderklift MA, Wernberg T, Thomsen MS. Gradients in the Number of Species at Reef-Seagrass Ecotones Explained by Gradients in Abundance. PLoS One. 2011;6(5). https://doi.org/10.1371/journal.pone.0020190.
Vonk JA, Christianen MJA, Stapel J. Abundance, edge effect, and seasonality of fauna in mixed-species seagrass meadows in Southwest Sulawesi, Indonesia. Mar Biol Res. 2010;6(3):282–91. https://doi.org/10.1080/17451000903233789.
Hammerschlag N, Morgan AB, Serafy JE. Relative predation risk for fishes along a subtropical mangrove-seagrass ecotone. Mar Ecol Prog Ser. 2010;401:259–67. https://doi.org/10.3354/meps08449.
Dolmer P, Christensen HT, Hansen BW, Vismann B. Area-intensive bottom culture of blue mussels Mytilus edulis in a micro-tidal estuary. Aquac Environ Interact. 2012;3(1):81–91. https://doi.org/10.3354/aei00053.
• Gross C, Donoghue C, Pruitt C, Trimble AC, Ruesink JL. Taxonomic and functional assessment of mesopredator diversity across an estuarine habitat mosaic. Ecosphere. 2017;8(4). https://doi.org/10.1002/ecs2.1792A seining survey that found edge habitats to be intermediate to sand and core habitats in terms of mesopredator community structure, with site effects generally exceeding edge effects.
Wong MC, Dowd M. Patterns in taxonomic and functional diversity of macrobenthic invertebrates across seagrass habitats: a case study in Atlantic Canada. Estuar Coasts. 2015;38(6):2323–36. https://doi.org/10.1007/s12237-015-9967-x.
• Gross C, Donoghue C, Pruitt C, Ruesink JL. Habitat use patterns and edge effects across a seagrass-unvegetated ecotone depend on species-specific behaviors and sampling methods. Mar Ecol Prog Ser. 2018;598:21–33. https://doi.org/10.3354/meps12609Seine and video data were used to assess mesopredator abundance and diversity in eelgrass-dominated landscape. Edge effects, relative to core and unvegetated sites, varied by species and sampling method based on available species pools and species-specific behavioral characteristics.
Matias MG, Coleman RA, Hochuli DF, Underwood AJ. Macrofaunal Responses to Edges Are Independent of Habitat-Heterogeneity in Experimental Landscapes. PLoS One. 2013;8(4). https://doi.org/10.1371/journal.pone.0061349.
Knights AM. Spatial variation in body size and reproductive condition of subtidal mussels: considerations for sustainable management. Fish Res. 2012;113:45–54.
Barnes RSK. Are seaward pneumatophore fringes transitional between mangrove and lower-shore system compartments. Mar Environ Res. 2017;125:99–109. https://doi.org/10.1016/j.marenvres.2017.01.008This paper found various macrobenthic assemblages across habitat ecotones, which shows consideration should be given to examining transitions between complex focal and matrix habitats.
Toscano BJ, Griffen BD. Predator size interacts with habitat structure to determine the allometric scaling of the functional response. Oikos. 2013;122(3):454–62. https://doi.org/10.1111/j.1600-0706.2012.20690.x.
Hammerschlag N, Heithaus MR, Serafy JE. Influence of predation risk and food supply on nocturnal fish foraging distributions along a mangrove-seagrass ecotone. Mar Ecol Prog Ser. 2010;414:223–35. https://doi.org/10.3354/meps08731.
Smith TM, Hindell JS, Jenkins GP, Connolly RM, Keough MJ. Edge effects in patchy seagrass landscapes: the role of predation in determining fish distribution. J Exp Mar Biol Ecol. 2011;399(1):8–16. https://doi.org/10.1016/j.jembe.2011.01.010.
Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(305-307) This comment highlights some of the issues with improper interpretation of results based on P values.
Sullivan GM, Feinn R. Using effect size - or why the P value is not enough. J Grad Med Educ. 2012;4:279–82.
• Beninger PG, Boldina I, Katsanevakis S. Stengthening statistical usage in marine ecology. J Exp Mar Biol Ecol. 2012;426–427:97–108 This review provides numerous examples of problems with using traditional statistics and assigning too much weight to P values. In particular, the authors point out the differences between statistical significance and biological relevance, and make suggestions for other methods of data analysis and interpretation.
Furman BT, Jackson LJ, Bricker E, Peterson BJ. Sexual recruitment in Zostera marina: a patch to landscape-scale investigation. Limnol Oceanogr. 2015;60:584–99.
Furman BT, Peterson BJ. Sexual recruitment in Zostera marina: Progress toward a predictive model. PLoS One. 2015;10:e0138206.
Espino F, Gonzalez JA, Haroun R, Tuya F. Abundance and biomass of the parrotfish Sparisoma cretense in seagrass meadows: temporal and spatial differences between seagrass interiors and seagrass adjacent to reefs. Environ Biol Fish. 2015;98(1):121–33. https://doi.org/10.1007/s10641-014-0241-z.
• Carroll JM, Furman BT, Jackson LJ, Hunter EA, Peterson BJ. Propagule risk in a marine foundation species: seascape effects on Zostera marina seed predation. J Ecol. 2019. https://doi.org/10.1111/1365-2745.13154The authors investigated seagrass seed predation across multiple spatial scales, and using structural equation modelling, demonstrated that within patch locations explained a small proportion of the variation in seed predation, with predation being increased at patch centers.
Baillie CJ, Fear JM, Fodrie FJ. Ecotone effects on seagrass and saltmarsh habitat use by juvenile nekton in a temperate estuary. Estuar Coasts. 2015;38(5):1414–30. https://doi.org/10.1007/s12237-014-9898-y.
Arroyave-Rincon A, Amortegui-Torres V, Blanco-Booksellers JF, Taborda-Marin A. Border effect on ble crab population Cardisoma guanhumi (Decapoda:Gecarcinidae) in the mangrove swamp of El Uno Bay, Uraba gulf (Colombia): an approach to its artisanal capture. Biol News. 2014;36:47–57.
Macreadie PI, Hindell JS, Jenkins CN, Connolly RM, Keough MJ. Fish responses to experimental fragmentation of seagrass habitat. Conserv Biol. 2009;23:644–52.
Travaille KL, Salinas-de-Leon P, Bell JJ. Indication of visitor trampling impacts on intertidal seagrass beds in a New Zealand marine reserve. Ocean Coast Manag. 2015;114:145–50. https://doi.org/10.1016/j.ocecoaman.2015.06.002.
Silliman BR, van de Koppel J, McCoy MW, Diller J, Kasozi GN, Earl K, et al. Degradation and resilience in Louisiana salt marshes after the BP-Deepwater horizon oil spill. Proc Natl Acad Sci. 2012;109:11234–9.
Caitano B, Dodonov P, Delabie JHC. Edge, area and anthropization effects on mangrove-dwelling ant communities. Acta Oecol. 2018;91:1–6. https://doi.org/10.1016/j.actao.2018.05.004A field survey approach for arboreal ant communities in fragmenting mangrove forests. They found no effect of distance to edge on ant abundance or community measures.
Acknowledgments
We would like to thank Dr. Kevin Hovel and Dr. Lenore Fahrig for inviting us to participate in this review. In addition, a number of people participated in initial ideas and discussions for this paper, including Dr. Bradley Peterson of Stony Brook University, Dr. Joel Fodrie at the University of North Carolina and Dr. Lauren Yeager at the University of Texas. Finally, we would like to thank Dr. Laura Treible from Georgia Southern University for providing feedback on this manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in determining the scope of this review. J.M.C. was responsible for overall literature searches, summarizing the shellfish literature and writing the initial draft. D.A.K. was responsible for summarizing the overall literature in terms of patterns and processes. Both B.T.F. and A.D.S. were involved in reviewing relevant literature in their study areas, and summarizing issues in scaling. All authors made significant contributions to the subsequent drafts and have given their final approval for publication.
Corresponding author
Ethics declarations
Conflict of Interest
John Carroll, Bradley Furman, Danielle Keller and Amber Stubler declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Landscape Ecology of Aquatic Systems
Electronic supplementary material
ESM 1
(DOCX 150 kb)
Rights and permissions
About this article
Cite this article
Carroll, J.M., Keller, D.A., Furman, B.T. et al. Rough Around the Edges: Lessons Learned and Future Directions in Marine Edge Effects Studies. Curr Landscape Ecol Rep 4, 91–102 (2019). https://doi.org/10.1007/s40823-019-00043-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40823-019-00043-7