Abstract
While considering important juvenile fish habitats individually, both seagrass and saltmarsh are often highly connected with other subtidal and intertidal habitats. As a result, juvenile fishes and crustaceans may utilize multiple habitats across tidal, diel, or seasonal cycles in a manner that makes interhabitat proximity an important driver of fish distribution and community composition. In this context, we examined the importance of seagrass (Zostera marina and Halodule wrightii) and saltmarsh (Spartina alterniflora) habitat characteristics in driving fish and crustacean catch rates and community composition in a temperate, polyhaline-euhaline, estuary. We found that habitats with highly connected seagrass and saltmarsh vegetation exhibited higher average catch rates of many recreationally and commercially valuable fish and crustacean species, as well as overall nekton catch rates and Shannon diversity (H), than habitats composed of either seagrass or saltmarsh habitat alone. Nekton-habitat associations varied temporally, showing strong seasonal trends which were potentially indicative of temporal shifts in relative habitat value. Catch rates of numerous recreationally and commercially targeted species were correlated with patch-scale variables, particularly seagrass canopy height, water temperature, and depth; however, regression analysis indicated that habitat type was more powerful in predicting overall nekton catch rates and Shannon diversity (H). We conclude that emergent properties (i.e., those operating at 10–100s m) are important drivers of nekton distributions among and within habitats. Considering the spatial and temporal scales at which humans are encroaching on estuarine ecosystems, our findings highlight the need for investigating organism-habitat associations at expanded spatial scales, as well as the need to adopt fishery and coastal management plans that consider habitat characteristics at multiple spatial scales to account for interhabitat connectivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuarine landscapes are generally composed of spatially heterogeneous but functionally connected habitat types. The abundance and distribution of organisms within a given habitat are thought to be inextricably linked to habitat structure at multiple spatial scales (Wiens 1989; Bell et al. 1991; Levin 1992). At small spatial scales (<10 m2), numerous studies have demonstrated the effects of within-patch habitat structural complexity, such as seagrass shoot density, epiphytic algal cover, and canopy height, on catch rates of marine organisms inhabiting these habitats (Orth and Heck 1980; Bell and Westoby 1986a; Worthington et al. 1991; Irlandi 1994). For example, Hovel et al. (2002) found that seagrass shoot biomass and relative wave exposure were the environmental factors exerting the greatest influence on invertebrate densities within seagrass beds in a temperate estuary. At broader scales, landscape ecology—functionally defined as the relationship between ecological function and spatial patterns—has received increased attention as scientists and managers have recognized the importance of environmental variables at expanded spatial scales on organism-habitat associations (Hovel et al. 2002; Grober-Dunsmore et al. 2004; Tanner 2006; Dorenbosch et al. 2007). As our understanding of the capacity for human activities to fragment, degrade, or destroy marine habitats increases, it seems likely that understanding the influence of processes at broad spatial scales will become more germane to future fishery management and conservation efforts.
Investigating organism-habitat associations at multiple spatial scales is crucial for ecosystem-based management plans given that multiscale approaches are likely necessary to adequately develop holistic understanding of fishery species’ niches (Sandel and Smith 2009, but see Chittaro 2004; Yeager et al. 2011). Studies focusing on landscape-scale processes have demonstrated the important role of habitat heterogeneity across 10–100 s of meters in maintaining species diversity and augmenting species abundance through modification of predator-prey interactions and impacts on growth rates as a result of different bioenergetic benefit-cost ratios associated with resource acquisition (Parrish 1989; Danielson 1991; Irlandi et al. 1995). For instance, fishes moving between mangrove and adjacent seagrass habitat will incur lower predation and smaller energetic expenditure than fishes moving across unstructured benthic habitat separating mangrove forests and seagrass meadows (Sheaves 2005). Indeed, studies in both terrestrial and marine environments have underscored the influence of habitat adjacency and configuration on community composition and organism movement (Belisle and Desrochers 2002; Robertson and Radford 2009; Ryan et al. 2012). Consequently, species distributions are often fundamentally different as a result of habitat context. As such, resource management would benefit from a more comprehensive understanding of how species abundance and nekton assemblage structure are impacted by the composition and configuration of habitat mosaics. To date, the significance of landscape and habitat-scale factors in structuring fish assemblages has been well documented in tropical ecosystems (Nagelkerken et al. 2002; Dorenbosch et al. 2004; Mumby 2006; Grober-Dunsmore et al. 2007, 2009), but less is known about how factors at broader scales influence faunal assemblages in temperate estuarine systems (but see Hovel et al. 2002; Boström et al. 2006).
Seagrass meadows, saltmarshes, tidal creeks, mudflats, and oyster reefs are the dominant shallow water benthic habitats in temperature estuaries. Structurally complex estuarine habitats are generally recognized as important for many of the world’s valuable fishery species largely because of their potential to increase the survival and growth of fishes and crustaceans during vulnerable early life stages compared to unstructured bottom (Boesch and Turner 1984; Heck and Thoman 1984; Hemminga and Duarte 2000; Beck et al. 2001; Stunz et al. 2001; Minello et al. 2003), although these effects are likely species dependent and spatially variable (Phelan et al. 2000; Elliott and Hemingway 2008). Factors at multiple spatial scales; however, mediate the value of benthic habitats as nurseries for estuarine-dependent fishes. At both patch and landscape scales, factors such as prey availability, foraging efficiency, and refuge from predators can vary widely according to species morphology and life history characteristics (Heck and Thoman 1984; Werner and Gilliam 1984; Levin 1994; Camp et al. 2011; Froeschke and Stunz 2012; Tait and Hovel 2012). For instance, Yeager et al. (2011) found that in subtropical systems, amount of seagrass at both patch and landscape scales was positively correlated with fish abundance within restored patch reefs; however, amount of seagrass at the landscape scale was the most important variable influencing differences in fish abundance. As many of the bottlenecks that are thought to regulate fish population size occur during early life stages, it is particularly critical to determine what constitutes high-value habitat for juveniles of fishery-targeted species (Kennedy et al. 2008).
Seagrass and saltmarsh habitats are often highly connected with each other or alternative estuarine habitats. As a result, individuals or species may utilize multiple habitats across tidal, diel, or seasonal cycles in a manner that makes habitat connectivity an important driver of fish distributions and community assemblages. Habitats that include ecotones, or habitat transition zones, may have appreciably different ecological processes and communities than interior habitat as some species and life stages may rely on edge habitat while others may preferentially utilize interior habitat (Yahner 1988; Fagan et al. 1999). For instance, pinfish (Lagodon rhomboides) were shown to be more than twice as abundant in intertidal marshes with adjacent seagrass beds than marsh adjacent to unvegetated bottom (Irlandi and Crawford 1997). Similarly, the presence of seagrass between oyster reef and saltmarsh habitat was shown to strongly influence macroinvertebrate abundance on oyster reefs due to seagrass acting as a nighttime corridor for predatory blue crabs (Callinectes sapidus) between marsh and reef patches (Micheli and Peterson 1999). These findings highlight the role of habitat composition and configuration in evaluating the functional role of seagrass-saltmarsh habitats for fishes and crustaceans.
The main objective of the present study was to examine how saltmarsh and seagrass habitat setting and abiotic characteristics influence juvenile fish abundance and community composition in a temperate estuary. A further purpose was to investigate whether structuring factors at patch (i.e., seagrass shoot density or canopy height and depth) or habitat scale (i.e., habitat composition and configuration at the 100 s m2 scale) had greater influence on fish abundance and community composition. Specifically, we asked the following questions regarding habitat utilization in shallow water estuarine habitats: (1) how do habitat composition and configuration affect nekton community characteristics and catch rates of individual nekton species, (2) what are the biotic (seagrass shoot density, canopy height) and abiotic (temperature, salinity, depth) factors that correlate with the observed catch rates of key species within and among habitats, and (3) are study-wide habitat effects on species catch rates and community composition temporally variable?
Methods
Study Area
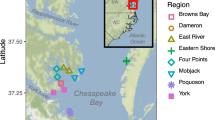
We surveyed fish communities within two sites in Back and Core Sounds, North Carolina, USA (Fig. 1). The first site, Middle Marsh, was located within the Rachel Carson component of the North Carolina National Estuarine Research Reserve (RCNERR), in Back Sound near Beaufort Inlet (Fig. 1). The second site, henceforth referred to as Core Sound, is approximately 10 km east of Middle Marsh in the southern extent of Core Sound, a 35-km water body oriented in a northeast-southwest direction (Fig. 1). Mean monthly salinity levels were ca. 32–34 ‰, and the tidal range was ca. 1 m at both of these sites. These locations were selected because they represent a range of environmental conditions (wave exposure and fetch direction, salinity, vegetation patchiness, etc.) and contained our target habitats with multiple ecotone alternatives represented for seagrass and saltmarsh areas. Within each site, expanding on the work by Irlandi and Crawford (1997), we identified multiple habitat types: (1) mudflat (MF), unvegetated sandy bottom absent of appreciable shell or drift algae spatially isolated from any vegetated habitat by greater than 200 m; (2) seagrass meadow (SG), which were composed of eelgrass Zostera marina, shoal grass Halodule wrightii, or of mixed composition, adjacent to unvegetated bottom and separated from any saltmarsh habitat by at least 200 m; (3) seagrass-saltmarsh interface (I) the interface between fringing Spartina alternaflora saltmarsh and an immediately adjacent seagrass meadow, consisting of Z. marina, H. wrightii, or of mixed composition, and not situated within a tidal creek; and (4) saltmarsh creek (SM), S. alternaflora bordered tidal creek with unvegetated sand or mud bottom absent of appreciable shell or drift algae. At the Core Sound site, we also sampled the following: (5) vegetated saltmarsh creek (MX), S. alternaflora bordered tidal creek with Z. marina, H. wrightii, or mixed seagrass composition bottom. For both SM and MX habitats, creeks were approximately 75 to 125 m wide, and sampling was conducted at least 100 m from the creek mouth. For each habitat type, we identified 3 replicate stations yielding 12 sampling stations (4 habitats × 3 replicate stations) in Middle Marsh and 15 sampling stations (5 habitats × 3 replicate stations) in Core Sound.
Study locations (left graph): Middle Marsh (top right graph), located in Back Sound, and southern Core Sound (bottom right graph), USA. Symbols indicate trawl locations, with filled triangles indicating mudflat (MF), open triangles representing isolated seagrass meadows (SG), filled circles indicating seagrass-saltmarsh interface (I), filled squares indicating vegetated saltmarsh creeks (MX), and open circles indicating unvegetated saltmarsh creeks (SM)
Sampling Methods
To determine the relative catch rates of fishes and decapod crustaceans within and among habitats, we conducted monthly surveys during daylight hours between July and November 2010, May and November of 2011, and May and June of 2012. This corresponded with the periods when most winter and spring spawned fishes inhabit shallow water estuarine habitats. Organisms were collected in all habitats using a 5-m otter trawl (15-m head rope, 2.0-cm body mesh, 0.6-cm cod end mesh, 0.3 × 0.7-m doors) with a 4-seam balloon design, with floating and lead lines but without a tickler chain. Otter trawling permits sampling of large areas and was shown to be an effective method of quantifying catch rates of both abundant and rare fishes in North Carolina seagrass beds (Petrik and Levin 2000). Owing to the reduced catch efficiency of otter trawls over saltmarsh vegetation (Zimmerman et al. 1985), sampling in saltmarsh creeks (SM and MX habitats) was conducted immediately adjacent to the edge of saltmarsh vegetation. Furthermore, we attempted to mitigate gear performance issues associated with erratic trawl flight by towing at speeds shown to maintain trawl mouth diameter and contact with bottom, as well as by visually monitoring the trawl during tows for indications of erratic behavior (Wathne 1977; Gibbs and Matthews 1982). At each station during each month, two trawls lasting 1.89 ± 0.02 min (mean ± 1 SE) were conducted behind a small (∼7 m) research vessel at a speed of 3.33 ± 0.02 km h−1. Sampling was conducted within 2 h of high tide with typical water depths >1.25 m (range 0.5–3.5 m). All organisms captured (Table 1) were enumerated and weighed to the nearest 1 g and released. Any unidentified specimens were euthanized by means of rapid cooling (IACUC ID 10-133.0-B) and returned to the lab for meristic identification by at least two technicians.
During each tow, we recorded salinity (‰), temperature (°C), and depth (m) using a Brix handheld analog refractometer, a Dwyer W-10 digital thermometer, and a sounding rod marked in 0.25-m increments, respectively. Depth measurements were taken at the inception of each tow and, due to the absence of appreciable sills or slopes at tow locations, were representative of the depth across the entire tow path. At each sampling station, mean seagrass shoot density was measured in June, 2012 by averaging shoot counts from three randomly placed 0.01-m2 quadrats. Within the same quadrats, we also measured the lengths (m) of up to 10 (if present) individual seagrass plants to determine average canopy height. Habitat characteristics and sampling effort are listed in Table 2. Overall, 741 individual, 111 ± 1-m-long tows were conducted covering a total linear distance of 82,710 m.
Data Analyses
Catch data were standardized to catch-per-unit-effort (fishes 100 m towed−1) and used for all subsequent analyses. Although the primary focus of our research was to examine the influence of habitat and ecotone types on fish community composition and species catch rates, we also considered patch-level biotic factors, abiotic factors, site and temporal factors our analyses to gauge the relative importance of factors at multiple spatial scales on the abundance of fishes and crustaceans. In our ensemble analysis, linear mixed effects models were used to analyze the effect of categorical independent variables on continuous response variables. More specifically, this included the effect of habitat/ecotone (MF, SG, I, MX, SM), site (Middle Marsh, Core Sound), and month (May, June, July, August, September, October, November) on overall catch rates, Shannon diversity index (H), and catch rates of numerically dominant and economically important species (e.g., catch rates of each fish species). We used R (R Core Team 2011) and nlme (Pinheiro et al. 2007) to perform linear mixed effects analyses for the relationship between response variables and independent variables. For our analysis of the effect of habitat type on catch rates and diversity, we entered habitat and site measured over time as fixed effects. Station was entered as a random effect nested within site. To investigate the temporal variability of catch rates among habitat types, habitat and month were entered as fixed effects, and station was entered as a random effect. To address the potential biases associated with temporal autocorrelation in times series data, our mixed effects models incorporated an autocorrelation structure with a continuous time covariate, function corCAR1 (Box et al. 2013). Post hoc multiple comparison analyses for fixed effects was conducted using the multcomp procedure which conducts simultaneous tests and confidence intervals for linear mixed effects models while mitigating issues associated with multiplicity by employing exact multivariate t distribution or asymptotic multivariate normal distribution (Hothorn et al. 2008; Bretz et al. 2010). Regression analyses were used to test for relationships between biotic (mean Z. marina density, mean H. wrightii density, mean canopy height) or abiotic (salinity, depth, temperature) factors and overall catch rates, Shannon diversity index (H), and catch rates of numerically dominant and commercially or recreationally targeted species. Analyses of the relationship between catch rates and seagrass characteristics were limited to the month of June 2012. Data were tested for normality with Shapiro-Wilk test prior to regression analyses. Normally distributed data were analyzed using Pearson correlation, and those that failed to meet the assumption of normality were analyzed using Spearman’s rank order correlation.
We used regression tree analyses to evaluate which factors were most powerful in determining overall nekton catch rates and Shannon diversity index (H). Regression trees explain variation of a single response variable using combinations of explanatory variables to repeatedly split data into increasingly homogenous groups and are increasingly being used as a valuable tool for analyzing ecological data (De’ath and Fabricius 2000; Sheaves 2006). In particular, regression trees have been shown to be an effective method for predicting species richness in shallow water seascapes (Pittman et al. 2007). Trees were grown using recursive partitioning, and over fitted trees were pruned using k-fold cross-validation. Cross-validation estimates the predicted error for trees of each size, and the tree with the lowest cross-validation predicted error is considered the optimal tree. Regression tree analyses were of particular importance in determining the spatial scale (and associated factors) which had the greatest effect on fish catch rates and species richness patterns, i.e., do patch scale, habitat scale, or regional scale (site) factors have the greatest influence on assemblage characteristics? The regression tree analyses for both total catch rates and Shannon index (run separately) considered seven independent variables: year, month, site (Middle Marsh or Core Sound), habitat, temperature, salinity, and depth. Temperature, salinity, and depth measurements were incorporated as individual measurements taken at the time of each tow. Separate regression tree analyses were conducted for June 2012 data catch data only that included seagrass characteristic variables. All regression tree analyses were run using R (R Core Team 2011) and rpart (Therneau et al. 2010).
Results
A total of 139,663 fishes and decapod crustaceans representing at least 71 species were captured during the course of this study. Pinfish (Lagodon rhombiodes) were by far the numerically dominant species, comprising 82 % of all individuals captured. The ten most abundant species accounted for greater than 98 % of our total catch (Table 2). There was a significant interaction between month and habitat on total fish catch rates (F 4,728 = 7.48, P < 0.0001). In the late spring and early summer (May, June, July pooled among years), all vegetated habitats had relatively high catch rates of nekton; however, seagrass (SG) showed an increasing trend in total catch rates while total catch rates in saltmarsh (SM) habitat decreased significantly between May and June before plateauing until the fall (Fig. 2). In late summer (August, September), fish catch rates began to decline in MF, SG, and I habitats, while catch rates in MX and SM habitats remained relatively stable (Fig. 2). Fish catch rates were relatively low across all habitats by October in both sampling years (Fig. 2).
Total catch rates were found to be significantly different among habitats (F 4,715 = 43.43, P < 0.001, Fig. 3, Tables 3 and 5). All vegetated habitats had significantly higher fish catch rates than mudflat sites by nearly an order of magnitude (Fig. 3). Overall, fish catch rates were significantly higher at I, MX, and SG habitats, with an average of 348.1, 276.2, and 202.4 fish 100 m towed−1, respectively, than at SM and MF habitats, which averaged 95.2 and 11.7 fish 100 m towed−1, respectively (Fig. 3). Among the habitats of highest catch rates, I habitat stations had significantly higher fish catch rates than SG habitat stations; however, neither was significantly different from MX habitat stations (Fig. 3). There was also a significant effect of habitat on mean Shannon diversity index (H) (F 4,715 = 84.16, P < 0.001, Table 5). Mean diversity (H) was significantly higher at MX, I, and SG stations, with an average of 0.73, 0.66 and 0.64, respectively, than that at SM stations (Tukey’s HSD, P < 0.05), which had an average Shannon diversity index of 0.45. These results suggest that habitats containing saltmarsh vegetation alone are characterized by lower diversity than habitats containing seagrass—either isolated or connected to saltmarsh habitat. Average Shannon diversity at MF sites was significantly lower than all other habitats with an average value of 0.14 (Tukey’s HSD, P < 0.05).
Habitat type significantly influenced the catch rates of many numerically dominant and economically valuable species. Lane snapper catch rates were significantly higher at SG stations than at any other habitat (F 4,715 = 6.02, P < 0.001, Fig. 4h, Table 3), by greater than an order of magnitude. Gag grouper catch rates were significantly higher at I habitat stations, by 390 %, than any other habitat sampled (F 4,715 = 14.86, P < 0.001, Fig. 4i, Table 3). Penaeid shrimp, gray snapper, and speckled trout catch rates were all significantly higher, by 240, 338, and 200 %, respectively, at MX stations than at any other habitat type (Fig. 4b, g, k, Table 3). Unvegetated saltmarsh creek (SM) and mudflat (MF) habitats were not characterized by significantly higher catch rates of any numerically dominant or economically important fish or crustacean species.
Average catch rate of numerically dominant and economically valuable juvenile a sheepshead, b blue crab, c spotted sea trout, d flounder, epinfish, f spot, g gray snapper, h lane snapper, i gag grouper, j penaeid shrimp, k pigfish, and l red drum among landscapes. MF mudflat, SG seagrass meadow, I seagrass-saltmarsh interface. MX saltmarsh creek with seagrass, SM saltmarsh creek without seagrass. Colored bars are Middle Marsh sites, and open bars are Core Sound sites. Data are shown as means ± 1 standard error. Different letters indicate significant differences from linear mixed effects models (Tukey, P < 0.01) among habitats (with Middle Marsh and Core Sound data pooled)
As expected, overall catch rates varied significantly throughout the year (F 1,736 = 45.48, P < 0.001, Table 4), with the highest catch rates in June, when fish catch rates were 2,071 % higher than catch rates in November, the month with the lowest catch rates. Pinfish catch rates strongly tracked overall monthly CPUE due to their numerical dominance, but we noted appreciable seasonal variability among other numerically dominant and economically valuable species (Table 4, Fig. 4a). The highest catch rates of pigfish, spot, penaeid shrimp, blue crab, gag grouper, sheepshead, and flounders (95 % gulf flounder Paralichthys albigutta based on continuing analysis) were observed in late spring and early summer (May, June, July) months (Table 4). Gray snapper, lane snapper, spotted sea trout, and red drum catch rates were highest in late summer and early fall (September, October, November) months (Table 4). Average diversity (H) was also significantly different among months, with the highest average diversity observed in July and the lowest average diversity observed in November (F 1,736 = 49.36, P = <0.0001, Table 4). Despite seasonal patterns, habitat remained a consistently important driver of catch rates across months.
Mean Z. marina density, H. wrightii density, and seagrass canopy height were all positively correlated with both overall nekton catch rate and Shannon diversity index (H) in June 2012 (Table 6). Among numerically dominant and targeted species, mean canopy height was the biotic factor which influences the catch rates of the greatest number of species; blue crab, penaeid shrimp, pinfish, spot, gag grouper, pigfish, and flounder catch rates were all positively correlated with mean canopy height in June 2012 (Table 5). We found a significant, positive correlation between Z. marina shoot density and catch rates of pinfish, pigfish, and flounders (Table 5). Only one species, pinfish, exhibited a positive relationship between catch rates and H. wrightii shoot density (Table 5). Catch rates of spotted sea trout, lane snapper, and gray snapper were too low in June 2012 to permit analysis of correlation with seagrass characteristics (Table 6). Temperature was found to be positively correlated with the catch rates of sheepshead, pinfish, spot, gag grouper, pigfish, flounders, overall catch rates, and Shannon diversity index and negatively correlated with catch rates of red drum (Table 5). Salinity was correlated with catch rates of sheepshead, pinfish, spot, pigfish, flounders, overall catch rates, and Shannon diversity index, all of which tended to have higher abundances in euhaline than polyhaline conditions. Blue crab, penaeid shrimp, pinfish, pigfish, spot, gray snapper, lane snapper, flounders, and overall catch rates as well as Shannon diversity index were negatively correlated with depth (Table 5).
Regression tree analyses were employed to determine the relative importance of spatial (site, habitat), temporal (year, month), and abiotic (depth, salinity, temperature) variables in determining overall nekton catch rates and Shannon diversity index (H). Regression tree analysis revealed that habitat type was the most powerful factor influencing overall fish and crustacean catch rate, partitioning SG, I, and MX into one node with significantly higher organism catch rates (275.3 fishes 100 m towed−1) than the node containing MF and SM (54.3 fishes 100 m towed−1) (Fig. 5). Further partitioning of the lower catch rate node revealed that habitat was again the most powerful explanatory factor influencing overall catch rates; SM habitat have significantly higher fish and crustacean catch rates (95.2 fishes 100 m towed−1) than MF habitat (11.7 fishes 100 m towed−1) (Fig. 5). Regression tree analysis revealed that habitat type was also the most powerful predictor of Shannon diversity index. In contrast with overall catch rate patterns, SM habitat was grouped with all other vegetated habitats (mean H = 0.60), and MF (mean H = 0.14) occupied a node by itself (Fig. 6). Within the lower diversity, MF node, the most powerful splitting factor was year, with 2010 (mean H = 0.27) having significantly higher Shannon diversity than 2011 or 2012 (mean H = 0.07) (Fig. 6). Within the higher diversity, vegetated habitat node, the most powerful splitting factor was month, with May, June, July, August, and September occupying a higher diversity (mean H = 0.75) node, and October and November occupying a lower diversity (mean H = 0.28) node.
Discussion
Regression tree analyses revealed that habitat type (with distinct ecotones) was an overarching factor influencing assemblages of fishes and crustaceans in our study region. While seagrass and saltmarsh have commonly been investigated separately as potential nursery habitats, the setting (i.e., interhabitat adjacency and configuration) of these habitats affected the catch rates of several ecologically dominant or economically valuable species. If these patterns correlate with higher survival and ultimately greater fish productivity, conservation efforts that overlook the broader “landscape” context within which a seagrass bed or saltmarsh is situated may fail to maximize their efficacy as essential fish habitat. Habitats composed of either seagrass (SG) or emergent saltmarsh vegetation (SM) both exhibited higher catch rates of fishes and crustaceans than unstructured bottoms (MF). That said, ecotone habitats composed of abutting seagrass and saltmarsh vegetation (I and MX) exhibited higher catch rates of many fishery species than habitats that lacked seagrass-saltmarsh boundaries. For instance, the presence of seagrass within tidal creek (MX) sites was correlated with significantly higher overall catch rates, diversity (H), and catch rates of fishery-targeted species than that in marsh creek sites without seagrass (SM). Notably, these results contrast previous experimental studies that determined that complex estuarine habitats such as seagrass, oyster reef, and saltmarsh were functionally redundant as juvenile fish habitat. In particular, experimentally restored oyster reefs (≤15 m2) placed adjacent to vegetated habitats did not augment juvenile fish catch rates over vegetated landscapes without reef habitats (Grabowski et al. 2005; Geraldi et al. 2009). Our data, collected within much larger habitat patches (>100 s m2), suggest that structured estuarine habitats are not necessarily functionally redundant when adjacent to each other. Rather, heterogeneous habitats with both seagrass and saltmarsh habitat outperformed more homogeneous habitats (even those that offer juvenile fish structure) as juvenile habitats. A potential explanation for this difference among studies is that previous oyster-reef restoration projects have occurred at patch scales (10–15 m2), while our survey study considered habitat context at much larger scales (100 s m2).
Although saltmarsh platforms are only accessible to fishes during a portion of the tidal cycle, species accessing saltmarsh habitat consume greater quantities of food and greater prey diversity than species without access to saltmarsh habitat (West and Zedler 2000; Hollingsworth and Connolly 2006). Fishes must balance the benefit of additional prey resources with refuge needs during saltmarsh emergence at low tide when predation intensity is high in tidal creeks as a result of concentration of predators and prey (Kneib 1984, 1987). As such, in habitat with a seagrass and saltmarsh ecotone, organisms may exploit the resources of both habitats without the need to traverse bare subtidal substrate, where predation risk may be elevated (Micheli and Peterson 1999). Hence, habitats with proximate seagrass and saltmarsh vegetation may reduce mortality and enhance growth for resident, mobile nekton (Irlandi and Crawford 1997). At the population and assemblage levels, these fitness advantages could translate in to elevated species catch rates as we observed in this study for a diverse assemblage of ecologically and economically important species.
Our results suggest that local habitat-scale attributes or processes may contribute to elevated catch rates of fishes and crustaceans, Shannon diversity indices, and catch rates of fishery target species in habitats with adjacent seagrass and saltmarsh vegetation. Specifically, that ecotone type in similar settings (i.e., saltmarsh-seagrass vs saltmarsh-sand within tidal creeks) may influence species abundance, community structure, and productivity. In our study, marsh creeks with seagrass (MX) had significantly higher catch rates of numerous commercially and recreationally important fish and crustacean species compared to marsh creek stations without seagrass (SM). Additionally, we found significantly higher total nekton catch rate and diversity in tidal creeks containing seagrass, indicative of interhabitat synergies. At stations with seagrass beds adjacent to fringing saltmarsh (I) total fish catch rate and catch rates of flounder species, gag grouper and pinfish were significantly greater than at isolated seagrass bed stations (SG), but the inverse was true for lane snapper and pigfish, a result potentially indicative of differences in species-specific mortality, cross-habitat subsidies, or movement at ecotones (Fagan et al. 1999).
Our work also demonstrates the potential for regulation of fish-habitat linkages across multiple spatial scales. Among vegetated habitats, for instance, stations with greater canopy height tended to have significantly higher diversity, overall fish catch rates, and higher catch rates of many economically valuable fish and crustacean species. While it has been theorized that increased canopy heights result in greater microhabitat and higher food availability (Connolly and Butler 1996), previous empirical studies have not necessarily supported this premise (Bell and Westoby 1986b; Connolly 1994). For the month, we sampled seagrass characteristics (June 2012); our data suggests that canopy height was an important factor influencing nekton catch rates, but further work is needed to examine whether this effect is temporally variable as well as the mechanism driving this positive relationship in our system. Seagrass shoot density influenced catch rates of fewer species of interest than expected in our study, as increasing structural complexity is generally observed to positively correlate with species catch rates at smaller spatial scales (Bell and Westoby 1986b), although we recognize our tows integrated fish distributions over 100 s of m2. We did note that Z. marina shoot counts in our surveys were significantly positively correlated with catch rates of pinfish, pigfish, and flounder which may benefit from reduced predation and/or greater prey availability in more structurally complex habitats. Other studies in North Carolina and the Chesapeake Bay have also found that juvenile flounder occupy shallow water Z. marina beds, particularly during spring and summer months when these habitats attract their prey (Adams 1976a, b; Lascara 1981). Pinfish catch rates were also significantly positively correlated with H. wrightii shoot density, and due to their numerical dominance likely resulted in overall catch rate being correlated with increased shoot density of both seagrass species. Stoner (1982) found that pinfish consumption of amphipods was higher in H. wrightii than other seagrass species. Furthermore, Crowder and Cooper (1982) postulated that prey densities should be highest at intermediate levels of habitat complexity where the balance between opportunities to forage and refuge from predators is maximized. In this vein, it seems plausible that the Z. marina, H. wrightii, and mixed composition seagrass beds we sampled that were characterized by higher shoot density may have provided greater feeding opportunities and refuge to pinfish, while generally remaining below the vegetation biomass density threshold at which feeding rates are strongly negatively impacted (Luczkovich 1988).
Over broad seasonal/regional scales, a majority of numerically dominant and economically valuable species catch rates were positively correlated with temperature. Whether these temporal trends are directly influenced by temperature or are the result of seagrass seasonality, availability of prey or some other factor merits further investigation. As many of these species (e.g., pinfish, spot, pigfish, flounder) are continental shelf spawners with peak larval immigration into estuaries during in the early months of the year (Taylor et al. 2009), the positive correlation with temperature may reflect increased catchability as individuals grew during the spring and summer and before moving to deeper water habitats as waters cool in the fall (Warlen and Burke 1990). Nearshore and estuarine spawners, such as blue crabs and penaeid shrimp species, exhibited higher catch rates earlier in the year than continental shelf spawners (van Montfrans et al. 1995). Previous work has shown that transformational stage snapper ingress much later in the year at Beaufort Inlet, between July and October, peaking in September, matching our observations that snapper catch rates peak in the early fall (Tzeng et al. 2003). Red drum and spotted sea trout spawn near inlets to estuaries in the late summer and early fall before dispersing to lower salinity habitats, which also matches our findings that peak abundances of these occur in the fall months, resulting in a significant negative correlation between red drum catch rates and temperature (Stewart and Scharf 2008).
The negative correlation between depth and catch rates of many numerically dominant or economically valuable species, overall catch rates, and diversity may have been the result of increased risk of predation in deeper areas (Ruiz et al. 1993; Miltner et al. 1995). For example, mortality rates of tethered daggerblade grass shrimp, killifish, and juvenile blue crabs were significantly higher in the Chesapeake Bay estuary in water depths over 70 cm, where large predators such as spot, large blue crabs, and Atlantic croaker are more abundant (Ruiz et al. 1993). It is plausible that reduced catch rates in deeper waters may have been influenced by reduced gear efficiency with increasing depth, but efforts were made to mitigate factors associated with the erratic trawl characteristics responsible for variability in towed net performance (see Methods section).
Although patch-level characteristics, particularly fine-scale plant structure, were shown to be significantly correlated with catch rates of numerous recreationally and commercially important species, regression tree analyses for June 2012 (not shown) revealed that habitat type was a more powerful predictor of overall catch rates and diversity than factors at patch-level scales. Over the course of our entire study, habitat type proved to be a more powerful predictor of overall catch rate and diversity than any abiotic factor or site differences (Middle Marsh vs Core Sound), underscoring that habitat scale effect persist across regional scales (>10 s of kilometers). Considering the spatial and temporal scale at which human encroachment on marine environments continues to take place, future application of landscape ecology approaches to investigate marine organism-habitat associations will likely be critical for successful ecosystem-based management.
We acknowledge that our trawling methodology, like all sampling approaches, is defined by potential biases that should be accounted for in interpreting survey results. First, our findings represent an assessment of nekton abundance during the higher end of the tidal range during the daytime. It is expected that community composition and species abundances vary across tidal and diel cycles. Higher densities of certain estuarine species have been observed in vegetated habitats at low tide due to the aggregating effect of receding waters; however, other nekton species may recede to adjacent deeper channels resulting in lower abundances in shallow water habitats, or in the case of intertidal areas, complete absence of fishes at low tide (Hettler 1989; Blaber et al. 1995; Marshall and Elliott 1998; Morrison et al. 2002). Similarly, studies comparing daytime and nighttime distribution of nekton have demonstrated that, in general, more species or higher densities of nekton are caught at night than during the day in seagrass habitats (Greening and Livingston 1982; Gray et al. 1998). Within species, larger individuals that reside in deeper channels during the day will often move to shallower habitats at night to feed on fishes and benthic organisms (Gray et al. 1998). Among species, diel differences in densities may result from species-specific habitat use decisions based on weighing the benefits of increasing prey availability at night against increased likelihood of encounters with piscivorous predators feeding in shallow water habitats (Mattila et al. 1999; Guest et al. 2003; Hammerschlag and Serafy 2010; Becker et al. 2011). For example, juvenile gray snapper has been observed to leave mangrove forests and bordering seagrass beds for deeper water habitats at sunset, likely as a result of increased predation risk during nighttime (Luo et al. 2009). Second, it is also likely that patterns of species abundance and community composition across habitats and ecotones were affected by the reduced effectiveness of towed nets in structurally complex habitats, such as seagrass and saltmarsh vegetation (Rozas and Minello 1997). If our results were indeed influenced by reduced catch efficiency in complex habitats, the findings of the study presented here would represent conservative estimates of differences among habitats in species abundances. Our observation that numerous species were positively correlated with shoot density and canopy height suggests that it is unlikely for reduced gear efficiency in more complex habitats to have been a serious limitation with our sampling approach. Third, we acknowledge some larger or highly mobile individuals (e.g., sciaenids) capable of eluding capture, as well as small cryptic species (e.g., gobiids) occupying burrows or able to pass through the trawl may have been undersampled. Due to these inherent gear limitations, our data must be viewed as approximations of species abundances across habitats.
Our findings highlight the importance of heterogeneity at both patch and ecotone scales in structuring faunal communities in seagrass and saltmarsh habitat in temperate, high-salinity estuarine waters. Additionally, they underscore the need to consider marine habitats as mosaics of interconnected habitats that interact dynamically as a function of their broader context, rather than discreet entities, and may have critical implications for management and conservation efforts, especially as managers begin to incorporate ecosystem-based approaches into fishery management plans and designation of protected areas (Beck et al. 2001). That habitat type was a more powerful predictor of juvenile species catch rate, total catch rate, and diversity than patch-level characteristics suggests that relative habitat value for juvenile fishes and crustaceans is likely influenced by processes at the scale of 100 s of meters and that greater focus on the mechanisms underlying observed habitat effects is merited. We suggest that effective coastal management requires consideration of the matrix of neighboring habitats as well as ecotone characteristics in habitat suitability on both overall community composition as well as a species-by-species basis (Tanner 2006). Central to the arguments surrounding the nursery role hypothesis debate is whether elevated juvenile abundances translate to increased production of adults, a methodologically difficult issue to address, but, until such a time as fishery research can definitively answer this question, prioritizing areas of high juvenile abundance for protection and restoration seems a sensible approach (Beck et al. 2001; Fodrie and Levin 2008). Furthermore, as estuarine habitats continue to be imperiled by human intervention, there is a very real need for increased attention to how species catch rates may be further impacted by habitat loss and fragmentation (Lotze et al. 2006).
References
Adams, S.M. 1976a. The ecology of eelgrass, Zostera marina (L.), fish communities. II. Functional analysis. Journal of Experimental Marine Biology and Ecology 22: 293–311.
Adams, S.M. 1976b. The ecology of eelgrass, Zostera marina (L.), fish communities. I. Structural analysis. Journal of Experimental Marine Biology and Ecology 22: 269–291.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.R. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51: 633–641.
Becker, A., P.D. Cowley, A.K. Whitfield, J. Järnegren, and T.F. Næsje. 2011. Diel fish movements in the littoral zone of a temporarily closed South African estuary. Journal of Experimental Marine Biology and Ecology 406: 63–70.
Belisle, M., and A. Desrochers. 2002. Gap-crossing decisions by forest birds: an empirical basis for parameterizing spatially-explicit, individual-based models. Landscape Ecology 17: 219–231.
Bell, J.D., and M. Westoby. 1986a. Importance of local changes in leaf height and density to fish and decapods associated with seagrasses. Journal of Experimental Marine Biology and Ecology 104: 249–274.
Bell, J.D., and M. Westoby. 1986b. Variation in seagrass height and density over a wide spatial scale—effects on common fish and decapods. Journal of Experimental Marine Biology and Ecology 104: 275–295.
Bell, S.S., E.D. McCoy, and H.R. Mushinsky. 1991. Habitat structure: the physical arrangement of objects in space, 1st ed. London: Chapman and Hall.
Blaber, S.J.M., D.T. Brewer, and J.P. Salini. 1995. Fish communities and the nursery role of the shallow inshore waters of a tropical bay in the gulf of Carpentaria, Australia. Estuarine, Coastal and Shelf Science 40: 177–193.
Boesch, D.F., and R.E. Turner. 1984. Dependence of fishery species on salt marshes—the role of food and refuge. Estuaries 7: 460–468.
Boström, C., E.L. Jackson, and C.A. Simenstad. 2006. Seagrass landscapes and their effects on associated fauna: a review. Estuarine, Coastal and Shelf Science 68: 383–403.
Box, G.E., G.M. Jenkins, and G.C. Reinsel. 2013. Time series analysis: forecasting and control. John Wiley & Sons.
Bretz, F., T. Hothorn, and P. Westfall. 2010. Multiple comparisons using R. CRC Press.
Camp, A.L., C.H. Ryer, B. Laurel, and K. Seals. 2011. Effect of nursery habitat on density-dependent habitat selection in juvenile flatfish. Journal of Experimental Marine Biology and Ecology 404: 15–20.
Chittaro, P.M. 2004. Fish-habitat associations across multiple spatial scales. Coral Reefs 23: 235–244.
Connolly, R.M. 1994. Removal of seagrass canopy—effects on small fish and their prey. Journal of Experimental Marine Biology and Ecology 184: 99–110.
Connolly, R.M., and A.J. Butler. 1996. The effects of altering seagrass canopy height on small, motile invertebrates of shallow Mediterranean embayments. Marine Ecology: Pubblicazioni della Stazione Zoologica di Napoli I 17: 637–652.
Crowder, L.B., and W.E. Cooper. 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Danielson, B.J. 1991. Communities in a landscape—the influence of habitat heterogeneity on the interactions between species. American Naturalist 138: 1105–1120.
De’ath, G., and K.E. Fabricius. 2000. Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81: 3178–3192.
Dorenbosch, M., M.C. van Riel, I. Nagelkerken, and G. van der Velde. 2004. The relationship of reef fish densities to the proximity of mangrove and seagrass nurseries. Estuarine, Coastal and Shelf Science 60: 37–48.
Dorenbosch, M., W.C.E.P. Verberk, I. Nagelkerken, and G. van der Velde. 2007. Influence of habitat configuration on connectivity between fish assemblages of Caribbean seagrass beds, mangroves and coral reefs. Marine Ecology Progress Series 334: 103–116.
Elliott, M., and K.L. Hemingway. 2008. Fishes in estuaries. John Wiley & Sons.
Fagan, W.F., R.S. Cantrell, and C. Cosner. 1999. How habitat edges change species interactions. The American Naturalist 153: 165–182.
Fodrie, F.J., and L.A. Levin. 2008. Linking juvenile habitat utilization to population dynamics of California halibut. Limnology and Oceanography 53: 799.
Froeschke, J.T., and G.W. Stunz. 2012. Hierarchical and interactive habitat selection in response to abiotic and biotic factors: the effect of hypoxia on habitat selection of juvenile estuarine fishes. Environmental Biology of Fishes 93: 31–41.
Geraldi, N.R., S.P. Powers, K.L. Heck, and J. Cebrian. 2009. Can habitat restoration be redundant? Response of mobile fishes and crustaceans to oyster reef restoration in marsh tidal creeks. Marine Ecology Progress Series 389: 171–180.
Gibbs, P., and J. Matthews. 1982. Analysis of experimental trawling using a miniature otter trawl to sample demersal fish in shallow estuarine waters. Fisheries Research 1: 235–249.
Grabowski, J.H., A.R. Hughes, D.L. Kimbro, and M.A. Dolan. 2005. How habitat setting influences restored oyster reef communities. Ecology 86: 1926–1935.
Gray, C.A., R.C. Chick, and D.J. McElligott. 1998. Diel changes in assemblages of fishes associated with shallow seagrass and bare sand. Estuarine, Coastal and Shelf Science 46: 849–859.
Greening, H., and R. Livingston.1982. Diel variation in the structure of seagrass-associated epibenthic macroinvertebrate communities. Marine Ecology Progress Series.
Grober-Dunsmore, R., T.K. Frazer, J. Beets, N. Funicelli, and P.D. Zwick. 2004. The significance of adjacent habitats on reef fish assemblage structure: are relationships detectable and quantifiable at a landscape-scale? Proceedings of the Fifty-Fifth Annual Gulf and Caribbean Fisheries Institute 713–734.
Grober-Dunsmore, R., T.K. Frazer, W.J. Lindberg, and J. Beets. 2007. Reef fish and habitat relationships in a Caribbean seascape: the importance of reef context. Coral Reefs 26: 201–216.
Grober-Dunsmore, R., S.J. Pittman, C. Caldow, M.S. Kendall, and T.K. Frazer. 2009. A landscape ecology approach for the study of ecological connectivity across tropical marine seascapes. Ecological Connectivity among Tropical Coastal Ecosystems 493–530.
Guest, M.A., R.M. Connolly, and N.R. Loneragan. 2003. Seine nets and beam trawls compared by day and night for sampling fish and crustaceans in shallow seagrass habitat. Fisheries Research 64: 185–196.
Hammerschlag, N., and J.E. Serafy. 2010. Nocturnal fish utilization of a subtropical mangrove-seagrass ecotone. Marine Ecology 31: 364–374.
Heck, K.L., and T.A. Thoman. 1984. The nursery role of seagrass meadows in the upper and lower reaches of the Chesapeake Bay. Estuaries 7: 70–92.
Hemminga, M.A., and C.M. Duarte. 2000. Seagrass ecology. Cambridge: Cambridge University Press.
Hettler Jr., W.F. 1989. Nekton use of regularly-flooded saltmarsh cordgrass habitat in North Carolina, USA. Marine Ecology Progress Series. Oldendorf 56: 111–118.
Hollingsworth, A., and R.M. Connolly. 2006. Feeding by fish visiting inundated subtropical saltmarsh. Journal of Experimental Marine Biology and Ecology 336: 88–98.
Hothorn, T., F. Bretz, and P. Westfall. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363.
Hovel, K.A., M.S. Fonseca, D.L. Myer, W.J. Kenworthy, and P.E. Whitfield. 2002. Effects of seagrass landscape structure, structural complexity and hydrodynamic regime on macrofaunal densities in North Carolina seagrass beds. Marine Ecology Progress Series 243: 11–24.
Irlandi, E.A. 1994. Large-scale and small-scale effects of habitat structure on rates of predation—how percent coverage of seagrass affects rates of predation and Siphon Nipping on an Infaunal Bivalve. Oecologia 98: 176–183.
Irlandi, E.A., and M.K. Crawford. 1997. Habitat linkages: the effect of intertidal saltmarshes and adjacent subtidal habitats on abundance, movement, and growth of an estuarine fish. Oecologia 110: 222–230.
Irlandi, E.A., W.G. Ambrose, and B.A. Orlando. 1995. Landscape ecology and the marine-environment—how spatial configuration of seagrass habitat influences growth and survival of the bay scallop. Oikos 72: 307–313.
Kennedy, B.P., K.H. Nislow, and C.L. Folt. 2008. Habitat-mediated foraging limitations drive survival bottlenecks for juvenile salmon. Ecology 89: 2529–2541.
Kneib, R. 1984. Patterns of invertebrate distribution and abundance in the intertidal salt marsh: causes and questions. Estuaries 7: 392–412.
Kneib, R. 1987. Predation risk and use of intertidal habitats by young fishes and shrimp. Ecology 68: 379–386.
Lascara, J. 1981. Fish predator–prey interaction in areas of eelgrass (Zostera marina). College of William and Mary.
Levin, S.A. 1992. The problem of pattern and scale in ecology. Ecology 73: 1943–1967.
Levin, P.S. 1994. Small-scale recruitment variation in a temperate fish—the roles of macrophytes and food-supply. Environmental Biology of Fishes 40: 271–281.
Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, C.H. Peterson, and J.B.C. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809.
Luczkovich, J.J. 1988. The role of prey detection in the selection of prey by pinfish < i > Lagodon rhomboides</i > (Linnaeus). Journal of Experimental Marine Biology and Ecology 123: 15–30.
Luo, J., J.E. Serafy, S. Sponaugle, P.B. Teare, and D. Kieckbusch. 2009. Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Marine Ecology Progress Series 380: 255–269.
Marshall, S., and M. Elliott. 1998. Environmental influences on the fish assemblage of the Humber Estuary, U.K. Estuarine and Coastal Marine Science 46: 175–184.
Mattila, J., G. Chaplin, M.R. Eilers, K.L. Heck Jr., J.P. O’Neal, and J.F. Valentine. 1999. Spatial and diurnal distribution of invertebrate and fish fauna of a Zostera marina bed and nearby unvegetated sediments in Damariscotta River, Maine (USA). Journal of Sea Research 41: 321–332.
Micheli, F., and C.H. Peterson. 1999. Estuarine vegetated habitats as corridors for predator movements. Conservation Biology 13: 869–881.
Miltner, R.J., S.W. Ross, and M.H. Posey. 1995. Influence of food and predation on the depth distribution of juvenile spot (Leiostomus xanthurus) in tidal nurseries. Canadian Journal of Fisheries and Aquatic Sciences 52: 971–982.
Minello, T.J., K.W. Able, M.P. Weinstein, and C.G. Hays. 2003. Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis. Marine Ecology Progress Series 246: 39–59.
Morrison, M.A., M.P. Francis, B.W. Hartill, and D.M. Parkinson. 2002. Diurnal and tidal variation in the abundance of the fish fauna of a temperate tidal mudflat. Estuarine, Coastal and Shelf Science 54: 793–807.
Mumby, P.J. 2006. Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biological Conservation 128: 215–222.
Nagelkerken, I., C. Roberts, G. Van Der Velde, M. Dorenbosch, M. Van Riel, E.C. De La Moriniere, and P. Nienhuis. 2002. How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Marine Ecology Progress Series 244: 299–305.
Orth, R.J., and K.L. Heck. 1980. Structural components of eelgrass (Zostera-Marina) Meadows in the Lower Chesapeake Bay—Fishes. Estuaries 3: 278–288.
Parrish, J.D. 1989. Fish communities of interacting shallow-water habitats in tropical oceanic regions. Marine Ecology Progress Series 58: 143–160.
Petrik, R., and P.S. Levin. 2000. Estimating relative abundance of seagrass fishes: a quantitative comparison of three methods. Environmental Biology of Fishes 58: 461–466.
Phelan, B., R. Goldberg, A. Bejda, J. Pereira, S. Hagan, P. Clark, A. Studholme, A. Calabrese, and K. Able. 2000. Estuarine and habitat-related differences in growth rates of young-of-the-year winter flounder (Pseudopleuronectes americanus) and tautog (Tautoga onitis) in three northeastern US estuaries. Journal of Experimental Marine Biology and Ecology 247: 1–28.
Pinheiro, J., D. Bates, S. DebRoy, and D. Sarkar. 2007. Linear and nonlinear mixed effects models. R Package Version 3: 57.
Pittman, S.J., J.D. Christensen, C. Caldow, C. Menza, and M.E. Monaco. 2007. Predictive mapping of fish species richness across shallow-water seascapes in the Caribbean. Ecological Modelling 204: 9–21.
R Core Team. 2011. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. Open access available at: http://cran.r-project.org.
Robertson, O.J., and J.Q. Radford. 2009. Gap-crossing decisions of forest birds in a fragmented landscape. Austral Ecology 34: 435–446.
Rozas, L.P., and T.J. Minello. 1997. Estimating densities of small fishes and decapod crustaceans in shallow estuarine habitats: a review of sampling design with focus on gear selection. Estuaries 20: 199–213.
Ruiz, G.M., A.H. Hines, and M.H. Posey. 1993. Shallow water as a refuge habitat for fish and crustaceans in non-vegetated estuaries: An example from Chesapeake Bay. Marine Ecology Progress Series 99: 1–16.
Ryan, M.R., S.S. Killen, R.S. Gregory, and P.V.R. Snelgrove. 2012. Predators and distance between habitat patches modify gap crossing behaviour of juvenile Atlantic cod (Gadus morhua, L. 1758). Journal of Experimental Marine Biology and Ecology 422: 81–87.
Sandel, B. & A.B. Smith 2009. Scale as a lurking factor: incorporating scale-dependence in experimental ecology. Oikos 118.9: 1284–1291.
Sheaves, M. 2005. Nature and consequences of biological connectivity in mangroves systems. Marine Ecology Progress Series 302: 293–305.
Sheaves, M. 2006. Scale-dependent variation in composition of fish fauna among sandy tropical estuarine embayments. Marine Ecology Progress Series 310: 173–184.
Stewart, C.B., and F.S. Scharf. 2008. Estuarine recruitment, growth, and first-year survival of juvenile red drum in North Carolina. Transactions of the American Fisheries Society 137: 1089–1103.
Stoner, A.W. 1982. The influence of benthic macrophytes on the foraging behavior of pinfish, Lagodon rhomboides (Linnaeus). Journal of Experimental Marine Biology and Ecology 58: 271–284.
Stunz, G.W., P.S. Levin, and T.J. Minello. 2001. Selection of estuarine nursery habitats by wild-caught and hatchery-reared juvenile red drum in laboratory mesocosms. Environmental Biology of Fishes 61: 305–313.
Tait, K.J., and K.A. Hovel. 2012. Do predation risk and food availability modify prey and mesopredator microhabitat selection in eelgrass (Zostera marina) habitat? Journal of Experimental Marine Biology and Ecology 426: 60–67.
Tanner, J.E. 2006. Landscape ecology of interactions between seagrass and mobile epifauna: the matrix matters. Estuarine, Coastal and Shelf Science 68: 404–412.
Taylor, J.C., W.A. Mitchell, J.A. Buckel, H.J. Walsh, K.W. Shertzer, G.B. Martin, and J.A. Hare. 2009. Relationships between larval and juvenile abundance of winter-spawned fishes in North Carolina, USA. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science 1: 12–21.
Therneau, T.M., B. Atkinson, and B. Ripley. 2010. rpart: recursive partitioning. R package version 3
Tzeng, M.W., J.A. Hare, and D.G. Lindquist. 2003. Ingress of transformation stage gray snapper, Lutjanus griseus (Pisces: Lutjanidae) through Beaufort Inlet, North Carolina. Bulletin of Marine Science 72: 891–908.
van Montfrans, J., C.E. Epifanio, D.M. Knott, R.N. Lipcius, D.J. Mense, K.S. Metcalf, E.J. Olmi III, R.J. Orth, M.H. Posey, and E.L. Wenner. 1995. Setilement of blue crab postlarvae in Western North Atlantic Estuaries. Bulletin of Marine Science 57: 834–854.
Warlen, S.M., and J.S. Burke. 1990. Immigration of larvae of fall/winter spawning marine fishes into a North Carolina Estuary. Estuaries 13: 453–461.
Wathne, F. 1977. Performance of trawls used in resource assessment. Marine Fisheries Review 39: 16–23.
Werner, E.E., and J.F. Gilliam. 1984. The ontogenetic niche and species interactions in size structured populations. Annual Review of Ecology and Systematics 15: 393–425.
West, J.M., and J.B. Zedler. 2000. Marsh-creek connectivity: fish use of a tidal salt marsh in southern California. Estuaries 23: 699–710.
Wiens, J.A. 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Worthington, D.G., M. Westoby, and J.D. Bell. 1991. Fish larvae settling in seagrass—effects of leaf density and an epiphytic alga. Australian Journal of Ecology 16: 289–293.
Yahner, R.H. 1988. Changes in wildlife communities near edges. Conservation Biology 2: 333–339.
Yeager, L.A., C.A. Layman, and J.E. Allgeier. 2011. Effects of habitat heterogeneity at multiple spatial scales on fish community assembly. Oecologia 167: 157–168.
Zimmerman, R.J., T.J. Minello, and S. Dent. 1985. Habitat-related growth of Penaeus-Aztecus in a Spartina-Alterniflora marsh. Estuaries 8: A9–A9.
Acknowledgments
We thank A. Poray, M. Brodeur, E. Voigt, S. Coleman, X. Chandler, M. Kenworthy, and R. Bouchillon for their assistance with field sampling. We also thank J. Grabowski, S. Scyphers, and two four anonymous reviewers for their helpful feedback on this manuscript. This research was supported by funding from North Carolina Marine Resources Fund (CRFL) and conducted under Institutional Animal Care and Use Committee (IACUC) ID 10–133.0-B.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bob Christian
Rights and permissions
About this article
Cite this article
Baillie, C.J., Fear, J.M. & Fodrie, F.J. Ecotone Effects on Seagrass and Saltmarsh Habitat Use by Juvenile Nekton in a Temperate Estuary. Estuaries and Coasts 38, 1414–1430 (2015). https://doi.org/10.1007/s12237-014-9898-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-014-9898-y