Abstract
The objective of the study is to assess the occurrence of the most frequently found contaminants such as fluoride, nitrate and trace metals in groundwater to decipher their potential health risk to the inhabitants of Shamirpet urban region, Hyderabad. Fifteen groundwater samples were collected according to the grid patter (3 × 3 km2) from Shamirpet urban regions to cover the entire study area, and analyzed the same to locate the target contaminants. The data showed that Na–Cl–SO4 and mixed Ca–Na–HCO3 type are the dominant hydrochemical facies found based on the anion and cation data of groundwater. According to the water quality index (WQI) values obtained, it demonstrated that most of the water samples (53%) were unsuitable for human consumption. Among the contaminants evaluated, fluoride content was higher in 13% of the samples, whereas 47% of the samples found to have more concentration of nitrate than permissible limits of WHO guidelines indicating that it is unfit for the purpose of drinking. Gibbs diagram explains the quality of the groundwater and its chemistry, which is influenced by the dissolution of rock and complex geochemical mechanisms. Potential health risk assessment of groundwater reveals that the adults in this region who consume this water are at high risk. This situation has further been substantiated by the hazard index values of groundwater for fluoride and nitrate. Therefore, local administration and policy makers should pay attention to supply protected groundwater to ensure and safeguard the human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater quality and its potential health risk assessment is vital in a rapidly growing urban region as it is directly linked to human health. Groundwater is a primary freshwater resource that is widely used for various purposes such as domestic, industrial, and agricultural activities. Groundwater resources in urban areas are highly vulnerable to contamination due to the letting out of untreated wastewater from industrial effluents, agricultural chemicals, etc. Water contamination affects not only the deterioration of water quality but also human health. The hydrogeochemical characteristics are useful in respect of the subsurface geologic practices convoluted in the chemical evolution of groundwater; in turn, it is useful to identify the source of pollution (Luo et al. 2018). Water quality plays a vital role in endorsing standards of human health as well as agricultural production as it is directly linked to human welfare (FAO 2017). In India, access to drinking water from groundwater resources has increased over the past decades leading to remarkable adverse impacts on human health due to the consumption of unsafe water (Bacquart et al. 2015). The scarcity of clean and potable drinking water has emerged in recent years as one of the most serious developmental issues in many parts of India one such example is Telangana state. Globally, around 748 million people still do not have access to an improved drinking water source, and its demand for manufacturing is expected to increase by 400 percent by 2050 (WWAP 2016). Inconsistency in groundwater quality majorly depends on the physical and chemical parameters of pollutants that are considerably influenced due to either natural processes or/and anthropogenic sources. Improper disposal of industrial, municipal solid waste, agricultural runoff, urbanization, and overexploitation have instigated the degradation of the groundwater quality (Aradhi and Kurakalva 2014; Aradhi et al. 2019; Re et al. 2014; Zhang et al. 2017). Additionally, increase in urbanization has an impact on the peri-urban area in terms of environmental resources in particular on groundwater (Prakash 2014). Eventually, contamination of groundwater and its health risk assessment on human beings in urban and peri-urban environments is of greater concern.
Water quality assessment is of a great environmental concern across the world that involves the evaluation and monitoring of a variety of physicochemical parameters including trace elements and major ions (Yadav et al. 2019a). Hydrogeochemical studies to ascertain the suitability of groundwater at a variety of aquifers such as alluvial groundwater, semi-confined aquifer and granitic aquifer were reported (Adimalla 2019; Rama Mohan and Keshav Krishna 2018) including statistical analysis and regulatory guideline values to find out its suitability for use in either drinking or irrigation use. The risk of polluting the groundwater environment depends on the intrinsic vulnerability of groundwater to pollution and hazard of the potentially polluting activity and its consequences (Alievi and Al-Khatib 2015). Further, assurance of drinking water safety is disparagingly important in protection of human health. Thereby studies related to the most significant inorganic pollutants such as fluoride and nitrate in groundwater were assessed for human health risk assessment using water quality data (Adimalla and Li 2019; Yadav et al. 2019a, b; Chen et al 2017a, b). Although a few works were reported in Hyderabad metropolitan city of Telangana state (Kurakalva et al. 2016; Srisailam et al. 2017), none of these studies focused on potential health risk assessment based on USEPA guideline values and GIS-based water quality index (Yadav et al. 2018). The objective of the present study is to characterize factors that are controlling groundwater chemistry, human health risk assessment and groundwater quality for its suitability for drinking and irrigation purposes in Shamirpet urban watershed of Medchal-Malkajgiri district in southern India. This study would help in the direction of proper management of groundwater resources. It will also create awareness among the public about the potential health problems due to the consumption of groundwater, which contains high concentration of fluoride, nitrate and trace elements.
Materials and methods
Description of the study area
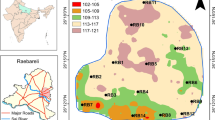
The study area, Shamirpet urban watershed falls under Rangareddy district, Telangana, India. The chosen area is bounded between latitudes 17°35′3.72′′ N and 17°41′56.47′′ N and longitudes 78°24′37.47′′ E and 78°24′59.58′′ E, with an extent of area 281.4 km2 as shown in Fig. 1. The study area is the principal town in the selected watershed considered to be the index area which is about 42 km far from Hyderabad city (Fig. 1a). The study area includes the peri-urban of Medchal-Malkajgiri district of Telangana State, India. The Shamirpet watershed households are dependent on agriculture and in some villages (Mollegudam, Gowdavalli, and Ravalkole) there is an acute water scarcity for domestic and irrigation purposes. Some villages (Rajabolaram and Kanukunta) of the study area have industrial activities that mainly include go downs to store the food items.
Geology
The study area is located on the granitic rock terrain as part of the Peninsular Gneissic Complex. The major rock types occurring in the study area are pink and grey granites. Dolerite dykes, primary enclaves, aplites, pegmatite, quartz veins, migmatites and alaskite traverse these granites (Sandhya 2014). However, intrusive dolerite dykes are also common in the area.
Hydrogeology
Ground water occurs under phreatic conditions in weathered zones and under semiconfined to confined conditions in the fractured zones. Ground water used to be exploited through shallow, large diameter dug wells until 1970 to meet domestic and irrigation requirements. Presently groundwater is principally exploited through shallow and deep bore wells with a depth ranging from 100–300 m (CGWB, 2013).
The major aquifers in the study area are weathered and fractured granites, basalts. Groundwater is designed in the study area under the conditions of consolidated formation or semi-consolidated formation and unconsolidated formation. Since the study area is composed of hard rock terrain (granitoid), groundwater is found either in contact zone or in the fractured zone, which directly connects to the aquifer or itself acts as an aquifer (consolidated formation). Depending on the principal aquifer, water level variation of different medium with minimum and maximum depth (meter) are given as basalt (1.9–69.5 m), granite (5.5–34.5 m), quartzite (6.3–12.2 m), sandstone (− 0.6–17.3 m), limestone (8.9–41.5 m), banded gneiss complex (1.6–54.8 m), gneiss (23.5–23.5 m), shale (3.3–14.8 m) and schist (9.1–9.1 m). The study area has been declared as overexploited with reference to the groundwater development. (CGWB 2007) The maximum depth of the weathered zone goes up to 29 m bgl (meters below ground level), while the deepest fracture is recorded up to 124.5 m bgl (CGWB 2019). The reported water level of 6.45 m bgl is predominant in the present study area, while it is between 2 and 40 m bgl in the state of Telangana. Besides, water level rise is observed between 2 and 4 m in the region. The long-term trend of water level is ranging between 0–2 m/year (1997–2016), according to CGWB (2017).
Climate
The climate of the study area was characterized by hot summers and is generally dry except during the South-west monsoon season. The area is dominant with dendritic drainage system and has an elevation ranging between 531 to 667 m above mean sea level. The elevation map along with stream order system from first order to higher fourth order is shown in Fig. 1b
Sample collection and preservation
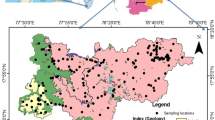
The study area was divided into an optimized grid size of 3 × 3 km using latitude–longitude as reference coordinates and identified 15 sampling stations for the proposed investigations. One groundwater sample from each grid was collected in pre-washed polypropylene (PP) bottles after pumping the tube wells for 20 min. Groundwater samples were collected in two sets; one sample with a volume of 1L and the other samples in a 60 ml bottle for trace metal determination, and they were preserved with 1% HNO3 (Suprapur® 65%, Merck India Pvt Ltd., Hyderabad, India) to obtain pH ~ 2. All the reagents used in this study were of analytical grade. Sampled groundwater was stored in an icebox in the field before they were transported to the Environmental Geochemistry Labs at CSIR-NGRI, Hyderabad. Soon after receipt in the lab, the sample bottles were preserved at a temperature of 4 °C till chemical analysis was conducted.
Analytical procedures
The groundwater samples were analyzed using the APHA (2017) standard methods as described. The physicochemical parameters such as pH, Electrical Conductivity, Total Dissolved Solids (Hanna combo HI981230, Hanna Instruments, Mumbai, India), and DO (Hanna DO HI9146, Hanna Instruments, Mumbai, India) were measured in the field using portable sensors. The other chemical constituents including total hardness (TH) as CaCO3 and bicarbonates (HCO3−) were determined following the standard procedures described (APHA 2005) by the titrimetric method. Water samples were filtered using 0.22 µm Millipore membrane filters (Merck Millipore, Mumbai India) to separate the suspended particulate matters for further Ion chromatographic analysis. The major ions, sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), fluoride (F−), chloride (Cl−), nitrate (NO3−), and sulfate (SO42−) were determined using ion chromatography (IC) (Metrohm, 882 IC, Metrohm AG, Herisau, Switzerland) in accordance with the standard procedures described (APHA 2005). Strict quality assurance and quality control programmes were implemented to ascertain a good quality of data. To achieve this, Ionic charge balance error (ICBE) was calculated using the data pertaining to the anions such as F−, Cl−, NO3−, SO42− and HCO3− while considering cations such as Na+, K+, Ca2+, and Mg2+. ICBE denoted was by E, and calculated from the Eq. (1) below:
Ionic Charge Balance Error (E) acceptable limit ± 10 percentage observed in this study.
Further, all the analytical methods applied in this investigations were validated based on standard reference materials used to construct calibration, accuracy, precision and to quantify each analyte detection limits.
Hydrogeochemical characteristics
Characterization of the hydrogeochemical profile was done through groundwater chemistry and the hydrogeochemical process. The analytical data of major ions were plotted on the Piper diagram using AquaChem software to know different water types, and Durov (1948) diagram was used for determining hydrochemical processes involved based on the percentage of major ions milliequivalents. Major ion data were plotted on Gibbs diagram (Gibbs 1970) to evaluate and comprehend the relationship between groundwater composition with a composition of recharging water and chemical weathering of aquifer rocks. To understand the groundwater composition and flow path (i.e., ion exchange mechanism), chloro-alkali indices (CAI-1 and CAI-2; Scholler 1977) were used and calculated using the following Eqs. (2) and (3).
Water quality was systematically assessed for dirking purposes in accordance with the WHO standards. Classification of groundwater for irrigation purpose was done using US Salinity diagram (USSL 1954), and Wilcox diagram (Wilcox 1955).
Health risk assessment
Studies on health risk assessment (HRA) have gained a lot of importance due to its ease of evaluation with the existing data available devoid of in situ toxicological dose assessment. As the water and air are the principal causes to pose health risks on human health (Aradhi and Kurakalva 2014; Aradhi et al. 2019), HRA has been widely used in risk assessment method. Identification and characterization of human health risk assessment is usually carried out based on ingestion, inhalation, and absorption of various trace metals. The health risk connected with oral ingestion of groundwater containing trace metals was calculated by means of average daily dose intake orally (ADDOral), and hazard quotient (HQ) adopted from USEPA (2012). The ADDOral can be calculated using Eq. (4) given below, which other researchers also used (Yadav et al. 2018).
where ADDoral is the average daily dose (mg/kg/day), C is the average concentration of each trace metal or each major ion in groundwater (mgL−1), IR is the groundwater ingestion rate L per day, EF denotes the exposure frequency (days/year), ED denotes the exposure duration (Years), ABW denotes body weight (Kg), and AET denotes the average time which can be calculated by multiplying EF \(\mathrm{and ED}\).
Hazard quotient (HQ) of each trace metal and major ions was assessed for the potential non-carcinogenic risk using the following Eq. (5)
RfD signifies toxicity reference dose oral (mg/kg/day).
Finally, risk assessment was evaluated based on the HQ values. The entire potential non-carcinogenic risk posed via metals in this study were calculated as per the following equation, which gives the hazardous index (HI), as stated below.
The average daily dose (ADDoral) exclusively for other non-carcinogenic pollutants such as F− and NO3− were also calculated using the Eq. (4). The hazard quotient was calculated using the Eq. (5) and considered the oral reference doses (RfD) value for F− and NO3− equal to 0.4 mg/kg/day and 1.6 mg/kg/day respectively. The values were obtained from the unified database of USEPA (2014). Exposure risks of F− and NO3− for drinking water was considered as reported in the literature (Chen et al. 2017a, b) and according to the following Eq. (7).
Water quality index (WQI)
WQI helps in the understanding of water quality issues and suggests the management plan by integrating extensive complex data and generating scores thereby a comprehensive depiction of the quality of the water resources can be obtained. It has been reported in the literature that WQI is a significant parameter for assessing groundwater quality for drinking purposes (Boateng et al. 2016). WQI provides the combination of various water quality parameters to ascertain the water quality of the area under investigation. Thirteen crucial water quality parameters include pH, EC, TDS, TH, HCO3−, F−, Cl−, NO3−, SO42−, Na+, K+, Ca2+, Mg2+, and are utilized for the calculation of WQI of each groundwater sample. Many researchers have used WQI to differentiate the types of water for quality and drinking purpose (Batabayal and Charaborty 2015; Chaurasia et al. 2018; Magesh et al. 2013; Raj and Shaji 2017; Vasanthavigar et al. 2010; Tiwari et al. 2018; Yadav et al. 2018). Weight value (wi) was assigned for each parameter range between 1–5, where TDS, F−, NO3− has the highest weight, wi = 5, pH, EC, SO−4 (4), HCO3−, Cl−, TH (3), Na+, K+, Ca2+(2) and Mg2+ has the lowest wi = 1, as shown in Table 1. The WQI was calculated using the equations and followed the three-step procedure.
Step 1. Relative weight formula
where Wi = relative weight, wi = parameters weight, n = number of parameters.
Step 2. Quality rating formula
where qi water quality rating. Ci measure concentration for each parameter in samples in mg/L, Si standard limit as per the WHO guideline value (mg/L) for each parameter.
Step 3. Sub-Indices is calculated by multiplying Eq. 8 and 9
Finally, after calculating SI values for every parameter, WQI (11) is calculated by summing all SI values for a particular sample that gives the quality classification according to the range and water types.
The calculated WQI value of each water sample was compared to the WQI classification to establish the type of water available in the study area (Tables 1 and 7).
Statistical and spatial analysis
The descriptive statistics such as range, mean, median, and mode of various water quality data was obtained and calculated using Microsoft Excel 2010. The spatial distribution of the groundwater quality parameters was processed using ArcGIS 10 software. In this study, an inverse distance weighted (IDW) interpolation technique was used to understand the spatial variations of various contaminants in groundwater. IDW interpolation method calculates the unknown values with respect to distance where the nearest point gets more weightage, then reduces with an increase in distance. Further, this technique is widely used by many researchers (Vaiphei et al. 2020; Zolekar et al. 2021; Tiwari et al. 2018; Singh and Hussain 2016) for spatial distribution maps of a variety of parameters. Interpolation maps do help to visualize the generalized view of hydrogeochemical processes along with the drainage pattern and stream order of the study area besides providing lucidity about groundwater quality to the people and decision-makers.
Results and discussion
Identification of hydrogeochemical process
The hydrogeochemical process in groundwater principally depends on rock-soil–water interactions as well as anthropogenic activities. Shamirpet watershed falls into the category of hard rock terrain apart being a part of urban settlements. Above all, the reaction between groundwater and aquifer minerals plays a substantial role in water quality that manifests the groundwater geochemistry of the study area.
Hydrochemistry
Analytical data of groundwater samples measured for physicochemical, anion, cations, and trace metals were statistically summarized and presented in Table 1. Besides, the measured parameters were compared following WHO permissible limits for drinking purposes. The average pH value is 7.4 that exhibited groundwater belongs to a neutral to a slightly alkaline. The electrical conductivity (EC) observed that 53% of samples were above the maximum permissible limit of 1500 µScm−1 recommended by WHO. The EC found in the range of 640 µScm−1 to 2400 µScm−1. The high values of EC further suggests that the dissolved solids/ion concentrations are more in the groundwater.
All the groundwater samples explained that the TDS values are above the desirable limit, whereas none of the samples is within the maximum permissible level of WHO (2011) drinking water standards, as shown in Table 2. Approximately 26.67% of the groundwater samples (Table 3) were slightly saline based on groundwater classification data of TDS that are not suitable for drinking purposes (Selvakumar et al. 2017).
Total hardness of the groundwater samples is found to range from 261 mgL−1 to 865 mgL−1 (average 535 mg L−1) and classified as a very hard category of water. This indicated the hardness of all of the samples exceeded the maximum allowable limit of WHO standards for drinking water. The dominance of anions was found in the following order Cl > HCO3 > NO3− > F− (Table 1), and their spatial distribution was shown in Fig. 2. Chloride ion concentration was found to be 56.5–785 mg/l, which might be owing to the precipitation/adsorption process where barring of other ions occurs. The high content of chloride in water causes salinity related problems. Bicarbonate concentration varies from 140 to 494 mg/l; perhaps due to carbonates dissolution, agricultural runoff, or infiltration, it might enhance groundwater concentration (Vázquez-Suñé et al., 2005). Among the cations, sodium had the highest concentration, and calcium and magnesium were less than sodium were observed in the study region. Cation exchange process (Shanmugam and Ambujam 2012) is a significant reason for how the Na+ concentration got increased, while Ca2+ and Mg2+concentrations decreased and this process was discussed in detail at “Ion Exchange”.
Groundwater types and evaluation
The hydrochemical facies of groundwater explains the relation between major anions and cations and their behaviour. Hydrochemical facies help in establishing the classification of different water types (Piper 1994) and in understanding the origin and geochemical evolution of groundwater. Therefore, we calculated hydrogeochemical facies of groundwater using the concentration of major anions (Cl−, SO42− and HCO3−) and cations (Ca2+, Mg2+, Na+, and K+) in meq/L through their concentration plotted in Piper diagram as shown in Fig. 3a. The mechanism for geochemical evolution generally represented into six different water types as Type-I (Ca-HCO3 type), Type-II (Na-Cl type), Type-III (mixed Ca-Na-HCO3 type), Type-IV (mixed Ca–Mg–Cl type), Type-V (Ca–Cl type) and Type-VI (Na-HCO3 type). The piper diagram shows that the two dominant hydrogeochemical facies, Na–Cl–SO4 and mixed Ca-Na-HCO3 type (Fig. 3a) were identified.
Durov diagram is a composite plot that reveals the samples with similar chemical composition their expedient relationship and properties for large sample groups. The ionic data which was plotted (Fig. 3b) showed that most of the samples fell into the dissolution mixing type of water.
Ion exchange
The chemical composition of groundwater along its flow path can be understood by studying the Chloro Alkaline Indices (CAI). Schoeller (1965, 1977) suggested that two types of Chloro Alkaline Indices (CAI1, 2) were useful for the interpretation of ion exchange between the groundwater and the host environment. In this investigations, CAI1 values ranged from − 0.12 to 1.37 with a mean of 0.48 while CAI2 values in the range of -0.05 to 3.35 with a mean value of 0.98 (Fig. 4). According to Chloro alkaline indices, 99% had positive value indicating an exchange of Na and K from the water with Mg and Ca of the rocks, and 1% had negative values when there was an exchange of Mg and Ca of the water with Na and K of the host rocks (Nagaraju et al. 2014).
Sources of dissolved constituents
Plotting the ionic data in Gibb’s diagram is useful to understand the source of dissolved constituents in the groundwater and their respective aquifer characteristics. These include rock dominance, precipitation dominance, and evaporation dominance. The ionic ratios between Cl−/(Cl− + HCO3−) and Na+ + K+/(Na+ + K+Ca2+) versus TDS values in groundwater were plotted in this study and presented in Fig. 5. It was observed that most of the samples were falling in zone belonging to the rock dominance cluster. This suggests that the groundwater chemistry controlled via dissolved ions. Further, similar findings were observed in north eastern Tunisia (Houatmia et al. 2016) where weathering of rocks was the principal mechanism that controls the groundwater chemistry. Recently, the relationship between physical and chemical occurrence of weathering was reported from a deep well (65-m) over earth’s critical zone (Holbrook et al. 2019). Based on the above observations, the chemistry of groundwater in Shamirpet watershed might be controlled by a complex geochemical mechanisms as described (Sajilkumar and James 2016).
Pearson correlation analysis
Understanding the magnitude of association or correlation between two variables was calculated using Pearson correlation analysis, as shown in Table 4. A strong correlation between EC and TDS confirms that good quantum of dissolved ion was noted in the groundwater. EC is also strongly correlated with Cl− (0.93), Ca2+ (0.86) Mg2+ (0.86) and TH (0.89). TDS is correlating with Cl−, Ca2+, Mg2+, and TH showed dissolution of salts in groundwater mainly due to natural sources such as parent rock. The poor correlation between total hardness with bicarbonate (0.19), but strongly correlated with calcium and magnesium concentrations indicated the presence of chloride salts of calcium and magnesium.
Health risk assessment
To calculate the average daily dose (ADDoral) for trace metals (Yadav et al. 2018), water ingestion rate (IR) as 2L/day, exposure frequency (EF) was 365 days, and exposure duration and average body weight 70Kg were considered. The ADD calculated for the mean concentration of Cu, Fe, Mn, and Zn, which is dependent on factors such as consumption of water, weight, and age of an individual. The RfD values were acquired from USEPA (2012) document. The dietary health risk assessment was carried out for trace metals through the ingestion rate, as shown in Table 5. The HQ values obtained for the studied metals (Table 5) demonstrated that the groundwater does not have any adverse health effects on human health (HQ ≤ 1).
The health risk was also evaluated for the dominant contaminants (fluoride and nitrate) which were observed in groundwater of the Shamirpet urban region. Besides, this study area falls in the endemic fluoride area as well as an overlay with urban and agricultural regions. In this study, the risk assessment was done for two categories: children and adults, according to USEPA (2012) guidelines. Water intake by adult and child was estimated as 2.5 L/day and 0.78 L/day, respectively (WHO 2011; USEPA 2014). For adults 64 years and for children 12 years were taken as exposure period for the assessment. AET values of 23,360 and 4,380, whereas ABW values were 65 and 15 kg were considered for adults and children, respectively (Adimalla 2019; Narsimha and Rajitha 2018; Yadav et al. 2019b). According to the classification of HQoral values if value is equal to 1 groundwater is safe, greater than 1, then it was considered a non-carcinogenic risk to human beings. The data obtained for F− and NO3− in this study showed in Table 5 demonstrated that the available groundwater posed high risk to adults while no risk was posed to children residing in this area.
Drinking water quality
Table 1 represents the evaluated groundwater quality data for various vital parameters following the WHO (2011) standard guideline values recommended for drinking purposes (Table 1). In the Shamirpet study area, the pH of the groundwater was found to be within the desirable limits; hence, it is suitable for drinking. Contrary to the WHO (2011) recommendations, Cl− HCO3−, NO3−, and F− were above the permissible limits in most of the samples indicating that most of the samples were unsuitable for drinking purposes. Calcium ion concentration varied from 26 to 960 mgL−1 while magnesium ion concentration varied from 48 to 164 mgL−1. Potassium ion concentration varied from 1.4 to 17 mgL−1 (Table 1). While comparing the World Health Organization (WHO 2011) guideline the values for cations revealed that Ca, Mg, and K concentrations (Table 1) were above the permissible limit in most of the samples.
Electrical conductivity and TDS
Based on the electrical conductivity (Table 6) it was noticed that about 50% of the samples fell in the permissible limits and the rest of the samples in the not permissible limits, respectively. The spatial distribution of the electrical conductivity was shown in Fig. 2e. TDS values obtained for groundwater samples in the study area were classified according to the Davis and Dewiest (1966) and presented in Table 6. The data represented that a few samples could be used for drinking without any risk (Table 6) as the data found to have < 500 mg L−1 of TDS values (Davis and DeWiest 1966). Besides, the data proved that 73% of groundwater samples were freshwater type, and remaining (27%) samples belonged to brackish water type as per the Freeze and Cherry (1979) classification (Table 3).
Total hardness
The total hardness of the groundwater represents the measurement of total multivalent cations. The hardness of the Shamirpet watershed was 261–865 mg L−1, with an average of 535 mg L−1 (Table 1). This data reveals that the TH showed higher concentration (WHO 2011) at sampling locations of GW-13, 6, and 2 through its spatial distribution map as shown in Fig. 2f. Table 3 indicates that all the groundwater samples fell in the very hard category as classified according to Sawyer and McCartly (1967). Further, the data concluded that the groundwater in the study area was unfit for the purpose of drinking.
Water quality index (WQI)
WQI which is useful to assess the groundwater quality ascertains its utilization for drinking purposes, which is a simple, stable, and reproducible index. Based on the range of WQI values, water was classified into five types such as excellent water having WQI < 50, good water (WQI range 50–100), poor water (WQI range 100–200), very poor water (WQI range 200–300) and unfit for drinking (WQI > 300) based on its use for drinking purposes. The WQI values for each sample were calculated and presented in Table 7. These values were found to be in the range between 80 and 271, with an average value of 165. The spatial variation of the water quality index values (Fig. 6) indicated that maximum number of groundwater samples in the Shamirpet region found unsuitable for drinking purposes. The WQI values of the present study indicated very poor quality (27%) of water in the area dominated by industrial activities (Sample IDs: GW-4, 6, and13). Poor water quality (60%) was observed in water-scarce zones due to over-exploitation of groundwater resources for agricultural use in the study area (Table 6). Irrigation is the most important sector of water usage accounting for about 70% of the global freshwater withdrawals and 90% of consumptive water uses. While the extent of irrigation and related water uses were estimated by model simulations, information on the source of irrigation water was scarce and very scattered (Siebert et al. 2010). It was also evident from the recent study (Jio et al. 2020) that irrigation activities yielded significant influence on the hydrochemistry of both river and groundwater. The remaining samples (13%) emerged in good water category. A comparison of WQI values from different urban and peri-urban regions were compared (Table 8). This data showed that there was a rapid exploitation of ground water resources in almost all the cities. This might be due to the burden from anthropogenic inputs. The WQI analysis concluded the fact that the groundwater of the Shamirpet urban region of Hyderabad requires treatment so that it could be used for the purpose of drinking as well as to protect it from further contamination.
Irrigation water quality
The quality of water used for irrigation is essential to ensure good crop yield, maintenance of soil productivity, and protection of the environment. Simultaneously, the irrigation water quality is largely influenced by the source constituents of the water (Oster 1994). Mineralization of water and its effects on soil and plants play a vital role in assessing groundwater suitability for irrigation purposes. Consequently, the indices such as USSL (1954) and Wilcox (1955) diagrams proposed by various agricultural and soil scientists were utilized to discuss groundwater suitability for irrigation purposes.
US salinity diagram
The sodium absorption ratio and electrical conductivity values of the groundwater were plotted in the USSL diagram (Fig. 7). The data demonstrated that the majority of the groundwater samples fell in the high salinity and low sodium hazard (C3-S1 type) category. It indicated that the groundwater quality was acceptable for irrigation. It further suggested that the studied groundwater could be used for irrigation purposes with salinity control. The maximum EC values of the groundwater in the study area was found up to 2400 μS/cm. The same results showed that the computed Na% versus EC values (Fig. 8) and the spatial distribution were shown in Fig. 2e.
Wilcox diagram
Wilcox's (1995) diagram is useful for the evaluation of groundwater for irrigation purposes, where the data can be used to plot the percentage of sodium against electrical conductance (Fig. 8). Twenty-seven percent of the groundwater samples were falling under the category of doubtful to unsuitable condition, whereas 60% of the samples fell in the good to a permissible field category in the study region. Then a few (13%) samples belonged to the very good to the good field.
Conclusions
Hydrogeochemical profile, potential health risk, and quality of groundwater was assessed and presented by way of investigating 15 sampling locations in the rapidly growing urban region of Hyderabad, south India. Gibbs diagram demonstrated that dissolved ions are the major controlling factors on groundwater chemistry in the study region. Groundwater quality was principally was influenced by natural weathering of rocks and controlled by the complex geochemical mechanism. The WQI values of the present study indicated very poor quality (27%) water in the area which is dominated by industrial activities (Sample IDs: GW-4,6 and13). Poor water quality (60%) was observed in water-scarce zones due to over-exploitation of groundwater for irrigation in the study area. Elevated concentrations of nitrate (47%) and fluoride (13%) were observed in groundwater making it unsuitable for drinking as it exceeded the WHO prescribed permissible values. Evaluation of human health risk to trace metals through the ingestion of contaminated water in adults showed that there was no adverse effect on human health. However, the data obtained for F− and NO3− health risk assessment demonstrated that groundwater poses a high risk to adults in the study area. The study results would be useful to all stakeholders, including policy makers, in understanding the extent of the situation and could serve as a basis in decision making for sustainable management of the study area’s groundwater resources.
References
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Heal. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N, Li P (2019) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess 25:81–103
Aliewi A, Al-Khatib IA (2015) Hzard and risk assessment of pollution on the groundwater resources and residents’ health of Salfit District, Palestine. J Hydrol Reg Stud 4:472–486. https://doi.org/10.1016/j.ejrh.2015.07.006
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edition. American Public Health Association, Washington, D.C.
Aradhi KK, Kurakalva RM (2014) Risk assessment of heavy metals and their source distribution in waters of a contaminated industrial site. Environ Sci Pollut Res 21:3653–3669. https://doi.org/10.1007/s11356-013-2359-5
Aradhi KK, Kurakalva RM, Banothu D (2019) Assessment of groundwater quality, toxicity and health risk in an industrial area using multivariate statistical methods. Environ Syst Res 8:26–34. https://doi.org/10.1186/s40068-019-0154-0
Bacquart T, Frisbie S, Mitchell E et al (2015) Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: arsenic, manganese, fluoride, iron, and uranium. Sci Total Environ 517:232–245. https://doi.org/10.1016/j.scitotenv.2015.02.038
Batabyal AK, Chakraborty S (2015) Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ Res 87:607–617. https://doi.org/10.2175/106143015x14212658613956
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality. Ghana Environ Earth Sci. https://doi.org/10.1007/s12665-015-5105-0
Central Ground Water Board (CGWB) (2007) Groundwater information, Ranga Reddy District, Andhra Pradesh. CGWB, Ministry of Water Resources, Government of India
Central Groundwater Board (CGWB) (2017) Groundwater year book of Telangana State, 2016-17, CGWB, Ministry of Water Resources, Government of India
Central Groundwater Board (CGWB) (2019) Groundwater year book of Telangana State, 2018-19, CGWB, Ministry of Water Resources, Government of India
Chaurasia AK, Pandey HK, Tiwari SK et al (2018) Groundwater quality assessment using water quality index (WQI) in parts of Varanasi District, Uttar Pradesh, India. J Geol Soc India 92:76–82. https://doi.org/10.1007/s12594-018-0955-1
Chen J, Qian H, Wu H, Gao Y, Xinyan Li (2017a) Assessment of arsenic and fluoride pollution in groundwater in Dawukou area, Northwest China, and the associated health risk for inhabitants. Environ Earth Sci 76:314–328
Chen J, Wu H, Qian H, Gao Y (2017b) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid Region of Northwest China. Expo Heal 9:183–195. https://doi.org/10.1007/s12403-016-0231-9
Davis SN, DeWiest RJ (1966) Hydrogeology. Weily, New York
Durov SA (1948) Classification of natural waters and graphical representation of their composition. Dokl Akad Nauk USSR 59:87–90
FAO (2017) Water pollution from agriculture: a global review. FAO, IWMI 35. http://www.fao.org/3/a-i7754e.pdf
Freeze RA, Cherry JA (1979) Groundwater, illustrate. Prentic-Hall, New Jersey
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170(80):1088–1090
Holbrook WS, Marcon V, Bacon AR et al (2019) Links between physical and chemical weathering inferred from a 65-m-deep borehole through Earth’s critical zone. Sci Rep. https://doi.org/10.1038/s41598-019-40819-9
Houatmia F, Azouzi R, Charef A, Bédir M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern, Tunisia. Environ Earth Sci. https://doi.org/10.1007/s12665-016-5441-8
Jia H, Qian H, Zheng L, Feng W, Wang H, Gao Y (2020) Alterations to groundwater chemistry due to modern water transfer for irrigation over decades. Sci Total Environ 717:13770
Kurakalva RM, Aradhi KK, Mallela KY, Venkatayogi S (2016) Assessment of groundwater quality in and around the Jawaharnagar municipal solid waste dumping site at greater Hyderabad, Southern India. Proc Environ Sci 35:328–336. https://doi.org/10.1016/j.proenv.2016.07.013
Luo W, Gao X, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the yuncheng basin, China—an area with complex hydrogeochemical conditions. PLoS One. https://doi.org/10.1371/journal.pone.0199082
Magesh NS, Krishnakumar S, Chandrasekar N, Soundranayagam JP (2013) Groundwater quality assessment using WQI and GIS techniques, Dindigul district, Tamil Nadu, India. Arab J Geosci 6:4179–4189. https://doi.org/10.1007/s12517-012-0673-8
Nagaraju A, Sunil Kumar K, Thejaswi A (2014) Assessment of groundwater quality for irrigation: a case study from Bandalamottu lead mining area, Guntur District, Andhra Pradesh, South India. Appl Water Sci. https://doi.org/10.1007/s13201-014-0154-1
Narsimha A, Rajitha S (2018) Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2018.1438176
Oster JD (1994) Irrigation with poor quality water. Agric Water Manag 25:271–297
Piper AM (1994) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Prakash A (2014) The periurban water security problem: a case study of Hyderabad in Southern India. Water Policy 16:454–469. https://doi.org/10.2166/wp.2013.140
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, Southern India. Geosci Front 8:117–124. https://doi.org/10.1016/j.gsf.2016.01.002
Rama Mohan K, Keshav Krishna A (2018) Assessment of groundwater quality and identification of hydrogeochemical process in hard rock terrain. In: Singh V, Yadav SYR (eds) Groundwater quality series. Springer, Singapore, pp 147–170
Re V, Sacchi E, Mas-Pla J et al (2014) Identifying the effects of human pressure on groundwater quality to support water management strategies in coastal regions: a multi-tracer and statistical approach (Bou-Areg region, Morocco). Sci Total Environ 500–501:211–223. https://doi.org/10.1016/j.scitotenv.2014.08.115
Sajil Kumar PJ, James EJ (2016) Identification of hydrogeochemical processes in the Coimbatore district, Tamil Nadu, India. Hydrol Sci J. https://doi.org/10.1080/02626667.2015.1022551
Sandya R (2014) Palaeomagnetism and anisotropy of magnetic susceptibility study on Dykes rocks of greater Hyderabad and Ranga Reddy Distract Andhra Pradesh Granitic Region. Osmania University, Hyderabad
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn. McGraw-Hill, New York
Schoeller H (1965) Qualitative evaluation of groundwater resources. Methods and techniques of groundwater investigation and development. UNESCO, Geneva, pp 44–52
Schoeller H (1977) Geochemistry of groundwater. In: Groundwater studies-an international guide for research and practice, pp 1–18
Selvakumar S, Chandrasekar N, Kumar G (2017) Hydrogeochemical characteristics and groundwater contamination in the rapid urban development areas of Coimbatore, India. Water Resour Ind 17:26–33. https://doi.org/10.1016/j.wri.2017.02.002
Shanmugam P, Ambujam NK (2012) A hydrochemical and geological investigation on the Mambakkam mini watershed, Kancheepuram District, Tamil Nadu. Environ Monit Assess 184:3293–3306. https://doi.org/10.1007/s10661-011-2189-1
Siebert S, Burke J, Faures JM, Frenken K, Hoogeveen J, Doll P, Portmann FT (2010) Groundwater use for irrigation—a global inventory. Hydrol Earth Syst Sci 14:1863–1880
Singh S, Hussain A (2016) Water quality index development for groundwater quality assessment of Greater Noida sub-basin, Utta Prades, India. Cogent Eng 3:1177155
Srisailam G, Narisinga DW, Vaseem Md, Kolli SK (2017) Assessment of ground water quality in Medchal-Malkajgiri district. Appl Sci Engg Technol 5:929–934
Tiwari AK, Singh AK, Mahato MK (2018) Assessment of groundwater quality of Pratapgarh district in India for suitability of drinking purpose using water quality index (WQI) and GIS technique. Sustain Water Resour Manag 4:601–616. https://doi.org/10.1007/s40899-017-0144-1
Todd DK, Mays LW (2005) Groundwater hydrology, 3rd edn. Wiley
USEPA (2012) Integrated risk information system
USEPA (2014) Human health evaluation manual, Supplemental Guidance. Update of Standard Default Exposure Factors-OSWER Directive 9200.1–120. pp 6. Washington DC
USSL (1954) Diagnosis and improvement of salinity and alkaline soil. In: U.S.Department of Agricutlure Hand Book No. 60
Vaiphei SP, Kurakalva RM, Sahadevan DK (2020) Water quality index and GIS based technique for assessment of groundwater quality in Wanaparthy watershed, Telangana, Inida. Environ Sci Pollut Res 27(36):45041–45062
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K et al (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
WHO (2011) Guidelines for drinking-water quality, 4th edn. WHO, Geneva, p 340
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture Circular 969, Washington DC
WWAP (2016) The united nations world water development report 2015: water for a sustainable world. Fut Food J Food Agric Soc 4:64–65
Yadav KK, Gupta N, Kumar V et al (2018) GIS-based evaluation of groundwater geochemistry and statistical determination of the fate of contaminants in shallow aquifers from different functional areas of Agra city, India: levels and spatial distributions. RSC Adv 8:15876–15889. https://doi.org/10.1039/c8ra00577j
Yadav KK, Kumar S, Pham QB, Gupta N, Rezania S et al (2019) Fluoride contamination, health probems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol Environ Saf 182:109362
Yadav KK, Kumar V, Gupta N, Kumar S, Rezania S, Singh N (2019) Human health risk assessment: study of a population exposed to fluoride through groundwater of Agra city, India. Regul Toxicol Pharmacol 106:68–80
Zhang X, Miao J, Hu BX et al (2017) Hydrogeochemical characterization and groundwater quality assessment in intruded coastal brine aquifers (Laizhou Bay, China). Environ Sci Pollut Res 24:21073–21090. https://doi.org/10.1007/s11356-017-9641-x
Zolekar RB, Todmal RS, Bhagat VS, Bhailume SA, Korade MS, Das S (2021) Hydro-chemical characterization and geospatial analysis of groundwater for drinking and agricultural usage in Nashik district in Maharashtra, India. Environ Dev Sustain 23:4433–4452. https://doi.org/10.1007/s10668-020-00782-2
Acknowledgements
The authors wish to express their thanks to Dr. V.M. Tiwari, Director, CSIR-NGRI, Hyderabad, India for permission and encouragement to publish this work. They are thankful to the summer trainees who assisted in sampling of groundwater. The authors also wish to acknowledge the support received from Environmental Geochemistry Labs at CSIR-NGRI, Hyderabad, India. Sincere thanks are due to the Editor and anonymous reviewers for their constructive comments and suggestions that improved the original version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a Topical Collection in Environmental Earth Sciences on Groundwater quality and contamination and the application of GIS, guest edited by Narsimha Adimalla and Hui Qian.
Rights and permissions
About this article
Cite this article
Kurakalva, R.M., Kuna, G., Vaiphei, S.P. et al. Evaluation of hydrogeochemical profile, potential health risk and groundwater quality in rapidly growing urban region of Hyderabad, South India. Environ Earth Sci 80, 383 (2021). https://doi.org/10.1007/s12665-021-09661-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09661-z