Abstract
Exposure to arsenic and fluoride through contaminated drinking water can cause serious health effects. In this study, the sources and occurrence of arsenic and fluoride contaminants in groundwater are analyzed in Dawukou area, northwest China, where inhabitants rely on groundwater as the source of drinking water. The triangular fuzzy numbers approach is adopted to assess health risk. The fuzzy risk assessment model incorporates the uncertainties that are caused by data gaps and variability in the degree of exposure to contaminants. The results showed that arsenic and fluoride in groundwater were mainly controlled by the dissolution–precipitation of Ca-arsenate and fluorite under weakly alkaline conditions. The arsenic and fluoride concentrations were higher in the shallow groundwater. The most probable risk values for arsenic and fluoride were 4.57 × 10−4 and 0.4 in the shallow groundwater, and 1.58 × 10−4 and 0.3 in the deep groundwater. Although the risks of fluoride were almost within the acceptable limit (<1.0), the risk values of arsenic were all beyond the acceptable levels of 10−6 for drinking water. Further, the local administration should pay more attention to the potential health risk through dietary intake and to the safety of deep water by ensuring it is not contaminated under prolonged pumping conditions. The fuzzy risk model treats the uncertainties associated with a quantitative approach and provides valuable information for decision makers when uncertainties are explicitly acknowledged, particularly for the variability in contaminants. This study can provide a new insight for solving data uncertainties in risk management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an essential resource that provides drinking water to billions of people around the world (UNESCO 2004). Assurance of drinking water safety is critically important to a healthy and viable community. Although tensions related to the quality of groundwater are expected to rise due to intensified human interventions, most large-scale sources of contamination in groundwater have been recognized as related to geological origins. The interaction of groundwater with aquifer minerals and the increased potential in aquifers for the generation of the physicochemical conditions are likely to escalate the release of geogenic contaminants (Smedley 2008; Li et al. 2013a, b).
Arsenic (As) and fluoride (F) are the most significant inorganic pollutants in drinking water that occur naturally in the Earth’s crust (Jianmin et al. 2015). Increased arsenic exposure has been reported to cause adverse health effects such as cardiovascular disease, diabetes, neoplastic respiratory changes, skin lesions, fetal anomaly, child development issues and various cancers (e.g., bladder, skin, kidney, lungs, liver and prostate cancers) (Smith et al. 1992; Zheng and Ayotte 2015). To protect consumers against the effects of chronic arsenic exposure, the World Health Organization (WHO) in 1993 adopted a stringent standard for arsenic in drinking water, having acceptable arsenic concentration reduced from 50 to 10 μg/L (WHO 2011). In comparison, the presence of fluoride in drinking water, at concentrations less than 1.0 mg/L, is generally beneficial to strengthen the apatite matrix of skeletal tissues and teeth (Maithani et al. 1998). Although fluorine has long been found to be an essential micronutrient for human, fluoride concentrations above the permissible limit of 1.5 mg/L in drinking water may result in dental fluorosis or, at its worst, crippling skeletal fluorosis (Ayoob and Gupta 2006; WHO 2011). In this context, exposure to geogenic arsenic and fluoride in groundwater has been a serious public health concern in many countries, such as China, Korea, Bangladesh, Mexico, Thailand, Argentina, Canada and the USA, where shallow or/and deep wells constitute the dominant sources of drinking water (Grantham and Jones 1977; Frost et al. 1993; Bundschuh et al. 2004; Smedley et al. 2005; Yu et al. 2007; Armienta and Segovia 2008; Ahn 2012; Li et al. 2014a).
The human health risk assessment (HHRA) model, which was proposed by the US Environmental Protection Agency (EPA 1989), is a prevalent and effective approach to estimate the nature and probability of adverse health effects in humans exposed to contaminants. The quantified risks of potential health hazards provide a basis in decision-making processes (Weisburger 1994; Li et al. 2014b; Chen et al. 2016). However, mean values or recommended values of parameters are often used to assess health risk based on the HHRA model. The imperfect knowledge includes ambiguities in contaminant concentration, fuzziness in ages, in weights and even in general health status. Due to the inherent limitations of the conventional model, uncertainties caused by data gaps and variability in the degree of exposure to contaminants inevitably harm the quality of data transmissions. Inaccurate or incomplete information will be provided to the concerned health professionals and regulatory agencies. An efficient approach is desired to assess human health risk under uncertainties.
Due to lack of sufficient information, uncertainty also appears as randomness inherent in other risk assessment models. To solve such problems, the concepts of fuzzy theory have been widely used for providing a mathematical framework to treat imprecise and uncertain data. Mujumdar and Sasikumar (2002) considered the seasonal variations of river flow to specify seasonal fraction removal levels for the pollutants and then developed a fuzzy optimization model for the seasonal water quality management of river systems. Branisavljevic and Ivetic (2006) used fuzzy set theory to address uncertain parameters in water distribution system simulation models. Jadidi et al. (2014) introduced the α-cut method as a measure of evidential support to describe the nature and level of spatial uncertainty in coastal erosion risk assessment. More related studies can be found in Cho et al. (2002), Li et al. (2007) and Hill et al. (2012). These studies reveal that uncertainties regarding the inadequate information can be expressed quantitatively and qualitatively based on the fuzzy theory. The triangular fuzzy numbers (TFN) approach is a concept of fuzzy set, which is conducive to solve problems under uncertainties and information imprecision (Zadeh 1965, 1976). This approach allows mathematical operators to be applied to the fuzzy domain and addresses uncertainties qualitatively and quantitatively (Jin et al. 2012). Systems based on this approach are flexible with respect to the available input data. As such, the uncertain parameters in the HHRA model can be represented by triangular fuzzy numbers. Nonetheless, there is a great concern in developing a coupling model based on the TFN approach and the conventional health risk assessment model. Considering the multiple uncertain sources with different levels of detail, it is necessary to identify the fuzziness and effectiveness in the coupling model. But in fact, there is a dearth of literature on the potential quantitative impact of the uncertainties and variabilities on the risk assessment.
Yinchuan Plain is labeled as a “high As and F groundwater” area in northwest China (Wen et al. 2013). According to previous studies (Li and Qian 2011; Han et al. 2013), groundwater with arsenic concentrations exceeding 10 μg/L and with extremely large concentrations of fluoride (maximum values of 5.2 and 4.9 mg/L in shallow and deep groundwater) was observed in the northern part of the plain. Dawukou area, located in the northern part of Yinchuan Plain, is the province’s second largest economic hub and is rich in mineral resources (including coal, silica, pyrite). Due to the limited availability of surface water, groundwater is the sole source of drinking water for the urban and rural communities in this area. Long-term exposure to arsenic and fluoride in drinking water may cause adverse health effects to the locate residents. Despite the importance of groundwater, what is less clear is the distribution of dissolved arsenic and fluoride in the groundwater in Dawukou area and the corresponding health hazards. Findings from previous large-scale studies (such as Qian et al. 2012; Wen et al. 2013; Han et al. 2013) are insufficient to provide specific insights to the local groundwater authorities.
The aims of the present study were to provide a detailed discussion about the status of arsenic and fluoride contamination in the aquifer system in Dawukou area. A methodology was developed by coupling TFN approach and the conventional HHRA model for assessing potential hazards associated with contaminated water consumption under uncertainties. Further, the potential impact of inadequate information on the health risk assessment was studied. This study will help to recognize the uncertainties on health risk assessment and provide insights into risk management.
Materials and methods
Location
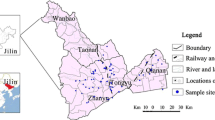
The study area is located in the north of Ningxia within longitudes 106°15′52″–106°37′21″E and latitudes 38°52′12″–39°9′15″N (Fig. 1). It covers an area of roughly 700 km2 and has a population of 0.48 million. The region has an arid climate with rare precipitation and intense evaporation. The annual average evaporation is about 1800 mm, which is ten times the annual average precipitation (about 180 mm). Approximately 60% of annual rainfall occurs during July–September.
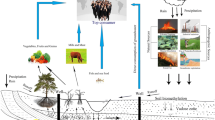
According to the hydrogeological characteristics of the area, the pore water in the Quaternary sediments can be divided into two aquifer systems (Qian et al. 2012) (Figs. 1, 2). Single phreatic aquifer is located in the west of the study area, which is mainly composed of gravel. From west to east, the grain size of aquifer materials decreases. The multiple layers of alluvial and lacustrine deposits include a phreatic aquifer with depths between 10 m and 40 m, an upper confined aquifer with depths between 25–60 and 140–160 m, and a lower confined aquifer with depths between 140 and 240–260 m (Han et al. 2013). These aquifers are separated by continuous aquitards. In this study, the phreatic aquifer shallower than 40 m depth is designated as the shallow aquifer. The depth from the groundwater surface to the water table is less than 4 m in the study area. When the depth is more than 40 m, it is designated as the deep aquifer.
Due to the relatively good quality of water, groundwater from the deep aquifer is the main source of potable water. No. 1 and No. 2 water source sites (Fig. 1) are two important water supply frameworks in this area. The depths of the supply wells are more than 100 m deep. Total groundwater abstractions are 7.72 million m3 per year for No. 1 water source and 6.39 million m3 per year for No. 2 water source, serving about 0.14 and 0.09 million people for drinking purposes, respectively. Apart from the public water supply system, other local communities primarily depend, for their drinking water supply, on the shallow groundwater that is pumped from private wells. Shallow groundwater is also used to irrigate crops in the plain.
Sampling and measurements
A total of 46 groundwater samples were collected from hand-pumping wells in March 2014. These wells were constructed by local drillers to a depth of 10–30 m at the request of the inhabitants. Additionally, 24 samples were collected from the output water supply wells at No. 1 and No. 2 water sources. Monitoring of the water supply wells (No. 1 and No. 2) was conducted from January to December 2013, and two samples were separately collected from the two water sources per month. To exclude the impacts of groundwater pumping that would be present in the samples from the water supply wells, ten more samples were collected from separately drilled monitoring wells that are far away from the water supply wells in the deep aquifer in March 2014.
The sample locations were recorded by a handheld GPS device in the field (Fig. 1). The samples were stored in a cooler box (below 4 °C) immediately after collection. For all the collected samples, pH, TDS and temperature were measured using portable pH and TDS meters in the field. Chemical analyses (Na+, K+, Ca2+, Mg2, SO4 2−, Cl−, HCO3 −, NO3 −, As and F) were performed at the Laboratory of Environmental, Geological, Monitoring and Survey Ministry (Ningxia) within 24 h of collection. All the samples were transported to the laboratory in a cooler box until performing physicochemical analysis. Each groundwater sample was analyzed by using technical guidelines established for environmental monitoring of groundwater by the State Environmental Protection Administration (2004). During the analysis, duplicates were introduced for QA/QC. Concentrations of arsenic and fluoride in the groundwater samples were determined by hydride generation atomic fluorescence spectrometry (HG-AFS) and ion chromatography (ICS-90A), respectively. The detection limit of arsenic was 0.2 μg/L, and it was 2.5 μg/L for fluoride.
Human health risk assessment
Triangular fuzzy numbers approach
A triangular fuzzy number is defined as \(\tilde{A} = (a_{1} ,a_{2} ,a_{3} )\) where a 1 < a 2 < a 3. The a 1, a 2 and a 3 denote the lower, expected and upper values of a fuzzy variable, respectively. Its membership function can be obtained using the following equations:
Triangular fuzzy numbers can be transformed to interval numbers corresponding to different confidence levels:
where α is the confidence level in the range of 0–1. \(\tilde{A}^{\alpha }\) represents a set of triangular fuzzy numbers that is associated with the confidence level not less than α. By the extension principle (Zadeh 1965), the fuzzy addition, subtraction, multiplication and division of triangular fuzzy numbers are also triangular fuzzy numbers. Let \(\tilde{M}\) = (m 1, m 2, m 3) and \(\tilde{N}\) = (n 1, n 2, n 3) be two TFNs. The following operational properties can be established:
Health risk assessment model based on TFN
In the present paper, the HHRA model was applied to estimate the adverse effects of contaminants on adults through ingestion. The carcinogenic risk of arsenic (R As) and non-carcinogenic risk of fluoride (HQ F) can be calculated by the following equations (EPA 1989; Li et al. 2016):
where D i is the exposure dose through ingestion of arsenic or fluoride (mg/kg per day); C i is the concentration of arsenic or fluoride in groundwater (mg/L); IR represents the ingestion rate (L/day); EF is the exposure frequency (days/year); ED is the exposure duration (years); BW represents the average body weight (kg); AT represents the averaging time of exposure (days); q As is the carcinogenic coefficient of carcinogenic pollutant arsenic by the drinking pathway (kg day mg−1); and RfD F is the reference dosage of non-carcinogenic pollutant fluoride (mg kg−1 day−1). According to the database of Integrated Risk Information System (EPA 2012), the reference values of q As and RfD F were taken as 1.5 kg day mg−1 and 0.06 mg kg−1 day−1, respectively (Li et al. 2016).
In Eq. (9), the EF value is 365 days/year for the residents by ingestion of chemicals in drinking water. Thus, Eq. (9) can be simplified as:
The USEPA typically uses a target reference risk range of 10−4–10−6 for carcinogens through ingestion, and the level of 10−6 has been seen as a gold standard for drinking water (Hunter and Fewtrell 2001). Adverse effects on human health may be caused by arsenic in groundwater when R As > 10−6. In addition, the permissible level for non-carcinogens is 1.0 (Li et al. 2016). If HQ F is greater than 1.0, it indicates the potential adverse systemic health effects for the exposed populations. When HQ F < 1.0, the risk of fluoride is within an acceptable level.
For a specific group, the adult weight and the daily water quantity are very likely to be specific. In this study, to provide valuable information and quantify the impact of data gaps and variability in contaminant on the health risk assessment, the arsenic and fluoride concentrations (C As and C F), adult weight (BW) and daily water quantity (IR) were all defined as triangular fuzzy numbers. Thus, the health hazard risk levels with different confidence levels could be calculated as follows:
According to Gardner and Altman (1986), and Nazir and Khan (2006), these variables (data in body weight and concentration of pollutants in water environment) generally followed Gaussian distribution or approximate Gaussian distribution. With this assumption, more than 95% of the data lie within the interval of the upper and lower limits, that is, mean ± twice the standard deviation. Accordingly, the lower, mean and upper values of triangular fuzzy numbers can be obtained based on mathematical analysis methods.
Results and discussion
As and F concentrations in the groundwater
In the study area, individual wells possessed considerable variability in arsenic concentrations at a local spatial scale (Fig. 3a). Due to the heterogeneity in the sediment texture, arsenic concentration below the detection limit was observed in 22 shallow groundwater samples (Tables 1, 2). Apart from these samples, the recorded arsenic concentration in the shallow wells ranged from 1 to 22 μg/L with a mean value of 7.48 μg/L. 13% of the shallow water samples had high arsenic concentrations exceeding the WHO safe drinking guideline (10 μg/L). For the deep wells, arsenic was observed only in the two water supply wells with a mean concentration of 6.7 μg/L (Fig. 3b). Interestingly, the seasonal variations of arsenic concentrations in the water supply wells were quite distinct. The arsenic concentrations were fairly stable in No. 1 water source throughout the year, while significant changes in arsenic levels were observed over time for No. 2 water source (Fig. 4). There was a nearly twofold increase in the arsenic concentrations for No. 2 water source during January–March.
The fluoride concentrations in most samples were within 1.5 mg/L except for three shallow water samples and one deep water sample (Table 1). The recorded fluoride values were in the range of 0.06–2.2 mg/L (the mean value of 0.59 mg/L) for the shallow groundwater and 0.1–2.8 mg/L (the mean value of 0.44 mg/L) for the deep groundwater, respectively. The distribution of fluoride in groundwater also showed distinct spatial heterogeneity. Fluoride concentration was found relatively higher in the northern part (Fig. 3c, d). The spatial variations of fluoride are very similar in No. 1 and No. 2 water sources, and the concentrations decreased during April–September and November (Fig. 4).
Sources and co-occurrence of As and F in groundwater
The mineralogical origins of arsenic in groundwater are commonly presumed to be arsenic-containing sulfide minerals (Ehrlich and Newman 2009). The recorded arsenic concentrations in the shallow Quaternary sediment in the northern part of Yinchuan Plain (4.2–49.8 mg/kg, Han et al. 2013) were beyond the mean levels in the earth (1.5–2.0 mg/kg) (Francisca and Perez 2009), suggesting As-enriched conditions in the area. As the redox condition of water exerts a significant control on its chemistry (Ohio EPA Ohio 2014), the presence of Fe (>0.1 mg/L) and NH4–N in groundwater commonly indicates anoxic conditions. In the study area, the shallow aquifer was primarily under Fe-reducing conditions as indicated by the observed Fe and NH4–N enrichment (Fig. 5a). Evidence of the negative oxidation–reduction potential (ORP) values from Han et al. (2013) also supports the anoxic conditions at the end of the flow path in the catchment of Yinchuan Plain. Consequently, the desorption of arsenic must have happened prior to the adsorption of arsenic to the iron minerals that are carrier phases. This is consistent with the observation of Rodríguez-Lado et al. (2013) that the natural arsenic in groundwater for China was primarily controlled by the reducing conditions. However, arsenic concentrations did not show significant correlation with Fe levels in the groundwater (Fig. 5b). It may be attributed to differential sequestration of As and Fe into sulfide minerals (McArthur et al. 2001; Buschmann and Berg. 2009), or the formation of other phases (e.g., siderite FeCO3) (Herbel and Fendorf 2006). Besides, due to the relatively lower Fe and NH4–N levels in the deep water, the differential sequestration is unable to explain the sources of arsenic in the water supply wells.
The saturation state of minerals affecting the arsenic and fluoride concentrations is determined using PHREEQC (Parkhurst and Appelo 2001). The study area is rich in mineral pyrite. Scorodite (FeAsO4·2H2O) is the main component of pyrite, and thus, it is considered as the potential source of arsenic in groundwater. In China, high concentrations of arsenic (>10 μg/L) were commonly found in the Ca-HCO3 type, indicating the geogenic origins (Guo et al. 2013). In the context, the saturation indices of Fe-arsenate (Scorodite, FeAsO4·2H2O) and Ca-arsenate (Ca3(AsO4)2·4H2O) were calculated (Fig. 6a, b). Groundwater samples had negative saturation indices of Fe-arsenate (Scorodite, FeAsO4·2H2O) and Ca-arsenate (Ca3(AsO4)2·4H2O), which were from −10.3 to −5.77 and from −14.95 to −9.66, respectively. When compared to Fe-arsenate, Ca-arsenate was the primary contributor to arsenic in groundwater in the study area. Figure 6b reveals positive logarithmic relationship between the arsenic levels and the saturation indices values. Although the correlation was not obvious for deep water due to the limited samples, the fitting curve shows a “best fit” to the data points for shallow water (Fig. 6b). Arsenic could be released from the minerals as follows:
Dissolution of fluorite (CaF2) is considered as the dominant mechanism responsible for fluoride in groundwater (Reaction 3). As shown in Fig. 6c, fluoride had strong positive correlation with the saturation indices of fluorite, indicating the contribution of fluorite dissolution. Importantly, small hydraulic gradients were beneficial for releasing fluoride from F-bearing materials through the greater impacts of water–rock interaction.
In view of hydrochemistry, groundwater with enriched arsenic and fluoride is commonly weakly alkaline (pH: 7.2–8.8) with high Na and HCO3 concentrations (Ahn 2012; Wen et al. 2013). The weakly alkaline conditions (pH values of 7–8.45) (Fig. 7a, b) observed in the study area suggested that the mobility of arsenic and hydrolysis (OH− in water exchanges for F−) of F-enriching silicates could be promoted under the conditions. Chloro-alkaline indices (CAI-I and CAI-II) proposed by Schoeller (1965) were used in the present study to reveal the occurrence of cation exchange in groundwater. Given that cation exchange was observed in the majority of the samples (Fig. 8), desorption of HAsO4 2− and F− was facilitated by the positive surface charge density around hydrous metal oxide sorption sites in the presence of Na. Desorption of arsenic oxyanions occurred in response to a change from Ca-rich to Na-rich pore water, and hence, arsenic was positively correlated with Na in high-arsenic groundwater (>10 μg) (Fig. 7c). The poor correlation between F and Na may be due to cation exchange and dissolution of other minerals (such as CaSO4, Na2SO4 and MgSO4) (Fig. 7d).
Additionally, high value of HCO3 − is another possible factor that caused the desorption of F− and HAsO4 2− by serving as a competitor for sorption sites. Positive correlations of HCO3 − with As and F (r 2 = 0.49 and 0.20, respectively) were reported in Yuncheng basin of China, where As and F levels were significantly high in groundwater (Wen et al. 2013). However, the effects of HCO3 − appeared insignificant in this study as suggested by the weak correlations shown in Fig. 7e, f. Therefore, despite the causal link between the co-occurrence of arsenic and fluoride and the favorable hydrogeochemical environment, arsenic and fluoride concentrations in groundwater are controlled by the combined interactions between geology, geomorphology and hydrochemistry.
Human health risk assessment
As stated before, the intervals of the upper and lower concentrations for arsenic and fluoride in groundwater were obtained. According to the statistical data (National Census Bureau 2010; Ningxia statistical Bureau 2012), the adult weight estimated for the people residing in the area was in the range of 45–63 kg. The water quantity for drinking ranged from 1.6 to 2.8 L/day (Yang et al. 2005). As such, the lower, expected and upper values of the variables were obtained (Table 3).
In Fig. 9, the interval values of health risk of ingesting water containing arsenic and fluoride are related to the confidence levels. The lower and upper health risks were the obtained values with respect to α = 0. Great fuzziness was observed in health risks when uncertainties were considered. Elevated fuzzy risks were closely linked to the decreased confidence levels. When α = 0, the interval values of the carcinogenic risks of arsenic in the shallow and deep groundwater were 5.33 × 10−5–1.39 × 10−3 and 1.33 × 10−5–2.41 × 10−4, respectively. The most probable values in the shallow and deep groundwater were equal to 4.57 × 10−4 and 1.58 × 10−4, respectively. For fluoride, the interval values (α = 0) of the non-carcinogenic risks in the shallow and deep groundwater were in the ranges of 0.04–1.13 and 0.06–1.10, respectively. The most probable values of fluoride in shallow and deep groundwater were 0.4 and 0.3, respectively. The health risks caused by arsenic and fluoride were higher in the shallow groundwater. The risk values of fluoride were almost less than 1.0, but all the values of risk levels of arsenic were above the acceptable levels of 10−6 as recommended by the USEPA and the Ministry of Environmental Protection of the People’s Republic of China (MEP) for drinking water. This implies that health problems may occur in the arsenic-affected areas, and the ingestion of groundwater from the No. 1 water supply wells may also increase the adverse health effects.
When interpreting the quality of results for decision-making purpose, a detailed qualitative evaluation of uncertainty analysis is helpful. In this study, the arsenic levels in deep groundwater were taken as an example. Table 4 lists seven scenarios (A1–A6) to assess the corresponding health hazards based on the TFN approach. For instance, in the case of A1, the mean value of arsenic and the triangular fuzzy numbers of W and Q were used to calculate the potential hazards to the public.
According to the results of various scenarios (Table 4), there was no difference among the most probable risks of arsenic. It implies that the most probable values of risks are only controlled by the expected values of parameters (mean values). When the confidence level α = 1 is required to make suitable decisions in risk management, it is more convenient to evaluate the health risks using the mean values of parameters. Unlike the most probable risks, the effects of variations in C As, BW and IR on the risk assessment are clearly distinct when α = 0. When the variabilities of BW and IR within the population were ignored, the lower and the upper values of risks of A6 were close to the calculated values for A2–A4. However, the calculation results between A1 and A2–A4 were quite different. More information was lost in A1, thus showing the smallest interval value of the carcinogenic risks (1.38 × 10−4–1.72 × 10−4). Similar effects of uncertainties on human health risks were also found for fluoride in deep groundwater (Table 5). There was a relatively narrower range of non-carcinogenic risk when the fluoride concentrations were fixed. According to the above discussion, the TFN approach helps to provide an adequate characterization of uncertainties from various sources. Most importantly, variability in contaminant was the determinant source of uncertainty in the evaluation of health risk based on the TFN approach.
Implications for groundwater management
Inorganic salts of arsenic and fluoride are tasteless and odorless, and thus, it is difficult to detect their toxicity to humans in daily life. Indeed, inhabitants are exposed to arsenic and fluoride not only through drinking water, but also through other approaches, such as dietary intake. The uptakes of arsenic and fluoride by grain crops (cereals, vegetables and fruits) are dependent on the related concentrations in agricultural soil and irrigated water, and on the species of plants (Brahman et al. 2014). Irrigating with As-contaminated water had been linked to higher levels of arsenic in rice and vegetables in Bangladesh. Williams et al. (2005) found that rice in the contaminated area had an average of ~200 μg/kg arsenic. If a 60-kg person consumed 0.5 kg of rice per day, rice would contribute 1.7 μg per kg body mass per day, which was close to the daily maximum tolerable intake of arsenic (2 μg per kg body mass per day). A positive correlation between the arsenic level in rice and the arsenic level in irrigation water (groundwater) was reported subsequently (Williams et al. 2006). Besides, the use of irrigation water containing high fluoride concentration has also resulted in fluoride accumulation in soil and vegetation (Poonam et al. 2013). In Dawukou area, groundwater is a vital water source for irrigation purpose, particularly during the dry periods. Yinchuan Plain has been one of the four grain production bases in northwest China. Thousands of shallow wells drilled in the plain are to ensure crop yields. Given the As-contaminated and F-contaminated groundwater, potential health risks associated with dietary intake remain a serious concern in the study area.
In recent years, due to intensified human activities, concerns have been raised about the sustainable development of deep groundwater (e.g., Radloff et al. 2011; Winkela et al. 2011; Park et al. 2014). Investigation in Mekong Delta region indicated that persistent deep groundwater extraction could cause interbedded clays to compact and consequently expel the water containing dissolved arsenic or arsenic-mobilizing solutes (e.g., dissolved organic carbon and competing ions) to deep aquifers over decades (Erbana et al. 2013). The water table can be lowered under prolonged pumping conditions. This implies that aquifer leakage from the phreatic aquifer to the confined aquifer seems easier to occur in the vicinity of cone for No. 2 water source. In the meantime, the seasonal variation in arsenic and fluoride concentrations in the two water sources revealed that decreasing rates of arsenic and fluoride concentrations were observed approximately during irrigation period (May–September and November) (Fig. 4). Dilution of minerals and mixing of water between the adjacent aquifers are more likely to occur. Under these conditions, deep groundwater is facing a growing threat associated with persistent pumping. Once deep groundwater is polluted, it is hard and costly to remove arsenic, fluoride and other contaminants. A detailed discussion among concerned authorities should be made on how high-capacity pumping affects the quality of deep groundwater in the area. Due to insufficient knowledge and poor management, public agencies have little power to regulate consumption of water from private wells. In this context, central water supply system could be beneficial to control contamination in drinking water. Furthermore, extensive monitoring is recommended for ensuring the supply of safe drinking water.
Conclusions
This study integrated a detailed investigation of arsenic and fluoride levels in the groundwater with a systematic evaluation of their potential risks in Dawukou area, northwest China. The result showed that 13% of the shallow groundwater was unsuitable for drinking due to high arsenic concentration exceeding the permissible limit of the WHO (10 μg/L). Arsenic concentration was within the permissible limit for the deep aquifer. Fluoride concentration was beyond the WHO drinking guideline (1.5 mg/L) in 6.5% of the shallow water sources and in 2.9% of the deep water sources. The arsenic and fluoride in groundwater were primarily contributed by the dissolution of Ca-arsenate and fluorite under the weakly alkaline conditions.
The triangular fuzzy numbers approach served as an effective tool for characterizing uncertainties in the health risk assessment and for managing drinking water safety. Lower risks were observed in water from deep aquifer. The most probable values of fluoride in shallow and deep groundwater were less than 1.0, but the values of arsenic were all above acceptable levels of 10−6 for drinking water that recommended by the USEPA and the MEP of China. To calculate the most probable risk (α = 1), the expected values of parameters in practice should be utilized. In the fuzzy risk model, the risks were highly sensitive to the variation in contaminant concentrations. Better decisions can be made using the risk assessment model when uncertainties are explicitly acknowledged, particularly for the contaminants. This study can provide a new insight for solving the problems of risk assessment which contains uncertainties. Further work is still necessary to evaluate the mechanisms that determine the occurrence of arsenic and influence the spatial variations of arsenic and fluoride in deep aquifers.
References
Ahn JS (2012) Geochemical occurrences of arsenic and fluoride in bedrock groundwater: a case study in Geumsan County, Korea. Environ Geochem Health 34:43–54. doi:10.1007/s10653-011-9411-5
Armienta MA, Segovia N (2008) Arsenic and fluoride in the groundwater of Mexico. Environ Geochem Health 30(4):345–353. doi:10.1007/s10653-008-9167-8
Ayoob S, Gupta AK (2006) Fluoride in drinking water a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Brahman KD, Kazi TG, Baig JA, Afridi HI, Khan A, Arain SS, Arain MB (2014) Fluoride and arsenic exposure through water and grain crops in Nagarparkar, Pakistan. Chemosphere 100:182–189
Branisavljevic N, Ivetic M (2006) Fuzzy approach in the uncertainty analysis of the water distribution network of Becej. Civ Eng Environ Syst 23:221–236. doi:10.1080/10286600600789425
Bundschuh J, Farias B, Martin R, Storniolo A, Bhattacharya P, Cortes J, Bonorino G, Albouy R (2004) Groundwater arsenic in the Chaco-Pampean Plain, Argentina: case study from Robles county, Santiago del Estero Province. Appl Geochem 19(2):231–243
Buschmann J, Berg M (2009) Impact of sulfate reduction on the scale of arsenic contamination in groundwater of the Mekong, Bengal and Red River deltas. Appl Geochem 24:1278–1286
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, northwest China. Expo Health 8(3):349–359. doi:10.1007/s12403-016-0208-8
Cho HN, Choi HH, Kim YB (2002) A risk assessment methodology for incorporating uncertainties using fuzzy concepts. Reliab Eng Syst Saf 78:173–183
Ehrlich HL, Newman DK (2009) Geomicrobiology, 5th edn. CRC Press, Boca Raton
EPA (1989) Risk assessment guidance for superfund: human health evaluation manual (part A). Office of Emergency and Remedial Response, Washington
EPA (2012) Integrated Risk Information System. United States Environmental Protection Agency. http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction¼iris.showSubstanceList. 03 Aug 2012
Erbana LE, Gorelicka SM, Zebkerb HA, Fendorf S (2013) Release of arsenic to deep groundwater in the Mekong Delta, Vietnam, linked to pumping-induced land subsidence. PNAS 110:13751–13756
Francisca FM, Perez MC (2009) Assessment of natural arsenic in groundwater in Cordoba Province, Argentina. Environ Geochem Health 31:673–682. doi:10.1007/s10653-008-9245-y
Frost F, Franke D, Pierson K, Woodruff L, Raasina B, Davis R, Davies J (1993) A seasonal study of arsenic in groundwater, Snohomish County, Washington, USA. Environ Geochem Health 15(4):209–214. doi:10.1007/BF00146744
Gardner MJ, Altman DG (1986) Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J 292:746–750. doi:10.1136/bmj.292.6522.746
Grantham DA, Jones JF (1977) Arsenic contamination of water wells in Nova Scotia. J Am Water Works Assoc 69(12):653–657
Guo M, Guo Q, Jia Y, Liu Z, Jiang Y (2013) Chemical characteristics and geochemical processes of high arsenic groundwater in different regions of China. J Earth Sci Environ 35(3):83–96 (in Chinese)
Han S, Zhang F, Zhang H, An Y, Wang Y, Wu X, Wang C (2013) Spatial and temporal patterns of groundwater arsenic in shallow and deep groundwater of Yinchuan Plain, China. J Geochem Explor 135:71–78
Herbel M, Fendorf S (2006) Biogeochemical processes controlling the speciation and transport of arsenic within iron coated sands. Chem Geol 228:16–32
Hill LJ, Sparks RSJ, Rougier JC (2012) Risk assessment and uncertainty in natural hazards. Cambridge University Press, Cambridge
Hunter PR, Fewtrell L (2001) Acceptable risk. In: Fetrell L, Bartram J (eds) Water quality: guidelines, standards and health. IWA Publishing, London, pp 207–227
Jadidi A, Mostafavi MA, Bédard Y, Shahriari K (2014) Spatial representation of coastal risk: a fuzzy approach to deal with uncertainty. ISPRS Int J Geo Inf 3:1077–1100. doi:10.3390/ijgi3031077
Jianmin B, Yu W, Juan Z (2015) Arsenic and fluorine in groundwater in western Jilin Province, China: occurrence and health risk assessment. Nat Hazards 77:1903–1914
Jin J, Wei Y, Zou L, Liu L, Fu J (2012) Risk evaluation of China’s natural disaster systems: an approach based on triangular fuzzy numbers and stochastic simulation. Nat Hazards 62:129–139
Li P, Qian H (2011) Human health risk assessment for chemical pollutants in drinking water source in Shizuishan City, Northwest China. Iran J Environ Health 8(1):41–48
Li JB, Huang GH, Zeng GM, Maqsood I, Huang YF (2007) An integrated fuzzy-stochastic modeling approach for risk assessment of groundwater contamination. J Environ Manag 82(2):173–188
Li P, Wu J, Qian H (2013a) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225. doi:10.1007/s12665-012-2049-5
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013b) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32(3):195–206. doi:10.1007/s10230-013-0234-8
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014a) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. doi:10.1007/s12665-013-2691-6
Li P, Wu J, Qian H, Lyu X, Liu H (2014b) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36:693–712. doi:10.1007/s10653-013-9590-3
Li P, Li X, Meng X, Li M, Zhang Y (2016) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health 8(3):361–379. doi:10.1007/s12403-016-0205-y
Maithani PB, Gurjar R, Banerjee R, Balaji BK, Ramachandran S, Singh R (1998) Anomalous fluoride in groundwater from western part of Sirohi district, Rajasthan and its crippling effect of human health. Curr Sci 74(9):773–777
McArthur JM, Ravenscroft P, Safiullah S, Thirlwall MF (2001) Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res 37:109–117
Mujumdar PP, Sasikumar K (2002) A fuzzy risk approach for seasonal water quality management of a river system. Water Resour Res. doi:10.1029/2000WR000126
National Census Bureau (2010) The 6th nationwide population census bulletin. www.stats.gov.cn/tjsj/tjgb/rkpcgb/
Nazir M, Khan FI (2006) Human health risk modeling for various exposure routes of trihalomethanes (THMs) in potable water supply. Environ Model Softw 21:1416–1429. doi:10.1016/j.envsoft.2005.06.009
Ningxia statistical Bureau (2012) Ningxia statistical yearbook. China Statistics Press, Beijing
Ohio EPA (2014) Reduction–oxidation (redox) control in Ohio’s ground water quality. J Environ Manag 139:97–108
Park DK, Bae G, Kim S, Lee K (2014) Groundwater pumping effects on contaminant loading management in agricultural regions. J Environ Manag 139:97–108
Parkhurst DL, Appelo CAJ (2001) User’s guide to PHREEQC (version 2)—a computer program for speciation, batch reaction, one dimensional transport, and inverse geochemical calculations. USGS water resource investigation report 994259
Poonam S, Suphiya K, Mamta B, Vinay S (2013) Mapping of fluoride endemic area and assessment of F−1 accumulation in soil and vegetation. Environ Monit Assess 185(2):2001–2008
Qian H, Li P, Howard KWF, Yang C, Zhang X (2012) Assessment of groundwater vulnerability in the Yinchuan plain, northwest China using OREADIC. Environ Monit Assess 184(6):3613–3628. doi:10.1007/s10661-011-2211-7
Radloff KA, Zheng Y, Michael HA, Stute M, Bostick BC, Mihajlov I, Bounds M, Huq MR, Choudhury I, Rahman MW, Schlosser P, Ahmed KM, Geen A (2011) Arsenic migration to deep groundwater in Bangladesh influenced by adsorption and water demand. Nat Geosci 4(11):793–798
Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA (2013) Groundwater arsenic contamination throughout China. Science 341:866. doi:10.1126/science.1237484
Schoeller H (1965) Qualitative evaluation of groundwater resources, in methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 53–83
Smedley PL (2008) Sources and distribution of arsenic in groundwater and aquifers. In: Arsenic in groundwater: a world problem. Appelo, CAJ (ed) Proceedings of an IAH seminar, Utrecht, pp 4–32
Smedley PL, Kinniburgh DG, Nicolli HB, Barros AJ, Tullio JO, Pearce JM, Alonso MS (2005) Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl Geochem 20(5):989–1016
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267
State Environmental Protection Administration (2004) The technical specification for environmental monitoring of groundwater, HJ/T 164-2004. China Environmental Science Press, Beijing (in Chinese)
United Nations Educational Scientific and Cultural Organization (UNESCO) (2004) Groundwater resources of the world and their use. IhP Series on groundwater. No. 6. Unesco
Weisburger JH (1994) Environmental hazards, lifestyles and disease prevention. J Hazard Mater 39:129–133
Wen D, Zhang F, Zhang E, Wang C, Han S, Zheng Y (2013) Arsenic, fluoride and iodine in groundwater of China. J Geochem Explor 135:1–21. doi:10.1016/j.gexplo.2013.10.012
WHO (World Health Organization) (2011) Guidelines for drinking water quality, vol 4. World Health Organization, Geneva
Williams PN, Price AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540. doi:10.1021/es0502324
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain As for regions of Bangladesh irrigating paddies with elevated As in groundwaters. Environ Sci Technol 40:4903–4908
Winkela LHE, Trangb PTK, Lanb VM, Stengela C, Aminia M, Ha NT, Vietb PH, Berg M (2011) Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. PNAS 108(4):1246–1251. doi:10.1073/pnas.1011915108
Yang X, Li Y, Ma G, Hu X, Wang J, Cui Z, Wang Z, Yu W, Yang Z, Zhai F (2005) Study on weight and height of the Chinese people and the differences between 1992 and 2002. China J Epidemiol 26:489–493
Yu G, Sun D, Zheng Y (2007) Health effects of exposure to natural arsenic from groundwater and coal in China: an overview of occurrence. Environ Health Perspect 115(4):636–642
Zadeh LA (1965) Fuzzy sets. Inf Control 8:338–353. doi:10.1016/S0019-9958(65)90341-X
Zadeh L (1976) A fuzzy-algorithmic approach to the definition of complex or imprecise concepts. Int J Man-Mach Stud 8:249–291. doi:10.1016/S0020-7373(76)80001-6
Zheng Y, Ayotte JD (2015) At the crossroads: hazard assessment and reduction of health risks from arsenic in private well waters of Northeastern United States and Atlantic Canada. Sci Total Environ 505:1237–1247
Acknowledgements
The research was supported by the National Natural Science Foundation of China (41572236 and 41172212), the Doctoral Postgraduate Technical Project of Chang’an University (2014G5290005) and the Foundation for the Excellent Doctoral Dissertation of Chang’an University (310829150002 and 310829165005). Anonymous reviewers are sincerely acknowledged for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Qian, H., Wu, H. et al. Assessment of arsenic and fluoride pollution in groundwater in Dawukou area, Northwest China, and the associated health risk for inhabitants. Environ Earth Sci 76, 314 (2017). https://doi.org/10.1007/s12665-017-6629-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6629-2