Abstract
The hydrochemical characterization of groundwater is important to bring out its nature and usefulness. The main objective of this paper was to discuss the major ion chemistry of groundwater in the Mambakkam mini watershed. Besides its semi-arid nature, rapid socioeconomic development encourages a greater demand for water, which leads to uncontrolled groundwater development. The groundwater of the study area is characterized by the dominance of alkaline earth (Ca2+, Mg2+) and strong acids (Cl−, SO −4 ) over alkalies (Na+, K+) and weak acids (HCO −3 , CO −3 ) during both post-monsoon and pre-monsoon seasons of the year 2010, based on the hydrochemical facies. These have been probably derived from natural chemical weathering of rock minerals, ion exchange and anthropogenic activities of the fertilizer source. The classification based on the total hardness reveals that a majority of groundwater samples fall in the hard to very hard category during the pre-monsoon season. Based on the values of EC, SAR and RSC and the diagrams of USSL and Wilcox, most of the groundwater samples range from excellent to permissible for irrigation purposes, with a low alkalinity and high salinity hazard, except for a few samples in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many parts of India, especially in the arid- and semi-arid regions, due to the failure of the monsoon and scarcity of surface water, dependence on the groundwater resource has increased tremendously in recent years. Groundwater is a vital source of clean drinking and irrigation water in many of the world's semi-arid regions. This is increasingly the case due to the effects of population growth and climate change, which are causing severe stress to surface water supplies in these areas (Edmunds 2003). India supports more than 16% of the world's population with only 4% of the world's freshwater resources (Singh 2003). In the last decades, the progressive increase of water requirements and the consequent scarcity of its availability, together with its qualitative deterioration, must certainly be included among the most serious environmental problems of developing countries. Spatial and temporal monitoring of groundwater quality, such as salinity, hardness and other parameters and identifying their changes, have become imperative for water management and regulation, especially, in a rapidly urbanizing environment (Tirumalesh et al. 2010). In view of the growing water scarcity and the adverse impact of global climate change on water resources, it is essential that groundwater be used efficiently, equitably, and in an ecologically sound manner for both the present and future generations, so as to ensure the sustainable utilization of this vital resource (Jha and Chowdhury 2010). Groundwater quality monitoring and evaluation is one of the most important aspects in groundwater studies to protect valuable groundwater sources and management (Schot and Van der Wal 1992; Al-Futaisi et al. 2007; Nisi et al. 2008; Ma et al. 2009). Changes in the groundwater quality are due to rock–water interaction and oxidation–reduction reactions during the percolation of water through the aquifers (Krishna Kumar et al. 2009; Prasanna et al. 2010; Aghazadeh and Mogaddam 2010). Undesirable and soluble constituents in the water cannot be controlled after it enters the ground (Johnson 1979; Sastri 1994). Sami (1992) has explained that leaching of surficial salts, ion-exchange processes and residential time of groundwater in the aquifer causes hydrogeochemical variations in the groundwater. The chemical composition of groundwater is controlled by several factors that include composition of precipitation, anthropogenic activities, geological structure and mineralogy of the watershed and aquifers and geological processes within the aquifer medium (Andre et al. 2005). The hydrochemical study reveals the quality of water that is suitable for drinking, irrigation and industrial uses (Hossain et al. 2010; Jalali 2007; Yesilnacar and Gulluoglu 2008; Ravikumar et al. 2010). Subramani et al. (2005) have studied the groundwater quality and its suitability for drinking and agricultural use in the Chittar basin, Tamil Nadu. They have compared the analytical results with the recommended limits suggested by the World Health Organisation (WHO 1971). High total hardness and TDS indicate the unsuitability of groundwater for drinking and irrigation purposes. Sadashivaiah et al. (2008) evaluated the groundwater quality in Tumkur Taluk, Karnataka. In this case, the methods proposed by Piper, Wilcox, Todd and USSL classification have been used to study critically the hydrochemical characteristics of groundwater.
Chennai is one of the major metropolitan cities in Tamil Nadu, India which experiences acute water scarcity due to the absence of perennial rivers. The Mambakkam mini watershed is situated adjacent to Chennai. The urban demand for water is increasing, and the groundwater within the city is not appreciable; the situation forced the transfer of groundwater from the urban peripherals in the form of tankers and packaged bottles.The southern part of Chennai City depends primarily on the groundwater available in the southern suburban area (study area) for drinking and domestic needs. The private water tanker suppliers and packaged water industries utilize the land and water resources of the nearby villages. Most of the agricultural wells in the study area villages act as a source of the water market, and raw groundwater from the agricultural wells by the private water suppliers and treated groundwater by the water industries is marketed for urban uses (Packialakshmi et al. 2010). Most of the people (agricultural well owners) in the villages here are involved in the groundwater marketing; its proximity to the city has induced severe changes in the land use system, such as reduction of agricultural land and increased settlements due to urbanization activities (Mahalingam et al. 2010). Hence, investigating the existing hydrogeological system and groundwater quality is the next step towards sustainable management and development of water resources in the study area. The present study aims to analyse the hydrogeochemical system, to assess the effects of natural and anthropogenic activities and increased human population on the groundwater. Furthermore, the study examines the hydrochemical constituents related to its suitability for domestic and agriculture use in the Mambakkam mini watershed, Tamil Nadu.
Study area
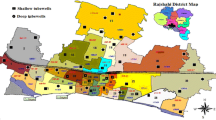
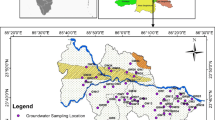
The Mambakkam mini watershed, Kancheepuram District of Tamil Nadu, India, is located south of Chennai City, and lies between the east longitudes 80°04′30″ to 80°15′35″ and the north latitudes 12°47′00″ to 12°56′30″. The aerial extent of the study area is 224 km2. The study area stretches from east to west for a width of 18 km from the sea coast towards the inland, and a length of 16 km along the north–south (Fig. 1). It comprises three blocks, namely Kattankulathur, St. Thomas Mount and Thiruporur of Kancheepuram district, covering 66 revenue villages. It lies on the east coast with plains, and slightly elevated areas on the west. The maximum and minimum elevations of the study area are between 2 and 10 m in the coastal plain, 10 and 25 m in the central region. The general slope is towards the east and north eastern direction. In the northwestern, central western and southwestern corners, there are hills and reserve forests. The trend of the coastal line is, more or less, uniform with a plain topography. There are no major rivers or system tanks other than some medium and small tanks, and some of the tanks are well connected and form a drainage network. Most of the surplus during the heavy monsoon floods these areas, and finally goes to the Pallikaranai swamp, stretching from north to south.
Almost all the drainage flows from west to east, except certain undulations due to topography, and finally end in the Bay of Bengal. Generally, a subtropical maritime monsoon-type climate prevails over the study area. The maximum temperature varies between 37°C and 44°C, and the minimum temperature varies from 18°C to 27°C. The annual rainfall of Tamil Nadu is 945.70 mm, but whereas the annual normal rainfall of the study area recorded in six rainfall station, the average varies from 883 in the year 2003 to a maximum of 2,133 mm in the year 2005 which is high when compared to the normal rainfall of Tamil Nadu as a whole (SG & SWDRC 2005). Though coastal aquifers of study area constitute an important source of freshwater supply but are often confronted with the problem of seawater intrusion. Population growth, agricultural, industrial and domestic requirements exploit the available groundwater with decreasing recharge areas. Regulation of groundwater extraction is essential to optimize the overdraft, reducing the risk of seawater intrusion. The study conducted by Sathish et al. (2009) to evaluate the extent of seawater intrusion in Chennai, Tamil Nadu indicates that the extent of seawater intrusion is comparatively higher in northern part than in southern part due to low groundwater usage. The act regarding the coastal regulation zone banned the uncontrolled extraction especially from the southern aquifer significantly controls the seawater intrusion in the study area.
Geological setting
The Mambakkam mini watershed consists mainly of pyroxene granulite, charnockite and coastal alluvium. Pyroxene granulite and charnockite occupy the western part of the watershed and the coastal alluvium occurs in the eastern part of the watershed (Fig. 2). The coastal alluvium is located between the Neelangarai and Kovalam tract, and is characterized by sand dunes and beach sand mainly.
The charockite of the archaen age is the most widespread rock type of this area. The exposures of charnockite are found along the western and southwestern periphery of the watershed in the form of low hills and hillocks. Isolated outcrops of charnockite are also noted, and several quarries present in the area and well cuttings reveal the disposition of this rock type. The charnockite are massive and dark-coloured rocks devoid of joints and fractures. The mineral composition of charnockites is basically blue quartz and plagiociase feldspar with pyroxene. On the surface, charnockite appears light coloured and exhibit foliation trend due to weathering.
Coastal alluvium deposits of the quaternary age occur along the Neelangarai to Kovalam stretch. These include sand dunes, beach ridges and beach sands. The alluvium comprises of sand (medium to coarse grained) silt, clay and broken shell pieces. The thickness of the coastal alluvium deposits range between 16 and 22 m along the sea shore.
Material and methods
To assess the groundwater chemistry, a total of 31 groundwater samples were collected from shallow open wells during the period of January 2010 (post-monsoon) and May 2010 (pre-monsoon) in the study area. The samples were collected in 500 ml sterilized polythene bottles. Then they were sealed and brought to the laboratory for analysis and stored properly (4°C). The water samples were analysed at the Wet Chemistry Laboratory, Centre for Water Resources, Anna University. The parameters analysed include major ions of Ca2+ and Mg2+ by Titrimetry, K+ and Na+ by Flame Photometer, Cl− and HCO3 by Titrimetry and SO −4 by spectrophotometer. The analyses were done, using standard procedures recommended by the American Public health Association (APHA 1995). The analytical precision for the measurements of cations and anions is indicated by the ionic balance error, which was computed on the basis of ions expressed in milliequivalent per litre. The values were observed to be within a standard limit of 5%. The lithology data collected from the Groundwater Resources Data Center (SG & SWDRC 2000), and primary field survey are used for an analysis of the hydrogeology of the aquifer.
Result and discussion
Hydrogeology
The lithological profile is presented for the selected location of hard rock, as well as coastal formation in Fig. 3, to understand the hydrogeological characteristics of the formation. The lithological profile is developed using the secondary data of lithological information and the primary field survey. Lithological profiles GP1 and GP3 are located in the coastal formation, whereas GP2, GP4, GP5 and GP6 are located in the hard rock formation. The rocks referred to as crystallines from a groundwater point of view in the investigated area, have negligible primary porosity. But, secondary porosity is generated by the processes of weathering and fracturing, leading to the development of aquifers. The clay materials reduce the hydraulic conductivity to some extent. The top soil/sand is in the range of 1–17 m and the weathered zone/fractured zone is in the range of 5–26 m with low (0.2 m/day) and high (50 m/day) hydraulic conductivity. The weathered rock at GP5 is at the 0–5 m, as it is located in the western hilly terrain of the study area. The depth of the sandy aquifer located in the coastal formation varies in the range of 13–17 m, and the massive/hard rock zone (zero hydraulic conductivity) lies between 17 and 30 m from the ground surface. The utilisation of groundwater in the area is through dug wells (circular and rectangular types) and bore wells.
Groundwater occurs in the weathered zone under water table conditions, whereas under semi-confined to confined conditions, it occurs in the fractured zone, as is observed elsewhere in the crystalline terrain (Subba Rao 1992). The depth of the water table in the pre-monsoon (May) is 5–14 m below ground level (b.g.l.), and it is 0–6 m b.g.l. in the post-monsoon (January), indicating a fluctuation of 5–8 m and also a significant change in the annual groundwater storage. Generally, the movement of the groundwater flow is in the direction of the ground slope. The hydrochemical constituent and quality of groundwater depends majorly on the geological profile of the aquifer medium. When the water level touches the weathered zone, it becomes brackish, whereas when the water level approaches the fractured zone, it is nearly fresh in general. Due to the low hydraulic conductivity, the water is in longer contact in the weathered zone, which aids the active dissolution of minerals and the promotion of ion exchange among the elements present in the soils and among the elements present in the circulating water during the weathering process (Hem 1991).
A long, dry climate with a low rainfall and high evapotranspiration causes a low freshwater exchange and the enrichment of salts in the groundwater of the weathered zone; in addition, the active dissolution and high anthropogenic activity in the area results in highly brackish water (Subba Rao 2002, 2003). In contrast, at the fractured zone, the conditions favour the retention of freshwater or low brackish water. Therefore, the changes in the state of the water levels can explain the variation in the quality of the groundwater, according to the differences in the hydrogeological environment in which the water level exists as well as the influence of artificial sources on the aquifer system.
Groundwater chemistry
The maximum and minimum concentrations of major ions present in the groundwater from the study area are presented in Table 1. Ionic concentrations were plotted in a Piper–Hill diagram (Piper 1944) to infer the hydrogeochemical facies (Figs. 4 and 5). These plots include two triangles: one for plotting the anions and the other for plotting the cations. The cation and anion fields are combined to show a single point in a diamond-shaped field, from which inference is drawn on the basis of the hydrogeochemical facies concept. These tri-linear diagrams are useful in bringing out the chemical relationships among the groundwater samples in more definite terms rather than with other possible plotting methods.
These diagrams reveal the analogies, dissimilarities and different types of waters in the study area, which are identified and listed in Table 2. The concept of hydrochemical facies was developed in order to understand and identify the water composition in different classes. It clearly explains the variations or domination of cation and anion concentrations during the pre-monsoon and post-monsoon. Groundwater hydrochemistry is primarily controlled by water–rock interaction and anthropogenic pollution (Byoung-Young et al. 2005). The Ca–Mg type of water predominated during post-monsoon and pre-monsoon. The percentage of samples falling under the Ca–Mg type (alkaline earth exceeded alkalies) was 93% during the post-monsoon season. A similar type of water predominated during the pre-monsoon also in 81% of the water samples. The SO4 + Cl type of water (strong acids exceeded than weak acids) predominated during the post-monsoon in 81% and during the pre-monsoon in 97%. This type of water causes salinity problems, and the factor of dilution reduces the salinity to some extent. Increased chloride concentration in the post-monsoon season may be due to the process of the removal of other ions from the system, either by precipitation or by adsorption (Chidambaram et al. 2008). In the study area, the major groundwater type is a mixed category (no one cation–anion pair exceeds 50%) during the pre-monsoon in 74% and the post-monsoon in 68%. During the post- and pre-monsoon, Ca is the major cation (Ca > Na > Mg) because of the dominance of alkaline earth over alkalies. Increased Na concentration and decreased Ca and Mg concentration when compared with the post-monsoon values with pre-monsoon can be explained by the probability of the loss of Ca and Mg and gain of Na+ by the cation exchange process. HCO3and Cl are the major anions during the post-monsoon (HCO3 > Cl > SO4), whereas in the pre-monsoon, SO4 is the major anion (SO4 > Cl > HCO3). HCO3 and Na concentrations come mainly from the weathering of alkali earth from rocks related to the recharge areas (Prasanna et al. 2010). Calcium and magnesium ions that combine with bicarbonates and the small amounts of carbonates in the groundwater cause the water to be temporarily hard (Anku et al. 2009). The various combinations of Ca–Mg–Na with HCO3-water types identified in this study, put the groundwater in the carbonate hardness category, and this can be removed by boiling. Calcium and magnesium are the dominant cations present in the groundwater followed by sodium, in the study area. The carbonates are also present in a considerable amount, whereas in the post-monsoon, sulphates and chlorides are dominant in the groundwater. The available carbonates and sulphates in the rocks might have been dissolved and added to the groundwater system during irrigation, rainfall infiltration and groundwater movement (Sunne et al. 2005). During the post-monsoon, most of the samples fall in the no dominant/mixed type, whereas during the pre-monsoon, some of the samples fall in the sulphate type of water (Figs. 4 and 5). There are no major significant changes in the hydrochemical facies during the study period (pre- and post-monsoon). This indicates the dominance of mineral constituents and rock weathering, in addition to recharge activities by precipitation, and induced anthropogenic activities, such as application of fertilizers, uncontrolled groundwater development that influences the groundwater chemistry.
Drinking water quality
The physical and chemical parameters of the analytical results of groundwater were compared with the standard guideline values recommended by the World Health Organisation (WHO 1971, 1983) for drinking and public health purposes. Table 3 shows the maximum desirable and most allowable limits for various parameters. The concentrations of ions such as Na+, Ca 2+, Mg2+ and Cl− are within the maximum allowable limits for drinking standards during pre- and post-monsoons. Calcium and magnesium ions present in the groundwater are particularly derived from the leaching of limestone, dolomites, gypsum and anhydrites, whereas the calcium ions are also derived from the cation exchange process (Garrels 1976). To ascertain the suitability of the groundwater for any purpose, it is essential to classify the groundwater depending upon its hydrochemical properties based on the total dissolved solids (TDS) values (Freeze and Cherry 1979). The value of TDS ranges from 247 to 1,500 mg/L and 397 to 1,960 mg/L during post- and pre-monsoons, respectively. The value of TDS in most of the groundwater samples is within the maximum allowable limit as per the standard, except eight samples during the pre-monsoon and one sample during the post-monsoon period. The possible source of sodium concentration in the groundwater is due to the dissolution of rock salts and weathering of sodium-bearing minerals.
The chloride concentration ranges from 30 to 500 mg/L and 40 to 240 mg/L with a mean value of 190 and 118 mg/L during post- and pre-monsoons, respectively. High chloride content has a deleterious effect on metallic pipes, structures and agricultural crops. Natural processes such as weathering, dissolution of salt deposits and irrigation drainage return flow are responsible for the chloride content in the groundwater, which is supported by the Cl/HCO3 ratio of 1:3.0 (Lusczynski and Swarzenski 1996). Though the chloride concentration in the groundwater exceeds the desirable limit of standards, the values are within the allowable limits during both the seasons (Table 3). The concentrations of sulphate vary from 36 to 264 mg/L, with a mean value of 104.5 mg/L and 88 to 792 mg/L, with a mean value of 347 mg/L during post- and pre-monsoons, respectively. Sulphate ion concentrations are probably derived from the weathering of sulphate and gypsum-bearing sedimentary rocks (Elango et al. 2003; Jeevanandam et al. 2006). The sulphate ion concentration is higher in a majority of the samples when compared with standards during the pre-monsoon periods. Six samples collected during the post-monsoon and five samples collected during the pre-monsoon exceed the maximum allowable limits. The classification of the groundwater based on total hardness (TH) shows that 77% of the groundwater samples fall in the hard and very hard water category during the post-monsoon, and all the samples fall under the hard and very hard water category during the pre-monsoon season (Table 4).
Irrigation water quality
The quality of groundwater is evaluated using residual sodium carbonate (RSC), EC, sodium absorption ratio (SAR) and %Na, and the results show that about 90% of the groundwater samples are found to be good for irrigation during both the pre- and post-monsoon periods. The classification of groundwater samples with respect to RSC is shown in Table 5. The RSC indicates the excess sum of carbonate and bicarbonate in the groundwater over the sum of calcium and magnesium.
where the concentrations are reported in milliequivalent per litre.
Based on the RSC values, all the samples fall in the class of excellent except one sample of the post-monsoon period. The classification of the groundwater samples with respect to %Na is shown in Table 6. The sodium percentage is calculated (Na%) using the formula given below.
where all the concentrations are expressed in milliequivalent per litre.
The Na% indicates that 97% of samples fall in the field of good to permissible during both pre- and post-monsoons. High concentrations of sodium in irrigation tend to be absorbed by clay particles, displacing Ca2+ and Mg2+ ions, and this exchange process reduces the permeability, which leads to poor internal drainage (Saleh et al. 1999; Subramani et al. 2005). A perusal of Wilcox's diagram (Wilcox 1955) with the values of the percentage of sodium and electrical conductivity shows that 93% of samples are excellent to good and good to permissible during the post-monsoon (Fig. 6), whereas in the pre-monsoon, only 61% of the samples fall under the excellent to good and good to permissible type. SAR is an important parameter for determining the suitability of the groundwater for irrigation, because it is a measure of the alkali/sodium hazard to crops (Table 7). During the pre- and post-monsoons, the SAR value of all the samples is found to be less than 10, and is classified as excellent for irrigation. It implies that there is no alkali hazard to the crops. The classification of water for irrigation can be determined by graphically plotting the values of electrical conductivity and SAR on the US salinity diagram (USSL). About 98% of the samples grouped within C2S1–C3S1 during the post-monsoon and C3S1–C4S1 during the pre-monsoon indicate medium to high salinity/low sodium, and high to very high salinity/low sodium type, respectively (Fig. 7).
Twenty-nine percent of the samples fall in the field of C4S1 during the pre-monsoon, indicating a high salinity and low alkalinity hazard. This water will be suitable for plants having good salt tolerance, but it restricts its suitability for irrigation, especially in soils with limited drainage. The classification of groundwater based on the salinity hazard is presented in Table 8. Based on the EC value (salinity hazard), only four samples are found to be unsuitable during the pre-monsoon.
Conclusion
The hydrochemical analysis of the study reveals that the groundwater in the Mambakkam mini watershed is hard to very hard, fresh to brackish and of an alkaline nature. The results of the Piper–Hill diagram for the post- and pre-monsoon seasons demonstrates an inverse relationship between the water level and the concentration of ions, i.e. an increase in the chemical composition of the water with a decrease in the water level. The percentage of samples falling under the Ca–Mg type (alkaline earth exceeded than alkalies) was 93% during the post-monsoon season. A similar type of water predominated during the pre-monsoon also, with 81% of the water samples. The major groundwater type is a mixed category (Non one cation anion pair exceeds 50%) during the pre-monsoon in 74% and the post-monsoon in 68%. The value of TDS, TH and sulphate ion concentration is higher in majority of the samples when compared with the WHO standard during the pre-monsoon periods. The value of Ca, Mg, Na and Cl ion concentration is within the limits in majority of the samples during both the seasons. The excess amount of total dissolved solids in the groundwater is due to anthropogenic factors and geological characteristics of the aquifer. Chloride and sulphate concentration in groundwater is due to weathering, dissolution of rock formations and irrigation drainage return flow. The suitability of water for irrigation is evaluated based on SAR, %Na, RSC and salinity hazards. Most of the samples in the Mambakkam mini watershed fall in the suitable range for irrigation purpose, either from the SAR, %Na or RSC values. About 90% of the samples are grouped under the C3S1 class in both the pre- and post-monsoon seasons (Figs. 6 and 7). A few samples fall in the C2S1 and C4S1 classes during the post-monsoon and pre-monsoon, respectively. The study concludes that the lowering of the groundwater level would accelerate the dissolved mineral constituents and deteriorate the water quality depending on the increase in the groundwater demand. The groundwater exploitation and management should be carried out carefully by taking the groundwater level–quality control measurements into consideration. The geographical distribution of the hydrochemical facies is an important parameter in determining new policies and management options for the resource. Such a result is of significant value in introducing solutions in the qualitative and quantitative management of the aquifer.
References
Aghazadeh, N. A., & Mogaddam. (2010). Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environmental Monitoring and Assessment. doi:10.1007/s10661-010-1575-4.
Al-Futaisi, A., Rajmohan, N., & Al-Touqi, S. (2007) Groundwater quality monitoring in and around Barka dumping site, Sultanate of Oman. The Second IASTED (The International Association of Science and Technology for Development) International Conference on Water Resources Management (WRM 2007). August 20–22, Honolulu, Hawaii, USA
Andre, L., Franceschi, M., Pouchan, P., & Atteia, O. (2005). Using geochemical data and modeling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitain Basin, south-west of France. Journal of Hydrology, 305, 40–62.
Anku, Y. S., Banoeng-Yakubo, B., Asiedu, D. K., & Yidana, S. M. (2009). Water quality analysis of groundwater in crystalline basement rocks, Northern Ghana. Environmental Geology, 58, 989–997. doi:10.1007/s00254-008-1578-4.
APHA. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington, DC: American public Health Association.
Byoung-Young, C., Seong-Taek, Y., Soon-Young, Y., Pyeong-Koo, L., Seong-Sook, P., Gi-Tak, C., et al. (2005). Hydrochemistry of urban groundwater in Seoul, South Korea: effects of land-use and pollutant recharge. Environmental Geology, 48, 979–990. doi:10.1007/s00254-004-1205-y.
Chidambaram, S., Senthil, K. G., Prasanna, M. V., John, P. A., Ramanthan, A. L., & Srinivasamoorthy, K. (2008). A study on the hydrogeology and hydrogeochemistry of groundwater from different depths in a coastal aquifer: Annamalai Nagar, Tamilnadu. India. Environ Geol DOI. doi:10.1007/s00254-008-1282-4.
Edmunds, W. M. (2003). Renewable and non-renewable groundwater in semi-arid regions. Developments in Water Science, 50, 265–280.
Elango, L., Kannan, R., & Kumar, S. (2003). Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram District, Tamil Nadu, India. Journal Environmental Geosciences, 10–4, 157–166.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater. Englewood Cliffs: Prentice Hall.
Garrels, R. M. (1976). A Survey of Low Temperature Water Mineral Relations, in Interpretation of Environmental Isotope and Hydrogeochemical Data in Groundwater Hydrology: Vienna, International Atomic Energy Agency, 65–84.
Hossain, G., Farhad, H. M., Lutfun, N., Syed, S. A., & Chowdhury, Q. (2010). Hydrochemistry and classification of groundwater resources of Ishwardi Municipal Area, Pabna District, Bangladesh. Geotechnical and Geological Engineering, 28, 671–679. doi:10.1007/s10706-010-9326-4.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water. Book 2254 (3rd ed.). Jodhpur, India: Scientific Publishers.
Jalali. (2007). Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah Basin in Western Iran. Environmental Monitoring and Assessment, 130, 347–364. doi:10.1007/s10661-006-9402-7.
Jeevanandam, M., Kannan, R., Srinivasalu, S., & Rammohan, V. (2006). Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore district, South India. Environmental Monitoring and Assessment, 132(1), 263–274. doi:10.1007/s10661-006-9532-y.
Jha, C. V. M., & Chowdhury, A. (2010). Groundwater assessment in Salboni Block, West Bengal (India) using remote sensing, geographical information system and multi-criteria decision analysis techniques. Hydrogeology Journal, 18, 1713–1728.
Johnson, C. C. (1979). Land application of water-an accident waiting to happen. Ground Water, 17(1), 69–72. doi:10.1111/j.1745-6584.1979.tb03277.x.
Kumar, K., Rammohan, V., Dajkumar, S. J., & Jeevanandam, M. (2009). Assessment of groundwater quality and hydrogeochemistry of Manimuktha river basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 159, 341–351. doi:10.1007/s10661-008-0633-7.
Lusczynski, N. J., & Swarzenski, W. V. (1996). Saltwater encroachment in Southern Nassu and SE Queen countries (p. 1613). Long Island, New York: USGS Paper.
Ma, J., Ding, Z., Wei, G., Zhao, H., & Huang, T. (2009). Sources of water pollution and evolution of water quality in the Wuwei basin of Shiyang River, Northwest China. Journal of Environmental Management, 90, 1168–1177.
Mahalingam .S, Chandrasekaran P., Packialakshmi S., Balasubramanian., Chelvaraajhan K.., and Kalphana KR (2010). Assessment on landuse changes causing hazards in groundwater aquifer in the new developing sub urban area, southern part of Chennai city Using Geomatics, National Conference On Climate Change: Coastal Ecosystems with SpecialSession on Planetary Geomatics and Annual Convention of Indian Society of Geomatics (ISG) February
Nisi, B., Buccianti, A., Vaselli, O., Perini, G., Tassi, F., & Minissale, A. (2008). Hydrogeochemistry and strontium isotopes in the Amo River Basin (Tuscany, Italy): constraints on natural controls by statistical modeling. Journal of Hydrology, 360, 297–313.
Packialakshmi, S., Ambujam, N. K., & Nelliyat, P. (2010). Groundwater market and its implications on agriculture and water resources in the southern peri-urban interface, Chennai, India. Journal of environment development and sustainability. doi:10.1007/s10668-010-9269-1.
Piper, A. M. (1944). A graphical procedure in the geochemical interpretation of water analysis. Transactions–American Geophysical Union, 25, 914–928.
Prasanna, M. V., Chidambaram, S., Senthil, K. G., Ramanathan, A. L., & Nainwal, H. C. (2010). Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arabian Journal of Geosciences. doi:10.1007/s12517-010-0191-5.
Ravikumar, P., Venkadesh, R. K., & Somashekar, R. K. (2010). Major ion chemistry and hydrochemical studies of groundwater of Bangalore South Taluk, India. Environmental Monitoring and Assessment, 163, 643–653. doi:10.1007/s10661-009-0865-1.
Sadashivaiah, C., Ramakrishnaiah, C. R., & Ranganna, G. (2008). Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State. India International Journal of Environmental Research Public Health, 5(3), 158–164.
Saleh, A., Al-Ruwaih, F., & Shehata, M. (1999). Hydrogeochemical processes operating within the main aquifers of Kuwait. Journal of Arid Environments, 42, 195–209.
Sami, K. (1992). Recharge mechanism and geochemical processes in a semi-arid sedimentary Basin. Eastern Cape, South Africa. Journal of Hydrology (Amsterdam), 139, 27–48. doi:10.1016/0022-1694(92)90193-Y.
Sastri, J. C. V. (1994) Groundwater chemical quality in river basins, hydrogeochemical modeling. Lecture notes-Refresher course, school of Earth Sciences, Bharathidasan Univ., Tiruchirapalli, Tamil Nadu, India.
Sathish S., Rajesh R., sarma and Elango L. (2009). Evaluation of extent of seawater intrusion using high resolution electrical resistivity tomography. Joint International Convention of 8th IAHS Scientific Assembly and 37th IAH Congress, Hyderabad, India
Schot, P. P., & Van der Wal, J. (1992). Human impact on regional groundwater composition through intervention in natural flow patterns and changes in land use. Journal of Hydrology, 134, 297–313.
Singh AK (2003) Water resources and their availability. In: Souvenir, National Symposium on Emerging Trends in Agricultural Physics, Indian Society of Agrophysics, New Delhi, 22–24 April, pp 18–29
SG & SWDRC (2000). Groundwater perspectives profile of Kancheepuram district, State Groundwater and Surface water Resources data Centre, Water Resources Organisation, Public Works department, Government of Tamil Nadu, Chennai
SG & SWDRC (2005). Groundwater perspectives profile of Chennai district, State Groundwater and Surface water Resources data Centre, Water Resources Organisation, Public Works department, Government of Tamil Nadu, Chennai
Subba Rao, N. (1992). Factors affecting optimum development of groundwater in crystalline terrain of Eastern Ghats, Visakhapatnam area, India. Journal of the Geological Society of India, 40(5), 462–467.
Subba Rao, N. (2002). Geochemistry of groundwater in parts of Guntur District, Andhra Pradesh, India. Environmental Geology, 41(5), 552–562.
Subba Rao, N. (2003). Groundwater prospecting and management in an agro-based rural environment of crystalline terrain of India. Environmental Geology, 43, 419–431. doi:10.1007/s00254-002-0659-z.
Subramani, T., Elango, L., & Damodarasamy, S. R. (2005). Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environmental Geology, 47, 1099–1110. doi:10.1007/s00254-005-1243-0.
Sunne Vazquez X., Villa Sanchez and Carrera. (2005). Introductory review of specific factors influencing urban groundwater, an emerging branch of hydrogeology, with reference to Barcelona, Spain. Hydrogeology Journal, Vol. No 13, pp 522–533.
Tirumalesh, K., Shivanna, K., Sriraman, A. K., & Tyagi, A. K. (2010). Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. Environmental Monitoring and Assessment, 163, 171–184. doi:10.1007/s10661-009-0825-9.
WHO. (1971). International standards for drinking water. Geneva: World Health Organisation.
WHO. (1983). Guidelines to drinking water quality (p. 186). Geneva: World Health Organisation.
Wilcox LV (1955) Classification and use of irrigation waters. USDA, Circular 969, Washington, DC, USA
Yesilnacar, M., & Gulluoglu, S. (2008). Hydrochemical characteristics and the effects of irrigation on groundwater quality in Harran Plain, GAP Project, Turkey. Environmental Geology, 54, 183–196. doi:10.1007/s00254-007-0804-9.
Acknowledgements
The present study was carried out under the Crossing Boundary Project and the authors are grateful to Saci WATERs, Hyderabad and the Wagningen University, Netherlands for providing the financial support. The authors thank Institute for Water Studies, government of Tamil Nadu for providing necessary data to carry out the present research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanmugam, P., N. K., A. A hydrochemical and geological investigation on the Mambakkam mini watershed, Kancheepuram District, Tamil Nadu. Environ Monit Assess 184, 3293–3306 (2012). https://doi.org/10.1007/s10661-011-2189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2189-1