Abstract
Groundwater represents a significant source of fresh water for drinking purposes and, therefore, preserving its availability and quality is extremely important. The hydrochemical characteristics and quality of groundwater in Ejisu-Juaben Municipality, Ghana have been evaluated based on different indices for assessing groundwater for drinking purposes. A total of 19 groundwater samples were collected and analyzed for major cations and anions using standard methods. The results show that the groundwater parameters were within the permissible limits of the World Health Organization except phosphate. The domination of major ions was in the order of Ca2+ > Mg2+ > NH4 + for cations and Cl− > HCO3 − > SO4 2− > PO4 2− > NO3 − > NO2 − in anions. The hydrochemical analysis suggest that the dominant ions were derived from ion exchange and silicate weathering process. According to Gibbs plot, the predominant samples fall in the rock–water interaction and precipitation dominance field. The R-mode factor analysis shows that the four factors extracted account for 83.9 % of the total variance. Groundwater quality index reveals that the majority of the samples falls under good to excellent category of water, suggesting that the groundwater is suitable for drinking and other domestic uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The availability of good quality water is vital for life, well-being, food and socio-economic development of mankind and it is generally obtained from two principal natural sources: surface water such as fresh water lakes, rivers, streams etc. and ground water such as borehole water and well water (McMurry and Fay 2004; Mendie 2005). Access to safe drinking water is a problem facing a large proportion of the inhabitant of the developing nations (Cosgrove and Rijsberman 2000; Gomez and Nakat 2002).
Groundwater accounts for over 60.9 % of the domestic supply in the Ejisu-Juaben Municipality of the Ashanti Region of Ghana (Ghana Statistical Service 2012). Unfortunately, in many countries around the world, including Ghana, water has become a scarce commodity as only a small proportion of the populace have access to treated water (IDLO 2011). In effect, communities situated in the Ejisu Juaben Municipality of the Ashanti Region of Ghana have resulted to the use of hand-dug wells as an alternative source of water supply. Hand-dug wells provide cheap and low technology solution to the challenges of rural and urban water supply. These areas are among the areas where local mining (Galamsay) and farming activities have been on rampant increase in recent times.
Water can be found both underground and on the surface of the earth (Aremu et al. 2011). Groundwater, surface waters, rain-water and springs are the main sources of water available to the rural dwellers in Ghana (Tay 2008). Both surface and ground water sources could become contaminated by biological and chemical pollutants arising from point and non-point sources (Rohul-Amin et al. 2012). Groundwater is a valuable natural resource for various human activities (Arya et al. 2012; Armon and Kitty 1994). It is believed to be comparatively much clean and free from pollution than surface water (Dahiya and Kaur 1999; Agbaire and Oyibo 2009; Efe 2002). Groundwater moves through pore-spaces within rocks and reacts with minerals that make up the rocks in the course of migration (Amadi et al. 2012). Groundwater quality in any locality takes after the chemical composition of the aquifer through which it migrates in accordance with the hydrological cycle and flow direction (Offodile 1983; Amadi et al. 2010). The chemical composition of groundwater is a measure of its suitability as a source of water for human and animal consumption, irrigation, industrial and other purposes (Babiker et al. 2007). However, it is susceptible to pollution and once polluted treatment is difficult and long term measures are needed (Henry and Heinke 2005). Therefore, systematic assessment of the physicochemical parameters, their sources and controlling hydrochemical processes are essential in maintaining the sustainable ecosystem.
Physicochemical parameters as well as hydrochemistry have been studied by many researchers to assess the characteristics of groundwater (Sanchez Martos et al. 1999; Subba Rao et al. 2002; Bhardwaj and Singh 2010). Increasing contamination of the surface as a result of increase in population, urbanization and industrialization has resulted in groundwater pollution especially those tapping water from shallow unconfined aquifers (Amadi 2011; Oluseyi et al. 2011). The most common source of groundwater pollutions are the discharge of sewage, industrial and agricultural waste, both organic and inorganic, mining, fertilizers and pesticides washed off the land by rain (Nwajei et al. 2012). The constituents in these chemicals are highly toxic even in minute amounts.
Comparatively, landfills in Ejusu Juaben Municipality are mostly in the form of uncontrolled dumps, where solid waste is filled up with little or no regard to the environment. Solid wastes deposited in landfills decompose and could pollute underlying groundwater through the infiltration of leachates. Since most of the surface waters are polluted by the anthropogenic activities of humans, majority of these people rely on groundwater by drilling boreholes and hand dug wells for their sustenance. Ejisu-Juaben is one of such urban areas that still has some deficit with respect to water coverage and so rely solely on hand dug wells and boreholes. The present study was carried out to evaluate the hydrochemical characteristics, groundwater quality and its suitability for domestic purposes in the Ejusu-Juaben Municipality.
Materials and method
Profile of the study area
Ejisu–Juaben Municipality lies within Latitudes 1°15′N to 1°45′N and Longitude 6°15′W to 7°00′W. The Municipality is located in the central part of the Ashanti Region and shares boundaries with six other Districts in the Region namely Kumasi, Kwabre, Afigya Sekyere, Asante Akim north, Asante Akim South and Bosomtwi Kwanwoma Districts. Due to its nearness to Kumasi, the Ashanti Regional Capital City, it has experienced rampant growth and its population has also increased tremendously. Ejisu stretches over an area of 637.2 Km2 with population around 143,762 (Ghana Statistical Service 2012). The mean monthly temperatures vary between 20 °C in August and 32 °C in March. The relative humidity ranged from 65 % in January to 85 % in August. The area usually experiences a wet semi-equatorial climate. Majority of the rainfall is from March to July and again from September to the later part of November, with an average of 1200 mm which is ideal for minor season cropping as a result of climatic changes and seasonal drought. Groundwater is an important water resource for drinking, agriculture, and industrial uses in the study area. Due to its high population density, resources such as water have become one of the major problems confronting the inhabitants and they are, therefore, compel to rely on groundwater as an alternative source of water for drinking and domestic purposes. Groundwater in study area are exposed to non-point source pollution such as leachates from dumpsites, open drains, agriculture wastes, urban run-off from storms, sewer pipes, indiscriminate human waste disposal in the fields and contents of septic tank. The area is characterized by rural setting and major occupation of the people is agriculture. The main food crops grown are plantain, cassava, maize and cocoyam. One of the major cash crops grown include cocoa, is the driving force of the economy. Oil palm is also another cash crop that is widely grown in the district.
Geology and hydrogeology
The Municipality falls within the forest dissected plateau terrain region and is underlain by pre-cambrian rocks of the Birimian and Tarkwaian formations which rises from about 240 to 300 m above sea level. The area is generally undulating and is drained by a number of rivers, notable among them being Oda, Anum, Bankro, Hwere and Baffoe. In the rainy season, occasional flooding is experienced in the inland valleys along the river basins. The geology and soils types in the Municipality offer vast opportunity for the cultivation of traditional and non-traditional cash crops and other staple food stuff and thus present the municipality as one of food basket case in Ghana.
Sampling
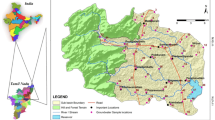
Groundwater samples of totally nineteen were collected from Ejisu communities in June, 2014. The sampling points are shown in Fig. 1. Water was collected from open wells using drawing buckets tied with ropes (Andrew et al. 2011). The samples were collected into acid pre-cleaned high-density 1.5 L polyethylene sampling bottles. Two sets of water samples were collected at each sampling points. One was for the analysis of anions and the other for cations. pH, electrical conductivity and total dissolved solids were measured in situ. The samples were kept over ice in an ice chest and transported to the Inorganic Laboratory in the Department of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi-Ghana for analysis.
Analytical methods
All chemicals and standards used during preparation and analysis were of the highest purity analytical grade supplied by BDH Chemical Ltd., UK. Deionized water was used throughout the analysis wherever applicable. Analytical methods used for the water samples varied depending on the parameters of interest. All field and laboratory determinations were carried out according to the standard methods (APHA 1998).
The pH was determined using model SENsion™ pH 31 Laboratory pH meter calibrated with buffers 4.01 and 7.00. The conductivity was determined using a JENWAY model 4510 conductivity meter. Calibrated EUTEC CON 2700 meter was used to measure the total dissolved solids. Alkalinity was determined by strong acid titration method. Turbidity was determined using Hach model 2100P turbidity meter. Calcium (Ca2+) and magnesium (Mg2+) were determined using the standard EDTA titrimetric methods according to APHA (1998). Total hardness was determined using standard EDTA titration with ammonia buffer and erichrome black ‘T’ indicator. Chloride determination was undertaken using the argentometric method (APHA 1998). Sulfates (SO4 2−) and Phosphate (PO4 3−) in the groundwater samples were analyzed by Palintest photometer (PT 515). Nitrates (NO3-N), ammonia (NH3-N) and nitrite (NO2-N) determination in the groundwater samples were carried out by DR 2700 spectrometer.
Water quality index method
Groundwater chemistry has been utilized as a tool to assess water quality for drinking purposes (Subba Rao 2006; Edmunds et al. 2002). Water quality index is an important parameter for assessing groundwater quality and its suitability for drinking purposes (Tiwari and Mishra 1985; Singh 1992; Subba Rao 1997; Mishra and Patel 2001; Naik and Purohit 2001; Avvannavar and Shrihari 2008). The water quality index (WQI) was calculated by assigning weight (w i ) to the physicochemical parameters according to its relative importance in the overall quality of water for drinking purposes. World Health Organization (2011) standards, assigned weight (w i ) and a relative weight (W i ) for each parameter are presented in Table 1.
A maximum weight of 5 was assigned to the parameters like nitrate, total dissolved solids, chloride, and sulfate due to their major importance in water quality assessment (Srinivasamoorthy et al. 2008). Bicarbonate and phosphate was given a minimum weight of 1 as they play an insignificant role in the water quality assessment. Other parameters such as calcium and magnesium were assigned a weight between 1 and 5 depending on their importance in the overall quality of water for drinking purposes (Ketata-Rokbani et al. 2011). The relative weight is determined using Eq. (1):
where W i is the relative weight, w i is the weight of each parameter, and n is the number of parameters.
According to World Health Organization (2011), the quality rating scale for each parameter is calculated using Eq. (2):
where q i is the quality rating, C i is the concentration (mg/L) of each chemical parameter, and S i is the World Health Organization (2011) standard for each chemical parameter.
For computing the WQI, SI is first determined for each chemical parameter using Eq. (3), which is then used to determine the WQI according to Eq. (4):
where SI i is the sub-index of ith parameter
where q i is the rating based on concentration of ith parameter and n is the number of parameters.
Computed WQI values are usually classified into five categories (Table 2): excellent, good, poor, very poor, and unsuitable for human consumption (Sahu and Sikdar 2008).
Statistical analysis
Analytical data were processed using IBM SPSS version 20 software. Basic statistics such as mean and standard deviation was computed along with multivariate analyses (principal component and cluster analysis) on the corresponding variables. Principal component analysis was used to identify the possible sources of heavy metals. Factor analysis was performed by varimax rotation (Howitt and Cramer 2005), which minimized the number of variables with a high loading on each component, thus facilitating the interpretation of PCA results. Cluster analysis was applied to identify groups of samples with similar heavy metal contents (Panda et al. 2006). CA was formulated according to the Ward-algorithmic method, and the rescaled linkage distance was employed for measuring the distance between clusters of similar metal contents. R-mode CA was used to determine the association of different water quality parameters and pollutant sources. Pearson’s correlation matrix was also used to identify the elements’ relationship.
Results and discussion
The physicochemical parameters of groundwater are considered as the most important principles in the identification of nature, quality and type of the water (Abdo 2005). The physicochemical characteristics of groundwater samples in Ejisu-Juaben municipality was statistically analyzed and the results such as maximum, minimum, average and standard deviation parameters are presented in Table 3.
The pH value of the groundwater samples ranged from 3.99 to 6.39 with an average value of 5.27. Low pH may be attributed to the acidic lateritic and humic soils of the area (Odoh et al. 2012; Reghunath et al. 2002). The permissible limit of pH for drinking water is 6.5–8.5 (WHO 2011). The pH levels from the groundwater samples were within the WHO permissible limit for drinking water. pH values lower than 6.5 are considered too acidic for human consumption and can cause health problems such as acidosis. The pH values greater than 8.5 are considered to be too alkaline for human consumption. Study done by Duncan et al. (2014), Cape Coast Metropolis, Ghana and Ansa-Asare et al. (2009), Volta Region indicated that pH values were higher compared to this study. However, the present study generally agrees with those reported by Kortatsi (2007a), Western Region of Ghana and Apau et al. (2014), Nyamebekyere.
The EC measurement indicates the ability of the water sample to allow an electric current to flow, which is related to the concentration of ionized substances in the water (Radojevic and Bashkin 2006). The EC ranged between 7.32 and 100.56 µS/cm. All the groundwater samples were within the permissible limit of 500 µS/cm (WHO 2011). Thus, a lower value of EC indicates the presence of a low concentration of dissolved ions, such as inorganic salt and organic matter in water (WHO 2011; Radojevic and Bashkin 2006). Results from this study were found to be comparable with study by Apau et al. (2014), Nyamebekyere. The levels were lower than study conducted by Akoto and Adiyiah (2007), Brong-Ahafo Region, Ghana.
The TDS values for the water samples ranged between 8.00 and 125.00 mg/L. The TDS values recorded fell within the WHO permissible limit of >500 mg/L in drinking water (WHO 2011). According to WHO (2008), there is no health based limit for TDS in drinking water, as TDS in drinking water at concentrations well within toxic effects may occur. According to Todd (1980), groundwater was classified using a TDS into very fresh (0–250 mg/L), fresh (250–1000 mg/L), brackish (1000–10,000 mg/L) and saline (10,000–100,000 mg/L). Using this categorization, all the groundwater samples fell under the very fresh water type.
Water hardness is primarily caused by the presence of cations such as calcium and magnesium; and of anions such as bicarbonate, chloride, and sulfate in the water (Ravikumar et al. 2010). The total hardness values recorded in the study area ranged between 18.00 and 90.00 mg/L. These values were within the WHO (2011) set standard of 300 mg/L for total hardness in drinking water. According to WHO (2004) grading standards of TH (as CaCO3), 52.6 % of the groundwater samples could be classified as soft water and 47.4 % as moderate soft. The range of TH obtained in the present study reveal that, about 36 % of the water samples is higher than TH value reported by Essumang et al. (2011) (19.21–32.98 mg/L) but lower than that reported by Mulla et al. (2012) for ground water (102–199.33 mg/L).
The turbidity values ranging from 0 to 3.73 NTU were within the WHO guideline value. The turbidity values from this study were lower that study conducted by Schafer et al. (2010), Ghana.
Magnesium and calcium ions are often present at significant concentrations in natural water. Calcium and magnesium are mainly originated from the carbonate minerals, such as calcite and dolomite. The major source of Mg in groundwater is due to ion exchange of minerals in rocks and soils by water. The concentration of calcium and magnesium ions ranged from 12.00 to 56.00 mg/L and 1.20 to 8.00 mg/L, respectively, (Table 3). The calcium and magnesium concentration in all the ground water samples were within the WHO (2011) permissible limit of 75 and 50 mg/L, respectively.
The concentration of chloride in the groundwater ranged from 28.40 to 227.20 mg/L. Chloride content was lower than the accepted limit of 250 mg/L at all the sampling sites. An excess of chloride in water is usually taken as an index of pollution and considered as tracer for groundwater contamination (Loizidou and Kapetanios 1993). According to Walker et al. (1991), Cl ion concentration in the groundwater normally arises from sources like solubility of Cl− bearing evaporation deposits and anthropogenic sources. High concentration of chloride gives salty taste to water and may result in hypertension, osteoporosis, renal stones and asthma (McCarthy 2004). Chloride levels from this study were lower than study conducted by Tay and Kortatsi (2007), Densu Basin and Kortatsi (2007b), Ankobra Basin.
The concentration of bicarbonate ranged between 20 and 40 mg/L. Bicarbonate is responsible for the alkalinity of groundwater. The bicarbonates are probably derived from weathering of silicate rocks, dissolution of carbonate precipitates, atmospheric and soil CO2 gas (Jeong 2001; Krishna Kumar et al. 2011).
Sulfates originate from natural sources as well as industrial effluents; however, the highest levels in groundwater are from natural mineral sources, such as barite (BaSO4), epsomite (MgSO4·7H2O) and gypsum (CaSO2·2H2O) (Jeevanandam et al. 2006; Krishna Kumar et al. 2009). The sulfate concentration ranged from 3.00 to 38.00 mg/L, with a mean value of 20.26 mg/L. The sulfate concentration in all the samples is within the permissible limit of 250 mg/L (WHO 2011). Higher concentration of sulfate in groundwater may be attributed to contamination of untreated industrial and domestic waste and their effluents (Baruah et al. 2008; Jeevanandam et al. 2012). Higher concentration of SO4 2− may cause gastrointestinal irritation particularly when Mg and Na are also present in drinking water resources (Suthar et al. 2009). The concentration of sulfate was lower than study reported by Tay and Kortatsi (2007), Densu Basin and Ansa-Asare et al. (2009), Volta Region.
The nitrate concentration ranged from 0.08 to 0.31 mg/L with a mean value of 0.15 mg/L. The concentration of nitrate is within the WHO-suggested permissible limit of 50 mg/L. Nitrate leaching is enhanced by high infiltration of soil layer and low runoff potential. The presence of high nitrate concentration in the drinking water increases the incidence of gastric cancer and other potential hazards to infants and pregnant women (Rao 2006). Nitrites occur as an intermediate product of conversion of ammonium ion to nitrate as well as in the nitrification process of ammonia (Eletta et al. 2010). Nitrites can be more harmful than nitrates in drinking water supply as nitrites can oxidize hamoglobin to methaemoglobin in the body and hinder the transportation of oxygen around the body (Alsabahi et al. 2009; Chapman 1992). The mean values of nitrites ranged between 0.06 and 0.27 mg/L. These values are within the WHO prescribed limit of 3 mg/L. The concentration of nitrate in the present study was lower than those reported by Tay and Kortatsi (2007), Densu Basin; Duncan et al. (2014), Cape Coast Metropolis, Ghana and Asare-Donkor et al. (2015), Obuasi.
Phosphate concentration in the water samples ranging from 9.00 to 26.00 mg/L with a mean value of 17.97 mg/L were above the WHO limit of 0.1 mg/L. Higher concentration of PO4 3− in the groundwater may be attributed to phosphate-rich sewage material from the communities (Akoto et al. 2010). It could also come from drains rich in detergents (Akpabli and Drah 2001; Sinha et al. 2000). The levels of PO4 3− from this study were higher than the study by Duncan et al. (2014), Cape Coast Metropolis, Ghana. NH4 + concentrations were between 0.04 and 0.12 mg/L as against a WHO guideline value of 35 mg/L.
Ionic dominance
The increasing order of major ions determined from the whisker and box plot (Fig. 2) are; the cationic dominance was Ca2+ > Mg2+ > NH4 + and anionic dominance was Cl− > HCO3 − > SO4 2− > PO4 2− > NO3 − > NO2 −. The trend was a characteristic of a freshwater system because of the dominance of Ca2+ and Cl−. The dispersion of ions in the water samples is related to the water–rock interaction that occurs as the water moves through the ground reacting to varying degrees with surrounding minerals and other constituents (Amadi et al. 2012). Moreover, the excess of alkali earth elements (Ca + Mg) over HCO3 − in some of the groundwater samples clearly indicates that they are supplied from silicate-weathering processes (Zhang et al. 1995).
Hydrogeochemical processes
Concentration of different major elements and their interrelationship was studied to understand and illustrate the hydrogeochemical processes that were involved during the evolution of groundwater facies. Major ion chemistry of groundwater provides basis to investigate the weathering reactions in the basin (Brijraj and Kaur 2007). Calcium and magnesium were the dominant cations and bicarbonate was the dominant anion in the Ejisu-Juaben Municipality. The Ca2+/Mg2+ ratio of 1 indicated dissolution of dolomite and of >2 reflected an effect of silicate minerals on the groundwater chemistry; it also suggested calcite dissolution for Ca2+–Mg2+ concentration in groundwater (May and Loucks 1995). All the groundwater samples have Ca2+/Mg2+ ratio >2, indicating calcite dissolution and effect of silicate minerals responsible for Ca2+/Mg2+ contribution (Fig. 3a). The scatter diagram of Ca2+ + Mg2+ vs. HCO3 − + SO4 2− (Fig. 3b) shows that majority of the samples fall below the equiline, indicating that silicate weathering was the primary process involved in the evolution of groundwater (Datta and Tyagi 1996). If bicarbonate and sulfate are dominating than calcium and magnesium, it reflects that silicate weathering was dominating and, therefore, was responsible for the increase in the concentration of HCO3 − in the groundwater (Elango et al. 2003; Elango and Kannan 2007). According to Cerling et al. (1989) and Fisher and Mulican (1997), if the points are below the median line in the plot, ion exchange was the active reaction leading to SO4 2−HCO3 − accumulation in groundwater. In Fig. 3b, 79 % of the samples occur below the median line and close to x axis, indicating that ion exchange was responsible for the higher HCO3 −SO4 2− concentration in groundwater.
The plot of Ca2+ + Mg2+ versus Cl− clearly indicated that the salinity decreased with an increase in Ca2+ + Mg2+, which may be due to ion exchange in the weathered layer (Fig. 3c). The ratio of Cl−/HCO3 − versus Cl− ranged from 0.71 to 11.36 and showed a strong positive linear relation to the Cl− concentration (r = 0.8393, p < 0.01) (Fig. 3d).
Gibbs plots
Gibbs plot (Gibbs 1970) is used to interpret the effect of hydrogeochemical processes such as precipitation, rock–water interaction and evaporation on groundwater geochemistry. The reaction between groundwater and aquifer minerals has a significant role in water quality which is useful to understand the genesis of water (Krishna Kumar et al. 2014). Gibbs ratio is calculated using TDS versus Cl−/(Cl− + HCO3 −) for anions. The sampling points mostly fall in the rock–water interaction and precipitation zone (Fig. 4). The rock domain suggests that rock–water interaction is the major source of dissolved ions over the control of groundwater chemistry. The rock–water interaction process includes the chemical weathering of rocks, dissolution–precipitation of silicates and ion exchange between water and clay minerals.
Hierarchical cluster analysis
The multiple water quality parameters analyzed can be clustered based on their interdependency (Routroy et al. 2013) or sampling locations can be clustered based on chemical similarity in samples collected from these locations (Singh et al. 2005; Shrestha and Kazama 2006). The cluster analysis classified the groundwater samples into two clusters based on the similarities among the chemical parameters involved in the groundwater quality (Fig. 5). Cluster 1 represents measures of Cl−. Cluster 2 represent measures of TDS, conductivity and turbidity. Cluster 3 represents PO4 3−, alkalinity, total hardness, Mg2+, Ca2+, SO4 2−, NO3 −, NO2 −, pH and NH4 +. The samples in clusters C1 and C3 had anionic and cationic composition that was dominated by Cl− and Ca2+. Therefore, the chemical compositions of these classes were characterized by the Ca–Cl water type, which indicated the slightly saline water.
Principal component
Principal components analysis (PCA) was useful for data reduction, to assess the continuity/overlap of clusters or clustering/similarities in the data and was used to determine the sources of variation between parameters (Guler et al. 2002). Factor analysis (FA) relates to principal component analysis in water quality evaluation studies, where various parameters are analyzed for particular causes leading to a change in the water quality (Helena et al. 2000; Reghunath et al. 2002; Yidana 2010). Results of factor analysis including factor-loading matrix, eigenvalues, total, cumulative variance and communities values are presented in Table 4. From the principal components analysis, four factors were extracted that accounted for 83.9 % of the total variance. The extracted factor results suggest that TH, TDS, turbidity, conductivity, Ca2+ and Mg2+ have high positive factor loadings in factor 1. Factor 1 source may be attributed to weathering and leaching of host rocks. Factor 2 which describes 21.74 % of the total variance has high positive loading for NO2 −, NO3 −, PO4 2− and NH4 +. Factor 2, therefore, could be said to reflect the influence of anthropogenic activities. pH and alkalinity have a high positive factor loading in factor 3 whereas chloride has a high positive loading in factor 4. Chloride (Cl−) is thought to be released from the leaching of upper soil layers, which receive waste water and sewage from domestic activities.
Correlation among parameters
The chemical composition of groundwater is characterized by major cations and anions. The correlation matrix of the physicochemical parameters is shown in Table 5. From the correlation matrix, most of the parameters were found to bear statistically significant correlation with each other indicating close association of these parameters with each other. Concentrations of the major ions and trace elements, except for NO2 −, NH4 +, NO2 −, HCO3 − and SO4 2−, were significantly and positively correlated with TDS. Significant positive correlation was seen between Ca2+ and TDS (r = 0.768, p < 0.01). Concentrations of NO2 −, NO3 −, NH4 + and PO4 3− were significantly related, with correlation coefficients ranging from 0.493 to 0.883, implying that there is possibly a common cause for an increase in these ions. Concentrations of Ca2+ and Mg2+ were significantly correlated with TH (p < 0.01), which indicates that hardness is an approximate measure of Ca2+ and Mg2+. TH was also significantly correlated with SO4 2− (r = 0.514, p < 0.05), implying that TH is essentially a permanent hardness. The correlation of SO4 2− was not as strong as that of Mg2+, so the dissolution of gypsum cannot be assumed as a main source of SO4 2−.
Water quality classification
Water quality index
The water quality assessment indices (WQIs) are aggregation and communication tools for monitoring water quality (Vasanthavigar et al. 2010). Water quality indices (WQI) were calculated for the samples using the concentrations of TDS, Ca2+, Mg2+, Cl−, HCO3 −, SO4 2−, PO4 2−, NO3 −, EC and pH at the various sample locations. The calculated WQI ranged from 20.71 to 57.45. Based on the groundwater quality index, 84.2 % of the samples fall under excellent and 15.8 % shows good water type (Table 6). The WQI values indicate that groundwater from the study area is of excellent quality for drinking purposes.
Multiple regression analysis using standardization of the coefficient was carried out to determine which of the quality parameters had a greater effect on the water quality index. From the results, TDS, Calcium and Magnesium had the most influence due to the highest standardized beta-coefficient. Therefore, TDS, Calcium and Magnesium are very important indicators in determining groundwater quality (Table 7).
Conclusion
Groundwater quality and its suitability for drinking purposes in Ejisu-Juaben municipality have been evaluated. Results of the hydrochemical analysis reveal that the groundwater is slightly acidic in nature. The analysis showed that all the physicochemical parameters except phosphate were within the WHO permissible limits of drinking water. Based on EC classification, the groundwater sample fell under fresh water type. Multivariate statistical analysis indicated that the four factors extracted explained 83.9 % of the total variance. The results from PCA suggested that the groundwater quality is primarily influenced by weathering, leaching and anthropogenic activities. In general, the dominant hydrogeochemical facies of groundwater were the Ca–Cl type. The influence of major anions and cations on groundwater quality was by ion exchange and silicate weathering. The Gibbs plot indicated that the hydrochemistry of these groups were mainly influenced by the rock-water and precipitate dominance field. WQI values suggest that the majority of the samples fall under excellent water quality classes. This study provides a baseline for the groundwater chemistry in Ejsu-Juaben municipality, which will help the policy makers to focus on the specific contaminant sources and its mitigation.
References
Abdo MH (2005) Physico-chemical characteristics of Abu Za’baal pond, Egypt. Egypt J Aquat Res 31(2):1–15
Agbaire PO, Oyibo IP (2009) Seasonal variation of some physicochemical properties of borehole water in Abraka, Nigeria. Afr J Pure Appl Chem 3(6):116–118
Akoto O, Adiyiah J (2007) Chemical analysis of drinking water from some communities in the Brong Ahafo Region, Ghana. Int J Environ Sci Technol 4(2):211–214
Akoto O, Bruce TN, Darko G (2010) Chemical and biological characteristics of streams in the Owabi watershed. Environ Monit Assess 161:413–422
Akpabli CK, Drah GK (2001) Water quality of the main tributaries of the Densu stream. JGSA 3(2):84–94
Alsabahi E, Abdulrahmi S, Zuhairi W, Al-Nozaily F, Alshaebi F (2009) The characteristics of leachate and groundwater pollution at municipal solid waste landfill of Ibb City, Yemen. Am J Environ Sci 5(3):256–266
Amadi AN (2011) Assessing the effects of Aladimma dumpsite on soil and groundwater using water quality index and factor analysis. Aust J Basic Appl Sci 5(11):763–770
Amadi AN, Yisa J, Okoye NO, Okunlola IA (2010) Multivariate statistical evaluation of the hydrochemical facies in Aba, Southeastern Nigeria. Int J Biol Phys Sci 15(3):326–337
Amadi AN, Nwankwoala HO, Olasehinde PI, Okoye NO, Okunlola IA, Alkali YB (2012) Investigation of aquifer quality in Bonny Island, Eastern Niger Delta, Nigeria using geophysical and geochemical techniques. J Emerg Trends Eng Appl Sci 3(1):180–184
Andrew AA, Jun S, Takahiro H, Kimpei I, George EN, Wilson YF, Gloria ETE, Ntankouo NR (2011) Evaluation of groundwater quality and its suitability for drinking domestic and agricultural uses in the Banana Plain (Mbanga Njombe Penja) of the Cameroon Volcanic Line. Environ Geochem Health 33:559–575
Ansa-Asare OD, Darko HF, Asante KA (2009) Groundwater quality assessment of Akatsi, Adidome and Ho districts in the Volta region of Ghana. Desalination 248:446–452
Apau J, Acheampong A, Bepule V (2014) Physicochemical and microbial parameters of water from hand-dug wells from Nyamebekyere, a surburb of Obuasi, Ghana. Int J Sci Technol 3(6):347–351
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health, Association (APHA), American Water Works Association (AWWA), and Water Pollution Control Federation (WPCF), New York
Aremu MO, Ozonyia GN, Ikokoh PP (2011) Physico-chemical properties of well, borehole and Stream waters in Kubwa, Bwari Area Council, FCT, Nigeria. Electron J Environ Agric Food Chem 10(6):2296–2304
Armon R, Kitty (1994) The Health dimension of groundwater contamination. In: Holler (ed) Groundwater contamination and control. Marcel Dekker Inc, New York
Arya S, Kumar V, Sharma S (2012) Analysis of water quality parameters of groundwater in and around Diamond Cement Industry, Jhansi, Central India. Int J Curr Res 4(3):75–77
Asare-Donkor NK, Kwaansa-Ansah EE, Opoku F, Adimado AA (2015) Concentrations, hydrochemistry and risk evaluation of selected heavy metals along the Jimi River and its tributaries at Obuasi a mining enclave in Ghana. Environ Syst Res 4:12. doi:10.1186/s40068-015-0037-y
Avvannavar SM, Shrihari S (2008) Evaluation of water quality index for drinking purposes for river Netravathi, Mangalore, South India. Environ Monit Assess 143:279–290
Babiker SI, Mohamed AA, Mohamed TH (2007) Assessing groundwater quality using GIS. Water Resour Manag 21:699–715
Baruah M, Bhattacharyya KG, Patgiri AD (2008) Water quality of shallow groundwater of core city area of Guwahati. In: Proceedings of sixteenth national symposium on environment, Haryana, India, pp 101–106
Bhardwaj V, Singh DS (2010) Surface and groundwater quality characterization of Deoria District, Ganga Plain, India. Environ Earth Sci 63:383–395. doi:10.1007/s12665-010-0709x
Brijraj K, Kaur P (2007) Geochemistry of surface and sub-surface waters of Rawalyar lakes, Mandi district, Himachal Pradesh: constraints on weathering and erosion. J Geol Soc India 69:1020–1030
Cerling TE, Pederson BL, Damn KLV (1989) Sodium-calcium ion exchange in the weathering of shales implication for global weathering budgets. Gelogy 17:552–554
Chapman D (1992) A guide to the use of BIOTA sediments and water in environmental monitoring water quality assessments UNESCO/WHO/UNEP. Chapman and Hall, London, pp 371–460
Cosgrove WJ, Rijsberman FR (2000) World water vision: making water everybody’s business. Earth Scan Publication Ltd, UK, p 108
Dahiya S, Kaur A (1999) Physico chemical characteristics of underground water in rural areas of Tosham subdivision, Bhiwani district, Haryana. J Environ Poll 6(4):281
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Duncan AE, Peprah MO, Marfo MO, Tetteh G, Opoku AE (2014) Groundwater Quality Analysis on selected hand-dug wells in the Cape Coast Metropolis, Ghana: a case study of Abura. J Basic Appl Sci 1(2):23–30
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico City. J Hydrol 258:1–24
Efe ST (2002) Urban warming in Nigerian cities. The case of Warri metropolis. Afr J Environ Stud 2(2):6
Elango L, Kannan R (2007) Rock–water interaction and its control on chemical composition of groundwater. Chap. 11. Dev Environ Sci 5:229–243
Elango L, Kannan R, Senthil Kumar M (2003) Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu. Environ Geosci 1(4):157–166
Eletta O, Adeniyi A, Dolapo A (2010) Physico-chemical characterisation of some ground water supply in a school environment in Ilorin, Nigeria. Afr J Biotechnol 9(22):3293–3297
Essumang DK, Senu J, Fianko J, Nyarko B, Adokoh C, Boamponsem L (2011) Groundwater quality assessment: a physicochemical properties of drinking water in a rural setting of developing countries. Can J Sci Ind Res 2(3):102–126
Fisher RS, Mulican WF (1997) Hydrogeochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Cnihvahvan desert. Trans-Pecos, Texas USA. Hydrogeol J 10(4):455–474
Ghana Statistical Service (2012) 2010 housing and population census report. Ghana Statistical Service, Accra
Gibbs RJ (1970) Mechanism controlling world water chemistry. Sciences 170:795–840
Gomez JD, Nakat AC (2002) Community participation in water and sanitation. Water Int 27:343–353
Guler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10(4):455–474
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34(3):807–816
Henry GJ, Heinke GW (2005) Environmental science engineering, 2nd edn. Prentice-Hall of India Private Limited, New Delhi
Howitt D, Cramer D (2005) Introduction to SPSS in psychology: with supplement for releases 10, 11, 12 and 13. Pearson, Harlow
IDLO (2011) Sustainable development law on climate change, International Development Law Organization Working paper series
Jeevanandam M, Kannan R, Srinivasalu S, Rammohan V (2006) Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore District, South India. Environ Monit Assess 132(1):263–274
Jeevanandam M, Nagarajan R, Manikandan M, Senthilkumar M, Srinivasalu S, Prasanna MV (2012) Hydrogeochemistry and microbial contamination of groundwater from Lower Ponnaiyar Basin, Cuddalore District, Tamil Nadu, India. Environ Earth Sci 67(3):867–887
Jeong CH (2001) Effects of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Ketata-Rokbani M, Gueddari M, Bouhlila R (2011) Use of geographical information system and Water Quality Index to assess groundwater quality in El Khairat Deep Aquifer (Enfidha, Tunisian Sahel). Iran J Energy Environ 2(2):133–144
Kortatsi BK (2007a) Groundwater quality in the Wassa West district of the western region of Ghana. West Afr J Appl Ecol 11:25–40
Kortatsi BK (2007b) Hydrochemical framework of groundwater in the Ankobra Basin, Ghana. Aquat Geochem 13:41–74
Krishna Kumar S, Ram Mohan V, Dajkumar Sahayam J, Jeevanandam M (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159(1–4):341–351
Krishna Kumar S, Chandrasekar N, Seralathan P, Godson PS, Magesh NS (2011) Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environ Monit Assess 184(7):4127–4139
Krishna Kumar S, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4:341–350
Loizidou M, Kapetanios EG (1993) Effect of leachate from landfills on groundwater quality. Sci Total Environ 128:69–81
May AL, Loucks MD (1995) Solute and isotope geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 170:795–840
McCarthy MF (2004) Should we restrict chloride rather than sodium? Med Hypotheses 63:138–148
McMurry J, Fay RC (2004) Hydrogen, oxygen and water. In: Hamann KP (ed) Chemistry, 4th edn. Pearson Education, New Jersey, pp 575–599
Mendie U (2005) The nature of water. The theory and practice of clean water production for domestic and industrial use. Lacto-Medals Publishers, Lagos, pp 1–21
Mishra PC, Patel RK (2001) Study of the pollution load in the drinking water of Rairangpur, a small tribal dominated town of North Orissa. Indian J Environ Ecoplan 5(2):293–298
Mulla J, Asif S, Abed S, Pardhan V (2012) Ground water quality assessment of Babalgaon District Latur. J Chem Biol Phys Sci 2(1):501–504
Naik S, Purohit KM (2001) Studies on water quality of river Brahmani in Sundargarh district, Orissa. Indian J Environ Ecoplan 5(2):397–402
Nwajei GE, Obi-Iyeke GE, Okwagi P (2012) Distribution of selected trace metals in fish parts from the River Nigeria. Res J Rec Sci 1(1):81–84
Odoh BI, Utom AU, Ezeh HN, Egboka BCE (2012) Hydrogeochemical properties in parts of Abakaliki city, Southeastern Nigeria. AAPG/Div Environ Sci 19(2):1–9
Offodile ME (1983) The occurrence and exploitation of groundwater in Nigeria basement rocks. J Min Geol 2:131–146
Oluseyi T, Olayinka K, Adeleke I (2011) Assessment of ground water pollution in the residential areas of Ewekoro and Shagamu due to cement production. Afr J Environ Sci Technol 5(10):786–794
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006) Application of factor and cluster analysis for characterization of river and estuarine water systems—a case study: Mahanadi River (India). J Hydrol 331:434–445
Radojevic M, Bashkin VN (2006) Practical environmental analysis. RSC Publishing, Cambridge
Rao NS (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51(4):631–645
Ravikumar P, Venkatesharaju K, Somashekar RK (2010) Major ion chemistry and hydrochemical studies of groundwater of Bangalore South Taluk, India. Environ Monit Assess 163:643–653
Reghunath R, Sreedhara Murthy TR, Raghavan BR (2002) The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka, India. Water Res 36:2437–2442
Rohul-Amin, Ali SS, Anwar Z, Khattak JZK (2012) Microbial analysis of drinking water and water distribution system in New Urban Peshawar. Curr Res J Biol Sci 4(6):731–737
Routroy S, Harichandran R, Mohanty JK, Panda CR (2013) A statistical appraisal to hydrogeochemistry of fluoride contaminated ground water in Nayagarh District, Odisha. J Geol Soc India 81:350–360
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata wetlands, West Bengal, India. Environ Geol 55:823–835
Sanchez Martos F, Pulido Bosch A, Calaforra JM (1999) Hydrogeochemical processes in an arid region of Europe (Almeria, SE Spain). Appl Geochem 14:735–745
Schafer AI, Rossiter HMA, Owusu PA, Richard BS, Awuah E (2010) Developing country water supplies: physico-chemical water quality in Ghana. Desalination 251:193–203
Shrestha S, Kazama F (2006) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475
Singh DF (1992) Studies on the water quality index of some major rivers of Pune, Maharashtra. Proc Acad Environ Biol 1(1):61–66
Singh PK, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques-a case study. Anal Chim Acta 538:355–374
Sinha AK, Srivastava KP, Sexena J (2000) Impact of urbanization on groundwater of Jaipur, Rajasthan. In: Sinha AK, Shrivastava PK (eds) Earth resources and environmental issues. ABD Publishers, Jaipur, pp 173–179
Srinivasamoorthy K, Chidambaram M, Prasanna MV, Vasanthavigar M, John Peter A, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain—a case study from Mettur taluk, Salem district, Tamilnadu, India. J Earth Syst Sci 117(1):49–58
Subba Rao N (1997) Studies on water quality index in hard rock terrain of Guntur district, Andhra Pradesh, India. National Seminar on Hydrology of Precambrian Terrains and hard rock areas. pp 129–134
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ Geol 49:413–429
Subba Rao N, Prakasa Rao J, John Devadas D, Srinivasa Rao KV, Krishna C, Nagamalleswara Rao B (2002) Hydrogeochemistry and groundwater quality in a developing urban environment of a semi-arid region, Guntur, Andhra Pradesh. J Geol Soc India 59:159–166
Suthar S, Bishnoi P, Singh S, Mutiyar PK, Nema AK, Patil NS (2009) Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J Hazard Mater 171:189–199
Tay CK (2008) Chemical characteristics of groundwater in the Akatsi and Ketu Districts of the Volta Region, Ghana. CSIR-Water Research Institute, P. O. Box AH 38, Achimota-Accra, Ghana
Tay C, Kortatsi B (2007) Groundwater quality studies. A case study of Densu Basin, Ghana. West Africa J Appl Ecol 12:81–99
Tiwari TN, Mishra MA (1985) A preliminary assignment of water quality index of major Indian rivers. Indian J Environ Prot 5:276–279
Todd DK (1980) Groundwater hydrology. Wiley, New York
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality for groundwater quality assessment: thirumanimuttar Sub basin, Tamil Nadu, India. Environ Monit Assess 171(1–4):595–609
Walker GR, Jolly ID, Cook PG (1991) A new chloride leaching approach to the estimation of diffuse recharge following a change in land use. J Hydrol 128:49–67
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
World Health Organization (2004) Guideline for drinking-water, vol. 1, Recommendations, 3rd edn. Geneva, Switzerland
World Health Organization (2008) Guidelines for drinking-water quality. Incorporating 1st and 2nd Addenda, vol. 1, Recommendations, 3rd edn. WHO, Geneva
Yidana SM (2010) Groundwater classification using multivariate statistical methods: Southern Ghana. J Afr Earth Sci 57:455–469
Zhang J, Huang WW, Letolle R, Jusserand C (1995) Major element chemistry of the Huanghe (Yellow River), China: weathering processes and chemical fluxes. J Hydrol 168:173–203
Acknowledgments
The authors are very grateful to the National Council for tertiary Education (NTCE), Ghana for a research Grant under the Teaching and Learning Innovation Fund (TALIF-KNUSTR/3/005/2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
All the authors contributed equally to the preparation of this manuscript. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Boateng, T.K., Opoku, F., Acquaah, S.O. et al. Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Ghana. Environ Earth Sci 75, 489 (2016). https://doi.org/10.1007/s12665-015-5105-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5105-0