Abstract
Ischemic diseases are life-threatening, and the incidence increases as people’s lifestyles change. Medications and surgical intervention offer limited benefit, and stem cell therapy has emerged as a potential approach for treating ischemic diseases. The exosomes secreted by stem cells have attracted more attention because they do not trigger the immune response and can be used as drug carriers. The non-coding RNA (ncRNA) carried by exosomes plays a key role in mediating exosome’s beneficial effect, which can be further enhanced when combined with nanomaterials to improve its retention time. Here, we review the downstream target molecules and signal pathways of ncRNA and summarize recent advances of some nanomaterials used to encapsulate exosomes and promote ischemic tissue repair. We highlight the imprinting of exosomes from parent cells and discuss how the inflammasome pathway may be targeted for the development of novel therapy for ischemic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic diseases such as cardiovascular disease and lower limb ischemic diseases resulting from vascular blockage seriously endanger human health, and the incidence gradually rises. Myocardial infarction (MI) is caused by the interruption of coronary blood flow and the hypoxic necrosis of myocardial cells, leading to reduced myocardial contractility and decreased cardiac output [1, 2]. The lower limb ischemic disease is caused by arterial occlusion of the lower limbs [3]. Cerebral ischemia and renal ischemia are also common tissue ischemic diseases. Although medical therapy and surgical treatment can alleviate ischemic disease symptoms, these approaches cannot regenerate damaged tissue. Stem cells can self-renew and differentiate into a variety of functional cells upon activation. Stem cell-derived exosomes also play an important role in the diagnostic and therapeutic processes of ischemia diseases. This review will summarize the effects of non-coding RNA (ncRNA) within stem cell-derived exosomes on ischemic tissue repair and the underlying mechanisms.
Exosomes Derived from Stem Cells
Stem cells have self-renewal capacity and can produce more than one type of highly differentiated progeny cells. Bone marrow mesenchymal stem cells (BMSCs) and induced pluripotent stem cells (iPSCs) have many medical applications. For example, the hematopoietic stem cells in the bone marrow have been used to treat leukemia [4]. In many animal model studies of MI, BMSCs and adipose-derived stem cells (ASCs) have been shown to improve heart function after infarction. A number of animal studies show that after injection of stem cells, the retention time and survival rate of stem cells in the body are very low. The therapeutic function of stem cells is mainly due to their paracrine effect because only a very small number of the injected stem cells differentiated into cardiomyocytes [5, 6].
There are many ways that cells can communicate with each other, including a direct connection between cells, electrical stimulation, extracellular matrix interaction, and the release of various chemical substances. The cells release extracellular vesicles (EVs) for remote interaction to deliver their messages. EVs carry a variety of molecules, including lipids, proteins, DNA, mRNA, and ncRNA [7, 8]. It is speculated that the transplanted stem cells promote the reconstruction and regeneration of distal tissues by releasing EVs [9]. Wang et al. [10] reported that percutaneous intracoronary injection of EVs from human plasma reduced infarction size in dogs with MI. The beneficial effect was mediated by EV-derived miR-486, which inhibits the expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) in cardiomyocytes and promote the activation of protein kinase B (AKT), thereby preventing apoptosis of cardiomyocytes. Furthermore, conjugation of EVs with cardiac homing peptide (CHP) effectively improved the retention time of EVs in mouse and canine hearts.
The EVs can be divided into exosomes (Exos), microvesicles (MVs), and apoptotic bodies (ABs) according to cell source and size [11,12,13] (Fig. 1). MVs are produced by the sprouting and division of membrane vesicles on the cell surface, with a diameter of 100 ~ 1000 nm, which can be shed from the plasma membrane from many different types of cells [14]. ABs are membrane vesicles with a diameter of 1000 ~ 5000 nm released by apoptotic cells [12]. Exosomes are produced through endocytosis by living cells [15]. The endocytosed cell membrane forms several small vesicles, and the small vesicles fuse to form an early endosome, which buds inward to form multivesicularbodies (MVBs). MVBs can either fuse with lysosomes for degradation or fuse with the cell membrane to release the intraluminal vesicles (ILVs) into the cell in the external environment, and the secreted ILVs are called exosomes [16]. Compared to other EVs, exosomes are the smallest, ranging in size from 30 to 100 nm.
Imprinting of Exosomes from Parent Cells and ncRNA
NcRNA is transcribed from DNA but not translated into proteins. It performs distinct functions at different stages during development. Understanding of the molecules and pathways at the RNA level has implications for the diagnosis and treatment of disease. Recently, various ncRNAs, including miRNA, lncRNA, and circRNA, have been discovered to play an important role in gene transcription and translation.
In myocardial infarction (MI), some cells produce angiogenic factors, anti-apoptotic factors, mitotic factors, growth factors, and exosomes, attempting to repair the infarcted myocardium at early stage. Exosomes in the heart and circulation contain a large number of cardiac-specific ncRNAs. These ncRNAs can be used as indicators of heart injury and have great diagnostic potential as biomarkers of MI. Exosomes derived from cardiac and non-cardiac stem/progenitor cells are involved in cardiac protection and regeneration. Exogenous ncRNA can be rapidly degraded by highly active ribonuclease in plasma, but exosome is an ideal carrier for ncRNA because of its stable nature and non-immunogenicity [17].
Li et al. proposed the theory of “imprinting of exosomes from parent cells” [18]. It means that the components within exosomes and the exerted functions are determined by the dominant or recessive imprinting of their parent cells, which are regulated by the microenvironment surrounding the cells. In addition, some exosome-derived ncRNAs or mRNAs may not be functional in the parent cells but act on the recipient cell. Therefore, it is important to understand how the imprinting of parent cells affects the function of exosomes.
Studies have demonstrated that exosomal miRNAs play a key role in cell migration, angiogenesis, and immune regulation [19]. The mechanisms whereby lncRNA regulates biological process is much more complex than that of miRNA [20] [21, 22], and the function of exosomal lncRNA in MI remains largely unknown. CircRNA acts as a miRNA sponge to compete with mRNA for miRNA binding sites in cells, thus improving the expression level of target genes [23,24,25]. Recent studies suggest that circRNA plays an important role in ischemic diseases, including MI and lower limb ischemia [26, 27]. It has been shown that exosome contains more circRNAs than their source cells, but their function remains to be defined [28]
Inflammasome
Inflammasomes are protein complexes of the innate immune system (Fig. 2) that mediate the inflammatory responses in ischemic tissue injury [29]. The activation of inflammasomes leads to the activation of caspase-1 or caspase-11, which in turn activates pro-inflammatory cytokines, such as IL-1β and IL-18, resulting in pyroptosis. Based on the activation of either caspase-1 or caspase-11, inflammasomes can be classified as either canonical or non-canonical [30]. Canonical inflammasomes include NLRP1, NLRP3, NLRC4, and AIM2, which initiate inflammatory responses by activating caspase-1, whereas the non-canonical inflammasomes activate caspase-11[31, 32]. Studies suggest that exosome-derived ncRNA can prevent the activation of NLRP3 signaling pathway [33,34,35,36], but its role in non-canonical inflammasomes require further investigation.
Classification and mechanisms underlying the activation of inflammasomes. Pingtan represents the inflammasome system; double-sided embroidery represents both sides of inflammasome; the balalaika represents the classical inflammasome NLRP3, AIM2, and NLRC4; pipa stands for non-classical inflammasome; the bridge represents the organ; the flow represents the circulation system delivering IL-1 and IL-18; Hanshan Temple bell represents the sound of healing bells
Acute inflammation (M type) is a beneficial process that destroys pathogens, removes dead cells, and initiates ischemic tissue repair. In contrast, chronic inflammation (N type) is a pathological condition that lasts for a long time and leads to ischemic tissue damage. Studies suggest that inflammation can be modulated by inflammasomes [37], which are potential targets for the prevention and treatment of ischemic disease. Drugs targeting IL-1 have entered clinical trials, which offered opportunities to treat chronic inflammatory diseases. The CANTOS trial demonstrated that a monoclonal antibody (Canakinumab) targeting IL-1β can reduce the rate of ischemic disease [38]. The IL-1 clinical trial is an important step toward clinical application; however, it also showed that canakinumab is associated with an increased incidence of fatal infection resulting from off-target effects [39]. Recent studies demonstrated that exosome-derived circRNA can repair the ischemic tissue by preventing the activation of NLRP3 [33, 34]. These findings opened a new avenue for treating ischemic diseases.
Repair of Ischemic Tissue Injury by Exosomes and Underlying Mechanisms
At present, the research on the repair of various ischemic tissues by exosomes is in full swing (Fig. 3).
Repair of MI by Exosomes
The current treatment strategies for MI include drug therapy, percutaneous coronary intervention, and coronary artery bypass grafting [40, 41]. Although these treatments may improve cardiac function to a certain extent, they can’t regenerate the damaged myocardium. With the development of novel technology, stem cell-derived exosomes have been shown to improve heart function by inhibiting inflammatory response, reducing cell death, and preventing fibrosis. Therefore, the delivery of exosomes is becoming a new option for the treatment of MI [42, 43].
Mechanisms Underlying the Action of Exosomal miRNA in MI
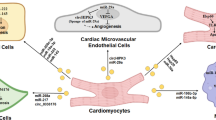
Many reports indicate that miRNAs in exosomes play a huge role in the repair and treatment of MI. Shao et al. [44] found that human umbilical mesenchymal stem cell-derived exosome (UMSC-Exo) is superior to UMSCs in preventing inflammation, cardiac fibrosis, and improving cardiac function in a rat model of MI. They demonstrated that UMSC-Exo and UMSCs have similar miRNA expression profiles, explaining why UMSC-Exo can replace UMSCs in cardiac repair. They further showed that knockout of HLA light chain β 2-microglobulin (B2M) in UMSCs by CRISPR/CAS9 can improve the survival rate of stem cells after transplantation by preventing the activation of CD8+T cells. In addition, exosome derived from B2M−UMSC is more effective in repairing tissue injury. MicroRNA sequencing and bioinformatics analysis revealed that Bim is the target of miR-24 [45].
Zhao et al. [46] showed that exosomes derived from mouse bone marrow mesenchymal stem cells (BMSC) cultured under hypoxia conditions are enriched with miR-125b, which mediates the cardioprotective effect. To improve tissue specificity, they conjugated the exosomes with an ischemic myocardium-targeted peptide and showed that these conjugated exosomes were able to home to injured hearts when injected intravenously. They further showed that miR-125b inhibits the expression of pro-apoptotic genes p53 and BAK1 in cardiomyocytes. Pan et al. [47] demonstrated that exosomes from adipose mesenchymal stem cells modified with miR-146a inhibited MI-induced apoptosis, inflammation, and fibrosis by suppressing EGR1 expression.
Mechanisms Underlying the Role of Exosomal lncRNA and circRNA in MI
The involvement of lncRNA and circRNA in MI is well documented [48, 49], but the mechanisms underlying the function of exosomal lncRNA and circRNA were rarely discussed.
Lin et al. [50] showed that lncRNA HCGl5 was enriched in exosomes from AC16 cardiomyocytes under hypoxia, which contributed to apoptosis of cardiomyocytes and inflammation via activation of NF-κB /p65 and p38 pathways. Furthermore, the overexpression of lncRNA HCGl5 aggravated MI injury in C57BL/6 J mice. Zhu et al. showed that the expression of lncRNA MALAT1 was decreased in damaged hears [51]. They injected the exosome into the mice treated with D-Gal, and the results showed that the exosomes alleviated the adverse effects of D-Gal on ejection fraction (EF) and fractional shorting (FS) in mice, and the beneficial effect was blocked when lncRNA MALAT1 was silenced, suggesting that lncRNA MALAT1 is the mediator of the protective effect afforded by the exosomes. Using a rat MI model, Li et al. showed that knockdown of circ-0001273 in exosomes led to impaired cardiac function by promoting apoptosis of cardiomyocytes [52].
Repair of Lower Limb Ischemia by Exosomes
Current treatment options of lower limb ischemic injury include drug therapy[53], endovascular intervention[54], and surgery [55], which can relieve symptoms to a certain extent but can’t cure the disease. Stem cell transplantation provides a new treatment option for some patients by promoting angiogenesis. But autologous stem cell transplantation is time-consuming and inconvenient, while allogeneic stem cell transplantation is associated with immune rejection. Therefore, the transplantation of stem cell-derived exosomes has emerged as a new alternative treatment of lower limb ischemic injury.
Mechanisms of Exosomal miRNA in Treating Lower Limb Ischemic Diseases
Using CRISPR/CAS9 technology, Zhang et al. [56] prepared UMSCs without human leukocyte antigen light chain β-2-microglobulin (B2M-UMSCs). The therapeutic potential of B2M-UMSCs was tested in a mouse hindlimb ischemia model. The results showed that transplantation of B2M-UMSCs resulted in enhanced perfusion and better running ability without causing immune rejection. The therapeutic effect was mediated by exosomes. MicroRNA sequencing identified miR-24 as the mediator of the beneficial effect. They further showed that Bim is the downstream target for miR-24.
MiR-126 is a miRNA highly expressed in endothelial cells, and it promotes angiogenesis by targeting inhibitors of angiogenic pathways. Ranghino et al. [57] found that endothelial progenitor-derived exosomes improved blood perfusion and neovascularization in a mouse femoral artery ligation model. They further showed that the exosomes are enriched with miR-126.
The Role of Exosomal lncRNA in Lower Limb Ischemic Injury
It was shown that pharmacological inhibition or genetic ablation of lncRNA MALAT1 reduced blood flow recovery and capillary density in a hindlimb ischemia model [58, 59]. Shyu et al. [60] found that hyperbaric oxygen (HBO) can promote neovascularization by upregulating lncRNA MALAT1, which counteracts the inhibitory effect of miR-92a on the expression of KLF2 in endothelial cells. Silence of LncRNA DLGAP1-AS1 can inhibit oxidative stress and apoptosis and reduce the levels of TNF-α by activating PI3K/Akt pathway in a rat model of acute lower limb ischemia reperfusion [61].
Mechanisms of Exosomal circRNA and NLRP3 in Lower Limb Ischemia
Using a mouse hindlimb ischemia model, Yan et al. [34] found that the expression of circHIPK3 in ischemic muscle was decreased. They showed that UMSC-derived exosomes improved muscle perfusion and function, and the effect was mediated by circHIPK3.They further showed that circHIPK3 inhibits inflammasome activation and pyroptosis by downregulating miR-421, leading to increased expression of FOXO3a.
In a study of skeletal muscle ischemic injury, Wang et al. [33] showed that tumor suppressor Rb1 acts as an inducer of NLRP3 inflammation. In ischemic muscle, the level of miR-29b increases, resulting in decreased expression of CDK6 expression and subsequent activation of Rb1. UMSC-derived exosomes improve blood perfusion and muscle motor function by releasing circRNA cPWWP2A, which inhibits the activity of miR-29b. This study not only discovered a new therapeutic target regulating NLRP3 inflammasomes but also demonstrated that exosomes can treat muscle injury by releasing circRNA, which has the potential for clinical application (Fig. 4).
Repair of Cerebral Ischemic Injury by Exosomes
Cerebral ischemic diseases are very difficult to treat due to the non-replicative nature of neurons and the presence of the blood–brain barrier. There is an urgent need to find specific methods and new drugs to repair ischemic neurons. Exosomes are ideal drug carriers for neurological diseases due to their ability to penetrate the blood–brain barrier [62].
At present, most studies on the effect and the mechanism of exosomes involved in cerebral ischemia repair focus on miRNAs, and studies on other ncRNAs are relatively few. Zhang et al. conjugated an c(RGDyK) peptide to exosomes to target ischemic brain [63, 64]. They loaded the exosomes with miR-210 to generate the RGD-exo, miR-210, which was then injected intravenously into a mouse model of transient middle cerebral artery occlusion (MCAO). They showed that miR-210 can be detected at the lesion area of the ischemic region. They further showed that the expressions of integrin β3, vascular endothelial growth factor, and CD34 were significantly upregulated, and the survival rate was also improved after 14 days treatment. Also using the MCAO model, Cai et al. [65] showed that MSC-derived exosomes contain miR-542-3p, which can attenuate ischemia-induced glial inflammation by inhibiting the activity of TLR4. Song et al. [66] found that M2 microglia-derived exosome contains miR-124, which inhibits ischemic brain injury and promotes neuronal survival by targeting USP14. The results of Chen et al. [67] showed that CircSHOC2 in exosomes secreted by ischemia-preconditoned astrocytes inhibited neuronal apoptosis and improved neuronal damage by regulating autophagy via the miR-7670-3p/SIRT1 axis.
Repair of Renal Ischemic Injury by Exosomes
Stem cell-derived exosomes have been shown to repair ischemic renal injury by inhibiting fibrosis, inflammation, and apoptosis. Using a rat model of acute renal ischemic injury, Li et al. [68] showed that human urine-derived stem cells (USCs) can restore renal function by releasing exosomes that contains miR-146a-5p, which targets the 3’UTR of IRAK1, leading to inhibition of NF-κB signaling pathway. Using a mouse renal ischemia reperfusion injury model, Cao et al. [69] demonstrated that exosomes derived from human umbilical cord mesenchymal stem cells (hucMSCs) exhibit tropism to ischemic kidney tissue and promote tubular repair by preventing cell cycle arrest and apoptosis through the miR-125b-5p/p53 pathway. Zhang et al. [70] showed that human urine-derived stem cell-derived exosomes (USCs -Exos) inhibit hypoxia/reoxygenation-induced apoptosis of human proximal tubular epithelial cells by releasing miR-216a-5p, which activates the Akt pathway by targeting PTEN.
Novel Biomaterials Improve Retention of Exosomes
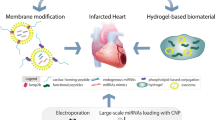
The exosomes have a short half-life in vivo, which reduces their efficacy in potential clinical treatment. Hydrogel biomaterials can overcome this problem by forming a microenvironment similar to the extracellular matrix in the tissue and prolonging the retention time of the exosomes. Various forms of hydrogels, such as chitosan/silk fibroin hydrogel, chitosan hydrogel, and imine crosslinked hydrogel, have been used to encapsulate exosomes and showed better therapeutic effects [71, 72] (Fig. 5).
Han et al. [73] found a decreased expression of miR-675 in damaged muscles, while the expression of transforming growth factor-β1 (TGF-β1) and p21 was increased. Injection of miR-675 into damaged muscle resulted in reduced expression of TGF-β1 and p21, suggesting that miR-675 is a modulator of TGF-β1 and p21. Using molecular approaches, they showed that TGF-β1 is the downstream target of miR-675, and TGF-β1 is an inducer of p21. These findings suggest that miR-675 inhibits the damaged process by targeting the TGF-β1/p21 signaling pathway. They further demonstrated that exosomes containing miR-675 wrapped in silk fibroin hydrogel promote blood perfusion of the ischemic hindlimb.
Moreover, Han et al. [74] developed a novel injectable self-assembled peptide amphiphile (PA) by adding a cardioprotective peptide and a matrix metalloproteinase-2 (MMP-2) degradable sequence to PA (PA-GHRPS). The gelatinization ability was further enhanced by adding peptide NapFF to form a PGN hydrogel, which was used to encapsulate exosomes. The PGN encapsulated exosomes were injected into the border zone of infarcted myocardium of rat hearts, and the results showed that PGN hydrogel could effectively encapsulate the exosome and ensure the stability and continuous release of the exosomes, which significantly improved myocardial function by reducing inflammation, fibrosis, and apoptosis and promoting angiogenesis.
The hydrogel formed by composite biomaterials such as silk fibroin (SF) and silk sericin (SS) is more suitable for tissue repair than that formed by a single biomaterial. Han et al. [75] investigated the effect of two different extraction methods on the ability of the composite hydrogel on tissue repair. SF-SS hydrogel was formed by extracting SF and SS proteins separately (LiBr dissolution of SF and hot water dissolution of SS). In contrast, the SF-SS hydrogel was formed by simultaneous extraction (LiBr dissolution of SF and SS proteins). The results showed that SF-SS hydrogel was more efficient in wrapping UMSC-Exo to promote wound healing and angiogenesis.
In addition, Zhao et al. [76] developed photosensitive delivery microcarriers (PDMs) for drug delivery. Under near-infrared light, the PDMs became shrank and triggered the release of drugs such as VEGF to promote angiogenesis. It would be interesting to know whether PDMs can be used to carry exosomes.
Conclusion
Stem cell-derived exosomes have been used to treat ischemic diseases in pre-clinical studies. New materials such as hydrogels have been used to improve the therapeutic potential of the exosome by prolonging its retention time. Many of the beneficial effects afforded by exosomes are mediated by ncRNAs. So far, most of the studies have been focused on miRNA, and there is only limited information on the role of lncRNA and circRNA in the literature. Emerging evidence suggests that inflammasomes are involved in the inflammatory reaction associated with ischemic tissue injury. Further understanding of the mechanisms responsible for the regulation of inflammasomes by exosomal ncRNA will lead to the identification of a novel therapeutic target for ischemic diseases.
References
Torabi, A., Cleland, J. G., Rigby, A. S., & Sherwi, N. (2014). Development and course of heart failure after a myocardial infarction in younger and older people. Journal of Geriatric Cardiology, 11(1), 1–12. https://doi.org/10.3969/j.issn.1671-5411.2014.01.002
Pan, W., Zhu, Y., Meng, X., Zhang, C., Yang, Y., & Bei, Y. (2019). Immunomodulation by exosomes in myocardial infarction. Journal of Cardiovascular Translational Research, 12(1), 28–36. https://doi.org/10.1007/s12265-018-9836-7
Chatterjee, S., Bashir, R., Lakhter, V., O’Murchu, B., O’Neill, B., & Aggarwal, V. (2017). Case of percutaneous extracorporeal femoro-femoral bypass for acute limb ischemia from large bore access. JACC. Cardiovascular Interventions, 10(12), e109–e110. https://doi.org/10.1016/j.jcin.2017.03.027
Turner, L., & Knoepfler, P. (2016). Selling stem cells in the USA: Assessing the direct-to-consumer industry. Cell Stem Cell, 19(2), 154–157. https://doi.org/10.1016/j.stem.2016.06.007
Chistiakov, D. A., Orekhov, A. N., & Bobryshev, Y. V. (2016). Cardiac extracellular vesicles in normal and infarcted heart. International Journal of Molecular Sciences, 17(1), 63. https://doi.org/10.3390/ijms17010063
Russo, V., Young, S., Hamilton, A., Amsden, B. G., & Flynn, L. E. (2014). Mesenchymal stem cell delivery strategies to promote cardiac regeneration following ischemic injury. Biomaterials, 35(13), 3956–3974. https://doi.org/10.1016/j.biomaterials.2014.01.075
Mathivanan, S., Fahner, C. J., Reid, G. E., & Simpson, R. J. (2012). ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Research, 40(Database issue), D1241-1244. https://doi.org/10.1093/nar/gkr828
Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O., & Skog, J. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature Communications, 2, 180. https://doi.org/10.1038/ncomms1180
Shao, H., Im, H., Castro, C. M., Breakefield, X., Weissleder, R., & Lee, H. (2018). New technologies for analysis of extracellular vesicles. Chemical Reviews, 118(4), 1917–1950. https://doi.org/10.1021/acs.chemrev.7b00534
Wang, H., Maimaitiaili, R., Yao, J., Xie, Y., Qiang, S., Hu, F., Li, X., Shi, C., Jia, P., Yang, H., Wei, M., Zhao, J., Zhou, Z., Xie, J., Jiang, J., Cai, H., Sluijter, J. P. G., Xu, Y., Zhang, Y., & Xiao, J. (2021). Percutaneous intracoronary delivery of plasma extracellular vesicles protects the myocardium against ischemia-reperfusion injury in Canis. Hypertension, 78(5), 1541–1554. https://doi.org/10.1161/HYPERTENSIONAHA.121.17574
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200(4), 373–383. https://doi.org/10.1083/jcb.201211138
Colombo, M., Raposo, G., & Thery, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Akers, J. C., Gonda, D., Kim, R., Carter, B. S., & Chen, C. C. (2013). Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of Neuro-oncology, 113(1), 1–11. https://doi.org/10.1007/s11060-013-1084-8
Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A., & D’Souza-Schorey, C. (2010). Microvesicles: Mediators of extracellular communication during cancer progression. Journal of Cell Science, 123(Pt 10), 1603–1611. https://doi.org/10.1242/jcs.064386
Farooqi, A. A., Desai, N. N., Qureshi, M. Z., Librelotto, D. R. N., Gasparri, M. L., Bishayee, A., Nabavi, S. M., Curti, V., & Daglia, M. (2018). Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnology Advances, 36(1), 328–334. https://doi.org/10.1016/j.biotechadv.2017.12.010
Wei, D., Zhan, W., Gao, Y., Huang, L., Gong, R., Wang, W., Zhang, R., Wu, Y., Gao, S., & Kang, T. (2021). RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Research, 31(2), 157–177. https://doi.org/10.1038/s41422-020-00409-1
Li, Y., Shen, Z., & Yu, X. Y. (2015). Transport of microRNAs via exosomes. Nature Reviews. Cardiology, 12(4), 198. https://doi.org/10.1038/nrcardio.2014.207-c1
Li, Y., Zhou, J., Song, Y. H., & Yu, X. Y. (2017). Dominant and recessive imprinting of exosomes from parent cells. Nature Reviews. Cardiology, 14(8), 491. https://doi.org/10.1038/nrcardio.2017.93
He, L., Zhu, W., Chen, Q., Yuan, Y., Wang, Y., Wang, J., & Wu, X. (2019). Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics, 9(26), 8206–8220. https://doi.org/10.7150/thno.37455
Ali, T., & Grote, P. (2020). Beyond the RNA-dependent function of LncRNA genes. Elife, 9, e60583. https://doi.org/10.7554/eLife.60583
Liu, C. Y., Zhang, Y. H., Li, R. B., Zhou, L. Y., An, T., Zhang, R. C., Zhai, M., Huang, Y., Yan, K. W., Dong, Y. H., Ponnusamy, M., Shan, C., Xu, S., Wang, Q., Zhang, Y. H., Zhang, J., & Wang, K. (2018). LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nature Communications, 9(1), 29. https://doi.org/10.1038/s41467-017-02280-y
Mao, Q., Liang, X. L., Zhang, C. L., Pang, Y. H., & Lu, Y. X. (2019). LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Research & Therapy, 10(1), 393. https://doi.org/10.1186/s13287-019-1522-4
Shi, Y., Jia, X., & Xu, J. (2020). The new function of circRNA: Translation. Clinical and Translational Oncology, 22(12), 2162–2169. https://doi.org/10.1007/s12094-020-02371-1
Liu, J., Xue, N., Guo, Y., Niu, K., Gao, L., Zhang, S., Gu, H., Wang, X., Zhao, D., & Fan, R. (2019). CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. Aging (Albany NY), 11(24), 12412–12427. https://doi.org/10.18632/aging.102580
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., & Kjems, J. (2013). Natural RNA circles function as efficient microRNA sponges. Nature, 495(7441), 384–388. https://doi.org/10.1038/nature11993
Fan, X., Weng, X., Zhao, Y., Chen, W., Gan, T., & Xu, D. (2017). Circular RNAs in cardiovascular disease: An overview. BioMed Research International, 2017, 5135781. https://doi.org/10.1155/2017/5135781
Bayoumi, A. S., Aonuma, T., Teoh, J. P., Tang, Y. L., & Kim, I. M. (2018). Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacologica Sinica, 39(7), 1100–1109. https://doi.org/10.1038/aps.2017.196
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., Chen, D., Gu, J., He, X., & Huang, S. (2015). Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Research, 25(8), 981–984. https://doi.org/10.1038/cr.2015.82
Srinivasula, S. M., Poyet, J. L., Razmara, M., Datta, P., Zhang, Z., & Alnemri, E. S. (2002). The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. Journal of Biological Chemistry, 277(24), 21119–21122. https://doi.org/10.1074/jbc.C200179200
Platnich, J. M., & Muruve, D. A. (2019). NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Archives of Biochemistry and Biophysics, 670, 4–14. https://doi.org/10.1016/j.abb.2019.02.008
Rathinam, V. A., Vanaja, S. K., & Fitzgerald, K. A. (2012). Regulation of inflammasome signaling. Nature Immunology, 13(4), 333–342. https://doi.org/10.1038/ni.2237
Shi, J., Zhao, Y., Wang, Y., Gao, W., Ding, J., Li, P., Hu, L., & Shao, F. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 514(7521), 187–192. https://doi.org/10.1038/nature13683
Wang, Y., Xie, W., Liu, B., Huang, H., Luo, W., Zhang, Y., Pan, X., Yu, X. Y., Shen, Z., & Li, Y. (2021). Stem cell-derived exosomes repair ischemic muscle injury by inhibiting the tumor suppressor Rb1-mediated NLRP3 inflammasome pathway. Signal Transduction and Targeted Therapy, 6(1), 121. https://doi.org/10.1038/s41392-021-00520-8
Yan, B., Zhang, Y., Liang, C., Liu, B., Ding, F., Wang, Y., Zhu, B., Zhao, R., Yu, X. Y., & Li, Y. (2020). Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics, 10(15), 6728–6742. https://doi.org/10.7150/thno.42259
Dai, Y., Wang, S., Chang, S., Ren, D., Shali, S., Li, C., Yang, H., Huang, Z., & Ge, J. (2020). M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. Journal of Molecular and Cellular Cardiology, 142, 65–79. https://doi.org/10.1016/j.yjmcc.2020.02.007
Noonin, C., & Thongboonkerd, V. (2021). Exosome-inflammasome crosstalk and their roles in inflammatory responses. Theranostics, 11(9), 4436–4451. https://doi.org/10.7150/thno.54004
Li, Y., Huang, H., Liu, B., Zhang, Y., Pan, X., Yu, X. Y., Shen, Z., & Song, Y. H. (2021). Inflammasomes as therapeutic targets in human diseases. Signal Transduction and Targeted Therapy, 6(1), 247. https://doi.org/10.1038/s41392-021-00650-z
Ridker, P. M., Everett, B. M., Thuren, T., MacFadyen, J. G., Chang, W. H., Ballantyne, C., Fonseca, F., Nicolau, J., Koenig, W., Anker, S. D., Kastelein, J. J. P., Cornel, J. H., Pais, P., Pella, D., Genest, J., Cifkova, R., Lorenzatti, A., Forster, T., Kobalava, Z., … Group, C. T. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. New England Journal of Medicine, 377(12), 1119–1131. https://doi.org/10.1056/NEJMoa1707914
Wu, D., Chen, Y., Sun, Y., Gao, Q., Li, H., Yang, Z., Wang, Y., Jiang, X., & Yu, B. (2020). Target of MCC950 in inhibition of NLRP3 inflammasome activation: A literature review. Inflammation, 43(1), 17–23. https://doi.org/10.1007/s10753-019-01098-8
Thuijs, D., Kappetein, A. P., Serruys, P. W., Mohr, F. W., Morice, M. C., Mack, M. J., Holmes, D. R., Jr., Curzen, N., Davierwala, P., Noack, T., Milojevic, M., Dawkins, K. D., da Costa, B. R., Juni, P., Head, S. J., & Investigators, S. E. S. (2019). Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet, 394(10206), 1325–1334. https://doi.org/10.1016/S0140-6736(19)31997-X
Head, S. J., Milojevic, M., Daemen, J., Ahn, J. M., Boersma, E., Christiansen, E. H., Domanski, M. J., Farkouh, M. E., Flather, M., Fuster, V., Hlatky, M. A., Holm, N. R., Hueb, W. A., Kamalesh, M., Kim, Y. H., Makikallio, T., Mohr, F. W., Papageorgiou, G., Park, S. J., … Kappetein, A. P. (2018). Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: A pooled analysis of individual patient data. Lancet, 391(10124), 939–948. https://doi.org/10.1016/S0140-6736(18)30423-9
Sahoo, S., & Losordo, D. W. (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research, 114(2), 333–344. https://doi.org/10.1161/CIRCRESAHA.114.300639
Chen, G. H., Xu, J., & Yang, Y. J. (2017). Exosomes: Promising sacks for treating ischemic heart disease? American Journal of Physiology. Heart and Circulatory Physiology, 313(3), H508–H523. https://doi.org/10.1152/ajpheart.00213.2017
Shao, L., Zhang, Y., Lan, B., Wang, J., Zhang, Z., Zhang, L., Xiao, P., Meng, Q., Geng, Y. J., Yu, X. Y., & Li, Y. (2017). MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Research International, 2017, 4150705. https://doi.org/10.1155/2017/4150705
Shao, L., Zhang, Y., Pan, X., Liu, B., Liang, C., Zhang, Y., Wang, Y., Yan, B., Xie, W., Sun, Y., Shen, Z., Yu, X. Y., & Li, Y. (2020). Knockout of beta-2 microglobulin enhances cardiac repair by modulating exosome imprinting and inhibiting stem cell-induced immune rejection. Cellular and Molecular Life Sciences, 77(5), 937–952. https://doi.org/10.1007/s00018-019-03220-3
Zhu, L. P., Tian, T., Wang, J. Y., He, J. N., Chen, T., Pan, M., Xu, L., Zhang, H. X., Qiu, X. T., Li, C. C., Wang, K. K., Shen, H., Zhang, G. G., & Bai, Y. P. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8(22), 6163–6177. https://doi.org/10.7150/thno.28021
Pan, J., Alimujiang, M., Chen, Q., Shi, H., & Luo, X. (2019). Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. Journal of Cellular Biochemistry, 120(3), 4433–4443. https://doi.org/10.1002/jcb.27731
Gao, L., Liu, Y., Guo, S., Yao, R., Wu, L., Xiao, L., Wang, Z., Liu, Y., & Zhang, Y. (2017). Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cellular Physiology and Biochemistry, 44(4), 1497–1508. https://doi.org/10.1159/000485588
Vausort, M., Wagner, D. R., & Devaux, Y. (2014). Long noncoding RNAs in patients with acute myocardial infarction. Circulation Research, 115(7), 668–677. https://doi.org/10.1161/CIRCRESAHA.115.303836
Lin, B., Chen, X., Lu, C., Xu, J., Qiu, Y., Liu, X., Song, H., Chen, A., Xiong, J., Wang, K., Yuan, Y., Shi, L., Zhong, L., & Jiang, X. (2021). Loss of exosomal LncRNA HCG15 prevents acute myocardial ischemic injury through the NF-kappaB/p65 and p38 pathways. Cell Death & Disease, 12(11), 1007. https://doi.org/10.1038/s41419-021-04281-8
Zhu, B., Zhang, L., Liang, C., Liu, B., Pan, X., Wang, Y., Zhang, Y., Zhang, Y., Xie, W., Yan, B., Liu, F., Yip, H. K., Yu, X. Y., & Li, Y. (2019). Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-kappaB/TNF-alpha signaling pathway. Oxidative Medicine and Cellular Longevity, 2019, 9739258. https://doi.org/10.1155/2019/9739258
Li, C. X., Song, J., Li, X., Zhang, T., & Li, Z. M. (2020). Circular RNA 0001273 in exosomes derived from human umbilical cord mesenchymal stem cells (UMSCs) in myocardial infarction. Eur Rev Med Pharmacol Sci, 24(19), 10086–10095. https://doi.org/10.26355/eurrev_202010_23228
Santistevan, J. R. (2017). Acute limb ischemia: An emergency medicine approach. Emergency Medicine Clinics of North America, 35(4), 889–909. https://doi.org/10.1016/j.emc.2017.07.006
Armstrong, E. J. (2017). Endovascular treatment of peripheral artery disease and critical limb ischemia. Interventional Cardiology Clinics, 6(2), xi. https://doi.org/10.1016/j.iccl.2017.01.001
Osterberg, K., Falkenberg, M., & Resch, T. (2014). Endovascular technique for arterial shunting to prevent intraoperative ischemia. European Journal of Vascular and Endovascular Surgery, 48(2), 126–130. https://doi.org/10.1016/j.ejvs.2014.04.007
Zhang, Y., Wang, Y., Shao, L., Pan, X., Liang, C., Liu, B., Zhang, Y., Xie, W., Yan, B., Liu, F., Yu, X. Y., & Li, Y. (2020). Knockout of beta-2 microglobulin reduces stem cell-induced immune rejection and enhances ischaemic hindlimb repair via exosome/miR-24/Bim pathway. Journal of Cellular and Molecular Medicine, 24(1), 695–710. https://doi.org/10.1111/jcmm.14778
Ranghino, A., Cantaluppi, V., Grange, C., Vitillo, L., Fop, F., Biancone, L., Deregibus, M. C., Tetta, C., Segoloni, G. P., & Camussi, G. (2012). Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. International Journal of Immunopathology and Pharmacology, 25(1), 75–85. https://doi.org/10.1177/039463201202500110
Michalik, K. M., You, X., Manavski, Y., Doddaballapur, A., Zornig, M., Braun, T., John, D., Ponomareva, Y., Chen, W., Uchida, S., Boon, R. A., & Dimmeler, S. (2014). Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circulation Research, 114(9), 1389–1397. https://doi.org/10.1161/CIRCRESAHA.114.303265
Zhang, X., Tang, X., Hamblin, M. H., & Yin, K. J. (2018). Long non-coding RNA Malat1 regulates angiogenesis in hindlimb ischemia. International Journal of Molecular Sciences, 19(6), 1723. https://doi.org/10.3390/ijms19061723
Shyu, K. G., Wang, B. W., Pan, C. M., Fang, W. J., & Lin, C. M. (2019). Hyperbaric oxygen boosts long noncoding RNA MALAT1 exosome secretion to suppress microRNA-92a expression in therapeutic angiogenesis. International Journal of Cardiology, 274, 271–278. https://doi.org/10.1016/j.ijcard.2018.09.118
Shen, G. H., Song, Y., Yao, Y., Sun, Q. F., Jing, B., Wu, J., Li, S. Y., Liu, S. Q., Li, H. C., Yuan, C., Liu, G. Y., Li, J. B., Liu, X. Y., & Wang, H. Y. (2020). Downregulation of DLGAP1-antisense RNA 1 alleviates vascular endothelial cell injury via activation of the phosphoinositide 3-kinase/Akt pathway results from an acute limb ischemia rat model. European Journal of Vascular and Endovascular Surgery, 59(1), 98–107. https://doi.org/10.1016/j.ejvs.2019.06.032
Hill, A. F. (2019). Extracellular vesicles and neurodegenerative diseases. Journal of Neuroscience, 39(47), 9269–9273. https://doi.org/10.1523/JNEUROSCI.0147-18.2019
Zhang, H., Wu, J., Wu, J., Fan, Q., Zhou, J., Wu, J., Liu, S., Zang, J., Ye, J., Xiao, M., Tian, T., & Gao, J. (2019). Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology, 17(1), 29. https://doi.org/10.1186/s12951-019-0461-7
Tian, T., Zhang, H. X., He, C. P., Fan, S., Zhu, Y. L., Qi, C., Huang, N. P., Xiao, Z. D., Lu, Z. H., Tannous, B. A., & Gao, J. (2018). Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials, 150, 137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Cai, G., Cai, G., Zhou, H., Zhuang, Z., Liu, K., Pei, S., Wang, Y., Wang, H., Wang, X., Xu, S., Cui, C., Sun, M., Guo, S., Jia, K., Wang, X., & Zhang, D. (2021). Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Research & Therapy, 12(1), 2. https://doi.org/10.1186/s13287-020-02030-w
Song, Y., Li, Z., He, T., Qu, M., Jiang, L., Li, W., Shi, X., Pan, J., Zhang, L., Wang, Y., Zhang, Z., Tang, Y., & Yang, G. Y. (2019). M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics, 9(10), 2910–2923. https://doi.org/10.7150/thno.30879
Chen, W., Wang, H., Zhu, Z., Feng, J., & Chen, L. (2020). Exosome-shuttled circSHOC2 from IPASs regulates neuronal autophagy and ameliorates ischemic brain injury via the miR-7670-3p/SIRT1 axis. Mol Ther Nucleic Acids, 22, 657–672. https://doi.org/10.1016/j.omtn.2020.09.027
Li, X., Liao, J., Su, X., Li, W., Bi, Z., Wang, J., Su, Q., Huang, H., Wei, Y., Gao, Y., Li, J., Liu, L., & Wang, C. (2020). Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics, 10(21), 9561–9578. https://doi.org/10.7150/thno.42153
Cao, J. Y., Wang, B., Tang, T. T., Wen, Y., Li, Z. L., Feng, S. T., Wu, M., Liu, D., Yin, D., Ma, K. L., Tang, R. N., Wu, Q. L., Lan, H. Y., Lv, L. L., & Liu, B. C. (2021). Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics, 11(11), 5248–5266. https://doi.org/10.7150/thno.54550
Zhang, Y., Wang, J., Yang, B., Qiao, R., Li, A., Guo, H., Ding, J., Li, H., Ye, H., Wu, D., Cui, L., & Yang, S. (2020). Transfer of microRNA-216a-5p from exosomes secreted by human urine-derived stem cells reduces renal ischemia/reperfusion injury. Front Cell Dev Biol, 8, 610587. https://doi.org/10.3389/fcell.2020.610587
Liu, X., Yang, Y., Li, Y., Niu, X., Zhao, B., Wang, Y., Bao, C., Xie, Z., Lin, Q., & Zhu, L. (2017). Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale, 9(13), 4430–4438. https://doi.org/10.1039/c7nr00352h
Xu, N., Wang, L., Guan, J., Tang, C., He, N., Zhang, W., & Fu, S. (2018). Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. International Journal of Biological Macromolecules, 117, 102–107. https://doi.org/10.1016/j.ijbiomac.2018.05.066
Han, C., Zhou, J., Liu, B., Liang, C., Pan, X., Zhang, Y., Zhang, Y., Wang, Y., Shao, L., Zhu, B., Wang, J., Yin, Q., Yu, X. Y., & Li, Y. (2019). Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Materials Science & Engineering, C: Materials for Biological Applications, 99, 322–332. https://doi.org/10.1016/j.msec.2019.01.122
Han, C., Zhou, J., Liang, C., Liu, B., Pan, X., Zhang, Y., Wang, Y., Yan, B., Xie, W., Liu, F., Yu, X. Y., & Li, Y. (2019). Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci, 7(7), 2920–2933. https://doi.org/10.1039/c9bm00101h
Han, C., Liu, F., Zhang, Y., Chen, W., Luo, W., Ding, F., Lu, L., Wu, C., & Li, Y. (2021). Human umbilical cord mesenchymal stem cell derived exosomes delivered using silk fibroin and sericin composite hydrogel promote wound healing. Front Cardiovasc Med, 8, 713021. https://doi.org/10.3389/fcvm.2021.713021
Zhao, X., Liu, Y., Shao, C., Nie, M., Huang, Q., Li, J., Sun, L., & Zhao, Y. (2019). Photoresponsive delivery microcarriers for tissue defects repair. Adv Sci (Weinh), 6(20), 1901280. https://doi.org/10.1002/advs.201901280
Funding
This work was supported by the National Natural Science Foundation of China (81870194 and 91849122 to Y Li, 81873528 to YH Song, and 91839101 to Z Shen), the Jiangsu Province Peak of Talent in Six Industries (BU24600117 to Y Li), the Introduction Project of Clinical Medicine Expert Team for Suzhou (No.SZYJTD201704), the project for the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Translational Research Grant of NCRCH (2020WSB07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Relevance
Ischemic disease is very harmful for human beings. This review summarizes the therapeutic effect and mechanism of stem cell-derived exosomes on ischemic diseases and expects to provide theoretical and technical information for clinical application.
Rights and permissions
About this article
Cite this article
Zhang, Y., Jiao, L., Lu, L. et al. The Mechanisms Underlying the Beneficial Effects of Stem Cell-Derived Exosomes in Repairing Ischemic Tissue Injury. J. of Cardiovasc. Trans. Res. 15, 524–534 (2022). https://doi.org/10.1007/s12265-022-10263-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10263-8