Abstract
Stem cell-based therapy for ischemic heart disease (IHD) has become a promising but controversial strategy during the past two decades. The fate and effects of stem cells engrafted into ischemia myocardium are still not fully understood. Stem cell-derived exosomes, a subcategory of extracellular vesicles with nano size, have been considered as an efficient and safe transporter for microRNAs (miRNAs) and a central mediator of the cardioprotective potentials of the parental cells. Hypoxia, pharmacological intervention, and gene manipulation could alter the exosomal miRNAs cargos from stem cells and promote therapeutic potential. Furthermore, several bioengineering methods were also successfully applied to modify miRNAs content and components of exosomal membrane proteins recently. In this review, we outline relevant results about exosomal miRNAs from stem cells and focus on the current strategies to promote their therapeutic efficiency in IHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease (IHD) is one of the leading causes of mortality worldwide and gave rise to approximately 1.7 million death of Chinese in 2016 [1, 2]. IHD is characterized by narrowed coronary arteries and reduced blood volume and oxygen supplements in the myocardium. Due to the poor self-renewal of cardiomyocytes, irreversible loss of the myocardium could result in cardiac remodeling. Prevailing therapy for IHD slows the progression but conclusively fails to reverse the decline of cardiomyocyte number [3]. Thus, the repairation and regeneration of damaged cardiomyocytes have become one of the hot topics in IHD treatment. With the self-renew and directional differentiation capability, the stem-cell transplanation was considered as a promising approach to replace necrotic cells and to replenish new cardiomyocytes. Due to ethical issues, adult stem cells and induced pluripotent cells (iPSC) were more widely used in clinical trials than embryonic stem cells (ESC). Therefore, various adult stem cells have been tested, such as bone marrow mesenchymal stem cells (BMSC), endothelial progenitor cells, and cardiac progenitor cells (CPC). Although stem cell transplantation has achieved beneficial effects on infarcted hearts in various animal models like mice [4], swine [5, 6], and non-human primates [7], accumulating studies revealed that the engrafted stem cells failed to generate new cardiac cells to compensate for the losses of cardiomyocytes [8, 9]. However, such beneficial effects may result from other mechnisms, such as the dying stem-cell hypothesis [10, 11] and paracrine hypothesis [12]. Exosomes are characterized by biocompatibility, penetration, and stability in extracellular space because of the lipid bilayer membrane and nano-size (30 to 150 nm) [13]. Thus, exosomes could be a good transporter to deliver modulators like miRNAs from stem cells to the infarcted hearts.
miRNAs are short non-coding RNAs with 18–25 nucleotides, regularly inhibiting the expression of objective genes via binding their mRNA 3′ untranslated regions [14]. One intriguing feature of miRNAs is that multiple miRNAs could target one gene, while one miRNA could target multiple genes. This particular multiple-target-multiple work model of miRNAs contributes to efficient gene regulation among a variety of pathophysiological processes [15]. By transferring exosomes, non-cardiomyocyte-derived miRNAs could modulate oxidative stress, apoptosis, fibrosis, and angiogenesis during myocardial infarction (MI) or myocardial ischemia–reperfusion (MI/R) injury [16, 17].
According to the Extracellular RNA Communication Consortium (ERCC), the optimizations of miRNA carriers are highly connected with future therapeutic applications [18]. As one of the carriers of miRNAs in the extracellular space, exosomes can be modified to elevate the transfer efficiency and therapeutic potential of exosomal miRNAs. Modifications targeting the stem cells, such as hypoxic stimulation and pharmacological intervention, are widely used. Direct engineering of exosomes is also a promising approach. Methods like electroporation, cellular nanoporation (CNP) biological chip, and membrane-peptide connection have successfully applied to improve the efficiency of exosome delivery. In this review, we introduced the advantages of exosomes as transporter to deliver stem cell-derived beneficial miRNAs and summarized several methods to modify or engineer the exosomes in order to gain higher therapeutic efficiency in IHD.
From Stem Cell Transplantation to Exosome Treatment
Insufficient Generation of Cardiomyocytes and Paracrine Hypothesis
Research on cardiomyocyte regeneration has been carried out for almost 30 years. Many studies have successfully transplanted stem cells into infarct hearts and restored myocardial functions in animal models [19]. In recent years, our perception of the therapeutic function of stem cells have undergone several shifts. Researchers initially reported stem cells to improve damaged tissues through direct cell replacement. However, a growing body of evidence suggests that stem cells primarily attenuate MI via paracrine factors, including exosomal miRNAs, not only by cell replacement. In 2001, Orlic et al. presented a milestone study that 9 days after the BMSC transplantation in a murine MI model, the engrafted cells generated newly endothelial cells and myocardiocytes to reduce the infarction zone[20]. However, Nygren et al. demonstrated that the BMSC engrafted into ischemic myocardium remained a hematopoietic fate. There was a small part of BMSC that could fuse with local cardiomyocytes [9]. This result is consistent with the conclusion from Manuel et al., which suggested that the fusion of BMSCs and cardiomyocytes was mistaken for the BMSC differentiation in infarct [8]. Cardiosphere-derived cells (CDC) are a group of cardiac-derived progenitor cells, which could be obtained from human cardiac tissue biopsy and expanded in vitro. The engraftment of allogeneic rodent CDCs could minimize adverse scar formation in the infarcted heart [21]. The cardioprotective effects of allogeneic CDC were also confirmed in a swine MI model, using contrast-enhanced MRI as a monitor [22]. It was convinced that the paracrine effect of CDC contributes to most of the therapeutic effects rather than direct CDC differentiation [23]. To our knowledge, no studies have provided clear evidence that the transplantation of BMSC or other stem cells leads to a robust generation of cardiomyocytes.
Besides the limited generation of cardiomyocytes, stem-cell transplantation also raises concerns of serval safety issues, including calcifications, tachyarrhythmias, and unexpected differentiation. Breitbach et al. observed calcifications and osteogenic differentiation in the infarct heart after engraftment of BMSC [24]. Romagnuolo et al. observed tachyarrhythmias in the injured pig heart after transplantation of human embryonic stem cell-derived cardiomyocytes [25]. Myocardial retention of stem cells is an important issue that should be concerned because the leak of stem cells may cause damage in other non-specific organs. After frequent injection of BMSCs, retention of BMSCs was observed in the lung [26]. The continued retention leads to pulmonary tissue damage accompanied by fibrosis. Further, Fischer et al. investigated the systemic circulations of several stem cells after intravenous injection, including BMSCs, neural stem cells (NSC), and multipotent adult progenitor cells (MAPCs). After testing the heart, liver, kidney, spleen, and lungs, the lungs intercept most of the stem cells [27].
The paracrine hypothesis was proposed with the finding that cytokines secreted from BMSC contribute to therapeutic effects on the ischemic rodent heart [28]. However, cytokines in the extracellular space suffered from rapid hydrolysis without a transporter [29, 30]. With accumulating researches carried out, extracellular vehicles such as exosomes appear to be an excellent transporter for delivering stem-cell cargos to the infarcted heart [31, 32]. Several basic studies have reported that stem cell-derived exosomes have beneficial effects on ischemic tissue and could efficiently recapitulate live cells’ therapeutic activities in animal models [33,34,35]. Given these results, the paracrine hypothesis may well explain the therapeutic effects of stem cells.
Exosomes as a Promising Alternative
Exosomes are nanoscale (30–150 nm) extracellular vesicles with a lipid bilayer membrane. Bioactive moderators like miRNAs can be selectively loaded into exosomes and shuttle from cell to cell, avoiding the degradation caused by extracellular enzymes [12]. As non-viable vesicles, exosomes are more accessible to stock than stem cells regarding clinical application. Compared with stem-cell transplantation, exosomes have a lower risk of calcifications and tachyarrhythmias [19]. Sun et al. assessed the safety of human umbilical cord MSC (hucMSC)-derived exosomes in various animals, including rats, pigs, and rabbits. By monitoring pyrogen, systemic anaphylaxis, and hemolysis in the animals, they pointed that intravenous injection of hucMSC-derived exosomes was safe with a therapeutic application [25]. What’s more, the contents of exosomes are dependent on the status of parental cells, which means pretreatments like hypoxia, gene modification, and pharmacological intervention could alter the cargos inside exosomes and confer better cardioprotective effects on infarcted hearts [17, 36].
However, as a transporter of miRNAs, exosomes also have disadvantages, especially in the retention issue. The systematic delivery of exosomes is also troubled by misplaced accumulation. The retention of exosomes appears principally in the liver and spleen, which contains a large number of macrophages. Since endocytosis significantly contributes to the absorption of exosomes into macrophages, downregulation of endocytosis-related protein such as clathrin heavy chain 1 (Cltc1) within exosomes could alleviate the retention in the liver and spleen [37]. CD47 is expressed on the exosomal membrane and could mediate the inhibition of phagocytosis. Overexpression of CD47 could also be helpful to increase the retention rate in target issues [38]. Although macrophages could weaken the efficiency of exosomes delivery, this kind of retention would not cause tissue damage or imperil the organ.
Collectively, transplantation of exosomes seems to be a safer way to deliver stem-cell cargos to infarcted hearts with lower side effects and negligible impact on other non-specific organs.
Pretreatments of Stem Cells Enhance Cardioprotective Effects of Exosomal miRNAs
The contents of exosomes are varied according to the cultural environment of their parental cells. Pretreatments targeting the stem cells like hypoxia, pharmacological intervention, and genetic manipulation have been reported to elevate the therapeutic miRNAs within exosomes. Here, we take BMSC (peripheral origin), CPC(cardiac in situ), and iPSC (induced stem cells) as examples.
BMSC-Derived Exosomal miRNAs
BMSC has drawn considerable attention as a stem-cell based therapy for various human diseases. By August 2021, over 300 BMSC-based clinical trials have been listed at www.clinicaltrials.gov. BMSCs are quickly isolated and suitable for large-scale culture in vitro. Thus, exosomes from BMSCs are productive, which is an attractive feature. BMSC-derived exosomes have been reported to improve myocardial injury via their miRNA cargoes with various biological effects, including pro-angiogenesis, regulation of autophagy and apoptosis, and immunomodulation. Scott W et al. analyzed the miRNA pathway and gene network of human BMSC. According to their sequencing data, the 23 most abundant miRNAs accounted for nearly 80 percent of the exosomal miRNA cargo. These miRNAs are highly enriched in angiogenesis and fibrosis-related signaling, including Wnt, TGF-beta, and PDGF signaling. By the use of electroporation technical, exosomes highly loaded with miR-130a-3p enhanced angiogenic effects in vitro. miR-130a-3p induced angiogenesis by downregulating homeobox genes GAX and HOXA5 in the recipient cells [17]. Joshua et al. reported that exosomal miR-21-5p from human BMSC increased calcium handling and contractility in human iPSC-derived cardiomyocytes in vitro [39]. Another research further confirmed the beneficial effect of exosomal miR-21-5p. Kristin et al. showed that mouse BMSC-derived exosomes comprising a high level of miR-21-5p can protect hypoxia-treated H9C2 and mediate cardioprotection against MI in vivo. The exosomes loaded with miR-21-5p significantly repressed apoptosis by decreasing the pro-apoptotic gene products like gene of phosphate and tension homology deleted on chromsome ten (PTEN) and programmed cell death 4 (PDCD4) in cardiomyocytes [40]. In addition, BMSC-derived exosomes could also attenuate myocardial injury by mediating the polarization of macrophages. The exosomal miR-182 targeted the toll-like receptor 4 (TLR4) and therefore increased the M2/M1 ratio of the macrophages [41]. BMSC-derived exosomal miRNA-125b could improve myocardial infarction, at least partially due to the inhibited autophagy activity. P53 is the target of miRNA-125b, which reduced autophagic flux through P53-Bnip3 signaling independently of the AMPK pathway in the process of autophagy during myocardial infarction [42]. These results summarize the cardioprotective effects exerted by BMSC exosomal miRNAs.
Many studies investigated various ways to increase the biological benefits of BMSC exosomes, and hypoxia is one of the most studied pretreatment methods. The oxygen level of physiological niches within the bone marrow is relatively low (< 1% O2). The normoxia culture condition (21% O2) differs significantly from the original environment of BMSCs. Therefore, hypoxic pretreatment may be beneficial for the BMSCs since a low level of oxygen tension (< 1% O2) is more similar to the original environment of MSCs in the bone marrow [43]. Zhu et al. found that exosomal miR-125b-5p could mediate the cardioprotection against MI via antiapoptotic effects on cardiomyocytes. They compared the miRNA content between exosomes derived from BMSCs with and without hypoxic pretreatment and found that miR-125b-5p was upregulated in the hypoxic group [44]. Further study by Zhu et al. showed that hypoxic pretreatment of rat BMCs significantly elevated the level of neutral sphingomyelinase 2 (nSMase2), which was documented as a critical factor in mediating the release and formation of exosomes. What’s more, the level of miR-210 in exosomes increases after hypoxia, which leads to better therapeutic efficacy in a rodent MI model, including angiogenesis, anti-apoptosis, and antifibrosis [45]. Another study using a mouse model confirmed the beneficial effects of BMSC-derived exosomal miR-210 on the ischemic heart. AIFM3 was found to be a downstream target of BMSC exosomal miR-210 in regulating the apoptosis of cardiomyocytes [46].
Besides hypoxia, genetic manipulations aiming at upregulating the beneficial miRNAs are also proved to be a promising method to increase the efficacy of exosomes. Antiapoptosis-related miRNAs (miR-19a, miR-451) were upregulated in BMSC exosomes after overexpression of GATA-4. MiR-451 could decrease cardiomyocytes’ apoptosis via targeting the RNA-binding protein CUGBP2, which inhibits cyclooxygenase 2 (COX-2) by combining AU-rich elements in 3′-untranslated regions [31, 47].
In addition, pharmacological pretreatment is also a promising method for improving the therapeutic efficacy of the exosomes. In prior researches, several pharmaceuticals (such as atorvastatin [48], oxytocin [49], rosuvastatin [50]) have been applied to enhance the effectiveness of BMSC transplantation in infarcted hearts. Recently, Huang et al. showed that pretreatment of atorvastatin enhanced the therapeutic effect of exosomes secreted by BMSCs in a rat MI model via up-regulating lncRNA H19 and miRNA-675[51].
In conclusion, hypoxic pretreatment, genetic manipulation, and pharmacological pretreatment could elevate the content of beneficial miRNAs within exosomes, which represent good ways to enhance the efficacy of stem cell-derived exosomes. Future studies are needed to optimize these methods and investigate new strategies to enhance the expression of beneficial miRNAs in BMSC exosomes.
CPC-Derived Exosomal miRNA
CPC are suggested to be a type of stem cell with beneficial effects on cardiac regeneration. In early studies, some researchers believed that the differentiation of CPC into cardiomyocytes in infarct heart principally contributes to the therapeutic efficacy [52]. However, further studies indicated that CPC could confer cardioprotection against myocardial infarction via paracrine, including exosomal miRNAs [53,54,55]. Chen et al. revealed that miR-451 in CPC-derived exosomes showed anti-apoptosis effects in infarct heart or H2O2-stimulated H9C2 [53]. During embryonic development, stem cells thrive and differentiate in comparative hypoxic circumstances. Thus, hypoxia was proposed to promote the therapeutic effects of CPC-derived exosomes. Xiao et al. reported that hypoxic pretreatment elevated the content of miR-21 within CPC-derived exosomes, which decreased the apoptosis of recipient H9C2 cardiomyocytes. The miR-21 in H9C2 under oxidative stress upregulated PDCD4 and therefore deteriorated apoptosis. Overexpressing exosomal miR-21 could rescue the deterioration partially [54]. Furthermore, a study by Gray et al. identified 11 exosomal miRNAs increased after hypoxic pretreatment of rodent CPC using microarray analysis. Seven of them were validated by qRT-PCR, of which six miRNAs had been reported to have a role in cardioprotection. The one left is miR-292, and there is no direct evidence to illustrate its role in heart disease, but system biology analysis revealed that it might mediate fibrosis. This research correlates exosomal miRNA levels with hypoxic pretreatment of CPC, indicating that oxidative stimulation could promote beneficial effects of the exosomes via varying its miRNA cargo [55]. In addition, another study examined the impact of hypoxia on exosomes derived from human c-kit positive CPC. The human samples were derived from pediatric cardiac surgeries in three age groups, including neonates, infants, and children. It turned out that hypoxia could enhance the antifibrosis effect of the exosomes from all three age groups. Using principal component regression and partial least squares regression (PLSR), the researcher identified several exosomal miRNAs as the potentially crucial mediator in response to hypoxia. These miRNAs were reported to mediate critical progress during myocardium infarction including fibrosis, cardiac hypertrophy, angiogenesis, and apoptosis [16].
iPSC-Derived Exosomal miRNAs
Patient-specific iPSCs are among the most promising cell-based strategies in regenerative medicine. Previous studies applied iPSC to cell regeneration in multiple areas, including cerebral ischemia [56] and spinal cord injury [57]. It was also shown that the culture medium of iPSC enhances the wound healing ability of human A549 alveolar epithelial-like cells. Wang et al. revealed that murine iPSC-exosome could confer protection against MI/R injury by intramyocardial injection into mice ischemic zone before reperfusion. Besides, murine iPSC-exosome contains miRNAs such as miR-21 and miR-210, which were reported to be cardioprotective [58]. In another study, overexpression of cyclin D2 (CCND2) in human iPSC altered the expression profile of its exosomal miRNAs and promoted hypoxic tolerance of the recipient cardiomyocytes. Apoptosis-related miRNAs decreased in exosomes derived from CCND2 overexpressed iPSC, including let-7c-5p, let-7d-3p, miR-133a-3p, miR-143-3p, miR-506-3p, and miR-584-5p. Exosomal miR-302b-3p in CCND2 overexpressed iPSC could activate the proliferation of cardiomyocytes in the swine MI/R model [59]. In addition, Josefson et al. compared exosomal miRNAs content from diverse origins of human iPSC. It turned out that compared to dermal-derived iPSC, cardiac fibroblast-derived iPSC has a decreased exosomal miR-22, which is identified as a regulator of cardiac hypertrophy. This study indicated that exosomes from different origins of iPSC could have diverse miRNAs content and therapeutic effects [60].
Following the general differentiation strategy, iPSC-derived cardiomyocytes (iPSC-CMs) were immature and more similar to those in the fetal state. iPSC-CMs and adult cardiomyocytes were different in electrophysiology, mitochondrial function, and morphology. Intracardiac injection of exosomes derived from iPSC-CMs was reported to ameliorate myocardial injury. Among the top 20 abundant miRNAs in iPSC-CMs exosomes, miR-30, miR-1, and miR-21 were suggested to be cardioprotective in former studies. To our surprise, the expressions of these miRNAs showed no significant difference between normoxia and hypoxia [61]. This result is inconsistent with the hypothesis that hypoxic pretreatment of stem cells promotes their therapeutic potential. iPSC-CMs may have different oxidative sensitivity from other stem cells because of the artificial differentiation procedure. Vaskova et al. showed that the combined use of sacubitril and valsartan could increase the production of iPSC-CMs exosomes. This pharmacological pretreatment could confer a downregulation of miR-181a, restoring the myocardial infarction in a rodent model [62]. Judging from the current data, it seems that hypoxic pretreatment has limited effects on the content of miRNAs in exosomes derived from iPSC-CMs. Pharmacological pretreatment and gene manipulation could be further studied to enhance the miRNAs cargo’s therapeutic potential in iPSC exosomes.
Direct Engineering of Exosomes
Loading miRNAs into Exosomes
Except for the general pretreatment methods above, there are also more precise strategies to engineer exosomes. To promote the therapeutic effects and cardiac-specific retention, modifications of exosome cargos and membrane are necessary. Cardioprotective miRNAs we have mentioned, especially those that were validated by animal models (Table 1), could be loaded in exosomes to promote functional effects.
Electroporation is the most widely used method to insert miRNA mimics or siRNAs into stem cell-derived exosomes. By electroporation technique, Ma et al. inserted miR-132 into BMSC-derived exosomes and transferred them to human umbilical vein endothelial cell (HUVEC), which enhanced angiogenesis. Exosomes loaded with miR-132 also enhanced angiogenesis in vivo [63]. The conventional electroporation method has relatively low efficiency and is not suitable for large-scale production. Recently, Yang et al. exploited a cellular nanoporation (CNP) biological chip, which enabled a monolayer culture of parental cells. Electrical pulses pass through the nanochannels within CNP and consequently shuttled nucleotide sequences (such as miRNA mimics) from the extracellular buffer to the parental cells of exosomes. CNP clearly has higher efficiency than electroporation, particularly in large-scale manufacturing [32]. In addition, electroporation could only transfer small artificial nucleotides like miRNA mimics, whereas CNP was able to transfer long endogenous RNAs.
Modifications of the Exosome Membrane
The most widely used modification of the exosome membrane is connecting short peptide motifs with transmembrane proteins or the external membrane of exosomes. Using phage display technology, Kanki et al. obtained several peptide sequences to target infarcted heart tissue, including CSTSMLKAC, a widely used cardiac homing peptide nowadays [64]. Subsequently, Wang et al. connected lysosomal-associated membrane protein 2 (lamp2b) with CSTSMLKAC and then created a recombinant protein with the ability to target the ischemia tissue. The expression of this recombinant protein lamp2b-CSTSMLKAC on the exosome membrane significantly elevated the accumulation of exosomes in the murine MI region [65]. Dioleoylphosphatidylthanolamine N-hydroxysuccinimide (DOPE-NHS) is a modified phospholipid with a fusogenic function. Employing DOPE-NHS, Vandergriff et al. tagged exosome membrane with CSTSMLKAC and thus increased exosome retention of the ischemia area [66]. Antes et al. exploited another phospholipid-based conjugation method, which consists of three parts: 1,2-bis (dimethylphosphino) ethane (DMPE), polyethylene glycol (PEG), and streptavidin(STVDN). Using DMPE-PEG-STVDN, the researchers successfully combined the membrane of exosomes with various molecules, including cardiac homing peptide, cell-specific antibody, and fluorescent protein. Discoidin domain receptor tyrosine kinase 2 (DDR2) is a specific membrane-enriched protein of cardiac fibroblasts. With the conjugation of DDR2 antibody and exosome surface, cardiac fibroblasts increased the uptake of exosomes. In this study, CSTSMLKAC was also applied to elevate cardiac retention of exosomes [67].
All three CSTSMLKAC-related strategies mentioned above have achieved cardioprotective effects on infarcted hearts. Other cardiac-specific peptides such as APWHLSSQYSRT [68] and WLSEAGPVVTVRALRGTGSW [69] also have been used to upgrade the efficiency of exosome delivery. In recent years, hydrogel-based biomaterial has successfully become a delivery platform of EVs and exosomes. Functional peptides (such as PA-GHRPS, NapFF) mixed with hydrogel offer a stabilized and continued release of exosomes and thus promote angiogenesis in the infarcted heart [70].
Conclusions
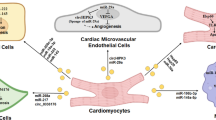
The carriers of miRNAs in the extracellular space are important for future clinical application. In this review, Moreover, we have discussed the advantages of exosomes as the carriers to transfer therapeutic miRNAs and summarized several methods to optimize the deliverability of exosomes(Fig. 1). However, each of the optimization methods we mentioned above has its shortcomings. No single modification could satisfy all clinical needs. Biomaterial-based strategies are often accomplished by intracardiac injection and suitable for patients requiring surgery. These strategies only focus on transferring efficiency or the content of exosomes and ignoring the tissue-specific problem. The discharge rate of the biomaterial should attract much more attention. Exosomes assembled with cardiac homing peptides are designed for systematic injection. “Time-consuming” issue of these artificial exosomes often has been ignored. Although there are plenty of endogenic exosomes traveling safely in peripheral blood, the report of artificial exosomal miRNAs’ impact on multiple organs is very limited. Thus, the leak of the manufactured exosomal miRNA in circulation is still risky, especially for patients with the multiorgan disorder. The engineering of exosomes as extracellular RNAs transporter could contribute to developing innovative treatment strategies for IHD in the future with the improvement of safety issues.
Physical and chemical methods are usually more stable than genetic modulation in bioengineering, especially when it comes to large-scale production. However, loading long nucleic fragments into nano-sized exosomes has technical difficulties. That is why miRNAs are the most used molecules in the studies of engineered exosomes. CNP, we mentioned above, is one technic that may overcome the difficulty of long nucleic fragments loading. With the help of CNP, other beneficial molecular like lncRNAs, mRNAs, and circular RNAs could also be artificially loaded into exosomes, which may diversify the treatments of IHD.
Abbreviations
- BMSC:
-
Bone marrow mesenchymal stem cells
- CCND2:
-
Cyclin D2
- CDC:
-
Cardiosphere-derived cells
- Cltc1:
-
Clathrin heavy chain 1
- CNP:
-
Cellular nanoporation biological chip
- COX-2:
-
Cyclooxygenase 2
- CPC:
-
Cardiac progenitor cells
- ERCC:
-
Extracellular RNA Communication Consortium
- ESC:
-
Embryonic stem cells
- hucMSC:
-
Human umbilical cord MSCs
- HUVEC:
-
Human umbilical vein endothelial cell
- IHD:
-
Ischemic heart disease
- iPSC:
-
Induced pluripotent cell
- Lamp2b:
-
Lysosomal-associated membrane protein 2
- MAPC:
-
Multipotent adult progenitor cells
- MI:
-
Myocardial infarction
- MI/R:
-
Myocardial ischemia–reperfusion
- NSC:
-
Neural stem cells
- nSMase2:
-
Neutral sphingomyelinase 2
- PCR:
-
Principal component regression
- PLSR:
-
Partial least squares regression
- PTEN:
-
Gene of phosphate and tension homology deleted on chromsome ten
- PDCD4:
-
Programmed cell death 4
References
Zhao, D., Liu, J., Wang, M., et al. (2019). Epidemiology of cardiovascular disease in China: Current features and implications. Nature Reviews. Cardiology, 16, 203–212. https://doi.org/10.1038/s41569-018-0119-4
Kaski, J.-C., Crea, F., Gersh, B. J., & Camici, P. G. (2018). Reappraisal of ischemic heart disease. Circulation, 138, 1463–1480. https://doi.org/10.1161/CIRCULATIONAHA.118.031373
van der Pol, A., van Gilst, W. H., Voors, A. A., & van der Meer, P. (2019). Treating oxidative stress in heart failure: Past, present and future. European Journal of Heart Failure, 21, 425–435. https://doi.org/10.1002/ejhf.1320
Bai, X., Yan, Y., Song, Y.-H., et al. (2010). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European Heart Journal, 31, 489–501. https://doi.org/10.1093/eurheartj/ehp568
Wang, J., Chen, Z., Dai, Q., et al. (2020). Intravenously delivered mesenchymal stem cells prevent microvascular obstruction formation after myocardial ischemia/reperfusion injury. Basic Research in Cardiology, 115, 40. https://doi.org/10.1007/s00395-020-0800-8
Gao, L., Gregorich, Z. R., Zhu, W., et al. (2018). Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation, 137, 1712–1730. https://doi.org/10.1161/CIRCULATIONAHA.117.030785
Shiba, Y., Gomibuchi, T., Seto, T., et al. (2016). Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature, 538, 388–391. https://doi.org/10.1038/nature19815
Alvarez-Dolado, M., Pardal, R., Garcia-Verdugo, J. M., et al. (2003). Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature, 425, 968–973. https://doi.org/10.1038/nature02069
Nygren, J. M., Jovinge, S., Breitbach, M., et al. (2004). Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine, 10, 494–501. https://doi.org/10.1038/nm1040
Thum, T., Bauersachs, J., Poole-Wilson, P. A., et al. (2005). The dying stem cell hypothesis. Journal of the American College of Cardiology, 46, 1799–1802. https://doi.org/10.1016/j.jacc.2005.07.053
Vagnozzi, R. J., Maillet, M., Sargent, M. A., et al. (2019). An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature, 577,. https://doi.org/10.1038/s41586-019-1802-2
Gomzikova, M. O., & Rizvanov, A. A. (2017). Current trends in regenerative medicine: From cell to cell-free therapy. Bionanoscience, 7, 240–245. https://doi.org/10.1007/s12668-016-0348-0
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367,. https://doi.org/10.1126/science.aau6977
Moghaddam, A. S., Afshari, J. T., Esmaeili, S. A., et al. (2019). Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis, 285, 1–9. https://doi.org/10.1016/j.atherosclerosis.2019.03.016
Hashimoto, Y., Akiyama, Y., & Yuasa, Y. (2013). Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE, 8, e62589. https://doi.org/10.1371/journal.pone.0062589
Agarwal, U., George, A., Bhutani, S., et al. (2017). Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell–derived exosomes from pediatric patients. Circulation Research, 120, 701–712. https://doi.org/10.1161/CIRCRESAHA.116.309935
Ferguson, S. W., Wang, J., Lee, C. J., et al. (2018). The microRNA regulatory landscape of MSC-derived exosomes: A systems view. Science and Reports, 8, 1–12. https://doi.org/10.1038/s41598-018-19581-x
Das, S., Abdel-Mageed, A. B., Adamidi, C., et al. (2019). The extracellular RNA communication consortium: Establishing foundational knowledge and technologies for extracellular RNA research. Cell, 177, 231–242. https://doi.org/10.1016/j.cell.2019.03.023
Wang, X., Tang, Y., Liu, Z., et al. (2021). The application potential and advance of mesenchymal stem cell-derived exosomes in myocardial infarction. Stem Cells Int, 2021,. https://doi.org/10.1155/2021/5579904
Orlic, D., Kajstura, J., Chimenti, S., et al. (2001). Bone marrow cells regenerate infarcted myocardium. Nature, 410, 701–705. https://doi.org/10.1038/35070587
Tseliou, E., Pollan, S., Malliaras, K., et al. (2013). Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. Journal of the American College of Cardiology, 61, 1108–1119. https://doi.org/10.1016/j.jacc.2012.10.052
Malliaras, K., Smith, R. R., Kanazawa, H., et al. (2013). Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation, 128, 2764–2775. https://doi.org/10.1161/CIRCULATIONAHA.113.002863
Chimenti, I., Smith, R. R., Li, T.-S., et al. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circulation Research, 106, 971–980. https://doi.org/10.1161/CIRCRESAHA.109.210682
Breitbach, M., Bostani, T., Roell, W., et al. (2007). Potential risks of bone marrow cell transplantation into infarcted hearts. Blood, 110, 1362–1369. https://doi.org/10.1182/blood-2006-12-063412
Sun, L., Xu, R., Sun, X., et al. (2016). Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy, 18, 413–422. https://doi.org/10.1016/j.jcyt.2015.11.018
Anjos-Afonso, F., Siapati, E. K., & Bonnet, D. (2004). In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. Journal of Cell Science, 117, 5655–5664. https://doi.org/10.1242/jcs.01488
Fischer, U. M., Harting, M. T., Jimenez, F., et al. (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev, 18, 683–691. https://doi.org/10.1089/scd.2008.0253
Takahashi, M., Li, T. S., Suzuki, R., et al. (2006). Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol - Hear Circ Physiol, 291, 886–893. https://doi.org/10.1152/ajpheart.00142.2006
Lee, K., Silva, E. A., & Mooney, D. J. (2011). Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. Journal of the Royal Society, Interface, 8, 153–170. https://doi.org/10.1098/rsif.2010.0223
Eppler, S. M., Combs, D. L., Henry, T. D., et al. (2002). A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans*. Clinical Pharmacology and Therapeutics, 72, 20–32. https://doi.org/10.1067/mcp.2002.126179
Yu, B., Kim, H. W., Gong, M., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360. https://doi.org/10.1016/j.ijcard.2014.12.043
Yang, Z., Shi, J., Xie, J., et al. (2020). Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng, 4, 69–83. https://doi.org/10.1038/s41551-019-0485-1
Arslan, F., Lai, R. C., Smeets, M. B., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res, 10, 301–312. https://doi.org/10.1016/j.scr.2013.01.002
Pêche, H., Heslan, M., Usal, C., et al. (2003). Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection1. Transplantation, 76, 1503–1510. https://doi.org/10.1097/01.TP.0000092494.75313.38
Lai, R. C., Arslan, F., Lee, M. M., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res, 4, 214–222. https://doi.org/10.1016/j.scr.2009.12.003
Yu, B., Gong, M., Wang, Y., et al. (2013). Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS ONE, 8, 1–11. https://doi.org/10.1371/journal.pone.0073304
Wan, Z., Zhao, L., Lu, F., et al. (2020). Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics, 10, 218–230. https://doi.org/10.7150/thno.38198
Kamerkar, S., LeBleu, V. S., Sugimoto, H., et al. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 546, 498–503. https://doi.org/10.1038/nature22341
Mayourian, J., Ceholski, D. K., Gorski, P. A., et al. (2018). Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circulation Research, 122, 933–944. https://doi.org/10.1161/CIRCRESAHA.118.312420
Luther, K. M., Haar, L., McGuinness, M., et al. (2018). Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. Journal of Molecular and Cellular Cardiology, 119, 125–137. https://doi.org/10.1016/j.yjmcc.2018.04.012
Zhao, J., Li, X., Hu, J., et al. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research, 115, 1205–1216. https://doi.org/10.1093/cvr/cvz040
Xiao, C., Wang, K., Xu, Y., et al. (2018). Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circulation Research, 123, 564–578. https://doi.org/10.1161/CIRCRESAHA.118.312758
Mohyeldin, A., Garzón-Muvdi, T., & Quiñones-Hinojosa, A. (2010). Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell, 7, 150–161. https://doi.org/10.1016/j.stem.2010.07.007
Zhu, L. P., Tian, T., Wang, J. Y., et al. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8, 6163–6177. https://doi.org/10.7150/thno.28021
Zhu, J., Lu, K., Zhang, N., et al. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells, Nanomedicine Biotechnol, 46, 1659–1670. https://doi.org/10.1080/21691401.2017.1388249
Cheng, H., Chang, S., Xu, R., et al. (2020). Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Research & Therapy, 11, 1–14. https://doi.org/10.1186/s13287-020-01737-0
Zhang, X., Wang, X., Zhu, H., et al. (2010). Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. Journal of Molecular and Cellular Cardiology, 49, 841–850. https://doi.org/10.1016/j.yjmcc.2010.08.007
Dai, G., Xu, Q., Luo, R., et al. (2015). Atorvastatin treatment improves effects of implanted mesenchymal stem cells: Meta-analysis of animal models with acute myocardial infarction. BMC Cardiovascular Disorders, 15, 1–6. https://doi.org/10.1186/s12872-015-0162-6
Kim, Y. S., Ahn, Y., Kwon, J. S., et al. (2012). Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells, Tissues, Organs, 195, 428–442. https://doi.org/10.1159/000329234
Xu, H., Yang, Y. J., Qian, H. Y., et al. (2011). Rosuvastatin treatment activates JAK-STAT pathway and increases efficacy of allogeneic mesenchymal stem cell transplantation in infarcted hearts. Circulation Journal, 75, 1476–1485. https://doi.org/10.1253/circj.CJ-10-1275
Huang, P., Wang, L., Li, Q., et al. (2020). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research, 116, 353–367. https://doi.org/10.1093/cvr/cvz139
Beltrami, A. P., Barlucchi, L., Torella, D., et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration we have documented the existence of cycling ventricular myocytes in the normal and pathologic adult mam. Cell, 114, 763–776.
Chen, L., Wang, Y., Pan, Y., et al. (2013). Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun, 431, 566–571. https://doi.org/10.1016/j.bbrc.2013.01.015
Xiao, J., Pan, Y., Li, X. H., et al. (2016). Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death & Disease, 7, 1–10. https://doi.org/10.1038/cddis.2016.181
Gray, W. D., French, K. M., Ghosh-Choudhary, S., et al. (2015). Identification of Therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation Research, 116, 255–263. https://doi.org/10.1161/CIRCRESAHA.116.304360
Chen, S. J., Chang, C. M., Tsai, S. K., et al. (2010). Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev, 19, 1757–1767. https://doi.org/10.1089/scd.2009.0452
Tsuji, O., Miura, K., Okada, Y., et al. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A, 107, 12704–12709. https://doi.org/10.1073/pnas.0910106107
Wang, Y., Zhang, L., Li, Y., et al. (2015). Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology, 192, 61–69. https://doi.org/10.1016/j.ijcard.2015.05.020
Zhao, M., Nakada, Y., Wei, Y., et al. (2021). Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction. Circulation, 210–228,. https://doi.org/10.1161/circulationaha.120.049497
Kurtzwald-Josefson, E., Zeevi-Levin, N., Rubchevsky, V., et al. (2020). Cardiac fibroblast-induced pluripotent stem cell-derived exosomes as a potential therapeutic mean for heart failure. International Journal of Molecular Sciences, 21, 1–15. https://doi.org/10.3390/ijms21197215
Lee, W. H., Chen, W.-Y., Shao, N.-Y., et al. (2017). Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells, 35, 2138–2149. https://doi.org/10.1002/stem.2669
Vaskova, E., Ikeda, G., Tada, Y., et al. (2020). Sacubitril/valsartan improves cardiac function and decreases myocardial fibrosis via downregulation of exosomal mir-181a in a rodent chronic myocardial infarction model. Journal of the American Heart Association, 9,. https://doi.org/10.1161/JAHA.119.015640
Ma, T., Chen, Y., Chen, Y., et al. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int, 2018,. https://doi.org/10.1155/2018/3290372
Kanki, S., Jaalouk, D. E., Lee, S., et al. (2011). Identification of targeting peptides for ischemic myocardium by in vivo phage display. Journal of Molecular and Cellular Cardiology, 50, 841–848. https://doi.org/10.1016/j.yjmcc.2011.02.003
Wang, X., Chen, Y., Zhao, Z., et al. (2018). Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. Journal of the American Heart Association, 7, 1–16. https://doi.org/10.1161/JAHA.118.008737
Vandergriff, A., Huang, K., Shen, D., et al. (2018). Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics, 8, 1869–1878. https://doi.org/10.7150/thno.20524
Antes, T. J., Middleton, R. C., Luther, K. M., et al. (2018). Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnology, 16, 1–15. https://doi.org/10.1186/s12951-018-0388-4
Kim, H., Yun, N., Mun, D., et al. (2018). Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochemical and Biophysical Research Communications, 499, 803–808. https://doi.org/10.1016/j.bbrc.2018.03.227
Mentkowski, K. I., & Lang, J. K. (2019). Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Science and Reports, 9, 10041. https://doi.org/10.1038/s41598-019-46407-1
Chen, C. W., Wang, L. L., Zaman, S., et al. (2018). Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovascular Research, 114, 1029–1040. https://doi.org/10.1093/cvr/cvy067
Acknowledgements
The authors thank Fangfei Wei (from University of Leuven, Belgium) for reviewing and modification of the manuscript.
Funding
The present study was funded by the National Natural Science Foundation of China (Nos. 81770394, 82000260) and Guangdong Basic and Applied Basic Research Foundation (2021A1515010755).
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Xue, R., Huang, P. et al. Modified Exosomes: a Good Transporter for miRNAs within Stem Cells to Treat Ischemic Heart Disease. J. of Cardiovasc. Trans. Res. 15, 514–523 (2022). https://doi.org/10.1007/s12265-022-10216-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10216-1