Abstract
Background
There is practice heterogeneity in the use, type, and duration of prophylactic antiseizure medications (ASMs) in patients with moderate–severe traumatic brain injury (TBI).
Methods
We conducted a systematic review and meta-analysis of articles assessing ASM prophylaxis in adults with moderate–severe TBI (acute radiographic findings and requiring hospitalization). The population, intervention, comparator, and outcome (PICO) questions were as follows: (1) Should ASM versus no ASM be used in patients with moderate–severe TBI and no history of clinical or electrographic seizures? (2) If an ASM is used, should levetiracetam (LEV) or phenytoin/fosphenytoin (PHT/fPHT) be preferentially used? (3) If an ASM is used, should a long versus short (> 7 vs. ≤ 7 days) duration of prophylaxis be used? The main outcomes were early seizure, late seizure, adverse events, mortality, and functional outcomes. We used Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology to generate recommendations.
Results
The initial literature search yielded 1998 articles, of which 33 formed the basis of the recommendations: PICO 1: We did not detect any significant positive or negative effect of ASM compared to no ASM on the outcomes of early seizure, late seizure, adverse events, or mortality. PICO 2: We did not detect any significant positive or negative effect of PHT/fPHT compared to LEV for early seizures or mortality, though point estimates suggest fewer late seizures and fewer adverse events with LEV. PICO 3: There were no significant differences in early or late seizures with longer versus shorter ASM use, though cognitive outcomes and adverse events appear worse with protracted use.
Conclusions
Based on GRADE criteria, we suggest that ASM or no ASM may be used in patients hospitalized with moderate–severe TBI (weak recommendation, low quality of evidence). If used, we suggest LEV over PHT/fPHT (weak recommendation, very low quality of evidence) for a short duration (≤ 7 days, weak recommendation, low quality of evidence).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Substantial heterogeneity exists in the clinical use of seizure prophylaxis for patients hospitalized with traumatic brain injury (TBI). Indeed, several surveys of practitioners have reported variability in the use, duration, and type of prophylactic antiseizure medications (ASMs) prescribed [1,2,3]. Recent concerns regarding cognitive side effects of ASMs have contributed to further inconsistencies in practice [4,5,6]. Currently, there are no guidelines available that address the utility of seizure prophylaxis in patients hospitalized with moderate–severe TBI.
In October 2019, the Neurocritical Care Society formed a subcommittee to develop seizure prophylaxis guidelines for neurocritically ill patients, including those with TBI, intraparenchymal hemorrhage, spontaneous nontraumatic subarachnoid hemorrhage, and supratentorial neurosurgery. Here, we present the guidelines for seizure prophylaxis following TBI.
The main questions we aimed to address were the following: (1) Should prophylactic ASM versus no ASM be used in patients hospitalized with TBI? (2) If an ASM is used, should levetiracetam (LEV) or phenytoin/fosphenytoin (PHT/fPHT) be preferentially prescribed? and (3) If an ASM is used, what is the appropriate duration of prophylaxis?
Methods
This guideline was developed in accordance with Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology [7, 8], and both panel co-chairs (JAF and SR) completed GRADE workshop training [9].
Panel Composition
The Seizure Prophylaxis Guideline Panel was formed in October 2019 and consists of nine members, including physicians, pharmacists, and nurses with subspecialty experience in neurocritical care, seizure management, trauma, and neurosurgery. In addition, a GRADE statistician (YY) performed statistical analyses. Organizational representation was present from the Neurocritical Care Society (JAF, EJG, DO, ET, SFZ, and SR) and the American Epilepsy Society (ELJ and AR). The panel consisted of six women and four men of diverse racial and ethnic backgrounds (Asian, South Asian, White, and Hispanic).
Disclosure and Management of Potential Conflicts of Interest
All panel members were required to comply with standard conflict of interest and commercial relationship disclosures, including review of any financial, intellectual, or other relationships that may be construed as a possible conflict of interest. Disclosures that were not directly related to the content of this article are listed in the Acknowledgments section. The chairs of the Neurocritical Care Society Guideline Committee that oversees the Seizure Prophylaxis Guideline Panel were responsible for vetting any potential conflicts of interest. All members of the Seizure Prophylaxis Guideline Panel were determined to be free of conflicts of interest. This study did not involve human study participants and was exempt from institutional review board review according to the New York University Institutional Review Board.
Population, Intervention, Comparison, and Outcomes Generation
Three specific questions were addressed for this guideline following the population, intervention, comparison, and outcomes (PICO) format [10]. The PICOs are as follows: (1) Should ASM versus no ASM be used in patients hospitalized for moderate–severe TBI with no history of clinical or electrographic seizures? (2) If an ASM is used, should LEV or PHT/fPHT be preferentially used for patients hospitalized with moderate–severe TBI with no history of clinical or electrographic seizures? and (3) If an ASM is used, should a long (> 7 days) versus short (≤ 7 days) duration of prophylaxis be used for patients hospitalized with moderate–severe TBI with no history of clinical or electrographic seizures?
The outcomes categorized as “critical” (indicating the highest level of importance) included the following: early seizure (either clinical or electrographic) occurring within 14 days of TBI, late seizure (either clinical or electrographic) occurring > 14 days from TBI, and adverse events associated with ASM use. A 14-day threshold for early versus late seizure was selected because some studies defined early seizure as within 7 days, others defined it as within 14 days, and still others defined it as seizure occurring during hospitalization. Because the threshold of 7 or 14 days is not biologically driven, we chose 14 days to be inclusive of the most studies. Additional outcomes such as mortality and functional (e.g., modified Rankin Scale scores [11], Glasgow Outcome Scale scores) and cognitive outcomes were rated as “important.” We chose to assess LEV versus PHT/fPHT, as opposed to another ASM, for PICO 2 for a variety of reasons. Although there are a substantial number of studies comparing valproic acid to PHT, the panel felt that comparison to a newer-generation ASM would be more relevant to practitioners. Acknowledging that there are side effects of LEV, particularly related to delirium in a critically ill population, and that other newer-generation ASMs may be preferred in certain circumstances (e.g., lacosamide), the panel chose to focus on LEV because of its widespread use, its intravenous formulation, and the extensive literature evaluating its use in the population of interest.
Study Population
This guideline pertains to patients hospitalized with moderate–severe TBI who do not have a prior history of seizure (clinical or electrographic) or ASM use prior to the index TBI. Several societies (American Academy of Neurology; American Congress of Rehabilitation Medicine; American Medical Society for Sports Medicine; Centers for Disease Control and Prevention; Department of Defense; Department of Veterans Affairs; Diagnostic Statistical Manual of Mental Disorders, Fifth Edition; International Conference on Concussion in Sport; Mayo Classification System; National Institute of Neurological Disorders and Stroke; and the World Health Organization) have offered variable definitions of TBI severity [12]. Some severity scales use the Glasgow Coma Score (GCS), loss of consciousness, cognitive tools, imaging criteria, and/or duration of neurological symptoms to categorize patients as having as mild, moderate, or severe TBI. In some literature, mild TBI implies concussion. For the purposes of this guideline and to be inclusive of the most clinically relevant literature, we defined moderate–severe TBI as injury with acute radiographic abnormalities (e.g., subarachnoid hemorrhage, subdural or epidural hematoma, contusion, intracerebral hemorrhage, intraventricular hemorrhage, skull fracture) requiring hospitalization. We did not require a GCS threshold or time frame for loss of conscious because we wanted to be as inclusive as possible in our search parameters, and many articles do not reference index clinical severity scores. Studies evaluating concussion or mild TBI not requiring hospitalization were excluded from analyses. Additional guidelines for ASM prophylaxis created for hospitalized patients with nontraumatic subarachnoid hemorrhage, intraparenchymal hemorrhage, and supratentorial neurosurgery are published separately.
Inclusion and Exclusion Criteria
Studies could be included if the following criteria were met: the article addressed prophylactic ASM use, the article included an adult population (aged > 18 years) hospitalized with TBI, and data were available on the primary outcomes of interest (early seizure, late seizure, adverse events, mortality, functional outcomes, and cognitive outcomes). Articles were excluded if they involved patients with a history of seizure, epilepsy, or ASM use prior to TBI; were not published in English; were nonhuman studies; were case series with fewer than ten patients; evaluated a pediatric population; or did not assess an outcome of interest. We excluded gray literature, including abstracts, conference proceedings, and non-peer-reviewed articles, as well as review articles and meta-analyses.
Search Strategy
A search of articles was conducted by an independent medical librarian from January 1, 1946, through July 10, 2020, using PubMed, Medline, Embase, Emcare, and Cochrane databases (Supplemental Table 1). Additional literature searches were performed by panel members between July 10, 2020, and November 1, 2022, to capture more recently published articles. Search terms included: “seizure,” “antiepileptic medication,” “antiseizure medication,” “levetiracetam,” “Keppra,” “lacosamide,” “Vimpat,” “phenytoin,” “Dilantin,” “fosphenytoin,” “Cerebyx,” “valproic acid,” “Depakote,” “carbamazepine,” “lamotrigine,” “prophylaxis,” “prevention,” “prophylactic,” “traumatic brain injury,” “TBI,” “mortality,” “death,” “functional outcome,” “function,” “modified Rankin,” “Glasgow Outcome score,” “cognition,” “cognitive,” “disability,” “activities of daily living,” “outcome,” “adverse events,” and “side effects.” Reference lists of published articles, review articles, and meta-analyses were also screened to identify additional articles.
Study Screening and Data Collection
Two reviewers independently screened each article title and abstract to determine inclusion eligibility. Full-text screening was performed in articles that passed the initial level of review. Screening was performed using DistillerSR software (Ottawa, Ontario, Canada), and all conflicts were adjudicated between reviewers prior to study inclusion. Data were extracted into a standardized tool and classified as randomized controlled trials versus nonrandomized studies, which could be observational studies using a retrospective, prospective, cross-sectional, or case series design.
Risk of Bias and Certainty of Evidence Evaluation
Risk of bias was assessed using the Cochrane Risk of Bias 2 (RoB-2) [13] tool for randomized trials and the Risk of Bias Instrument for Non-randomized Studies of Interventions (ROBINS-I) tool [14] for nonrandomized studies. These tools were selected based on recommendations from GRADE and the types of articles evaluated. Final risk of bias scores were adjudicated by two reviewers (EJG and SR). RoB-2 scoring specifically addresses randomization bias, bias related to deviation from intended interventions, bias due to missing outcome data, bias in measurement of outcome, and bias in selection of the reported result. The ROBINS-I assessment accounts for bias in confounding, bias in patient selection, bias in classification of interventions, deviations from intended interventions, bias from missing data, bias in measurement of outcomes, and bias in selection of the reported result.
The certainty of evidence assessment was performed using GRADEPro Guideline Development Tool (GDT) software (McMaster University and Evidence Prime Inc) according to GRADE methodology [15]. In brief, studies that address a specific outcome of interest can be assessed as a group to determine the certainty with which the evidence leads the panel to make a recommendation. The certainty of evidence may be reduced by the risk of bias, inconsistency (heterogeneity across different studies, typically signified by high I2 values), indirectness (how closely the studies pertain to the PICO), imprecision (unclear effect size due to low event rates, small sample sizes, or wide confidence intervals [CIs]), and publication bias. The certainty of evidence could be increased by a large effect size, a dose–response gradient, or residual confounding that favors the comparator. A final level of confidence rating is generated from this process, ranging from very low to high confidence in the estimate of effect (Fig. 1).
GRADE methodology for rating certainty of evidence, level of confidence, and determining the strength of recommendation. Strong recommendations use the term “recommend”, while weak recommendations use the term “suggest.” Unrestricted use of this figure was granted by the US GRADE Network. GRADE Grading of Recommendations Assessment, Development, and Evaluation
Statistical Analyses
All analyses were outcome based and performed by one study statistician (YY). For each outcome of interest (early seizure, late seizure, adverse events, functional and cognitive outcomes, mortality), we stratified the analysis by ASM type and study design (randomized versus nonrandomized studies) and tested their differences. The summary statistic used for dichotomous data was relative risk, and the mean difference or standardized mean difference was used, when applicable, for continuous data. Studies that reported adjusted odds ratios were pooled using the method of inverse variance. All meta-analyses were conducted using random-effects models. Substantial heterogeneity was defined as I2 ≥ 50%. All analyses are presented in forest plots and were performed using Revman 5.4 software (Cochrane, London, UK).
Development of Recommendations
Assessments of judgment for each PICO were performed using GRADEPro GDT software (McMaster University and Evidence Prime Inc). Final recommendations were based on consideration of the importance of the PICO, the certainty and confidence level of the evidence, the balance between the desirable and undesirable effects of the intervention, patient values, and the acceptability and feasibility of the recommendation (Fig. 1). Consensus of all panel members was required for final recommendations. Independent members of the Neurocritical Care Society guideline committee reviewed all recommendations. Strong recommendations, which imply that the majority of stakeholders would want to adopt the prescribed guidance and that policy makers may use the guideline in most situations, are indicated by the phrase “we recommend.” Conditional recommendations, which imply that most stakeholders would want to adopt the recommendation, though many might not, and that shared decision-making between patient and practitioner is likely required, are indicated by the verbiage “we suggest.” The overall quality (certainty) of evidence was averaged across outcomes for each PICO and could be categorized as very low, low, moderate, or high. The limitations in the current body of literature and proposals for future avenues of research are discussed with each PICO.
Recognizing the inherent restrictions of formulated guidelines, the panel has included an “in our practice” section following the formal GRADE-based recommendation and justification. This section highlights current practices (such as dosing, use of Electroencephalogram (EEG) etc.) that might not be specifically covered in the recommendation and do not meet the specific criteria for “good/best practice statements” within GRADE [16]. The pragmatic details of this section were arrived at after anonymous surveys of panel members followed by discussion and represent expert consensus. A caveat to this section is that panel members primarily represent academic centers and reflect current practice in the United States. As such, these suggestions may not be generalizable to all settings.
Results
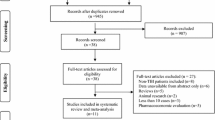
The initial literature search yielded 1998 articles, of which 33 formed the basis of the recommendations and 26 were included in meta-analyses (Supplemental Fig. 1). Below we address each PICO and expound on the relevant literature for each outcome of interest.
TBI PICO 1: Should ASM Versus No Antiseizure Medication be Used as Seizure Prophylaxis for Patients Hospitalized with Moderate–Severe TBI?
To Prevent Early Seizure (≤ 14 Days from TBI Onset or During Hospitalization)
A total of 11 studies that included 9024 patients were included in a meta-analysis evaluating the outcome of early seizure [17,18,19,20,21,22,23,24,25,26,27]. Of these, four studies evaluated LEV versus no ASM (N = 2457) [18, 20, 25, 27], six studies evaluated PHT/fPHT versus placebo or no ASM (N = 3311) [19, 21,22,23,24,25], and two studies included a variety of ASMs (PHT, fPHT, valproic acid, phenobarbital, and LEV) compared to no ASM (N = 3256) [17, 26]. Of note, one study had three arms and compared both LEV and PHT to no ASM [25]. When this study was entered in the analysis, stratified by ASM type, the control group was split to avoid double counting. Only two of these studies were randomized controlled trials [23, 24], and both were placebo controlled. Two studies that evaluated early seizure but did not have a control group were excluded from analysis [28, 29]. Overall, there was no significant reduction in early seizure with ASM use (including PHT or LEV) versus no ASM or placebo (Risk Ratio (RR) 0.72, 95% CI 0.40–1.29, P = 0.27). There was significant heterogeneity across studies (I2 = 68%, P = 0.001), though there was no significant heterogeneity between randomized and nonrandomized studies (I2 = 0%, P = 0.42) or between studies evaluating different ASMs (I2 = 0%, P = 0.54; Fig. 2a, b).
TBI PICO 1 Anti-seizure medication (ASM) versus no ASM for early seizure outcome. Meta-analysis of early seizure outcome among patients with TBI comparing ASM versus no ASM stratified by randomized versus nonrandomized study design (a) and ASM type using a random-effects model (b). When stratified by ASM type, the control group for one study that had three arms (levetiracetam, phenytoin and no ASM) was split in half to avoid double counting [25] RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
To Prevent Late Seizure (> 14 Days from TBI Onset or Post Hospitalization)
Five studies evaluated late seizure (N = 2741) [17, 23, 30,31,32], including two randomized trials [23, 32] (N = 482). Three studies compared PHT to placebo [23, 32] or no ASM [31] (N = 544), one compared LEV to no ASM [30] (N = 86), and one compared a variety of ASMs to no ASM [17] (N = 2111). The total duration of ASM prophylaxis administration ranged from 30 days [30] to 12 months [23] to 18 months [32] and was unspecified in two studies [17, 31]. The duration of follow-up varied from 6 months [31] to 18 months [32] to 2 years [23, 30] and was unspecified in one study [17]. Overall, there was no significant effect of ASM for preventing late seizures (RR 0.75, 95% CI 0.37–1.52, P = 0.42). There was significant heterogeneity among all studies (I2 = 67%, P = 0.02), and there was substantial heterogeneity between randomized and nonrandomized trials (I2 = 68%, P = 0.08) but not between studies using different ASMs (I2 = 0%, P = 0.65; Fig. 3a, b).
TBI PICO 1 Anti-seizure medication (ASM) versus no ASM for late seizure outcome. Meta-analysis of late seizure outcome among patients with TBI comparing ASM versus no ASM stratified by randomized versus nonrandomized study design (a) and ASM type using a random-effects models (b). RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
Adverse Events Rates in ASM Versus no ASM Groups
Only one study evaluated adverse events in both the ASM and control group (N = 404) [23], though there was no specification regarding which adverse events were collected, how adverse events were defined, if patients were systematically screened for events, or if there was a data safety monitoring board (DSMB). In this study, adverse reactions, including rash, leukopenia, and elevated liver enzymes, occurred in 37 of 208 (18%) patients in the PHT group and 25 of 196 (13%) patients in the placebo group (RR 1.39, 95% CI 0.87–2.23, P = 0.16). Another study [30] reported adverse events only in the ASM (LEV) group (including headache, fatigue, drowsiness/somnolence, memory impairment, amnesia, pain, irritability, dizziness, emotional lability, insomnia, cognitive changes, ataxia, depression, hostility, vertigo, nausea, cough, nervousness, paresthesia, and weight gain). Two study participants discontinued LEV therapy because of drug-related toxicity (somnolence, fatigue, irritability, and headache), 40% experienced depression, and one experienced transient suicidal ideation.
Mortality/Functional Outcomes in ASM Versus No ASM Groups
Six studies assessed mortality in patients who had received ASM compared to no ASM [17, 23, 24, 26, 30, 32] (N = 4149). Three studies compared PHT to placebo or no ASM [23, 24, 32], one compared LEV to no ASM [30], and two compared a variety of ASMs to no ASM [17, 26]. Three randomized trials [23, 24, 32] and three nonrandomized studies [17, 26, 30] were included. The duration of follow-up ranged from 7 days [24, 26] to 18 months [32] to two years [23, 30] and was not specified in one study [17]. Overall, there was no significant effect of ASM on mortality (RR 1.14, 95% CI 0.69–1.89, P = 0.52). There was significant heterogeneity across studies (I2 = 81%, P < 0.0001); however, there was no significant heterogeneity between randomized and nonrandomized studies (I2 = 0%, P = 0.75) and across studies of different ASMs (I2 = 0%, P = 0.90; Fig. 4a, b). Randomized trials showed neutral effects of ASM on mortality. The strong signal favoring no ASM in one nonrandomized study [17] (RR 2.30, 95% CI 1.73–3.04) may reflect treatment bias because sicker patients with higher mortality rates may have been more likely to receive ASM. One retrospective study of 1145 patients with TBI aged > 65 years found significantly lower 7-day mortality rates in adjusted analysis in those who received an ASM compared to those who did not (Hazard Ratio (HR) 0.48, 95% CI 0.28–0.81, P = 0.006), despite the fact that there were significantly more moderate and severe TBIs (compared to less severe TBI) in the ASM group and that more patients in the ASM group required mechanical ventilation [26]. Mortality rates in the ASM group remained significantly lower than those in the no ASM group at 30 days and 1 year. The two groups had similar rates of withdrawal of life-sustaining measures or changes in do-not-resuscitate status. There were no studies that compared ASM to no ASM and evaluated functional outcomes, such as modified Rankin Scale or Glasgow Outcome Scale scores.
TBI PICO 1 Anti-seizure medication (ASM) versus no ASM for Mortality Outcome. Meta-analysis of mortality outcome among patients with TBI comparing ASM versus no ASM stratified by randomized versus nonrandomized study design (a) and ASM type using a random-effects models (b). RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
One randomized controlled trial evaluated cognition among PHT and placebo patients at 1 month and 1–2 years post TBI using standardized neuropsychological metrics [6]. At 1 month, half of the study participants who were comatose at presentation (GCS ≤ 8) were unable to complete neuropsychological testing (78% of those receiving PHT compared to 47% of those receiving placebo could not be tested). Among those who could complete testing, those who had received PHT had significantly worse scores across a range of neuropsychological tests and domains. Among study participants with GCS > 8 at presentation, there were no differences in any neuropsychological metric among those who received PHT compared to placebo. These differences at 1 month were attributed to a larger proportion of patients with GCS ≤ 8 who had received PHT and were untestable. At 12 months, there were no differences in any neuropsychological metric between the PHT and placebo groups, irrespective of whether patients had GCS ≤ 8 or > 8 at presentation. However, between 12 and 24 months’ follow-up, those who had been exposed to PHT for 12 months and subsequently discontinued medication had significantly faster rates of improvement than placebo patients on a variety of metrics in domains of attention, memory, verbal and performance IQ, and return to work at 24 months (overall rank-sum type test P < 0.05). These data suggest that the adverse cognitive effects of PHT may be reversible once the drug is discontinued.
Limitations in the Literature
There are several limitations that should be mentioned. First, the definitions for TBI severity varied across studies, and most studies predated modern TBI severity rating scales [12]. Indeed, most articles did not describe index neurological severity or duration of loss of consciousness in their inclusion criteria. Whereas the majority of studies evaluated patients with moderate–severe TBI defined as brain imaging demonstrating contusion, subdural hematoma, epidural hematoma, depressed skull fracture, penetrating head wound, unconsciousness for ≥ 6–24 h, major focal neurological deficits, and/or GCS ≤ 10 [17,18,19,20,21, 23, 24, 30,31,32], two studies used different TBI severity scores. One defined TBI severity based on the Marshall computed tomography (CT) score [22], and another used the head Abbreviated Injury Score [25] However, all studies included hospitalized patients with acute brain imaging abnormalities, hence meeting our inclusion criteria for moderate–severe TBI. Additionally, two studies [23, 30] specified inclusion criteria of seizure within 24 h of TBI, though it is unclear if this seizure occurred prior to initiation of the study ASM.
Second, there were multiple different time points used to classify early and late seizure. Seven studies specified early seizure as occurring within ≤ 7 days from TBI onset [18, 19, 21,22,23,24,25], whereas two studies included any seizure during the index hospitalization for TBI [17, 20]. Late seizure was defined as ≥ 8 days and up to 2 years post TBI in two studies [23, 30], as ≥ 8 days up to 18 months post TBI in one study [32], and as > 14 days in one study [31], and one study did not specify a time frame for late seizure [17]. In addition to variable follow-up times, the duration of ASM administration varied widely from 7 days to up to 18 months [32].
Third, the duration of follow-up varied from study to study. Whereas some studies had minimal loss to follow-up, others had attrition of as many as 50% of study participants. In the study by Wohns and Wyler, 50% of patients were lost to follow-up after 14 days [31]. Temkin reported a 57% follow-up rate in both the ASM and control groups at 1 year and a 53% follow-up rate in both groups at 2 years [23]. In contrast, Young et al. [32] reported an 84% follow-up rate at 18 months, and Klein et al. [30] reported a 79% follow-up rate at 2 years.
Fourth, there was detection bias in measuring seizure outcomes (e.g., clinical detection only versus electrographic seizures or both). Only two studies reported adjunct use of EEG when a subclinical or nonconvulsive seizure was suspected [18, 23]. However, neither had a standardized protocol for EEG monitoring, neither specified how many study participants in each group underwent EEG monitoring, and the number of clinical versus electrographic seizures in each group was not reported in either study. Because up to 52% of seizures post moderate–severe TBI are nonconvulsive and can only be detected by EEG monitoring [29], it is likely that event rates in both the ASM and the no ASM groups were grossly underestimated, particularly in patients receiving sedation or those with coma or limited neurological examinations. Additionally, the duration of EEG monitoring was not specified in any study. Because the sensitivity of EEG increases with increasing duration of monitoring [33, 34], particularly among comatose patients, it is likely that trials using short-duration EEG underestimate subtle or nonconvulsive seizure rates.
Fifth, there was likely to be treatment biases in the nonrandomized studies that were included. Patients with more severe TBI or coma may have been more likely to receive ASM. Conversely, when management was deemed futile or if life-sustaining therapy was withdrawn, ASM may not have been used. None of the studies that reported mortality rates divulged the number of patients who underwent withdrawal of life-sustaining therapy. Because most studies were unblinded, rates of withdrawal may have been unbalanced between ASM and no ASM groups.
Sixth, whereas several studies specified ASM dosing and noted that dosing was titrated to achieve therapeutic levels [19, 21, 23, 24, 30,31,32], two studies did not titrate dosing to blood levels [18, 21], and several studies neither provided dosing information nor assessed drug levels [17, 20, 25,26,27]. Among those that did titrate ASMs to achieve therapeutic levels, 15–74% of patients were subtherapeutic during the study time frame [19, 22, 24, 30, 32]. Additionally, only one study of LEV used weight-based dosing and monitored drug levels [30]. Using a dosage of 55 mg/kg/day divided in two doses, 85% of patients were within the therapeutic range throughout the 30 days of drug administration [30]. Two studies evaluating LEV versus no ASM did not report dosing information, and neither monitored drug levels [20, 25]. One study using low-dosage LEV (500 mg twice daily) did not monitor levels [18]. Pharmacokinetic studies suggest that systemic clearance of LEV is faster and the terminal elimination half-life is shorter in critically ill neurological patients compared to heathy adults [35]. Consequently, LEV dosages of 1000 mg every 8 h or 1500–2000 mg every 12 h were found to have the highest probability of achieving therapeutic trough concentrations [35]. Because ASM levels may have been subtherapeutic in a substantial proportion of patients in multiple studies, the effect size for both benefit and harm may be underestimated. Though one retrospective study of 866 patients with TBI suggested no difference in the cumulative incidence of early posttraumatic seizures among patients who received LEV ≤ 1000 mg/day, 1500 mg/day, or ≥ 2000 mg/day, those in the higher dosage groups were more than twice as likely to have EEG monitoring, and it is possible that seizures were underdiagnosed in the lowest dosage tertile [36]. Additionally, per institutional protocol, patients with creatinine clearance < 30 mL/min were prescribed ≤ 1000 mg/day, whereas those with creatinine clearance ≥ 30 mL/min were prescribed 1000 mg twice daily. Because LEV levels were not checked, it is difficult to know if patients in the lowest dosage group were, in fact, more often in the therapeutic range than those receiving higher dosages. Lastly, this study is confounded by prescriber bias and did not account for up-titration of LEV dosing in response to seizure occurrence.
Lastly, there were very limited data regarding adverse events in ASM compared to no ASM groups. Indeed, Medical Dictionary for Regulatory Activities (MedDRA) was not developed until 1994 [37], and the National Institutes of Health (NIH) did not require Data Safety Monitoring Boards (DSMBs) for NIH-sponsored phase III clinical trials until 1998 [38]. All three randomized trials used in this analysis were conducted prior to 1990 [23, 24, 32], and two nonrandomized studies [21, 31] were conducted prior to the release of a standardized definitions of adverse events.
Certainty of Evidence
The certainty of evidence, including risk of bias assessment and effect size, is shown for each outcome of interest (early seizure, late seizure, adverse events, and mortality), stratified by trial design (randomized versus nonrandomized; Table 1). The risk of bias for each article can be found in Supplemental Table 1 and 2, stratified by article type (randomized controlled trial versus nonrandomized controlled trial).
Recommendation
We suggest that either prophylactic ASM (initiated during index hospitalization) or no ASM could be used in patients hospitalized with moderate–severe TBI (weak recommendation, low quality of evidence; Fig. 5).
Justification: Across all outcomes of interest, we did not detect any significant positive or negative effect of ASM compared to no ASM on the outcomes of early seizure, late seizure, adverse events, or mortality (all meta-analyses’ CIs crossed 1.0). These findings did not differ by study design (randomized versus nonrandomized) nor by ASM type (PHT versus LEV). Both the desirable (seizure prevention, mortality) and undesirable (adverse events) effect sizes were trivial across studies, and the overall certainty of the evidence was low. Larger randomized controlled trials that include both electrographic and clinical seizure outcomes, as well as functional and cognitive outcomes, are needed. Study drugs should be titrated to therapeutic levels to avoid underdosing, which can minimize effect size. Subgroup analyses in patients with mild versus moderate–severe TBI should be considered, and adverse events should be collected in a systematic fashion using MedDRA definitions under the oversight of DSMBs.
TBI PICO 2: Should LEV Versus PHT/fPHT be Used for Seizure Prophylaxis in Patients Hospitalized with Moderate–Severe TBI?
To prevent Early Seizure (≤ 14 Days from TBI Onset or During Hospitalization)
A total of ten studies that included 1741 patients were included in a meta-analysis evaluating LEV versus PHT for the treatment of early seizure [39,40,41,42,43,44,45,46,47,48]. Of these, three studies (N=342) were randomized controlled trials [39, 45, 48], and seven (N=1399) were nonrandomized controlled trials [40,41,42,43,44, 46, 47]. Overall, there was no significant reduction in early seizure with the use of LEV compared to PHT/fPHT (RR 1.07, 95% CI 0.84–1.36, P=0.58). There was no significant heterogeneity across studies (I2=0%, P=0.52) or between randomized and nonrandomized studies (I2=0%, P=0.52; Fig. 6). One additional randomized controlled trial (N=379) [49] excluded from the aforementioned analysis compared valproate to PHT and saw no significant difference in the rate of early seizures between the two ASMs (RR 2.94, 95% CI 0.66–13.06, P = 0.16).
TBI PICO 2 Levetiracetam versus phenytoin/fosphenytoin for early seizure outcome. Meta-analysis of early seizure outcome among patients with TBI comparing levetiracetam to phenytoin/fosphenytoin using a random-effects model. RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
To Prevent Late Seizure (> 14 Days of TBI Onset or Post Hospitalization)
A total of three studies that included 208 patients were included in a meta-analysis evaluating LEV versus PHT for the treatment of late seizure [17,18,19,20,21,22,23,24,25]. Of these, one study (N=135) was a randomized controlled trial [48], and two (N=73) were nonrandomized controlled trials [41, 44]. Overall, across studies there was marginal significance (RR = 0.54, 95% CI 0.30–1.00, P=0.05) favoring LEV, with the randomized controlled study significantly favoring LEV (0.36, 95% CI 0.15–0.86, P=0.02). There was no significant heterogeneity across studies (I2=0%, P=0.41) or between randomized and nonrandomized studies (I2=39.7%, P=0.20; Fig. 7). Two additional randomized controlled trials [32, 49] were excluded from the aforementioned analysis because they compared valproate to PHT (N=379) [49] or PHT to phenobarbital (N=105) [32]. Neither saw a significant difference in the rate of late seizures between the two ASMs (RR 1.23, 95% CI 0.72–2.08, P = 0.45 in ref. [23]; RR 1.29, 95% CI 0.31–5.38, P = 0.72 in ref. [32]).
TBI PICO 2 Levetiracetam versus phenytoin/fosphenytoin for late seizure outcome. Meta-analysis of late seizure outcome among patients with TBI comparing levetiracetam to phenytoin/fosphenytoin using a random-effects model. RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
Adverse Events Rates in PHT/fPHT Versus LEV Among Patients with TBI
Four studies evaluated adverse events in both LEV and PHT/fPHT groups (N=1169) [39, 41, 42, 47], though there was heterogeneity regarding which adverse events were collected, how adverse events were defined, if patients were systematically screened for events, or if there was a DSMB in the case of one randomized controlled trial [39]. Adverse reactions, including fever, infection, sepsis/systemic inflammatory response syndrome, acute respiratory distress syndrome, decreased level of consciousness, neurological worsening, cognitive problems, increased intracranial pressure (ICP), fatigue, vomiting, gastrointestinal upset, ileus, gastrointestinal bleed, decreased appetite, rash, dermatologic events, neutropenia, leukopenia, thrombocytopenia, hematologic events, deep vein thrombosis, pulmonary embolus, vertigo, drug intolerance, atrial fibrillation, myocardial infarction, hypotension, acute kidney injury, diabetes insipidus, and elevated liver enzymes, occurred in 89 of 561 (15.9%) patients in the PHT group and 57 of 608 (9.4%) patients in the LEV group (RR 0.49, 95% CI 0.23–1.07, P=0.07). There was significant heterogeneity across studies (I2=78%, P=0.003) but not between randomized and nonrandomized studies (I2=57.5%, P=0.13; Fig. 8). A retrospective study of 200 patients with TBI compared neurobehavioral side effects (agitation, aggression, hostility, emotional lability, and inappropriate behavior) of LEV to those of PHT and found no significant difference between the ASMs, though the incidence was high in both groups (80% in PHT group versus 71% in LEV group, P=0.189) [50]. Another retrospective study found higher rates of dizziness (24% versus 8%; P=0.018) and longer length of stay among patients who received PHT compared to LEV [51].
TBI PICO 2 Levetiracetam versus phenytoin/fosphenytoin for adverse events outcome. Meta-analysis of adverse events outcome among patients with TBI comparing levetiracetam to phenytoin/fosphenytoin using a random-effects model. RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
In a randomized controlled trial [39], there was no difference in rates of fever for PHT (55.6%, n = 10) as compared to LEV (52.9%, n = 18). In one nonrandomized controlled study (N = 813), leukocytosis and longer length of stay were more common in patients receiving LEV compared to PHT (1.2% vs. 9.6% [P = 0.001] and 11.8 vs. 7.5 days [P = 0.001], respectively), whereas adverse events resulting in a change in ASM were more common in the PHT group (0% vs. 2.9%; P = 0.001) [42]. In another study [41], medication-related complications were significantly higher in the PHT group (78.6% [n = 11] vs. 20% [n = 1]; P = 0.038), and the PHT group had a higher rate of days with fever (0.2 ± 0.22 vs. 0; P = 0.014).
Mortality/Functional Outcome in PHT/fPHT Versus LEV
Three studies assessed mortality in patients who had received PHT/fPHT compared to LEV (N = 974) [39, 42, 46]. One randomized trial (N = 52) [39] and two nonrandomized studies (N = 922) [42, 46] were included. The duration of follow-up ranged from 7 days [39, 42, 46] to 6 months [39]. Overall, there was no significant difference between PHT/fPHT and LEV when evaluating the outcome of mortality (RR 1.06, 95% CI 0.66–1.72, P = 0.80). There was no significant heterogeneity across studies (I2 = 7%, P = 0.34) or between randomized and nonrandomized studies (I2 = 0%, P = 0.99; Fig. 9). Two studies [39, 41] evaluated functional outcomes using either the Glasgow Outcome Scale–Extended (GOSE) or the Disability Rating Scale (DRS) or both, but only one study reported ranges [39], and therefore data could not be pooled. In the study by Szaflarski et al. [39], surviving patients on LEV experienced better long-term outcomes than those on PHT (DRS: at 3 months 5 vs. 11 [P = 0.006], and 6 months 6 vs. 3 [P = 0.037]; GOSE at 6 months: 5 vs. 3 [P = 0.016], respectively) [39]. When controlling for disease severity (GCS on admission), there was no difference in DRS at discharge (P = 0.47), but at 3 and 6 months, the DRS was 5.2 points lower (95% CI 0.2–10.3, P = 0.42) and 3.7 points lower (95% CI − 1.0 to 8.5, P = 0.118), respectively, among patients treated with LEV compared to those treated with PHT. Similarly, when controlling for admission GCS, the GOSE was not different at discharge or 3 months between the two groups, but at 6 months, it was 1.5 points higher for those treated with LEV as compared to PHT (95% CI 0.1–3, P = 0.039). These data suggest that LEV has better long-term outcomes as measured with GOSE and DRS and may be a suitable alternative to PHT in seizure prevention in patients with TBI. Conversely, the study by Gabriel and Rowe [41] did not find a difference in GOSE scores at 6 months or more after injury for patients randomized to LEV versus PHT (5.6 vs. 5.1; P = 0.58) [41]. However, there were only 19 patients in the study, and the LEV group (n = 5) had a statistically higher median GCS at presentation (14 vs. 3; P = 0.016) and ICU discharge (15 vs. 14; P = 0.044), and the PHT group (n = 14) had a significantly longer time, in days, between onset of injury and GOSE assessment (808.8 ± 146.0 vs. 484 ± 152.6, P = 0.001), which could have biased the results.
TBI PICO 2 Levetiracetam versus phenytoin/fosphenytoin for mortality outcome. Meta-analysis of mortality outcome among patients with TBI levetiracetam to phenytoin/fosphenytoin using a random-effects model. RCT=Randomized Controlled Trial; M-H=Mantel-Haenszel; LEV=levetiracetam; PHT=phenytoin/fosphenytoin; CI=confidence interval
Limitations in the Literature
There are several limitations that should be mentioned. First, the severity of TBI varied across studies. Whereas some studies specifically evaluated patients with moderate–severe TBI defined as brain imaging demonstrating contusion, subdural hematoma, epidural hematoma, depressed skull fracture, penetrating head wound, or GCS ≤ 10 [39, 42, 43, 45, 46, 52], other studies included hospitalized patients with International Classification of Diseases, Ninth Revision codes for TBI with unspecified brain imaging abnormalities. Additionally, several studies specified inclusion criteria of TBI within 12 [45] or 24 [39, 41, 43, 48, 52] hours of admission, but only a few specified that no seizure could occur prior to initiation of the study ASM [41] or study enrollment [42, 46].
Second, most studies only looked at early seizures defined as ≤ 7 days from TBI onset [39, 42, 43, 45, 46, 48]; however a few studies looked at late seizures classified as ≥ 8 days [41, 44] or any seizure during the hospitalization [47]. In addition, one study also evaluated seizures in the first 24 h but was not powered to assess ASM differences within the different time window subgroups [44].
Third, very few studies incorporated the use of EEG monitoring in the diagnosis of electrographic or nonconvulsive seizures [40, 43, 47, 48], and only three studies required EEG monitoring, albeit of varying duration: in one study, up to 72 h of continuous EEG or if awake and following commands (N = 52) [39]; in another, routine EEG (n = 42, 46.7%) [40] or continuous EEG (n = 48, 53.3%); and the duration of EEG was unspecified in one study (N = 27) [43]. Though EEG findings were reported in some studies, variable and sometimes vague terminology was used, including “abnormal” [43], “status epilepticus” [40], “seizure activity” [40, 43, 48], “electrographic seizures” [47], “seizure tendency” [43], and “periodic epileptiform discharges” [40]. As previously noted, more than half of seizures following moderate–severe TBI are nonconvulsive and can only be detected by EEG monitoring [29]. Hence, the true frequency of seizure events is likely underestimated in both the LEV and PHT groups, especially in patients receiving sedation or those with coma or limited neurological examinations.
Fourth, whereas a few studies specified ASM dosing and noted that dosing was titrated to achieve therapeutic levels in the case of PHT/fPHT [39, 41, 42, 47], several studies did not titrate dosing to blood levels [40, 43, 48, 52] or provide dosing information [41, 45, 46]. Among those that did titrate ASMs to achieve therapeutic levels, one [40] reported time to the therapeutic level (median 30 h, interquartile range 11–56), percentage of patients with initial therapeutic levels (52%, n = 37 of 71), and duration the therapeutic level was maintained (median 2 days, interquartile range 1–5), whereas another reported a low percentage of time spent in the therapeutic range (47.2%, n = 42) [46]. Additionally, though some studies used weight-based loading doses for PHT/fPHT [39, 42, 47, 48], none of the studies used weight-based maintenance dosing or monitored drug levels for LEV. A few studies used either fixed low-dosage LEV (500 mg twice daily in ref. [43]; 500 mg once or twice daily in ref. [41]) or a dosing range that included low-dosage LEV (500–1000 mg twice daily) [47, 48] and did not monitor levels. Because ASM levels may have been subtherapeutic in a substantial proportion of patients, the effect size for both benefit and harm may be underestimated. Additionally, there were very limited data regarding adverse events in the PHT compared to LEV groups.
Finally, there was likely to be treatment biases in the nonrandomized studies that were included, as mentioned in the PICO 1 section. Rates of withdrawal of life-sustaining therapy or limitations in treatment were not described in any study.
Certainty of Evidence
The certainty of evidence, including risk of bias assessment and effect size, is shown for each outcome of interest (early seizure, late seizure, adverse events, and mortality), stratified by trial design (randomized versus nonrandomized; Table 2).
Recommendation
If a prophylactic ASM is used in patients hospitalized with moderate–severe TBI, we suggest LEV should be used over PHT/fPHT for seizure prophylaxis (weak recommendation, very low quality of evidence; Fig. 10).
Justification: We did not detect any significant positive or negative effect of PHT/fPHT compared to LEV for early seizures or mortality. However, there were signals suggesting fewer late seizures (RR 0.54, 95% CI 0.30–1.00, P = 0.05) and fewer adverse events (RR 0.49, 95% CI 0.11–1.12, P = 0.07) with LEV compared to PHT/fPHT. These findings did not differ by study design (randomized versus nonrandomized). However, in general, the desirable (seizure prevention, mortality) and undesirable (adverse events) effect sizes were small across studies, and the overall certainty of the evidence was very low (Fig. 11).
TBI PICO 3: Should a Short (≤ 7 Days) or Long (> 7 Days) Duration of ASM be Used for Seizure Prophylaxis in Patients Hospitalized with Moderate–Severe TBI?
To Prevent Late Seizure (≥ 14 Days from TBI Onset up to 6 Months, 18 Months, or 2 Years)
One single-center randomized study of 90 patients with TBI compared PHT for 7 days versus 21 days and found no difference in the incidence of seizures over the 21-day study, nor were there differences in reported adverse events [53]. Another randomized, double-blinded, controlled study compared PHT for 7 days (n = 132) to valproic acid for 1 month (n = 120) or to valproic acid for 6 months (n = 121) [49]. All patients were observed for 24 months and had ASM levels checked every 3 months while on study medication. Paradoxically, patients randomized to a longer duration of ASM had more late seizures, though this difference did not reach statistical significance. Over the 2-year study, late seizures (> 7 days from TBI) occurred in 15% in the PHT for 7 days group, 16% in the valproic acid for 1 month group, and 24% in the valproic acid for 6 months group (RR 1.4, 95% CI 0.8–2.4, P = 0.19). Notably, 16% of patients were noncompliant with medications at 1 month post TBI, and 21% were noncompliant at 6 months. Of those who were compliant, 90% were in the therapeutic range at 1 month and 85% were in the therapeutic range at 6 months.
A variety of studies that used varying durations of ASM, ranging from 1 month [30] to 18 months [32] post TBI, did not show benefit of ASM compared to placebo over follow-up periods ranging from 6 months [31] to 2 years [23, 30]. These data imply that longer duration of ASM use does not impact late seizure occurrence. In one study [41], PHT was administered for a mean of 10.6 days (n = 14) compared to 4.6 days (n = 5) for LEV (P = 0.112), and there was no difference in late seizures (from 8 days to 6 months post TBI) between the PHT patients (late seizure in 14%) and the LEV group (late seizure in 0, P = 0.53).
Adverse Events Rates in Short- Versus Long-Duration ASM
In one study, the incidence of serious adverse events was similar in patients who received 7 days of PHT, 1 month of VPA, and 6 months of VPA [49].
Mortality/Functional Outcomes in Short- Versus Long-Duration ASM
One study found higher mortality rates (though not significant) over 2 years in study participants who received valproic acid for 1 month (15 of 120, 13%) or 6 months (17 of 121, 14%) compared to those who received PHT for 7 days (9 of 132, 7%; P = 0.07), even after adjusting for index injury severity [49]. There was no difference in mortality between those who took 1 month and those who took 6 months of valproic acid.
A randomized controlled study that evaluated cognitive outcomes in patients with TBI who received PHT for 12 months versus placebo found that PHT was associated with worse cognitive function at 1 month, but after 12 months, the groups were similar [6]. Between 12 and 24 months, the PHT group (which had now discontinued PHT) had more rapid gains in neuropsychological testing, which allowed these patients to effectively catch up cognitively with the control group [6]. These data suggest that there is a deleterious cognitive effect of PHT, and shorter dosing periods may be less disruptive to cognitive function than longer durations of ASM use. Conversely, there were no differences in 1-, 6-, or 12-month cognitive or neuropsychiatric metric scores when comparing patients who were randomized to either 7 days of PHT, 1 month of valproic acid, or 6 months of valproic acid, even after adjusting for index severity of illness and demographics [5].
Limitations in the Literature
Only one study directly evaluated the duration of ASM use in patients with TBI [53]. Though another randomized controlled trial compared two different ASMs (VPA and PHT) used for different durations, it did not routinely use EEG to diagnose seizure, it did not use standardized adverse event definitions, and a substantial proportion of patients were noncompliant with ASM at 1 and 6 months’ follow-up [49]. Additionally, there are no trials that address the risk of late seizures based on ictal-interictal phenomenon on EEG or the presence of epileptiform discharges. The appropriate duration of prophylaxis in these patients is unclear. However, the 2HELPS2B scoring system, which uses EEG findings to predict seizure in critically ill patients, included 142 of 4772 (2.6%) patients with TBI for model development [54]. According to this model, the presence of brief ictal rhythmic discharges, lateralized periodic discharges, lateralized rhythmic delta, bilateral independent discharges, or sporadic epileptiform discharges; a frequency > 2 Hz for any periodic or rhythmic pattern; and the presence of any superimposed fast, rhythmic, or sharp activity (“plus features”) all increase the risk of future seizure. Subsequent retrospective studies of hospitalized patients requiring ≥ 12 h of continuous EEG monitoring found that 1 h of EEG allowed for stratification into 2HELPS2B risk categories with < 5% calibration error, and 24 h of monitoring was recommended for patients with highly epileptiform patterns on the initial 1-h EEG [55]. It is unclear how many patients in this study had acute TBI; however, 35% were comatose and 43% had acute structural brain injury [55]. Another study found that 20% of comatose patients did not have EEG evidence of seizure until after 24 h of EEG monitoring, suggesting that ≥ 48 h of monitoring may be needed in this population [33]. Further study using the 2HELPS2B score to guide the use and duration seizure prophylaxis in patients with TBI is needed. Because we imputed the impact of longer duration of ASM use across trials assessing ASM given for variable time frames versus placebo, our recommendations are indirect. Additionally, there were very limited data regarding the long-term side effects of ASM, particularly for newer-generation ASM, such as LEV, and no study evaluated adverse events according to standardized reporting systems. Last, there are no studies evaluating the duration of use of LEV or more modern ASMs (such as lacosamide) in TBI populations.
Certainty of Evidence
The certainty of evidence, including risk of bias assessment and effect size, is shown for each outcome of interest (late seizure, adverse events, mortality, and cognition), stratified by trial design (randomized versus nonrandomized; Table 3).
Recommendation
If a prophylactic ASM is used in patients hospitalized with moderate–severe TBI, we suggest a short duration of use (≤ 7 days) versus a longer duration of use (> 7 days) (weak recommendation, low quality of evidence; Table 3).
Justification: One study evaluating PHT administered for 7 versus 21 days post TBI did not find any differences in seizures between groups over a 21-day follow-up period [53]. A randomized controlled trial comparing PHT × 7 days versus VPA × 1 month or VPA × 6 months identified nonsignificant trends toward higher rates of seizure and mortality in the groups that received a longer duration of ASM [49]. Additionally, in another randomized controlled trial, cognitive outcomes were worse in patients receiving PHT compared to placebo and improved once PHT was discontinued, suggesting a deleterious effect of long-term PHT use [23]. Similarly, we did not detect any difference in late seizures with longer duration of ASM use compared to short duration across different studies. Overall, the desirable effects of longer duration of ASM use were deemed to be trivial, whereas the undesirable effects were small to moderate. The overall certainty of evidence was low, and this recommendation was based, in part, on indirect data evaluated across studies.
Discussion
In an effort to add pragmatic clinical guidance and contextualize the evidence-based GRADE recommendations, the committee developed consensus expert opinion statements termed “in our practice” to frame prophylactic ASM use for each PICO.
PICO 1: Use of ASM Versus No ASM in Our Practice
Only one randomized, placebo-controlled trial was found to have low risk of bias across all categories that were assessed (Supplemental Table 2) [23]. This well-conducted trial found a significant reduction in early seizure with PHT prophylaxis compared to placebo [23]. In long-term follow-up, patients exposed to PHT had worse cognitive outcomes at 1 month post TBI but were similar to the placebo group at 1 year and had substantially improved cognition once exposure to PHT was removed [6]. The results of this positive trial were attenuated in meta-analyses that included studies with substantial methodological issues. Based on these data, many practitioners may choose to use short-term prophylaxis. An alternative strategy may be to use continuous EEG monitoring and only use prophylactic ASM in patients with high-risk EEG features (e.g., brief ictal rhythmic discharges, lateralized periodic discharges, lateralized rhythmic delta, bilateral independent periodic discharges, sporadic epileptiform discharges, frequency > 2 Hz for any periodic or rhythmic pattern, and/or the presence of superimposed rhythmic, sharp, or fast activity) [54]. The latter option depends on access to continuous EEG monitoring and frequent interpretation by epileptologists with expertise in ICU EEG. Generally, the risks of seizure and the estimated severity of postseizure sequelae (e.g., elevated ICP), as well as the risk of adverse events related to ASM use (e.g., sedation, fever, delirium), should be assessed on a case-by-case basis. Among patients with elevated ICP or significant space-occupying lesions who are at risk for brainstem herniation, it may be reasonable to opt for empiric prophylaxis because a seizure could lead to significant increases in ICP.
PICO 2: LEV Versus PHT/fPHT in Our Practice
If a prophylactic ASM is used, we generally prefer LEV to PHT/fPHT because of fewer drug interactions, less albumin binding (and hence less fluctuation in levels over time), and lower risk of fever and sedation. We typically use a loading dose of LEV followed by maintenance dosages of at least 750–1000 mg twice daily. The higher maintenance dosage is suggested because the terminal half-life of LEV is shorter in critically ill patients, leading to more rapid drug metabolism and systemic clearance, than in noncritically ill patients [35]. In a meta-analysis of 30 studies that evaluated LEV for seizure prophylaxis in patients with TBI, subarachnoid hemorrhage, intracerebral hemorrhage (ICH), and supratentorial neurosurgery, the authors found no significant differences in seizure events among those prescribed LEV prophylaxis versus no seizure medication [56]. However, 37% of studies used low LEV dosages (250–500 mg BID), with 500 mg BID (BID = twice daily) being the most commonly prescribed dosage [56]. This negative result may stem, in part, from the fact that many LEV patients may not have received a therapeutic dose. Indeed, a pharmacokinetic study of neurocritically ill patients suggested that LEV dosages of 500 mg BID have less than 25% probability of achieving therapeutic levels and that dosages as high as 3000–4000 mg/day may be required [35]. In a prospective study of adult neurocritically ill patients (including TBI, subarachnoid hemorrhage, intracerebral hemorrhage (IPH), and supratentorial neurosurgery), use of LEV dosed at 750–1000 mg BID was associated with a two-fold increased odds of achieving target drug levels and a 68% lower odds of clinical or electrographic seizure compared to low-dosage LEV (500 mg BID) [57]. Lower dosages of LEV (500 mg once or twice daily) may be considered in patients with creatinine clearance < 30 mL/min or in patients requiring renal replacement therapy. Redosing of LEV post dialysis may be necessary. Additionally, LEV may not be preferred in patients with depression, agitation, or other psychiatric features, as these are known LEV side effects. Alternate ASMs (e.g., lacosamide) may be considered in these contexts; however, evaluation of these medications was beyond the scope of this PICO.
PICO 3: Duration of ASM Use in Our Practice
If a prophylactic ASM is used, we prefer a limited time course of 7 days. We favor use of continuous EEG monitoring to risk stratify patients according to the 2HELPS2B score to determine whether prophylaxis should be continued for a longer duration. It may be reasonable to continue ASM beyond hospital discharge in patients with moderate- to high-risk 2HELPS2B scores ≥ 1 or in patients with high-risk EEG features [54, 55, 58]. Indeed, epileptiform activity, including sporadic epileptiform discharges, significantly increase the risk of post-TBI epilepsy [59, 60]. In patients with clinical or electrographic seizures despite ASM prophylaxis, we prefer to continue ASM beyond hospital discharge with a short-term outpatient follow-up (1–3 months) and repeat outpatient EEG to readdress duration of ASM use.
Conclusions
A summary of recommendations is listed in Table 4. Overall, the available data are limited by failure to consistently use continuous EEG monitoring for seizure detection, use of low-dose ASMs and/or lack of adequate testing for therapeutic drug levels [56], and inconsistent tracking of adverse events related to ASM use. The ideal duration of prophylactic ASM use, particularly in the context of epileptiform discharges or ictal-interictal continuum phenomenon, is unknown. There may be cost implications related to the decision to use ASM prophylaxis; however, this outcome was beyond the scope of this guideline. Well-designed randomized controlled trials evaluating modern ASMs are needed to better quantify the risks and benefits of prophylactic use in patients with TBI.
References
Mee H, Kolias AG, Chari A, Ercole A, Lecky F, Turner C, et al. Pharmacological management of post-traumatic seizures in adults: current practice patterns in the UK and the Republic of Ireland. Acta Neurochir. 2019;161(3):457–64.
Huijben JA, Volovici V, Cnossen MC, Haitsma IK, Stocchetti N, Maas AIR, et al. Variation in general supportive and preventive intensive care management of traumatic brain injury: a survey in 66 neurotrauma centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Crit Care. 2018;22(1):90.
Hickman ZL, Spielman LA, Barthelemy EJ, Choudhri TF, Engelman B, Giwa AO, et al. International survey of antiseizure medication use in patients with complicated mild traumatic brain injury: a New York Neurotrauma Consortium Study. World Neurosurg. 2022;6:66.
Naidech AM, Beaumont J, Muldoon K, Liotta EM, Maas MB, Potts MB, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46(9):1480–5.
Dikmen SS, Machamer JE, Winn HR, Anderson GD, Temkin NR. Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology. 2000;54(4):895–902.
Dikmen SS, Temkin NR, Miller B, Machamer J, Winn HR. Neurobehavioral effects of phenytoin prophylaxis of posttraumatic seizures. JAMA. 1991;265(10):1271–7.
GRADE. Available from: https://www.gradeworkinggroup.org/.
Schunemann HJB, J.; Guyatt, G.; Oxman, A. GRADE Handbook 2022. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
GRADE Guidelines Workshop + focus on implementation. Available from: https://gradeconf.org/2022-11/.
Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans. Washington, DC; 2019.
Higgins JPS, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor. Cochrane handbook for systematic reviews of interventions version 63: Cochrane; 2022.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Dewidar O, Lotfi T, Langendam MW, Parmelli E, Saz Parkinson Z, Solo K, et al. Good or best practice statements: proposal for the operationalisation and implementation of GRADE guidance. BMJ Evid Based Med. 2022;6:66.
Inglet S, Baldwin M, Quinones AH, Majercik S, Collingridge DS, MacDonald J. Seizure prophylaxis in patients with traumatic brain injury: a single-center study. Cureus. 2016;8(8): e753.
Khor D, Wu J, Hong Q, Benjamin E, Xiao S, Inaba K, et al. Early seizure prophylaxis in traumatic brain injuries revisited: a prospective observational study. World J Surg. 2018;42(6):1727–32.
Ohimor SF, Falcone RE. Phenytoin prophylaxis in posttraumatic head injury. J Pharm Technol. 1996;12:160–4.
Pruitt P, Naidech A, Van Ornam J, Borczuk P. Seizure frequency in patients with isolated subdural hematoma and preserved consciousness. Brain Inj. 2019;33(8):1059–63.
Rish BL, Caveness WF. Relation of prophylactic medication to the occurrence of early seizures following craniocerebral trauma. J Neurosurg. 1973;38(2):155–8.
Sundararajan K, Milne D, Edwards S, Chapman MJ, Shakib S. Anti-seizure prophylaxis in critically ill patients with traumatic brain injury in an intensive care unit. Anaesth Intensive Care. 2015;43(5):646–51.
Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323(8):497–502.
Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JR. Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. J Neurosurg. 1983;58(2):231–5.
Zangbar B, Khalil M, Gruessner A, Joseph B, Friese R, Kulvatunyou N, et al. Levetiracetam prophylaxis for post-traumatic brain injury seizures is ineffective: a propensity score analysis. World J Surg. 2016;40(11):2667–72.
Glaser AC, Kanter JH, Martinez-Camblor P, Taenzer A, Anderson MV, Buhl L, et al. The effect of antiseizure medication administration on mortality and early posttraumatic seizures in critically Ill older adults with traumatic brain injury. Neurocrit Care. 2022;37(2):538–46.
Pease M, Zaher M, Lopez AJ, Yu S, Egodage T, Semroc S, et al. Multicenter and prospective trial of anti-epileptics for early seizure prevention in mild traumatic brain injury with a positive computed tomography scan. Surg Neurol Int. 2022;13:241.
Patanwala AE, Kurita A, Truong E. Low-dose levetiracetam for seizure prophylaxis after traumatic brain injury. Brain Inj. 2016;30(2):156–8.
Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91(5):750–60.
Klein P, Herr D, Pearl PL, Natale J, Levine Z, Nogay C, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Arch Neurol. 2012;69(10):1290–5.
Wohns RN, Wyler AR. Prophylactic phenytoin in severe head injuries. J Neurosurg. 1979;51(4):507–9.
Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JR. Failure of prophylactically administered phenytoin to prevent late posttraumatic seizures. J Neurosurg. 1983;58(2):236–41.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–8.
Scozzafava J, Hussain MS, Brindley PG, Jacka MJ, Gross DW. The role of the standard 20 minute EEG recording in the comatose patient. J Clin Neurosci. 2010;17(1):64–8.
Spencer DD, Jacobi J, Juenke JM, Fleck JD, Kays MB. Steady-state pharmacokinetics of intravenous levetiracetam in neurocritical care patients. Pharmacotherapy. 2011;31(10):934–41.
Ohman K, Kram B, Schultheis J, Sigmon J, Kaleem S, Yang Z, et al. Evaluation of levetiracetam dosing strategies for seizure prophylaxis following traumatic brain injury. Neurocrit Care. 2022;6:66.
MedDRA. MedDRA History. Available from: https://www.meddra.org/about-meddra/history.
Van Norman GA. Data safety and monitoring boards should be required for both early- and late-phase clinical trials. JACC Basic Transl Sci. 2021;6(11):887–96.
Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–72.
Caballero GC, Hughes DW, Maxwell PR, Green K, Gamboa CD, Barthol CA. Retrospective analysis of levetiracetam compared to phenytoin for seizure prophylaxis in adults with traumatic brain injury. Hosp Pharm. 2013;48(9):757–61.
Gabriel WM, Rowe AS. Long-term comparison of GOS-E scores in patients treated with phenytoin or levetiracetam for posttraumatic seizure prophylaxis after traumatic brain injury. Ann Pharmacother. 2014;48(11):1440–4.
Inaba K, Menaker J, Branco BC, Gooch J, Okoye OT, Herrold J, et al. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J Trauma Acute Care Surg. 2013;74(3):766–71; discussion 71–3.
Jones KE, Puccio AM, Harshman KJ, Falcione B, Benedict N, Jankowitz BT, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25(4):E3.
Kancharla TRRV, Mohan R, Nagesh A. Comparing the efficacy of phenytoin, levetiracetam and sodium valproate in prevention of post-traumatic seizures in brain injury. Int Res J Pharm. 2019;10(4):66.
Khan SA, Bhatti SN, Khan AA, Khan Afridi EA, Muhammad G, Gul N, et al. Comparison of efficacy of phenytoin and levetiracetam for prevention of early post traumatic seizures. J Ayub Med Coll Abbottabad. 2016;28(3):455–60.
Kruer RM, Harris LH, Goodwin H, Kornbluth J, Thomas KP, Slater LA, et al. Changing trends in the use of seizure prophylaxis after traumatic brain injury: a shift from phenytoin to levetiracetam. J Crit Care. 2013;28(5):883e9–13.
Radic JA, Chou SH, Du R, Lee JW. Levetiracetam versus phenytoin: a comparison of efficacy of seizure prophylaxis and adverse event risk following acute or subacute subdural hematoma diagnosis. Neurocrit Care. 2014;21(2):228–37.
Younus SM, Basar S, Gauri SA, Khan AA, Imran M, Abubakar S, et al. Comparison of phenytoin versus levetiracetam in early seizure prophylaxis after traumatic brain injury, at a Tertiary Care Hospital in Karachi, Pakistan. Asian J Neurosurg. 2018;13(4):1096–100.
Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999;91(4):593–600.
Nguyen JV, Yaw T, Gratton H. Incidence of neurobehavioral side effects associated with levetiracetam compared to phenytoin in traumatic brain injury patients. Brain Inj. 2021;35(8):902–6.
Harris L, Hateley S, Tsang KT, Wilson M, Seemungal BM. Impact of anti-epileptic drug choice on discharge in acute traumatic brain injury patients. J Neurol. 2020;267(6):1774–9.
Kancharla TR. Comparing the efficacy of phenytoin, levetiracetam and sodium valproate in prevention of post-traumatic seizures in brain injury. Int Res J Pharm. 2019;10(4):156–60.
Kumar S, Bharti AK, Prasad RS, Kumari S, Singh A, Yadav G. Efficacy of phenytoin for 7 days versus 21 days as prophylactic anticonvulsant in traumatic brain injury patients—a comparative study. J Fam Med Prim Care. 2022;11(8):4805–10.
Struck AF, Ustun B, Ruiz AR, Lee JW, LaRoche SM, Hirsch LJ, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurol. 2017;74(12):1419–24.
Struck AF, Tabaeizadeh M, Schmitt SE, Ruiz AR, Swisher CB, Subramaniam T, et al. Assessment of the validity of the 2HELPS2B score for inpatient seizure risk prediction. JAMA Neurol. 2020;77(4):500–7.
Fang T, Valdes E, Frontera JA. Levetiracetam for seizure prophylaxis in neurocritical care: a systematic review and meta-analysis. Neurocrit Care. 2022;36(1):248–58.
Valdes E, Fang T, Boffa M, Frontera JA. Optimal dosing of levetiracetam for seizure prophylaxis in critically ill patients: a prospective observational study. Crit Care Med. 2023;6:66.
Jones FJS, Sanches PR, Smith JR, Zafar SF, Blacker D, Hsu J, et al. Seizure prophylaxis after spontaneous intracerebral hemorrhage. JAMA Neurol. 2021;78(9):1128–36.
Kong THJ, Abdul Azeem M, Naeem A, Allen S, Kim JA, Struck AF. Epileptiform activity predicts epileptogenesis in cerebral hemorrhage. Ann Clin Transl Neurol. 2022;9(9):1475–80.
Kim JA, Boyle EJ, Wu AC, Cole AJ, Staley KJ, Zafar S, et al. Epileptiform activity in traumatic brain injury predicts post-traumatic epilepsy. Ann Neurol. 2018;83(4):858–62.
Acknowledgements
We would like to acknowledge the work of our independent medical librarian, Thomasin Adams-Webber, MLS, MA, Information Specialist, Health Search Library and Information Services University Health Network. The American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Neurotrauma and Critical Care affirms the educational benefit of this document. The American Epilepsy Society (AES) Board of Directors approved Affirmation of Value for this guideline on October 24, 2023.
Funding
Support for Distiller software and medical librarian assistance was provided by the Neurocritical Care Society.
Author information
Authors and Affiliations
Contributions
Drafting, analysis, and revision of the manuscript: JAF, EJG; data analysis and revision of the manuscript: ELJ, DO, AR, ET, JU, SFZ, and SR; data analyses, statistical analyses, and data interpretation: YY.
Corresponding author
Ethics declarations
Conflicts of interest
The panel was required to be free of content-related commercial conflicts of interest for participation in this committee. Disclosures unrelated to the content of this article are listed as follows: JAF receives grant funding for COVID-related research from the NIH National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Aging (NIA), and National Heart, Lung, and Blood Institute. JAF has received consulting feeds from BrainCool, FirstKind Medical, Lumosa, and PER-Physician Education Resource. JAF has been compensated travel expenses by Alexion and receives publication royalties from Thieme. EJG receives grant funding for TBI-related research from the NIH/NINDS. EJG has received consulting fees from UCB and AAN. EJG has been compensated travel expenses by American Academy of Neurology (AAN). ELJ has received consulting fees from EpiWatch and receives grant funding from the NIH/NIA. DO receives funding as the editor for the Journal of Neuroscience Nursing and has received research funding from the NIH/NINDS and the Agnes Marshall Walker Foundation. ET has received consulting fees from Medical Insights Group. SFZ receives grant funding from the NIH/NINDS. SFZ is a clinical neurophysiologist for CortiCare. AR, JU, YY, and SR report no disclosures.
Ethical approval/informed consent
This study did not involve human subjects and was exempt from institutional review board review according to the New York University Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frontera, J.A., Gilmore, E.J., Johnson, E.L. et al. Guidelines for Seizure Prophylaxis in Adults Hospitalized with Moderate–Severe Traumatic Brain Injury: A Clinical Practice Guideline for Health Care Professionals from the Neurocritical Care Society. Neurocrit Care 40, 819–844 (2024). https://doi.org/10.1007/s12028-023-01907-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01907-x