Abstract
Background
Levetiracetam is commonly used for seizure prophylaxis in patients with intracerebral hemorrhage (ICH), traumatic brain injury (TBI), supratentorial neurosurgery, and spontaneous subarachnoid hemorrhage (SAH). However, its efficacy, optimal dosing, and the adverse events associated with levetiracetam prophylaxis remain unclear.
Methods
A systematic search of PubMed, Embase, and Cochrane central register of controlled trials (CENTRAL) database was conducted from January 1, 2000, to October 30, 2020, including articles addressing treatment with levetiracetam for seizure prophylaxis after SAH, ICH, TBI, and supratentorial neurosurgery. Non-English, pediatric (aged < 18 years), preclinical, reviews, case reports, and articles that included patients with a preexisting seizure condition or epilepsy were excluded. The coprimary meta-analyses examined first seizure events in (1) levetiracetam versus no antiseizure medication and (2) levetiracetam versus other antiseizure medications in all ICH, TBI, SAH, and supratentorial neurosurgery populations. Secondary meta-analyses evaluated the same comparator groups in individual disease populations. Risk of bias in non-randomised studies - of interventions (ROBINS-I) and risk-of-bias tool for randomized trials (RoB-2) tools were used to assess risk of bias.
Results
A total of 30 studies (n = 6 randomized trials, n = 9 prospective studies, and n = 15 retrospective studies), including 7609 patients (n = 4737 with TBI, n = 701 with SAH, n = 261 with ICH, and n = 1910 with neurosurgical diseases) were included in analyses. Twenty-seven of 30 (90%) studies demonstrated moderate to severe risk of bias, and 11 of 30 (37%) studies used low-dosage levetiracetam (250–500 mg twice daily). In the primary meta-analyses, there were no differences in seizure events for levetiracetam prophylaxis (n = 906) versus no antiseizure medication (n = 2728; odds ratio [OR] 0.79, 95% confidence interval [CI] 0.53–1.16, P = 0.23, fixed-effect, I2 = 26%, P = 0.23 for heterogeneity) or levetiracetam (n = 1950) versus other antiseizure prophylaxis (n = 2289; OR 0.84, 95% CI 0.55–1.28, P = 0.41, random-effects, I2 = 49%, P = 0.005 for heterogeneity). Only patients with supratentorial neurosurgical diseases benefited from levetiracetam compared with other antiseizure medications (median 0.70 seizure events per-patient-year with levetiracetam versus 2.20 seizure events per-patient-year for other antiseizure medications, OR 0.34, 95% CI 0.20–0.58, P < 0.001, fixed-effects, I2 = 39%, P = 0.13 for heterogeneity). There were no significant differences in meta-analyses of patients with ICH, SAH, or TBI. Adverse events of any severity were reported in a median of 8% of patients given levetiracetam compared with 21% of patients in comparator groups.
Conclusions
Based on the current moderately to seriously biased heterogeneous data, which frequently used low and possibly subtherapeutic doses of levetiracetam, our meta-analyses did not demonstrate significant reductions in seizure incidence and neither supports nor refutes the use of levetiracetam prophylaxis in TBI, SAH, or ICH. Levetiracetam may be preferred post supratentorial neurosurgery. More high-quality randomized trials of prophylactic levetiracetam are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ever since its Food and Drug Administration approval in 1999, levetiracetam has been a preferred agent for seizure prophylaxis among patients with intracerebral hemorrhage (ICH), traumatic brain injury (TBI), spontaneous subarachnoid hemorrhage (SAH), and supratentorial neurosurgery. Some meta-analyses report levetiracetam to be safer and as effective, or more effective, than other antiseizure medications for prophylaxis in patients with TBI [1, 2] and postsupratentorial neurosurgery [3, 4]. However, increasing evidence indicates that use of antiseizure medications may not be necessary unless there is a strong suspicion that a seizure may have already occurred [5, 6]. Heterogenous study methodologies and the use of low doses of levetiracetam, which may not have induced therapeutic levels, have created confusing and conflicting results regarding the efficacy of prophylactic levetiracetam. Given the limitations in the existing literature, we conducted a systematic review and meta-analysis to assess the use of levetiracetam compared with no antiseizure medication or with a different antiseizure medication for the prevention of first seizure across a varied population of patients with neurocritical illness. Secondary aims were to determine adverse event rates associated with levetiracetam, compared with other antiseizure medications.
Methods
Identification and Selection of Studies
All procedures used in this systematic review were consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A systematic search of PubMed, Embase, and Cochrane (CENTRAL) database was conducted from January 1, 2000 (levetiracetam was Food and Drug Administration-approved November 30, 1999), through October 30, 2020, to include publications on patients treated with levetiracetam for seizure prophylaxis after SAH, ICH, TBI, or supratentorial neurosurgery. The following search terms and their combinations were used: levetiracetam, Keppra, antiepileptic drug, serum concentration, seizure prophylaxis, prevention, intracranial tumor, TBI, stroke, intracranial hemorrhage, ICH, subdural hematoma, SAH, aneurysm, trauma, nontraumatic, craniotomy, craniectomy, and neurosurgery. The references of included studies were manually reviewed to identify any additional relevant studies that were potentially missed in the database search.
We included studies that reported seizure events (clinical and/or electrographic) among adults (aged ≥ 18 years) with spontaneous SAH, ICH, TBI, or supratentorial neurosurgery who received levetiracetam prophylaxis. We excluded studies of patients with preexisting seizure disorders, studies of levetiracetam in the management of epilepsy, studies that combined levetiracetam with other antiseizure medications, case reports and series with < 10 cases, studies that did not report a comparator group, non-English studies, editorials/commentaries, duplicate publications, nonpeer reviewed publications/gray literature, meta-analyses, review articles, pediatric studies (age < 18), and animal or preclinical studies.
Outcomes
The following PICO questions were addressed: (1) does prophylactic levetiracetam after SAH, ICH, TBI, or supratentorial neurosurgery reduce the occurrence of first seizure compared with no antiseizure medications, and (2) does prophylactic levetiracetam after SAH, ICH, TBI, or supratentorial neurosurgery reduce the occurrence of first seizure compared with other antiseizure medications. The primary outcome was the proportion of patients with seizure events and seizure events per-patient-year (EPPY) of follow-up. The number of seizure EPPYs was calculated for all studies using the duration of follow-up (in years) multiplied by the number of patients in each study. EPPY were calculated to compare seizure rates across studies with variable follow-up periods. The secondary outcome was the proportion of patients experiencing adverse antiseizure medication effects.
Data Collection and Synthesis
Two review authors (TF and EV) independently reviewed abstracts, full text articles, and references for study inclusion and carried out data extraction and risk of bias assessments. Each article was abstracted for the disease state for which levetiracetam was indicated, the dose of levetiracetam that was administered, levetiracetam levels (if reported), adverse event occurrences, and the percentage of patients with new seizure events. When multiple sources reported data from the same population, the original report with the most complete data was used.
Risk of Bias Assessment
One reviewer independently assessed the quality of included studies in terms of risk of bias. If randomized controlled trials (RCTs) were identified, revised Cochrane risk of bias 2 tool (Rob 2) was used, which grades the risk of selection, performance, attrition, detection, and reporting bias. Risk of bias of the nonrandomized studies was evaluated with the risk of bias in non-randomised studies - of interventions (ROBINS-I) tool with adaptations as appropriate. The quality assessment was reviewed by a second author. Using this tool, each of the items were rated low, moderate, serious, or critical risk of bias [7, 8].
Statistical Analyses
We conducted two coprimary meta-analyses: levetiracetam versus no antiseizure medication or placebo and levetiracetam versus other antiseizure medications across all disease populations (TBI, SAH, ICH, and supratentorial neurosurgery). In secondary analyses, we performed the same meta-analyses in each specific disease population (e.g., TBI, SAH, ICH, and supratentorial neurosurgery). Heterogeneity between studies was examined using Higgins’s I2 statistic and a χ2 test (I2 value ≥ 50% or P value < 0.1 is considered indicative of possible heterogeneity) [9]. Data across the included studies were pooled, and meta-analysis methods were selected based on the heterogeneity and the number of trials included; fixed-effects models were used when the parameter estimates were homogeneous and random-effects models when heterogeneity was detected [10]. Studies were weighted by the Mantel–Haenszel methods for the reported outcome. Publication bias (i.e., assessment of bias across studies) was graphically evaluated with funnel plots. Sensitivity analyses were performed by excluding studies with serious risk of bias (Supplemental Tables 1 and 2).
Seizure EPPY were compared between groups using Mann–Whitney U-tests. Secondary outcomes of adverse event rate were also analyzed, but because adverse event definitions varied across studies, a meta-analysis of this outcome was not conducted. The Review Manager software program (RevMan 5.4; the Nordic Cochrane Center, Copenhagen, Denmark), provided by the Cochrane Collaboration, was used for meta-analyses and graphical representation of the pooled data. Other analyses were performed using IBM SPSS Statistics for Mac version 26 (IBM Corp, Armonk, NY).
Results
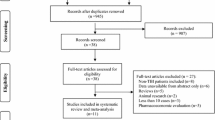
The initial database search identified 534 articles (Fig. 1). After the exclusion of duplicated studies, pediatric studies, review articles, preclinical studies, non-English articles, case reports and studies without a comparator group, 30 studies (n = 7609 patients) remained. Among these 30 articles (Supplemental Table 3), there were 6 RCTs, 9 prospective studies, and 15 retrospective studies. The number of articles per study population were: TBI, n = 13 studies (n = 4737 patients), SAH, n = 4 studies (n = 701 patients), ICH, n = 3 studies (n = 261 patients) and supratentorial neurosurgery, n = 10 studies (n = 1910 patients). Seizure events were coded for clinical events only in 17 (57%) studies and for clinical or electrographic seizure events in 13 (43%) studies. The median duration of follow-up across all studies was 7 days (range 2–730 days).
The risk of bias of included studies is summarized in Supplemental Tables 1 and 2. There were only three studies rated as low risk of bias, whereas 11 studies were rated as serious risk of bias, and 16 studies were rated as moderate risk of bias. We did not detect substantial publication bias as indicated by funnel plots (Supplemental Figs. 1 and 2) for the comparison of levetiracetam versus placebo or no antiseizure mediation or for levetiracetam versus another antiseizure mediation.
Levetiracetam Dosing
Levetiracetam dosing regimen was reported in 23 of 30 (77%) trials [2, 5, 6, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] and 500 mg twice daily was the most common dosage used in 11 of 23 (48%) studies [15,16,17,18,19,20,21,22,23,24,25]. A range of doses (250–1500 mg twice daily) was allowed in 6 of 23 (26%) of studies [4, 12, 14, 31,32,33] and 6 of 23 (26%) of studies [2, 5, 11, 28,29,30] required weight-based dosing or doses ≥ 1000 mg twice daily. Three studies evaluated serum levetiracetam levels for therapeutic range dosing [5, 11, 19]. However, one checked levels following a loading dose, rather than at steady state [11, 19], and another did not report the timing of levels nor the dosing associated with these levels [5]. The third study, which utilized 55 mg/kg/day dosing of levetiracetam, documented mean trough levels in the therapeutic range (19.6–26.7 μg/mL) on days 2–30 post initiation [11].
Seizure Events
Overall, across all disease populations and study types the median seizure EPPY for levetiracetam was 1.1 EPPY (interquartile range [IQR] 0.4–5.3), compared with a median 0.59 seizure EPPY (IQR 0.1–1.8) among patients who received either no antiseizure medication or placebo (P = 0.269), and a median 2.5 seizure EPPY (IQR 0.8–9.4) among those who received phenytoin or valproic acid prophylaxis (P = 0.180). Alternately, comparing the raw percentages of seizures in each group, a median of 4% of patients who received levetiracetam had seizures, compared with 4% who received either no antiseizure medication or placebo (P = 0.792) and 8% who received phenytoin or valproic acid (P = 0.155). However, the follow-up time varied for each of these groups, making raw percentages less informative than EPPY. Seizure EPPY varied across disease populations. The median seizure EPPY for levetiracetam was 1.9 (IQR 0.9–7.4) in patients with TBI, 2.6 (IQR 0.2–7.1) in patients with SAH, 9.3 (IQR range 5.5–9.3) in patients with ICH, and 0.4 (IQR 0.1–0.9) in patients who had supratentorial neurosurgery (P = 0.032). Seizure EPPY also differed substantially depending on whether clinical seizures alone were recorded (median seizure EPPY for levetiracetam 0.8, IQR 0.2–1.5) or if electrographic seizures were also included in seizure outcomes (median seizure EPPY for 5.5, IQR 1.6–13.0, P = 0.019). In prospective or randomized trials, the median seizure EPPY for levetiracetam prophylaxis was 1.6 (IQR 0.1–7.4), while in retrospective studies the median seizure EPPY was 1.1 (IQR 0.6–3.9, P = 1.00).
Meta-analyses of Levetiracetam Versus No Antiseizure Medication or Placebo
In the coprimary meta-analysis of levetiracetam versus no antiseizure medication or placebo across all disease categories, 8 studies (n = 1 RCT, n = 4 prospective, and n = 3 retrospective) were included (n = 3634 patients) [5, 11, 12, 20, 22, 26, 34, 35]. Across all disease categories (TBI, SAH, ICH, and supratentorial neurosurgery) there was no significant benefit for levetiracetam versus no antiseizure medication for prevention of first seizure. Overall, 41 of 906 (4.5%) had seizures in the levetiracetam group with a median of 0.63 seizure EPPY versus 105 of 2728 (3.8%) had seizures in the no antiseizure medication group with a median 0.59 seizure EPPY (odds ratio [OR] 0.79, 95% confidence interval [CI] 0.53–1.16, P = 0.23 based on fixed-effects meta-analysis). There was low heterogeneity across trials (I2 = 26%, P = 0.23 for heterogeneity, Fig. 2a, Supplemental Fig. 1).
Primary meta-analyses comparing levetiracetam to placebo or no antiseizure medication (n = 8 studies and n = 3634 patients; panel (a) and levetiracetam compared with other antiseizure medications (n = 23 studies and n = 4239 patients; panel (b) in patients with traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage, and supratentorial neurosurgery. AED antiepileptic drug; MH Mantel–Haenszel test; CI confidence interval; df degree of freedom
In the supratentorial neurosurgery group, n = 4 studies (n = 2 prospective, n = 2 retrospective, and n = 777 patients) [5, 12, 26, 36] compared levetiracetam to no antiseizure medication. In the levetiracetam group 24 of 315 (7.6%) had seizures with a median 0.12 seizure EPPY versus 66 of 462 (14.2%) patients with seizure in the no antiseizure medication group with a median 0.11 seizure EPPY (OR 0.52, 95% CI 0.24–1.13, P = 0.10 based on a random-effects meta-analysis, I2 = 48%, P = 0.13 for heterogeneity, Fig. 3a).
Meta-analyses in the supratentorial neurosurgery subgroup comparing levetiracetam to placebo or no antiseizure medication (n = 4 studies, and n = 777 patients; panel (a) and levetiracetam compared with other antiseizure medications (n = 7 studies and n = 1306 patients; panel (b). AED antiepileptic drug; MH Mantel–Haenszel test; CI confidence interval; df degree of freedom
In subgroup analysis of patients with TBI, four studies (n = 1 RCT, n = 1 prospective, n = 2 retrospective) [11, 20, 34, 35] compared levetiracetam versus no antiseizure medication in n = 2888 patients. 22 of 619 (3.6%) in the levetiracetam group had seizures with a median 1.7 seizure EPPY versus 60 of 2269 (2.2%) patients had seizures in the control group with a median 1.5 seizure EPPY (OR 0.93, 95% CI 0.54–1.62, P = 0.80 based on a fixed-effects meta-analysis, I2 = 0%, P = 0.65 for heterogeneity, Fig. 4a).
Meta-analyses in the traumatic brain injury subgroup comparing levetiracetam to placebo or no antiseizure medication (n = 4 studies, and n = 2888 patients; panel (a) and levetiracetam compared with other antiseizure medications (n = 10 studies and n = 2057 patients; panel (b), and meta-analysis of levetiracetam versus other antiseizure medications in patients with spontaneous subarachnoid hemorrhage (n = 4 studies and n = 673 patients; panel (c), and intracerebral hemorrhage (n = 2 studies and n = 119 patients; panel (d). AED antiepileptic drug; MH Mantel–Haenszel test; CI confidence interval; df degree of freedom
There were no studies in the SAH population and only one study in the ICH population comparing levetiracetam versus no antiseizure medication or placebo [22]. Hence, meta-analyses were not performed in these subgroups.
Meta-analyses of Levetiracetam Versus Other Antiseizure Medications
In the coprimary meta-analysis of levetiracetam versus other antiseizure medications across all disease categories, n = 23 studies (n = 5 RCT, n = 5 prospective, and n = 13 retrospective) with n = 4239 patients were analyzed. Of these, n = 21 studies [2, 13,14,15,16,17,18,19, 21, 23,24,25, 27, 29, 30, 35,36,37,38,39,40] evaluated phenytoin as the comparator and n = 2 evaluated valproic acid [6, 28]. Seizure events occurred in 135 of 1950 (6.9%) or a median of 1.45 seizure EPPY in the levetiracetam group versus 184 of 2289 (8.0%) or a median of 2.45 seizure EPPY in the comparator antiseizure medication group, (OR 0.84, 95% CI 0.55–1.28, P = 0.41 based on random-effects meta-analysis, I2 = 49%, P = 0.005 for heterogeneity, Fig. 2b and Supplemental Fig. 2).
Among patients who had neurosurgery, n = 6 studies (n = 2 RCT, n = 4 retrospective) comparing levetiracetam to phenytoin and n = 1 retrospective study comparing levetiracetam to valproic acid were analyzed (n = 1306 patients) [6, 18, 19, 23, 36, 37, 39]. Seizure events occurred in 17 of 452 (3.8%) in the levetiracetam group with a median 0.70 seizure EPPY compared with 82 of 854 (9.6%) with a median 2.20 seizure EPPY in the comparator group (OR 0.34, 95% CI 0.20–0.58, P < 0.001 based on fixed-effects meta-analysis, I2 = 39%, P = 0.13 for heterogeneity, Fig. 3b).
In subgroup analysis of patients with TBI there were n = 10 studies (n = 3 RCT, n = 3 prospective, n = 4 retrospective) comparing levetiracetam to phenytoin including n = 2057 patients [2, 13, 14, 16, 21, 24, 29, 30, 35, 40]. Seizure events occurred in 90 of 1034 (8.7%) patients with a median of 3.7 seizure EPPY in the levetiracetam group versus 96 of 1023 (9.4%) with a median 5.5 seizure EPPY in the phenytoin group, (OR 1.02, 95% CI 0.72–1.45, P = 0.89 based on fixed-effects meta-analysis, I2 = 0%, P = 0.70 for heterogeneity, Fig. 4b). In a sensitivity analysis, we included only studies that evaluated the dual outcome of clinical and electrographic seizures in patients with TBI who received levetiracetam or phenytoin (n = 7 studies, n = 771 patients) [13, 14, 16, 21, 24, 30, 40]. Overall, mores seizures were detected, but there were no significant differences between levetiracetam compared with other antiseizure medications. Seizure occurred in 73 of 343 (21.2%) patients who were given levetiracetam, with a median of 7.3 seizure EPPY, and 81 of 428 (18.9%) patients who were given phenytoin with a median of 9.0 seizure EPPY (OR 1.05, 95% CI 0.70–1.56, P = 0.23 based on fixed-effects meta-analysis, I2 = 0%, P = 0.69 for heterogeneity).
In patients with SAH, n = 3 retrospective studies with phenytoin and n = 1 prospective study with valproic acid as the comparator (total n = 673 patients) were analyzed [17, 25, 28, 38]. Seizures occurred in 25 of 308 (8.1%) with a median of 2.6 seizure EPPY in the levetiracetam group versus 22 of 365 (6.0%) patients with seizures and a median of 2.2 seizure EPPY in the comparator group, (OR 0.91, 95% CI 0.21–3.85, P = 0.90 based on random-effects meta-analysis, I2 = 67%, P = 0.05 for heterogeneity, Fig. 4c).
In patients with ICH, n = 2 studies (n = 1 prospective, n = 1 retrospective) using phenytoin as the comparator, n = 119 patients) were included in analysis [15, 27]. Seizures occurred in 3 of 72 (4.2%) of the levetiracetam group with a median 13.0 seizure EPPY versus 6 of 47 (12.8%) seizure events with a median 9.5 EPPY in the phenytoin group, (OR 0.45, 95% CI 0.03–8.09, P = 0.59 based on random-effects meta-analysis, I2 = 64%, P = 0.09 for heterogeneity, Fig. 4d).
Sensitivity Analysis Excluding Studies with Serious Risk of Bias
We identified 11 studies with a “serious” risk of bias (Supplemental Tables 1 and 2) and conducted sensitivity meta-analyses removing these studies. For the coprimary end point of levetiracetam vs. no antiseizure medication or placebo across all disease populations, the original meta-analysis generated the following results: OR 0.79 (95% CI 0.52–1.16), P = 0.23, I2 = 26%. After excluding one study with a serious risk of bias [20], the meta-analysis yielded similar results (OR 0.72, 95% CI 0.47–1.10, P = 0.13, I2 = 28%; Supplemental Fig. 3). For the other coprimary end point of levetiracetam vs. other antiseizure medication across all disease populations, the original meta-analysis generated the following results: OR 0.84, 95% CI 0.55–1.28, P = 0.41, I2 = 49%. Again, after excluding those studies with serious risk of bias [15, 16, 24, 27, 28, 38, 39, 41, 42], the meta-analysis yielded similar results (OR 0.81, 95% CI 0.60–1.09, P = 0.17, I2 = 45%; Supplemental Fig. 4). Finally, we performed a sensitivity analysis excluding studies with a serious risk of bias evaluating levetiracetam versus other antiseizure medications among patients with supratentorial neurosurgery. The original meta-analysis found a significant benefit for levetiracetam compared with other medications (OR 0.34, 95% CI 0.20–0.58, P < 0.001, I2 = 39%). After excluding one study with a serious risk of bias [39], the meta-analysis showed similar results (OR 0.33, 95% CI 0.19–0.57, P < 0.001, I2 = 49%; Supplemental Fig. 5).
Adverse Events
Adverse events related to antiseizure medications were reported 10 of 30 (33%) studies (Table 1). Adverse events of any severity were reported in a median of 8% (range 0–58%) of patients given levetiracetam compared with 21% (range 5–38%) of patients in the comparator groups.
Discussion
In this study, we did not detect a benefit for the prophylactic use of levetiracetam compared with no antiseizure medication or compared with other antiseizure medications in pooled cohorts of patients with TBI, ICH, SAH, or supratentorial neurosurgery. Similarly, there were no significant differences in subgroup meta-analyses for TBI, ICH, or SAH. Conversely, levetiracetam significantly reduced seizure events among patients who underwent supratentorial neurosurgery compared with patients who received phenytoin or valproic acid. Compared with no antiseizure medication, however, there was no significant difference in seizure events in the supratentorial neurosurgery subgroup. Although prior meta-analyses have evaluated a variety of different prophylactic antiseizure medications (some in heterogenous mixed populations of TBI, supratentorial neurosurgery, SAH, ICH [1, 43]) ours is the first, to our knowledge, to comprehensively compare levetiracetam versus no antiseizure medication and versus other antiseizure medications in individual populations of patients with TBI, ICH, SAH, and supratentorial neurosurgery, as well as in an aggregate population of patients with neurocritical illness.
Overall, most studies had a moderate to serious risk of bias and we detected substantial heterogeneity across trials. Even among the supratentorial neurosurgery group that appeared to benefit from prophylactic levetiracetam, patients had pathologies that ranged from brain tumors to intracranial hemorrhage, which may impact subsequent seizure risk. We identified variability in outcome measures of seizure, wherein some studies included only clinical seizures and others also included electrographic seizures. Indeed, in a sensitivity analysis of TBI studies that included only studies with both electrographic and clinical seizure end points, we found higher rates of seizures in both levetiracetam and other antiseizure medication groups, compared with our original meta-analysis that also included studies with only clinical seizure end points (7.3 EPPY for levetiracetam and 9.0 EPPY for other antiseizure medications for studies with electrographic and clinical end points versus 3.7 EPPY for levetiracetam and 5.5 EPPY for other antiseizure medications for studies with clinical end points). These differences are explained by subclinical and subtle seizure detection afforded by electroencephalography monitoring. Future studies should include electrographic seizure monitoring as an outcome measure when testing the efficacy of antiseizure medications in patients with critical illness to increase the likelihood of capturing subtle or nonconvulsive seizures.
Although most studies measured seizure events more than 7 days (21 of 30 or 70% of included studies), the follow-up time varied not only between studies, but within comparator groups of the same study. To account for variable duration of follow-up, we calculated seizure EPPY in each study. Overall, the seizure EPPY for levetiracetam was lower than that of other antiseizure medications (median 1.1 EPPY versus 2.5 EPPY, P = 0.180), but trended higher than no antiseizure medication at all (1.1 EPPY versus 0.59 EPPY, P = 0.269). This may be explained, in part, by treatment bias since many nonrandomized trials were included. Patients most at risk for seizure may have been more likely to receive antiseizure prophylaxis. Additionally, recall bias for seizure events may occur in retrospective studies leading to underestimation of seizures in control groups. In the one RCT that compared levetiracetam versus placebo, the seizure rate for levetiracetam was half that of placebo (EPPY 0.05 versus 0.1011). This further underscores the biases contributed by nonrandomized studies.
Another major limitation among included studies was the use of low doses of levetiracetam, which may not generate therapeutic levels in average size adults. Although weight-based dosing of levetiracetam is not required in adults, for children the recommended maintenance dosage is 50–60 mg/kg/day in divided doses (equivalent to 1750–2100 mg BID for a 70 kg adult). By contrast, for the average 70 kg adult, 500 mg twice daily would amount to 14 mg/kg/day. In one retrospective study, a median levetiracetam dosage of 20 mg/kg/day (equivalent to ~ 750 mg twice daily for a 70 kg person) generated levetiracetam levels in therapeutic range (6–20 μg/mL) [44]. However, pharmacokinetic studies have demonstrated that, among patients with neurocritical illness, systemic clearance of levetiracetam is faster and the terminal elimination half-life is shorter [45]. In a study of patients receiving prophylactic levetiracetam for SAH, subdural hematoma or TBI, the highest probability of achieving target trough concentrations (6–20 μg/mL) was with doses of 1000 mg every 8 h or 1500–2000 mg every 12 h [45]. Only one study included in our analysis reported steady state levetiracetam troughs. This study [11] used a dosage of 55 mg/kg/day in divided doses (equivalent to 1925 mg BID for a 70 kg person) based on maximally effective antiepileptic doses in animal models [46]. This study documented therapeutic levels (19.6–26.7 μg/mL) in all patients between 2 and 30 days after levetiracetam initiation. While there is not robust evidence demonstrating a strong correlation between levetiracetam trough levels and seizure events (hence the wide therapeutic range of 6–20 μg/mL), it seems reasonable to tailor medication regimens to individual patient size and weight to maintain levels at least within the lower range of target trough levels.
Seizure prophylaxis is currently recommended by a variety of guidelines, such as the Fourth Edition of “Guidelines for the Management of Severe Traumatic Brain Injury” [47] and Quebec Institut National d’Excellence for the management of TBI. SAH guidelines suggest that prophylactic anticonvulsants other than phenytoin can be considered for a short period of time (3–7 days), but this was a weak recommendation based on very low quality evidence [48]. Conversely, neurosurgical guidelines recommend against routine use of antiseizure medications post craniotomy in patients who are seizure-free with brain metastases [49]. A recent Cochrane Review found limited, low certainty data that did not confirm efficacy of prophylactic antiseizure medications for the prevention of early or late seizures post-craniotomy [50]. Similarly, ICH guidelines recommend against administration of antiseizure medication [51]. However, many of these guidelines are based on data generated using phenytoin or valproic acid as prophylactic antiseizure medications. Levetiracetam’s favorable side effect profile might make it an attractive alternative agent. Indeed, we identified fewer adverse medication side effects with levetiracetam compared with other antiseizure medications (7% vs. 20%).
There were limitations to our study. First, we combined early (within 7 days) and late seizures (> 7 days) as an outcome variable because there were few studies with follow-up periods exceeding 7 days and most studies that had longer follow-up (typically for the purposes of long-term functional and cognitive assessments), did not report whether seizure events occurred early or late after the index injury. Furthermore, since many studies were retrospective, follow-up durations varied between comparator groups within same study, in some instances. We utilized EPPY to adjust for variable outcome periods, but it is important to note that the risk of early and late seizure is not constant over time, hence the EPPY is only an estimation of event incidence. Studies with longer follow-up periods would likely provide more accurate event rate estimations. Second, due to nonstandardized reporting of adverse events across a variety of studies, a meta-analysis for the adverse events and drug-drug reactions was not conducted. Third, individual patient-level meta-analysis would be more robust but challenging given that some of the included studies date back three decades or more.
Conclusions
Based on the current moderately to seriously biased heterogeneous data, our meta-analysis did not demonstrate significant reductions in incident seizure and neither supports nor refutes the use of levetiracetam prophylaxis in TBI, SAH, ICH, or supratentorial neurosurgery. However, our data suggest that levetiracetam may be superior to other seizure medications following supratentorial neurosurgery. There are major limitations in the existing literature, including the use of low-dosage levetiracetam, which may not have provided therapeutic levels of antiseizure protection, variable durations of follow-up with limited data on functional and cognitive outcomes, and variable measures of seizure events (clinical, electrographic or both), all of which substantially weaken meta-analyses. More robust randomized trials using documented therapeutic levetiracetam dosing strategies and examining both clinical and electrographic seizure events are warranted.
Source of support
This study was not funded.
References
Chaari A, Mohamed AS, Abdelhakim K, Kauts V, Casey WF. Levetiracetam versus phenytoin for seizure prophylaxis in brain injured patients: a systematic review and meta-analysis. Int J Clin Pharm. 2017;39(5):998–1003.
Khan SA, Bhatti SN, Khan AA, et al. Comparison of efficacy of phenytoin and levetiracetam for prevention of early post traumatic seizures. J Ayub Med Coll Abbottabad. 2016;28(3):455–60.
Pourzitaki C, Tsaousi G, Apostolidou E, Karakoulas K, Kouvelas D, Amaniti E. Efficacy and safety of prophylactic levetiracetam in supratentorial brain tumour surgery: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(1):315–25.
Lee CH, Koo HW, Han SR, Choi CY, Sohn MJ, Lee CH. Phenytoin versus levetiracetam as prophylaxis for postcraniotomy seizure in patients with no history of seizures: systematic review and meta-analysis. J Neurosurg. 2019;130(6):1–8.
Garbossa D, Panciani PP, Angeleri R, et al. A retrospective two-center study of antiepileptic prophylaxis in patients with surgically treated high-grade gliomas. Neurol India. 2013;61(2):131–7.
Lee YJ, Kim T, Bae SH, et al. Levetiracetam compared with valproic acid for the prevention of postoperative seizures after supratentorial tumor surgery: a retrospective chart review. CNS Drugs. 2013;27(9):753.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. 2016;355:i4919.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2019;366:l4898.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Klein P, Herr D, Pearl PL, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Arch Neurol. 2012;69(10):1290–5.
Liang S, Ding P, Zhang S, Zhang J, Zhang J, Wu Y. Prophylactic levetiracetam for seizure control after cranioplasty: a multicenter prospective controlled study. World neurosurgery. 2017;102:284–92.
Younus SM, Basar S, Gauri SA, et al. Comparison of phenytoin versus levetiracetam in early seizure prophylaxis after traumatic brain injury, at a tertiary care hospital in Karachi. Pak Asian J Neurosurg. 2018;13(4):1096–100.
Radic JA, Chou SH, Du R, Lee JW. Levetiracetam versus phenytoin: a comparison of efficacy of seizure prophylaxis and adverse event risk following acute or subacute subdural hematoma diagnosis. Neurocrit Care. 2014;21(2):228–37.
Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40(12):3810–5.
Jones KE, Puccio AM, Harshman KJ, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25(4):E3.
Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after subarachnoid hemorrhage. World Neurosurg. 2011;75(2):269–74.
Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71(9):665.
Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry. 2015;86(10):1158–62.
Khor D, Wu J, Hong Q, et al. Early seizure prophylaxis in traumatic brain injuries revisited: a prospective observational study. World J Surg. 2018;42(6):1727–32.
Gabriel WM, Rowe AS. Long-term comparison of GOS-E scores in patients treated with phenytoin or levetiracetam for posttraumatic seizure prophylaxis after traumatic brain injury. Ann Pharmacother. 2014;48(11):1440–4.
Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med. 2018;46(9):1480–5.
Fuller KL, Wang YY, Cook MJ, Murphy MA, D’Souza WJ. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia. 2013;54(1):45–57.
Caballero GC, Hughes DW, Maxwell PR, Green K, Gamboa CD, Barthol CA. Retrospective analysis of levetiracetam compared to phenytoin for seizure prophylaxis in adults with traumatic brain injury. Hosp Pharm. 2013;48(9):757–61.
Kodankandath TV, Farooq S, Wazni W, et al. Seizure prophylaxis in the immediate post-hemorrhagic period in patients with aneurysmal subarachnoid hemorrhage. J Vasc Interv Neurol. 2017;9(6):1–4.
Kamenova M, Stein M, Ram Z, et al. Prophylactic antiepileptic treatment with levetiracetam for patients undergoing supratentorial brain tumor surgery: a two-center matched cohort study. Neurosurg Rev. 2020;43(2):709–18.
Taylor S, Heinrichs RJ, Janzen JM, Ehtisham A. Levetiracetam is associated with improved cognitive outcome for patients with intracranial hemorrhage. Neurocrit Care. 2011;15(1):80–4.
Mink S, Muroi C, Seule M, Bjeljac M, Keller E. Levetiracetam compared to valproic acid: plasma concentration levels, adverse effects and interactions in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2011;113(8):644–8.
Inaba K, Menaker J, Branco BC, et al. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. J Trauma Acute Care Surg 2013;74(3):766–71 (discussion 71–3).
Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–72.
Zachenhofer I, Donat M, Oberndorfer S, Roessler K. Perioperative levetiracetam for prevention of seizures in supratentorial brain tumor surgery. J Neurooncol. 2011;101(1):101–6.
Gokhale S, Khan SA, Agrawal A, Friedman AH, McDonagh DL. Levetiracetam seizure prophylaxis in craniotomy patients at high risk for postoperative seizures. Asian J Neurosurg. 2013;8(4):169–73.
Yang Y, Zheng F, Xu X, Wang X. Levetiracetam versus phenytoin for seizure prophylaxis following traumatic brain injury: a systematic review and meta-analysis. CNS Drugs. 2016;30(8):677–88.
Candy N, Tsimiklis C, Poonnoose S, Trivedi R. The use of antiepileptic medication in early post traumatic seizure prophylaxis at a single institution. J Clin Neurosci. 2019;69:198–205.
Zangbar B, Khalil M, Gruessner A, et al. Levetiracetam prophylaxis for post-traumatic brain injury seizures is ineffective: a propensity score analysis. World J Surg. 2016;40(11):2667–72.
Kutteruf R, Yang JT, Hecker JG, Kinney GA, Furman MA, Sharma D. Incidence and risk factors for intraoperative seizures during elective craniotomy. J Neurosurg Anesthesiol. 2019;31(2):234–40.
Höhne J, Schebesch KM, Ott C, Brawanski A, Lange M. The risk of hypotension and seizures in patients receiving prophylactic anti-epileptic drugs for supratentorial craniotomy. J Neurosurg Sci. 2018;62(4):418–22.
Karamchandani RR, Fletcher JJ, Pandey AS, Rajajee V. Incidence of delayed seizures, delayed cerebral ischemia and poor outcome with the use of levetiracetam versus phenytoin after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21(9):1507–13.
Kern K, Schebesch KM, Schlaier J, et al. Levetiracetam compared to phenytoin for the prevention of postoperative seizures after craniotomy for intracranial tumours in patients without epilepsy. J Clin Neurosci. 2012;19(1):99–100.
Kruer RM, Harris LH, Goodwin H, et al. Changing trends in the use of seizure prophylaxis after traumatic brain injury: a shift from phenytoin to levetiracetam. J Crit Care. 2013;28(5):883.e9-13.
Kruer RM, Harris LH, Goodwin H, et al. Changing trends in the use of seizure prophylaxis after traumatic brain injury: a shift from phenytoin to levetiracetam. J Crit Care. 2013;28(5):883.
Murphy-Human T, Welch E, Zipfel G, Diringer MN, Dhar R. Comparison of short-duration levetiracetam with extended-course phenytoin for seizure prophylaxis after subarachnoid hemorrhage. World Neurosurg. 2011;75(2):269.
Zafar SN, Khan AA, Ghauri AA, Shamim MS. Phenytoin versus Leviteracetam for seizure prophylaxis after brain injury—a meta analysis. BMC Neurol. 2012;12:30.
Cotta MO, Abdul-Aziz MH, Frey OR, Sime FB, Roberts JA, Roehr AC. What are the predictors for achieving therapeutic levetiracetam serum concentrations in adult neurological patients? Ther Drug Monit. 2020;42(4):626–30.
Spencer DD, Jacobi J, Juenke JM, Fleck JD, Kays MB. Steady-state pharmacokinetics of intravenous levetiracetam in neurocritical care patients. Pharmacotherapy. 2011;31(10):934–41.
Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62(4):668–700.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Fourth Edn Neurosurg. 2017;80(1):6–15.
Diringer MN, Bleck TP, Claude Hemphill J, III, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care 2011;15(2):211–40.
Chen CC, Rennert RC, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of prophylactic anticonvulsants in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84(3):E195–7.
Greenhalgh J, Weston J, Dundar Y, Nevitt SJ, Marson AG. Antiepileptic drugs as prophylaxis for postcraniotomy seizures. Cochr Database System Rev 2020;4(4):Cd007286.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the american Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60.
Author information
Authors and Affiliations
Contributions
TF performed the literature search, performed the statistical meta-analysis, risk of bias assessments, and drafted the article. EV performed the literature search, abstracted data from included studies, and critically revised the article. JAF designed the study, oversaw data analysis, and critically revised the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval/Informed Consent
This study was exempt from institutional review board review because it is nonhuman research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, T., Valdes, E. & Frontera, J.A. Levetiracetam for Seizure Prophylaxis in Neurocritical Care: A Systematic Review and Meta-analysis. Neurocrit Care 36, 248–258 (2022). https://doi.org/10.1007/s12028-021-01296-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01296-z