Abstract
Selenoproteins and selenium (Se) play important roles in the immune system. Selenoprotein expression in the immune system of mammals is sensitive to dietary Se levels; however, little is known about the expression of selenoproteins and their immune functions in the chicken thymus. We assessed selenoprotein gene expression and cytokine content in the chicken thymus in this study. The animals were randomly assigned to two groups as follows: the Se-deficient group (L group) was fed a diet containing 0.033 mg Se/Kg, and the control group was fed the same basal diet supplemented with Se at 0.15 mg/kg (sodium selenite). Real-time qPCR was used to investigate the expression level of selenoproteins on days 15, 25, 35, 45, and 55, and ELISA was used to evaluate the cytokine content on days 15, 35, and 55. The messenger RNA (mRNA) levels of Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, GPx1, GPx2, GPx3, Gpx4, Sepp1, Selo, Sep15, Sepx1, Sels, Seli, Selu, Selh, and SPS2 were all significantly decreased (P < 0.05) in the L group compared to the control group. A significant decrease in IL-2, IL-10, IL-17, IL-1β, IFN-α, and IFN-β was observed in the L group, and there was also a significant increase in IL-6, IL-8, IFN-γ, and TNF-α in the L group. In summary, Se deficiency results in significant changes in the expression of selenoproteins, which may cause oxidative stress in the chicken thymus tissue. Moreover, immunological changes and immune stress may occur because of Se deficiency in the chicken thymus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biological trace element selenium (Se) is critical for various forms of life [1]. Se deficiency can impair immune function and cause DNA damage in chickens [2]. Studies demonstrated that Se deficiency negatively impacts immune cells during activation, differentiation, and proliferation, which causes protein misfolding and calcium flux in immune cells [3]. The thymus is a primary lymphoid organ of the immune system, and loss of the thymus at an early age results in severe immunodeficiency, which leads to high susceptibility to infection. Thymus growth inhibition and oxidative stress occur due to Se deficiency [4], and the Se levels can also affect selenoproteins and the immune function of the thymus.
Se is incorporated into selenoproteins as the amino acid selenocysteine [5]. A total of 25 selenoproteins were identified in chickens [6, 7]. The expression of selenoproteins, such as Selw and Selt, is influenced by the Se level in the muscles and myoblasts of chickens [8, 9]. Se deficiency significantly decreases the levels of Selt, SPS1, and SecS in the chicken immune organs and causes oxidative stress [4]. In addition, Se deficiency constrains the transformation of T4 to T3 in chicken thyroids and decreases the expression of selenoproteins, such as Txnrd2, Dio1, Dio2, Dio3, Seli, Selu, Gpx1, and Gpx2 [10]. The decreased expression of Txnrd2, Gpx1, Gpx3, Seli, Dio1, Sepp1, Sepw1, Selo, Selt, Selm, Sepx1, and SPS2 was also detected in the pancreas of chicken with Se deficiency [11]. Furthermore, the messenger RNA (mRNA) levels of Gpx1, Gpx2, Gpx3, Gpx4, Dio3, Sepp1, Selt, Seli, and Selu were decreased in the visceral adipose tissues of chickens with Se deficiency [12]. These data indicate that the levels of various selenoproteins in different tissues are affected by the Se level in chickens. However, no information is available on the levels of selenoproteins in chicken thymus with Se deficiency.
Se deficiency increases the risk of bacterial and viral infections and impairs host immune responsiveness [13]. Cytokines are involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis, and are important mediators of the inflammatory response [14]. Se deficiency increases the amount of pro-inflammatory cytokines, and the structure of the white pulp and red pulp are disorganized because of the effect on the cytokine content in the chicken immune organs [2]. Damage to the brain tissues and changes in cytokines are observed during Se deficiency [15]. As shown in previous studies, both selenoproteins and cytokines are sensitive to the Se concentration in the tissues; however, little information about the effect of dietary Se on the level of selenoproteins and immune function in chicken thymus is available. In this study, we determined the effect of Se deficiency on the mRNA expression of selenoproteins and immune functions in chicken thymus. The results indicated that Se deficiency downregulates the mRNA expression of selenoproteins and suppresses immune function in chicken thymus.
Materials and Method
The Institutional Animal Care and Use Committee of Northeast Agricultural University approved all procedures. A total of 300 (Weiwei chicken breed Co. Ltd., Harbin, China) 1-day-old chickens were randomly divided into two groups (control group and Se deficiency group [L group]). The L group was fed a Se-deficient corn-soy basal diet (corn and soy were produced in the Se-deficient region of Heilongjiang Province, China, and the diet contained 0.033 mg Se/kg). The control group was fed the same basal diet supplemented with Se at 0.15 mg/kg (sodium selenite). The feeding experiment lasted for 55 days, and the feed and water were supplied ad libitum. Thymus tissues were collected at days 15, 25, 35, 45, and 55. All collected samples were stored at −80 °C.
Determination of mRNAs Levels by Quantitative Real-Time PCR
Total RNA was isolated from the tissue samples (50 mg tissue; n = 3/diet group) using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, China). Standard PCRs were first performed to confirm the specificity of the primers (Table 1). The PCR products were electrophoresed on 2 % agarose gels, extracted, cloned into the pMD18-T vector (TaKaRa, China), and sequenced. First-strand complementary DNA (cDNA) was synthesized from 50 μg of total RNA using oligo dT primers and superscript II reverse transcriptase according to the manufacturer’s instructions (Invitrogen, China). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use. Real-time quantitative reverse-transcription PCR was used to detect the expression of selenoprotein genes in the chicken thymus tissues using an ABI PRISM 7500 real-time PCR system (Applied Biosystems, USA). The reaction mixtures consisted of the following: 10 μl of ×2 SYBR Green I PCR Master Mix (TaKaRa, China), 2 μl of diluted cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of ×50 ROX reference Dye II, and 6.8 μl of PCR-grade water. The PCR program for the selenoproteins and GADPH was 1 cycle at 95 °C for 30 s, 40 cycles at 95 °C for 15 s, and 60 °C for 30 s. The melting-curve analysis showed only one peak for each PCR product. A dissociation curve was run for each plate to confirm the production of a single product. The amplification efficiency for each gene was determined using the DART-PCR program. The expression levels of the selenoprotein mRNAs were analyzed based on the Ct values of the PCR products expressed as the fold-change relative to the GADPH gene, by the 2−△△CT method.
Determination of Immune Function
A total of 100 mg of thymus tissues was rinsed with ×1 PBS, homogenized in 1 ml of ×1 PBS, and stored overnight at −20 °C. Two freeze-thaw cycles were performed to break the cell membranes, and the homogenates were centrifuged for 5 min at 5000×g at 4 °C. The supernatants were removed and assayed immediately using an ELISA kit according to the protocol provided by the manufacturer (CUSABIO BIOTECH Co., Ltd.). Pre-diluted standards (50 μl) were pipetted in duplicate, followed by samples of the same amount in the remaining wells. The plate was incubated for 2 h at room temperature for maximum binding. After the incubation, the solution was decanted and washed five times with the wash solution provided in the kit. Following this, 100 μl of pre-diluted detector solution was added to each well, and the samples were incubated at room temperature for 1 h. The plate was then washed and blotted five times. Next, 100 μl of TMB substrate solution was pipetted into each well, and the samples were incubated in the dark at room temperature for 30 min. After 30 min, 50 μl of stop solution was added to each well, and the absorbance was determined at 450 nm using a calibrated plate reader; the change in reading between the two wavelengths was obtained for analysis.
Statistical Analysis
Statistical analyses of the selenoprotein mRNA levels and cytokine content were performed using SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). A significant value (P < 0.05) was determined using a two-tailed paired Student’s t test. The data are expressed as the mean ± standard deviation.
Result
Effect of Se on Selenoprotein mRNA Abundance in Chicken Thymus
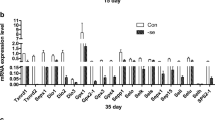
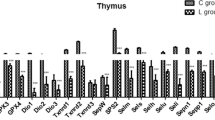
The effects of Se deficiency on selenoprotein mRNA abundance were determined by qPCR in chicken thymus. The selenoprotein mRNA abundance is shown in Fig. 1a–e. The mRNA abundances of the selenoproteins Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, Dio3, GPx1, GPx2, GPx3, Gpx4, Sepp1, Selo, Selk, Sep15, Sepx1, Sels, Seli, Selu, Selh, and SPS2 were significantly decreased (P < 0.05) in the L group compared to the control group from days 15 to 55 in the chicken thymus. The expression levels of Txnrd1, Txnrd2, Gpx1, Gpx3, Dio1, Dio3, Sepx1, Sep15, Sepp1, Selh, and SPS2 were significantly decreased in the L group. The results showed that Se deficiency downregulates selenoproteins in the chicken thymus.
Effects of Se deficiency on the selenoprotein mRNA levels in the thymus. a Selenoprotein mRNA levels on day 15. b Selenoprotein mRNA levels on day 25. c Selenoprotein mRNA levels on day 35. d Selenoprotein mRNA levels on day 45. e Selenoprotein mRNA levels on day 55. *P < 0.05 indicates that there are significant differences between the control group and the L group at the same time point. Each value represents the mean ± SD, n = 3
Effect of Se Deficiency on Cytokine Content in Chicken Thymus
The effects of Se deficiency on the cytokine content were determined using ELISA in the chicken thymus. The cytokine content is shown in Figs. 2a–e and 3a–e. The amounts of IL-2, IL-10, IL-17, IL-1β, IFN-α, and IFN-β were decreased (P < 0.05) in the L group compared to the control group on days 15, 35, and 55, and the amounts of IL-6, IL-8, IFN-γ, and TNF-α were increased (P < 0.05) in the L group compared to the control group on days 15, 35, and 55. The results demonstrate that Se deficiency affects immune function in the chicken thymus.
Effects of Se deficiency on IL-2, IL-10, IL-17, and IL-1β, IFN-α, and IFN-β in the thymus. a. IL-2. b IL-10. c IL-17. d IL-1β. e IFN-α. f IFN-β. *P < 0.05 indicates that there are significant differences between the control group and the L group at the same time point. Each value represents the mean ± SD, n = 3
Discussion
In chickens, different types of selenoproteins have been identified, such as Txnrds, Gpxs, Dios, Selk, Selw, Sels, Selp, Seln1, Selt, Sepp1, Selo, SPS2, Slem, Selu, Selh, Sep15, Sepx1, Seli, and SelPb, decreases of which may cause various injuries and diseases [16, 17]. Txnrds (Txnrd1, Txnrd2, and Txnrd3) work as an antioxidant system in the body and against oxidative stress by regulating the protein dithiol/disulfide balance. Murine T cell sulfur thioredoxin reductase (TR) was low in the livers and spleens of the Se-deficient group, with a significant decrease of Txnrds [18]. Gpxs are enzymes involved in the catabolism of peroxide. Se deficiency decreased the amount of four Gpxs in chicken livers [19]. Dio1 is the most abundant of the three Dios and is expressed in the kidney, thyroid, liver, and pituitary of mammals. Dio2 has same function as Dio1. Dio3 has a regulatory function in thyroid-hormone metabolism and catalyzes the inactivation of T4 into T3. Se deficiency decreases the expression of Dios in the thyroids of chickens [10]. We found that Se deficiency decreased Txnrds (Txnrd1, Txnrd2, and Txnrd3), Dios (Dio1, Dio2, Dio3), and Gpxs (Gpx1, Gpx2, Gpx3, Gpx4) in the chicken thymus. A similar observation was found in rats and turkeys [20].
Sepp1 is as biomarker of Se status and transports Se into the brain and testes. Selk is a selenoprotein with antioxidant properties that is localized in the endoplasmic reticulum membrane. Expression of Sels is modulated by glucose metabolism and endoplasmic reticulum stress [21]. Sels is a transmembrane protein that is widely expressed in a variety of tissues [22] Glutathione peroxidase activity is decreased and lipid peroxidation is increased in chicken muscles, along with decreased Selk, Sels, Sepn1, and Selt, during Se deficiency [8]. The other selenoproteins have special functions. Our results showed that the mRNA levels of Sepp1, Selo, Selk, Sepx1, Sep15, Seli, Selu, Selh, and SPS2 were decreased due to Se deficiency. Among the detected selenoproteins, Txnrd1, Txnrd2, Gpx1, Gpx3, Dio1, Dio3, Sepx1, Sep15, Sepp1, Selh, and SPS2 were significantly decreased in the thymus. Furthermore, another study described relationships among the decrease in selenoproteins, tissue injury, physiological disorder, and oxidative stress in Se-deficient chicken [11]. In addition, the significantly downregulated expression of several selenoproteins causes muscle atrophy during Se deficiency [23]. Consistent with these previous studies, we found that Se deficiency induces the decreased expression of various selenoprotein mRNAs, which may cause oxidative stress in the chicken thymus.
Se is also required for the functions of macrophages, T lymphocytes, and other immunological cells for the proper functioning of the immune system and production of anti-inflammatory molecules in the systemic inflammatory response syndrome [24, 25]. T cell, B cell, and NK cell functions are regulated by Se. Se deficiency affects both the maturation of specific lymphocyte subpopulations and the functional and proliferative capabilities of peripheral lymphocytes in chickens [26].
Cytokines are involved with the control of the host immune response to different foreign antigens and have crucial protective effects on the immune organs of chickens [27]. Se influences the anti-oxidation and immune function in broiler chickens [28]. IL-2 responds to pathogenic challenges in the host [29], and the decrease in IL-2 may cause diapauses during the development of the thymus with a low-Se diet by arresting the cell cycle [30]. IL-1β is a mediator of the inflammatory response involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis [14]. Studies have showed that the decrease in IL-1β and IL-2 caused immune lesions during Se deficiency [2]. The decreases of the IL-2 and IL-1β contents in the chicken thymus under Se deficiency were determined in this study. The decreased level of IL-10 caused inflammatory bowel disease and arthritis in mice [31]. Hangalapura et al. found that the expression of IL-10 was decreased during cold stress by a specific antibody in chickens [32]. Similarly, we found that the IL-10 content was significantly decreased during Se deficiency.
IL-17 plays an important role in regulating the Th2 response in the mucosa. IL-17 is associated with autoimmune mucosal diseases and the immune response to extracellular bacterial pathogens in the mucosal surfaces of the intestinal tract and lungs [33]. Increased high mortality due to deficiency of the IL-17 receptor was observed in mouse lungs after bacterial infection [34]. Under cold stress exposure, IL-17 was decreased and caused intestinal tract injury [35]. In this study, decreased IL-17 was observed during Se deficiency in the chicken thymus. IFN-α and IFN-β may limit viral infection [36, 37]. Our results showed that the levels of IFN-α and IFN-β were reduced in Se deficiency. As a pro-inflammatory cytokine, high levels of TNF-α cause tissues damage [38] and produce oxidative stress in Se-deficient chickens [2]. TNF-α contributes to inflammatory bowel disease (IBD) and also plays important roles in regulating epithelial barrier function and gut homeostasis [39]. IFN-γ also plays a role in the modulation of immune cells [40]. Rabinovitch showed that pro-inflammatory cytokines, such as TNF-α, IFN-γ, and free radicals, are inflammatory mediators that lead to inflammation and the lesion of pancreatic islets [41]. Liu showed that immuno-toxicity and immuno-suppression occur in pigeon spleens with increased levels of TNF-α and IFN-γ [42]. Another experiment demonstrated that the levels of TNF-α and IFN-γ were significantly increased in the pancreas of the untreated diabetes mellitus group compared to the normal control group [43]. IL-6 is a cytokine with various functions that plays a central role in host defense because of its wide range of immune and hematopoietic activities and its potent ability to induce the acute phase response [44]. Increased concentrations of IL-6 caused the induction of an inflammatory response and liver injury [45]. We observed that the levels of TNF-α, IFN-γ, and IL-6 were increased in Se-deficient thymus tissues. Similar results were found in another study, which showed that aflatoxin B1 induced high expression of TNF-α, IFN-γ, and IL-6 and then caused immunological changes and oxidative stress in chicken spleens [46]. Chicken IL-8 attracts neutrophils and naive T lymphocytes. IL-8 also displays chemo-tactic activity toward chicken peripheral blood mononuclear cells and may act as a mitogen of fibroblasts. We found that the content of IL-8 was increased in chicken thymus under Se deficiency. This result was similar to that of Lee, who showed that IL-8 and IFN-γ were upregulated in unvaccinated chickens challenged with IBDV in the bursa [47]. We also determined that the increased amount of IL-8 recruits heterophils and naive T lymphocytes to the thymus.
Conclusion
In summary, our results show that Se deficiency causes a decrease in overall selenoprotein expression, which may cause oxidative stress in various tissues. The decrease in IL-2, IL-10, IL-17, IL-1β, IFN-α, and IFN-β expression and the increase in IL-6, IL-8, IFN-γ, and TNF-α expression indicate that immunological changes and immune stress occur in the chicken thymus due to Se deficiency.
References
Loflin J, Lopez N, Whanger PD et al (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100:1679–1684
Zhang ZW, Wang QH, Zhang JL et al (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149:352–361
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16:705–743
You L, Liu C, Yang ZJ et al (2014) Prediction of selenoprotein T structure and its response to selenium deficiency in chicken immune organs. Biol Trace Elem Res 160:222–231
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Mariotti M, Ridge PG, Zhang Y et al (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7:e33066
Yao HD, Liu W, Zhao WC et al (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. Rsc Adv 4:64032–64042
Yao HD, Wu Q, Zhang ZW et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143:613–619
Yao HD, Wu Q, Zhang ZW et al (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830:3112–3120
Lin SL, Wang CW, Tan SR et al (2014) Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to triiodothyronine (T3) in chicken thyroids. Biol Trace Elem Res 161:263–271
Zhao X, Yao H, Fan R et al (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161:341–349
Liang Y, Lin SL, Wang CW et al (2014) Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res 160:41–48
Pighetti GM, Eskew ML, Reddy CC et al (1998) Selenium and vitamin E deficiency impair transferrin receptor internalization but not IL-2, IL-2 receptor, or transferrin receptor expression. J Leukoc Biol 63:131–137
Brigelius-Flohe R, Banning A, Kny M et al (2004) Redox events in interleukin-1 signaling. Arch Biochem Biophys 423:66–73
Sheng PF, Jiang Y, Zhang ZW et al (2014) The effect of Se-deficient diet on gene expression of inflammatory cytokines in chicken brain. Biometals 27:33–43
Pappas AC, Zoidis E, Surai PF et al (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151:361–372
Lescure A, Rederstorff M, Krol A et al (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790:1569–1574
Hoffmann PR (2008) Selenium and asthma: a complex relationship. Allergy 63:854–856
Liu CP, Fu J, Lin SL et al (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159:192–198
Sunde RA, Hadley KB (2010) Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood) 235:23–31
Kim KH, Gao Y, Walder K et al (2007) SEPS1 protects RAW264.7 cells from pharmacological ER stress agent-induced apoptosis. Biochem Biophys Res Commun 354:127–132
Ye Y, Shibata Y, Yun C et al (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429:841–847
Yao H, Zhao W, Zhao X et al (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161:318–327
Kiremidjian-Schumacher L, Roy M (1998) Selenium and immune function. Z Ernahrungswiss 37(Suppl 1):50–56
Hoffmann PR (2007) Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp (Warsz) 55:289–297
Kiremidjian-Schumacher L, Roy M, Wishe HI et al (1992) Regulation of cellular immune responses by selenium. Biol Trace Elem Res 33:23–35
Liu LL, Zhang JL, Zhang ZW et al (2014) Protective roles of selenium on nitric oxide-mediated apoptosis of immune organs induced by cadmium in chickens. Biol Trace Elem Res 159:199–209
Zhang ZW, Zhang JL, Gao YH et al (2013) Effect of oxygen free radicals and nitric oxide on apoptosis of immune organ induced by selenium deficiency in chickens. Biometals 26:355–365
Bonham M, O'Connor JM, Hannigan BM et al (2002) The immune system as a physiological indicator of marginal copper status? Br J Nutr 87:393–403
Peng X, Cui H, Yuan J et al (2011) Low-selenium diet induces cell cycle arrest of thymocytes and alters serum IL-2 content in chickens. Biol Trace Elem Res 144:688–694
Netea MG, Joosten LA, Lewis E et al (2006) Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med 12:650–656
Hangalapura BN, Kaiser MG, Poel JJ et al (2006) Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Dev Comp Immunol 30:503–511
Crhanova M, Hradecka H, Faldynova M et al (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun 79:2755–2763
Ye P, Rodriguez FH, Kanaly S et al (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527
Zhao FQ, Zhang ZW, Yao HD et al (2013) Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res Vet Sci 95:146–155
Sheridan PA, Zhong N, Carlson BA et al (2007) Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr 137:1466–1471
de Andres C, Aristimuno C, De Las Heras V et al (2007) Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J Neuroimmunol 182:204–211
Wang CX, Shuaib A (2002) Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol 67:161–172
Olson TS, Reuter BK, Scott KG et al (2006) The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med 203:541–552
Yeh HY, Winslow BJ, Junker DE et al (1999) In vitro effects of recombinant chicken interferon-gamma on immune cells. J Interferon Cytokine Res 19:687–691
Rabinovitch A (1994) Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation? Diabetes 43:613–621
Liu C, Li M, Cao Y et al (2014) Effects of avermectin on immune function and oxidative stress in the pigeon spleen. Chem Biol Interact 210:43–50
Zeng J, Zhou J, Huang K (2009) Effect of selenium on pancreatic proinflammatory cytokines in streptozotocin-induced diabetic mice. J Nutr Biochem 20:530–536
Simpson RJ, Hammacher A, Smith DK et al (1997) Interleukin-6: structure-function relationships. Protein Sci 6:929–955
Hinton DM, Myers MJ, Raybourne RA et al (2003) Immunotoxicity of aflatoxin B1 in rats: effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol Sci 73:362–377
Li Y, Ma QG, Zhao LH et al (2014) Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int J Mol Sci 15:5649–5662
Lee CC, Kim BS, Wu CC et al (2014) Bursal transcriptome of chickens protected by DNA vaccination versus those challenged with infectious bursal disease virus. Arch Virol 160:69–80
Acknowledgments
The authors thank the members of the Veterinary Internal Medicine Laboratory, College of Veterinary Medicine, and Northeast Agriculture University for their help with sample collection.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No.31472161).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoso, P.A., Yang, Z., Liu, C. et al. Selenium Deficiency Downregulates Selenoproteins and Suppresses Immune Function in Chicken Thymus. Biol Trace Elem Res 167, 48–55 (2015). https://doi.org/10.1007/s12011-015-0282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0282-y