Abstract
Selenoprotein T (SelT) is associated with the regulation of calcium homeostasis and neuroendocrine secretion. SelT can also change cell adhesion and is involved in redox regulation and cell fixation. However, the structure and function of chicken SelT and its response to selenium (Se) remains unclear. In the present study, 150 1-day-old chickens were randomly divided into a low Se group (L group, fed a Se-deficient diet containing 0.020 mg/kg Se) and a control group (C group, fed a diet containing sodium selenite at 0.2 mg/kg Se). The immune organs (spleen, thymus, and bursa of Fabricius) were collected at 15, 25, 35, 45, and 55 days of age. We performed a sequence analysis and predicted the structure and function of SelT. We also investigated the effects of Se deficiency on the expression of SelT, selenophosphate synthetase-1 (SPS1), and selenocysteine synthase (SecS) using RT-PCR and the oxidative stress in the chicken immune organs. The data showed that the coding sequence (CDS) and deduced amino acid sequence of SelT were highly similar to those of 17 other animals. Se deficiency induced lower (P < 0.05) levels of SelT, SPS1, and SecS, reduced the catalase (CAT) activity, and increased the levels of hydrogen peroxide (H2O2) and hydroxyl radical (–OH) in immune organs. In conclusion, the CDS and deduced amino acid sequence of chicken SelT are highly homologous to those of various mammals. The redox function and response to the Se deficiency of chicken SelT may be conserved. A Se-deficient diet led to a decrease in SelT, SecS, and SPS1 and induced oxidative stress in the chicken immune organs. To our knowledge, this is the first report of predictions of chicken SelT structure and function. The present study demonstrated the relationship between the selenoprotein synthases (SPS1, SecS) and SelT expression in the chicken immune organs and further confirmed oxidative stress caused by Se deficiency. Thus, the information presented in this study is helpful to understand chicken SelT structure and function. Meanwhile, the present research also confirmed the negative effects of Se deficiency on chicken immune organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential nutritional trace element [1]. According to news reports, Se has been considered a preventive therapy for immune-mediated skin disease [2]. One of its main and important functions is protection against disease associated with increased free radical activity [3]. However, Se deficiency has been shown to induce several diseases and injuries, including inhibiting the immune system, neurologic diseases, muscle disease, and epilepsy [4–6]. Animal studies have demonstrated the importance of Se in both innate immunity and adaptive immune systems [7, 8]. These studies showed that Se deficiency could inhibit the proliferation of T and B lymphocytes [9]. Furthermore, Se deficiency inhibited the growth of the bursa of Fabricius and thymus and induced atrophy and loose structure in chicks [10]. In addition, severe Se deficiency impaired the host immune ability, which further increased the risk of bacterial and viral infections as well as certain types of cancers [11]. The important role Se plays in enhancing the immune function at the molecular level is only beginning to be understood, primarily via the action of selenoproteins [8, 12]. However, the relationship between selenoproteins and the immune system in chickens remains unclear.
Se exerts its biological effects via the incorporation as selenocysteine into various selenoproteins. Previous studies showed that the biosynthesis of selenoproteins and regulation by selenium levels may be associated with selenocysteine synthase (SecS) and selenophosphate synthetase (SPS) [13]. SecS was found to be required for the biosynthesis of Sec. SecS can convert the serine attached to transfer RNA (tRNA) [Ser] Sec to Sec [14, 15]. SPS is incorporated into protein in all three lines of descent, eukaryota, archaea, and eubacteria. SPS initially was thought to play a role in selenophosphate synthesis, which is used to synthesize selenocysteine in mammals. Approximately 25 selenoproteins have previously been characterized and are indicated to play crucial roles in mammals [16]. Some of the identified selenoproteins have been found to exhibit several functions: redox regulation, calcium mobilization, ER stress, Se transportation, and storage [17, 18]. However, the roles of many selenoproteins remain unclear, including selenoprotein T (SelT). SelT is conserved from plants to humans and localizes to the endoplasmic reticulum [19]. Due to its characteristics, mammalian SelT has been identified as a member of the Rdx protein family, which shows a sequence similar to that of the thioredoxin-like fold and a conserved CxxU motif [18, 20]. SelT is ubiquitously expressed in many tissues in mice, including the liver, kidney, spleen, intestine, brain, and heart. SelT messenger RNA (mRNA) is highly expressed in mice immune organs [21]. Although the distribution, function and structure of SelT in mammals have been well studied, studies of the exact function of chicken SelT and the SelT homology between chickens and other animals are unfortunately lacking, and the effects of Se deficiency on the SelT mRNA expression in the immune organs of birds remain unclear. Hence, we analyzed the homology, structure, and function of SelT. We also detected the effects of Se deficiency on the expression of SelT, SecS, and selenophosphate synthetase-1 (SPS1) mRNA and measured biomarkers of oxidative stress in chicken immune organs.
Materials and Methods
Poultry and Diets
All procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. A total of 150 1-day-old chickens were randomly allocated to two groups (control group and Se-deficient group, 75 chickens each group, each group with three replicate pens consisting of 25 chickens). Over the entire experimental period, the chickens were allowed ad libitum consumption of feed and water. The chickens were maintained either on a Se-deficient diet or on sodium selenite for 55 days. The Se-deficient diet group (L group) containing 0.02 mg Se/kg was purchased from the Se deficiency region of Heilongjiang Province in China. The sodium selenite diet group (C group) containing 0.2 mg Se/kg was from Weiwei Co. Ltd. (Harbin, China). 25 chickens per group were euthanized at 15, 25, 35, 45, and 55 days old, respectively (five chickens per pen per time point). The immune tissues (spleen, thymus, and bursa of Fabricius) were quickly removed, minced, and stored at −80 °C in order to determine the index of oxidative stress and isolate the RNA. The remaining tissues were used in the preliminary experiment and served as standby tissues.
Determination of the SelT, SPS1, and SecS mRNA Level by Quantitative RT-PCR
Total RNA was isolated from the tissue samples using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, China). The dried RNA pellets were resuspended in 50 μl of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were spectrophotometrically determined at 260 nm/280 nm according to the spectrophotometer (Gene Quant 1300/100, General Electric Company, USA). First-strand cDNA was synthesized from 5 μg of total RNA using oligo dT primers and superscript II reverse transcriptase according to the manufacturer’s instructions (Roche, USA). The synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use.
The Primer Premier Software (PREMIER Biosoft International, USA) was used to design primers specific to SelT, SPS1, and SecS based on the known chicken sequences (Table 1). Quantitative real-time PCR was performed on an ABI PRISM 7500 Detection System (Applied Biosystems, USA). The reactions were performed in a 20-μl reaction mixture containing 10 μl of 2× SYBR Green I PCR Master Mix (TaKaRa, China), 2 μl of either diluted cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of 50× ROX reference dye II and 6.8 μl of PCR-grade water. The PCR procedure for SelT, SPS1, and SecS consisted of 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. The amplification efficiency for each gene was determined using the DART-PCR program [22]. The mRNA-relative abundance was calculated according to the method of Pfaffl [23], which accounts for gene-specific efficiencies and was normalized to the mean expressions of SelT, SPS1, and SecS.
Determination of Antioxidant Enzyme Activities
The tissues (thymus, spleen, and bursa of Fabricius) were homogenised (1:10 w/v) with a glass Teflon homogenizer (Heidolph SO1 10R2RO) in physiological saline. The homogenate was centrifuged at 700 × g for 30 min at 4 °C to obtain the postmitochondrial supernatant for measuring CAT activity, –OH contents, and H2O2 levels.
The catalase (CAT) activity (CAT; EC1.11.1.6) was determined according to the method of Weissman [24]. Tris–HCl (1 M), 5 mM EDTA (pH 8.0), 10 mM H2O2, and H2O were mixed at 37 °C, and the rate of H2O2 consumption was measured at 230 nm in order to quantitatively determine the CAT activity. An extinction coefficient of 22 for H2O2 at 230 nm was used to calculate the enzyme activity. The hydrogen peroxide (H2O2) was determined according to the method of Zhou et al. [25]. Absorbance was recorded at 505 nm by using microplate reader. The hydroxyl radicals (–OH) was determined according to the method of Pascual and Romay [26]. The absorbance was recorded at 550 nm using visible spectrophotometer. The protein content of the samples was measured by the method of Bradford [27] using bovine serum albumin as the standard.
Sequence Analysis, 3D Molecular Modeling, and Phylogenetic Analysis
The cDNA sequence and deduced amino acid sequence of chicken SelT were analyzed using the BLAST algorithm (http://www.ncbi.nlm.nih.gov/blast) and the Expert Protein Analysis System (http://www.expasy.org/). The protein domains were identified using the InterProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan/). The 3D structure and biological function of chicken SelT were predicted using the I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/); the evolutionary and tree homology analysis were constructed using the dnaSTAR software MegAlign module Clustal W method.
Statistical Analysis
All data were statistically analyzed using the SPSS statistical software for Windows (version 13; SPSS, Chicago, IL, USA). The data were analyzed further when a significant difference (P < 0.05) was identified using a one-way analysis of variance. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honest significant difference test for post hoc multiple comparisons. The relationship between the Se concentrations in tissues and the abundance of SelT mRNA were assessed using Pearson’s correlation coefficient. The data are expressed as the mean ±standard deviation. Differences were considered to be significant at P < 0.05.
Results
Homology Analysis and Phylogenetic Analysis of Chicken SelT
The DNA STAR software MegAlign module Clustal W method was applied to analyze the homology of 18 species. The amino acid sequence of chicken SelT shares 90.1 % identity with that of Macaca and Mus musculus, while it shares 89.6 % and 88.5 % identity with Sus scrofa and Cavia porcellus, respectively (Fig. 1).
To determine the position of the chicken SelT gene in evolution, 18 SelT sequences from birds, mammals, and aquatic invertebrates were analyzed to construct a phylogenetic tree (Fig. 2a, b). The phylogenetic analysis provided evidence that chicken SelT is derived from a common ancestor with other SelT family proteins and that it is a novel selenoprotein that differs from the SelT of mammals and aquatic invertebrates.
Predicted Secondary Structures of Chicken SelT
Predicting the protein secondary structure is generally considered to be the first step in protein structure prediction. Bioinformatics approaches identified the chicken SelT, and the complete nucleotide sequence of the gene encodes 199 amino acids (Fig. 3). Helix and h (H) represents an α helix, Sheet and e (S) represents a β fold, and Coil and c (C) represents a random coil. The prediction of the chicken SelT protein secondary structure indicates that the SelT model includes a β1-α1-β2-α2-β3-β4-α3 secondary structure pattern, wherein β1 and β2 are parallel strands forming a classical β1-α1-β2 motif, which is also observed in thioredoxin-like fold proteins. Thus, SelT contains a CXXU motif. The presence and location of the CXXU motif suggested its various redox functions.
Predicted Domains of Chicken SelT
As shown in Fig. 4, the 44-184 aa domain of SelT is analogous to Rdx, the 45-183 aa domain of SelT is analogous to CXXU, and the 45-183 aa domain of SelT is like Thioredoxin-like. Thus, SelT contains a CXXU motif. The domain of the CXXU motif suggested its redox in regulation.
Predicted Spacial Structure of Chicken SelT
The 3D structure and biological function of chicken SelT were predicted using the I-TASSER server (Fig. 5). The N-terminal part begins with the β1 strand, resulting in shorter β strands in the classical β1-α1-β2 motif with respect to SelW. Based on the sequence data and structure data, SelT is proposed to possess a thioredoxin-like fold and a conserved CxxU (U is Sec) motif, suggesting a redox function.
Correlation Analysis of SecS, SPS1 mRNA Expression, and SelT mRNA Expression in Chicken Immune Organs
As shown in Table 2, the calculations of the Pearson’s correlation coefficients indicated strong correlations (P < 0.05) between SecS and SelT as well as SPS1 and SelT in the bursa of Fabricius and thymus. In contrast, the mRNA amounts of SelT did not correlate with those of SecS and SPS1 in other organs (P > 0.05).
Effect of Se Deficiency on the mRNA Levels of SelT, SecS, and SPS1 in Immune Organs of Chicken
The effects of Se deficiency on the mRNA levels of SelT, SecS and SPS1 in chicken immune organs are shown in Figs. 6, 7 and 8, respectively. At each sampling point, the SelT, SecS, and SPS1 mRNA levels in the immune tissues of chickens that were fed the Se-deficient basal diet were lower than those of chickens that were fed a sodium selenite diet.
Effects of Se deficiency on the mRNA expression of SelT, SecS and SPS1 of Bursa of Fabricius in chicken. a Result of effects of Se deficiency on mRNA level of SelT. b Result of effects of Se deficiency on mRNA level of SecS. c Result of effects of Se deficiency on mRNA level of SPS1. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
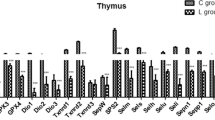
Effects of Se deficiency on the mRNA expression of SelT ,SecS and SPS1 of Thymus in chicken. a Result of effects of Se deficiency on mRNA level of SelT. b Result of effects of Se deficiency on mRNA level of SecS. c Result of effects of Se deficiency on mRNA level of SPS1. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
Effects of Se deficiency on the mRNA expression of SelT, SecS and SPS1 of Spleen in chicken. a Result of effects of Se deficiency on mRNA level of SelT. b Result of effects of Se deficiency on mRNA level of SecS. c Result of effects of Se deficiency on mRNA level of SPS1. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
Figure 6 shows the effects of Se deficiency on the mRNA expression of SelT, SecS, and SPS1 in the bursa of Fabricius. Diet without supplementation of Se significantly decreased the SelT and SPS1 mRNA relative to the C group on day 15 (P < 0.05) and day 25 (P < 0.01). Notably, the most significant decrease in SelT and SPS1 among the groups was observed on days 35, 45, and 55 in the L group (P < 0.001). However, the SecS mRNA level decreased in the L group on day 15 compared to that in the C group (P < 0.05), and a conspicuous decrease is evident on days 25, 35, and 45 (P < 0.001).
In the thymus (Fig. 7), the SelT, SecS, and SPS1 mRNA levels in the L group were significantly lower at each sampling time point. Compared with the C group, the levels of SelT, SecS and SPS1 mRNA at the first sampling time point (day 15) in the L group were low among all groups in the present study (P < 0.01). Moreover, these mRNA levels significantly decreased (P < 0.001) in the L group after day 35.
In the spleen (Fig. 8), the SelT mRNA levels in the L group significantly decreased on days 15 and 25 (P < 0.01) compared with the C group. The decrease in mRNA on day 35 was greater than those on the other days (P < 0.001). The levels of SecS and SPS1 mRNA were decreased (P < 0.01) on day 15 compared with the C group. Significant decreases (P < 0.001) in the levels of SecS and SPS1 mRNA were detected in the L group on days 25–55 compared to the C group.
Effects of Se Deficiency on CAT Activity Immune Organs of Chicken
The effects of Se deficiency on the CAT activity in immune organs at each time point are shown in Fig. 9. The CAT activities in the spleen and thymus of the L group chickens were significantly lower than those of the control group (P < 0.001). This significant difference was noted on day 55. The CAT activity in the bursa of Fabricius was also significantly lower in the L group than in the controls on day 15 (P < 0.01).
Effects of Se deficiency on CAT activity of immune organs in chicken. a Result of effects of Se deficiency on CAT activity in bursa of Fabricius. b Result of effects of Se deficiency on CAT activity in thymus. c Result of effects of Se deficiency on CAT activity in spleen. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
Effects of Se Deficiency on the H2O2 and –OH Levels of Immune Organs in Chicken
The effects of Se deficiency on the –OH and H2O2 levels in immune organs at each time point are shown in Figs. 10 and 11, respectively. The –OH and H2O2 levels in the examined tissues of the L group chickens were significantly lower than those of the control group at first and then significantly increased.
Effects of Se deficiency on –OH activity of immune organs in chicken. a Result of effects of Se deficiency on –OH activity in bursa of Fabricius. b Result of effects of Se deficiency on –OH activity in thymus. c Result of effects of Se deficiency on –OH activity in spleen. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
Effects of Se deficiency on content of H2O2 of immune organs in chicken. a Result of effects of Se deficiency on content of H2O2 in bursa of Fabricius. b Result of effects of Se deficiency on content of H2O2 in thymus. c Result of effects of Se deficiency on content of H2O2 in spleen. Each value represented the mean ± SD (n = 3). *a–c indicate that there are significant differences (P < 0.05) between the C group and the L group at the same time point
In Fig. 10, Se deficiency significantly decreased the –OH levels relative to the C group in the spleen on day 15 (P < 0.05) and day 25 (P < 0.001). However, the levels of –OH were significantly higher than those of the C group chickens after day 35 (P < 0.001). Compared with the C group, the –OH levels in the L group significantly decreased on day 15 (P < 0.01) and day 25 (P < 0.001) in the bursa of Fabricius. The –Se diet subsequently produced a nearly linear increase after day 35 (P < 0.01). In the thymus, the –OH levels in the L group significantly decreased on day 15 (P < 0.05) and day 25 (P < 0.01) compared with the C group. Then –Se diet subsequently produced a nearly linear increase after day 35 (P < 0.01).
Figure 11 shows that Se deficiency in the chicken significantly decreased the level of H2O2 relative to the C group in the spleen on day 15 (P < 0.01) and day 25 (P < 0.001). However, the level of H2O2 was significantly higher than that of the C group chickens after day 35 (P < 0.001). Compared with the C group, the H2O2 levels in the L group significantly decreased on days 15 and 25 (P < 0.001) in bursa of Fabricius. Then –Se diet subsequently produced a nearly linear increase after day 35 (P < 0.001). In the thymus, the H2O2 levels in the L group significantly decreased on day 15 (P < 0.05) and day 25 (P < 0.001) compared with the C group. Then –Se diet subsequently produced a nearly linear increase after day 35 (P < 0.001).
Discussion
Similar to its mammalian homolog, chicken SelT may play a role in redox regulation, as defined using bioinformatics. The coding sequence (CDS) and deduced amino acid sequence were very similar to the SelT sequences from other animals in the following descending order: Macaca, Mus musculus, Pongo, Bos taurus>Sus scrofa, Canis>Cavia. In the present study, the results showed that Se-deficiency decreased the expression levels of SelT, SecS, and SPS1 mRNA in the bursa of Fabricius, thymus, and spleen. The expression levels of the three genes correlate. The similar structure and the response to Se deficiency may indicate that roles of SelT may be conserved in chickens.
SelT was found to reside in the endoplasmic reticulum and participate in the regulation of calcium homeostasis and neuroendocrine secretion [19]. Previous studies have shown that a lack of SelT in mouse fibroblasts changes the cell adhesion and enhances the expression of several oxidoreductase genes, suggesting the involvement of SelT in redox regulation and cell fixation [28]. Using the DNA STAR software MegAlign module Clustal W method to analyze the homology between chicken SelT and that of 17 other animals, we found that the amino acid sequence of chicken SelT shares 90.1 % identity with Macaca and Mus musculus. The high homology of SelT observed in this study indicated chicken SelT was derived from a common ancestor. The chicken SelT was identified using the I-TASSER server, and the complete nucleotide sequence of the gene encodes 199 amino acids. The chicken SelT protein secondary structure and 3D structure prediction indicated that the SelT model includes a β1-α1-β2-α2-β3-β4-α3 secondary structure pattern, wherein β1 and β2 are parallel strands forming a classical β1-α1-β2 motif. Finn L predicted that mouse SelT possesses a thioredoxin-like fold, suggesting a role for SelT in redox regulation [29]. The 3D structure of chicken SelT was similar to that of mouse SelT, which indicated they might exhibit similar functions. This finding may guide further studies to the roles of chicken SelT.
In the present study, we also examined the response of SelT to Se deficiency. Our result showed that Se deficiency resulted in decreases in the levels of SelT, SecS, and SPS1 mRNA in immune organs, and the most sensitive organ was the bursa of Fabricius, followed by the thymus and spleen. Yao et al. [30] confirmed that Se deficiency correlated with the level of SelT mRNA in the pectoral muscle. Furthermore, Huang et al. [31] described that dietary Se-regulated SelT in chick livers and pectoral muscles. Similar to these results, the present study showed that the response of chicken SelT is similar to that observed in other animals. Previous studies have shown that the expression of selenoprotein was associated with two important enzymes, SecS and SPS [32]. Bacterial SecS is a pyridoxal phosphate (PLP)-dependent protein that converts the serine attached to tRNA [Ser] Sec to Se [33]. SecS is involved in Se metabolism and the biosynthesis of Sec or insertion into protein. SPS1 and SPS2 are two genes initially thought to play a role in selenoprotein synthesis in mammals [34, 35]. Previous studies have shown that the levels of Se could influence the expression of these selenoprotein synthesis enzymes. Sun et al. [32] reported that high Se level upregulated the mRNA expression of SecS but not SPS1 in the livers of avian species. This study also showed that the mRNA levels of SecS were slightly enhanced by adding Se to the diet, but this addition had little effect on SPS1 in the pancreatic tissue of birds [34]. Yao et al. [30] indicated that Se deficiency affected the levels of SecS and SPS1 in muscles, which further influenced the mRNA levels of SelN, SelT, SelK, and SelS. To examine whether Se deficiency also influenced the levels of these crucial enzymes in chicken immune organs, we measured the expression of SecS and SPS1 in chicken immune organs. Furthermore, we analyzed the correlation of these enzymes with SelT. The results showed that Se deficiency decreased the expression of SecS and SPS1 in immune tissue. However, in the present study, the results showed that Se deficiency decreased the expression of SecS and SPS1 in the immune tissue. Similar to previously published results, Se deficiency can reduce the expression of SecS. However, SPS1 levels were significantly lower than C group induced by selenium deficiency in chicken immune organs, which is different from that of the previous studies. The effect of Se on the levels of these enzymes may depend on the animal or organs. The studies have also demonstrated that the presence of the two selenoprotein synthetases (SecS and SPS1) has a potential synergistic effect in the immune organs. The decreased levels of these crucial enzymes, SecS and SPS1, may also influence the levels of selenoproteins [36] and further cause oxidative damage. Thus, the effect of Se on the levels of these enzymes depends on the animals or organs.
SelT was knocked down in murine cells, which enhances the expression of several oxidoreductase genes, indicating the involvement of SelT in redox regulation [28]. SelT may serve as an oxidoreductase to eliminate free radicals. In addition, Se can reduce the accumulation of free radicals and enhance antioxidant enzyme activities [3]. The measurement of biomarkers of oxidative stress is a well-established method to assess the extent of oxidative damage by free radicals [37]. The major antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) [38], and the lipid peroxidations include malondialdehyde (MDA), –OH, and H2O2. Many studies have been related to the relationship between selenium and oxidative stress. For instance, SelW siRNA-transfected cells were more sensitive to the oxidative stress induced by treatment with H2O2 [39]. The overexpression of SelW cells markedly decreased the sensitivity to H2O2-induced oxidative stress [40]. Zhang et al. [41] demonstrated that a low-Se diet caused a decrease in the activities of SOD and GSH-Px of chicken immune organs as well as an increase in the MDA contents and then confirmed the negative effects of oxidative stress on the immune functions. CAT activity changes in the immune organs of Se-deficient chickens have not yet been reported; thus, the measurements from our study can be used to determine the CAT activity. CAT is one of the most important enzymes of the cell antioxidant defense system [42]. A decreased CAT activity indicates an increased generation of oxygen free radicals and related tissue damage, which is consistent with Se-GPx. Numerous oxidases produce reactive oxygen intermediates, which include H2O2 and –OH [43]. This scavenging of H2O2 is important, because falling to scavenge, this species may yield –OH, which can result in the nonspecific oxidation of DNA and protein [44]. H2O2 is extensively referred to as one of the major oxidative stimuli in antioxidation [43]. Recent studies have shown that H2O2 impairs cell membranes and the nucleus in neuronal cells, such as PC12. The activities of certain antioxidant enzyme also decrease, such as CAT. CAT prevents mitochondrial permeability transitions by removing H2O2 in mitochondria. The relationship between the CAT and H2O2 is antagonistic, and H2O2 can induce oxidative damage. In agreement with previous reports, the present research indicated that the H2O2 and –OH levels increased in response to Se deficiency in chicken immune organs after day 35. CAT partially inhibited the concentration increase of H2O2. As the activity of CAT decreased, the contents of H2O2 increased. The released H2O2 is readily converted into highly toxic –OH via the Fenton reaction [45]; thus, both the levels of H2O2 and –OH increased. Our results are in good agreement with previous reports mentioned above. However, their levels appeared to decrease at first, which suggests compensatory responses.
In conclusion, the data suggested that the CDS and deduced amino acid sequence of chicken SelT were very similar to the sequences of 17 other species. Similar to its mammal homology, the redox regulation function and response to the Se deficiency of chicken SelT may be conserved. In addition, the strong correlation of SecS and SPS1 with the expression of SelT was conserved in the bursa of Fabricius and the thymus but not in the spleen. Thus, SecS and SPS1 may play similar roles in chicken immune organs. Furthermore, the response of chicken SelT to Se deficiency may be related to the levels of SecS and SPS1. Moreover, Se deficiency increased the lipid peroxidation levels and reduced the CAT activity in the immune organs of chickens. To our knowledge, this is the first report of predictions of chicken SelT structure and function. The present study demonstrated the relationship between the selenoprotein synthases (SPS1, SecS) and SelT expression in the chicken immune organs and further confirmed oxidative stress caused by Se deficiency. Thus, the information presented in this study is helpful to understand chicken SelT structure and function. Meanwhile, the present research also confirmed the negative effects of Se deficiency on chicken immune organs.
References
Lenz M, Lens PN (2009) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407(12):3620–3633
Nazıroğlu M, Yıldız K, Tamtürk B et al (2012) Selenium and psoriasis. Biol Trace Elem Res 150(1–3):3–9
Fatmi W, Kechrid Z, Nazıroğlu M et al (2013) Selenium supplementation modulates zinc levels and antioxidant values in blood and tissues of diabetic rats fed zinc-deficient diet. Biol Trace Elem Res 152(2):243–250
Lei C, Niu X, Ma X et al (2011) Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health 33(2):183–188
Robinson M, Campbell D, Sutherland W et al (1983) Selenium and risk factors for cardiovascular disease in New Zealand. New Zealand Med J 96(741):755–757
Demirci S, Kutluhan S, Nazıroğlu M et al (2013) Effects of selenium and topiramate on cytosolic Ca2+ influx and oxidative stress in neuronal PC12 cells. Neurochem Res 38(1):90–97
McKenzie RC, Rafferty TS, Beckett GJ (1998) Selenium: an essential element for immune function. Immunol Today 19(8):342–345
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Wilson CL, Mann J, Walsh M et al (2014) Quiescent hepatic stellate cells functionally contribute to the hepatic innate immune response via TLR3. PLoS One 9(1):e83391
Oda SS, El-Maddawy ZK (2012) Protective effect of vitamin E and selenium combination on deltamethrin-induced reproductive toxicity in male rats. Exp Toxicol Pathol 64(7):813–819
Willett W, Steven Morris J, Pressel S et al (1983) Prediagnostic serum selenium and risk of cancer. Lancet 322(8342):130–134
Hoffmann PR (2007) Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp 55(5):289–297
Papp LV, Lu J, Holmgren A et al (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806
Leinfelder W, Zehelein E, MandrandBerthelot M et al (1988) Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine
Hatfield DL, Tsuji PA, Carlson BA et al (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends in Biochemical Sciences
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Reeves M, Hoffmann P (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66(15):2457–2478
Pappas A, Zoidis E, Surai P et al (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151(4):361–372
Grumolato L, Ghzili H, Montero-Hadjadje M et al (2008) Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 22(6):1756–1768
Dikiy A, Novoselov SV, Fomenko DE et al (2007) SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry 46(23):6871–6882
Hoffmann PR, Höge SC, Li P-A et al (2007) The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res 35(12):3963–3973
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31(14):e73–e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45–e45
Weissman SM (1976) Red cell metabolism. a manual of biochemical methods. Yale J Biol Med 49(3):310
Zhou B, Wang J, Guo Z et al (2006) A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul 49(2–3):113–118
Pascual C, Romay C (1992) Effect of antioxidants on chemiluminescence produced by reactive oxygen species. J Biolumin Chemilumin 7(2):123–132
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Sengupta A, Carlson BA, Labunskyy VM et al (2009) Selenoprotein T deficiency alters cell adhesion and elevates selenoprotein W expression in murine fibroblast cells. Biochem Cell Biol 87(6):953–961
Aachmann FL, Fomenko DE, Soragni A et al (2007) Solution structure of selenoprotein W and NMR analysis of its interaction with 14-3-3 proteins. J Biol Chem 282(51):37036–37044
Yao H-D, Wu Q, Zhang Z-W et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of SE-deficient chicks. J Nutr 143(5):613–619
Huang J-Q, Li D-L, Zhao H et al (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141(9):1605–1610
Sun B, Wang R, Li J et al (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143(3):1516–1523
Schmidt RL, Simonović M (2012) Synthesis and decoding of selenocysteine and human health. Croat Med J 53(6):535–550
Wang R, Sun B, Zhang Z et al (2011) Dietary selenium influences pancreatic tissue levels of selenoprotein W in chickens. J Inorg Biochem 105(9):1156–1160
Xu X, Carlson B, Irons R et al (2007) Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem J 404:115–120
Wang K-T, Wang J, Li L-F et al (2009) Crystal structures of catalytic intermediates of human selenophosphate synthetase 1. J Mol Biol 390(4):747–759
Özcelik D, Nazıroglu M, Tunçdemir M et al (2012) Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res 150(1–3):342–349
Massafra C, Gioia D, De Felice C et al (2000) Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol 167(3):447–452
Yu D, Zhang Z-w, Yao H-d et al (2014) Antioxidative role of selenoprotein W in oxidant-induced chicken splenic lymphocyte death. BioMetals 27(2):277–291
Han Y-H, Zhang Z-W, Su J et al (2012) Effects of chicken selenoprotein W on H2O2-induced apoptosis in CHO-K1 cells. Biol Trace Elem Res 147(1–3):395–402
Zhang Z-w, Wang Q-h, Zhang J-l et al (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361
Pigeolet E, Corbisier P, Houbion A et al (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51(3):283–297
Tate D, Miceli MV, Newsome DA (1995) Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 36(7):1271–1279
Hansford RG, Hogue BA, Mildaziene V (1997) Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr 29(1):89–95
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J Recept Sig Transd 32(3):134–141
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Grant No. 31320103920).
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, L., Liu, C., Yang, Zj. et al. Prediction of Selenoprotein T Structure and Its Response to Selenium Deficiency in Chicken Immune Organs. Biol Trace Elem Res 160, 222–231 (2014). https://doi.org/10.1007/s12011-014-0049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0049-x