Abstract
Selenoprotein has many functions in chicken, and the expression of selenoproteins is closely associated with the selenium (Se) level. However, little is known about the expression patterns of selenoproteins in chicken immune organs. Here, we investigated the effect of dietary Se deficiency on the expressions of 23 selenoproteins in broiler immune organs. In this study, 150 broilers were randomly divided into two groups (75 chickens per group). The chickens were maintained either on a diet supplemented with Se through the addition of 0.2 mg/kg of Se (C group) via sodium selenite or on a Se-deficient granulated diet (L group) until the broilers exhibited an onset of exudative diathesis (ED). Following euthanasia, the samples from the immune tissues (including the spleen, thymus, and bursa of Fabricius) were quickly collected, and the messenger RNA (mRNA) expression levels of 23 selenoproteins were examined by real-time quantitative PCR and analyzed using principal component analysis. The results showed that Se deficiency decreased the mRNA levels of 23 selenoproteins in the thymus, spleen, and bursa of the Fabricius tissues of broiler chickens. Furthermore, we found that among 23 selenoproteins, the mRNA levels of Dio1 in the thymus, Txnrd2 in the spleen, and Txnrd3 in the bursa of Fabricius decreased significantly (90.9 %, 83.3 %, and 96.8 %, respectively). In addition, the principal component analysis (PCA) results suggested that Se deficiency mainly influenced the expression of antioxidative selenoproteins, especially glutathione peroxidases (Gpxs), thioredoxin reductases (Txnrds), and iodothyronine deiodinases (Dios) in chicken immune organs. The results of this study are valuable for understanding the relevance of selenoprotein activity in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential nutritional trace element for many organisms, and it plays an important role in the normal physiology of a wide range of species, including birds. Se deficiency induces a variety of health problems in animals, including effects related to chemoprevention [4, 15], oxidant defense [28, 30], neurobiological functions [29], aging [23], and reproduction [12]. In chicken, Se is essential for the normal function of the immune system [10, 24], and Se deficiency can also cause ED, pancreas atrophy, diarrhea, reproductive dysfunction, and immune or nerve damage. The immune system is particularly sensitive to Se deficiency. Previous studies have shown that Se-deficient diets can inhibit bursal and thymic growth [22] and reduce T cell numbers [13]. Moreover, compounds that affect Se have been reported to affect the incorporation of Se into the spleen and lymph nodes [8].
Se is present in the body and exerts its biological functions mainly in the form of selenoproteins [3, 27]. Selenoproteins can be divided into two groups on the basis of the position of selenocysteine (Sec). One group has a Sec near the C-terminus of selenoproteins, for example, in thioredoxin reductases (Txnrds), SelI, SelK, SelO, and Seps1, and the other group has a Sec near the N-terminus of selenoprotein L (Sel), for example, glutathione peroxidases (Gpxs), iodothyronine deiodinases (Dios), SelH, SelM, Sepn1, SelT, Sepw1, SPS2, and Sep15 [1, 25]. These selenoproteins include the well-characterized enzyme families, Gpxs, Dios, and Txnrds [21]. Txnrds (Txnrd1, Txnrd2, and Txnrd3) function as antioxidant molecules in the body by regulating the protein dithiol/disulfide balance. Gpxs are enzymes that are involved in the catabolism of peroxide. Recently, Liu et al. [19] found Se deficiency decreased the amount of four Gpxs in chicken livers. Selenoprotein expression is regulated by the Se level in humans and animals. An effect of Se deficiency on selenoproteins was found in mammals. Se deficiency influenced the expressions of selenoproteins in human, pig, and rat [11, 18]. When there is a lack of Se in the diet, the expression levels of selenoproteins can be altered in a variety of chicken organs, including the spleen [34], pancreas [35], and the adipose tissue [16].
Although knowledge of the effects of Se deficiency has increased markedly within the past several years, such studies have mainly focused on the effect of Se deficiency on the expressions of a few selenoproteins. The effects of Se deficiency on all selenoproteins are unknown. It is also unknown whether selenoproteins differentially respond to Se deficiency or whether there is a different response to Se deficiency in the immune organs. Thus, we examined 23 selenoproteins in broiler immune tissues (thymus, spleen, bursa of Fabricius) and analyzed and compared their expression patterns following Se deficiency.
Materials and Methods
Poultry and Tissue Collection
All of the procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. One hundred and fifty broiler chicks (1 day old; Weiwei Co. Ltd., Harbin, China) were randomly divided into two groups (75 chickens per group). The chickens were maintained either on a diet supplemented with Se through the addition of 0.2 mg/kg Se (C group) via sodium selenite or on a Se-deficient granulated 76 diet (L group, from the Se-deficient region of Heilongjiang Province in China, containing 0.008 mg/kg Se) until the broiler chicks exhibited ED onset. Feed and tap water were supplied ad libitum. When the symptoms of Se deficiency occurred in the L group at 20 days, the birds were euthanized, and the immune tissues (including the spleen, thymus, and bursa of Fabricius) were quickly collected. The tissues were excised immediately on an ice-cold plate, washed in a physiological saline solution, and then divided into three portions; one potion was stored at −80 °C until the isolation of RNA.
Design of Primers and Quantitative PCR
Total RNA was isolated from the tissue samples using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, China). The dried RNA pellets were resuspended in 50 μl of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were spectrophotometrically determined at 260 nm/280 nm according to the spectrophotometer instructions (Gene Quant 1300/100, General Electric Company, USA). First-strand complementary DNA (cDNA) was synthesized from 5 μg of total RNA using oligo dT primers and superscript II reverse transcriptase according to the manufacturer’s instructions (Roche, USA). The synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use.
Primer Premier software (PREMIER Biosoft International, USA) was used to design specific primers for Hsps based on known chicken sequences (Table 1). Real-time quantitative PCR (qPCR) was performed on an ABI PRISM 7500 Detection System (Applied Biosystems, USA). The reactions were performed in a 20-μl reaction mixture containing 10 μl of 2× SYBR Green I PCR Master Mix (TaKaRa, China), 2 μl of either diluted cDNA, 0.4 μl of each primer (10 μM), 0.4 μl of 50× ROX reference Dye II, and 6.8 μl of PCR-grade water. The PCR procedure for Hsps was 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 60 °C for 30 s. Melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify the primer specificity and product purity. The relative messenger RNA (mRNA) abundance was calculated according to the ΔΔCt method, which accounts for gene-specific efficiencies and was normalized to the mean expression of the abovementioned index.
Statistical Analysis
Statistical analysis of all of the data was performed using SPSS for Windows (version 19, SPSS Inc., USA). Differences between the Se deficiency group and the control group were considered to be significant at P < 0.05. The data are expressed as the mean ± SD. In addition, principal component analysis (PCA) was used to define the most important parameters, which could be used as key factors for individual variations using the Statistics 6.0 program (version 19, SPSS Inc., Chicago, IL, USA).
Result
The Effect of Se Deficiency on the mRNA Expression Levels of Selenoproteins in Broiler Chicken Immune Organs
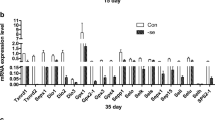
In this study, we examined the mRNA expression levels of selenoproteins using real-time quantitative PCR. The results in Figs. 1, 2, and 3 show that compared with the C groups, the mRNA levels of 23 selenoproteins were lower in the thymus, spleen, and bursa of Fabricius tissues of broiler chickens in the L groups. The results (Figs. 1, 2, and 3) indicated that 22, 20, and 23 selenoproteins were significantly decreased in the thymus, spleen and bursa of Fabricius tissues, respectively, from the L group (P < 0.001), with the exception of Txnrd3 in the thymus (P > 0.05) and Gpx4, Txnrd1 (P < 0.05), and Sepp1 (P > 0.05) in the spleen. As shown in Fig. 1, in the thymus tissues of broiler chickens, the mRNA level of SPS2 was highest in both of the C group and L group, and at the same time, the expression levels of Txnrd1 and Gpx2 were lowest, respectively, in the C group and L group. Figure 2 shows that in the spleen tissues, the highest and lowest expression levels of selenoproteins in the C group were Sepx-15 and Txnrd1, respectively, and that the selenoproteins with a similar expression in the L group were SelM and Txnrd2. Figure 3 shows that in the bursa of Fabricius, the mRNA level of Txnrd3 was highest in the C group and lowest in the L group, the SelI mRNA level was the lowest in the C group, and the Sepn1 mRNA level was highest in the L group.
Effects of Se-deficiency on Sels mRNA expression in the broiler thymus. The results are from at least five independent experiments. Data are represented as the mean ± SD (n = 6). Bars with different superscript letters represented statistically significant differences (P < 0.05) between the control group and the low-dose group
Effects of Se-deficiency on Sels mRNA expression in broiler spleen. The results are from at least five independent experiments. Data are represented as the mean ± SD (n = 6). Bars with different superscript letters represented statistically significant differences (P < 0.05) between the control group and the low-dose group
Effects of Se deficiency on Sels mRNA expression in the broiler bursa of Fabricius. The results are from at least five independent experiments. Data are represented as the mean ± SD (n = 6). Bars with different superscript letters represented statistically significant differences (P < 0.05) between the control group and the low-dose group
The Expression Pattern of Selenoproteins in Broiler Chickens Immune Organs
Tables 2, 3, and 4 show that the expression levels of the selenoproteins were different from each other. Compared to the C group, we found that the mRNA levels of Dio1, SelI, and Txnrd2 were the most significantly reduced (90.9, 86.7, and 76.6 %, respectively) and that Txnrd3, Gpx3, and SepW had the minimum differences (12.6, 34.3, and 40.2 %, respectively). We also observed that Txnrd2, Gpx2, and Sepx-15 were mostly decreased in the spleen tissues (83.3, 81.1, and 76.0 %, respectively) and Sepp1, Gpx4, and Txnrd1 were changed the least (14.1, 27.1, and 27.4 %, respectively). Table 4 shows that Txnrd3, Txnrd2, and SelO had the most reduced mRNA levels (96.8, 94.6, and 94.3 %, respectively) and Sepx1, Gpx1, and Sepp1 had the least reduced expression (52.4, 54.0, and 63.6 %, respectively) in the bursa of Fabricius tissues.
Principal Component Analysis
Using PCA, all of the parameters were distinguished on ordination plots corresponding to the first and second principal components (71.26 and 12.23 % in the thymus, 83.2 and 10.54 % in the spleen, and 79.44 and 16.73 % in the bursa of Fabricius, respectively) (Figs. 4, 5, and 6). In addition, the observed relationships among the parameters were confirmed and quantified by Spearman’s test (Tables 5, 6, and 7). The results show that there were positive correlations between different selenoproteins, except that of SelW and Gpx2 in the thymus, Sepp1 and SelM in the spleen, and Sepp1 and SelH in the bursa of Fabricius. Furthermore, as shown in Figs. 4, 5, and 6, most of selenoproteins were grouped in the rotating component matrix. In response to Se deficiency, selenoproteins generally showed a similar expression pattern. After analyzing the component matrix, we found that in the thymus organs, Sepx1 had a strong positive correlation with component 1, but was not correlated with component 2 (Table 8); Gpx3, Dio1, SelH, and SelU had different positive correlation between components 1 and 2 (Table 9) in the spleen tissues; and SelM was correlated with component 1, but not with component 2 (Table 10).
Discussion
Much of the beneficial influence of Se on animal health is attributed to its presence within selenoproteins. Moreover, different families of selenoproteins have been identified, and decreases of these proteins may cause various impairments and diseases in chicken [14, 26]. Previous studies revealed that selenoproteins in animals respond to dietary Se. In broiler chicken, Se deficiency influenced the expressions of more than 20 selenoproteins in the liver [19], thyroid tissues [17], and adipose tissues [16]. These previous studies suggest that selenoproteins show different expression patterns and responses to Se deficiency. However, it is still unclear which selenoproteins play major roles in different organs. Therefore, we examined the mRNA expression levels of 23 selenoproteins in the broiler chicken thymus, spleen, and bursa of Fabricius tissues. Similar with the aforementioned studies, our results showed that diet Se deficiency could decrease the mRNA expression levels of selenoproteins in broiler chicken immune organs.
Our results showed that under normal conditions, the mRNA levels of Gpxs, Txnrds, Dios, SelW, SelH, SelS, SelM, and SelI were high in broiler chicken immune organs. The abovementioned selenoproteins are parts of antioxidative selenoproteins, so it indicates that antioxidative selenoproteins may heavily exist in and exert biological functions in broiler immune organs. In the present study, the mRNA expressions of selenoproteins were decreased by Se deficiency, and among these selenoproteins, Gpxs, Txnrds, Dios, SelW, SelH, SelS, and SelM belong to antioxidative selenoproteins [2]. Thus, antioxidative selenoproteins may play the major roles in broiler chicken immune organs for response to Se deficiency. Even though some selenoproteins exert the similar function, they may preserve different status in different tissues. In the chicken liver, Se deficiency decreased the expressions of the selenoproteins Gpx1, Gpx3, Gpx4, Txnrd1, Txnrd3, Dio1, Dio2, SPS2, Sepp1, Txnrd2, Dio3, SelH, and SelS [19]. In adipose tissues, more than 20 selenoproteins were decreased by Se deficiency in a time-dependent manner, even among which Gpxs, Dio3, SelI, SelU, Sepw1, and Sepn1 were highly expressed [16]. However, in thyroid tissues, selenoproteins, Dios, Txnrds, SelU, and SelO were highly expressed than other selenoproteins, and Gpxs family was relatively less expressed [17]. From these prior studies, we can see that selenoproteins preserve different expression levels in different chicken tissues. Yao HD et al. [32] indicated that selenoproteins Gpx3, Gpx4, and Sepw1 were highly expressed in chicken muscles, and muscles preserved a higher percentage of antioxidative selenoproteins that were highly expressed. Similar with their study, we found the mRNA levels of Gpxs, Txnrds, Dios, and SelI were high in broiler chicken immune organs. So, antioxidative selenoproteins may possess a crucial status in broiler chicken immune organs. This inference was in accord with our previous study, which indicated that Se deficiency could induce oxidative damage in immune organs of broiler chicken [31].
In recent years, several selenoprotein families have been cloned and partially characterized according to their function or structure. In general, there are three selenoprotein families, the Gpxs family (including Gpx1–Gpx6), the Txnrds family (including Txnrd1, Txnrd2, and Txnrd3), and the Dios family (including Dio1, Dio2, and Dio3) [21]. Gpxs and Txnrds play important roles in antioxidant defense and redox regulation. Gpxs are a family of peroxide catabolism enzymes. Txnrds (Txnrd1, Txnrd2, and Txnrd3) also play roles in the antioxidant activities of the body and the oxidative stress response by regulating the protein dithiol/disulfide balance. In addition, other selenoproteins play important roles in the body, such as SelH, SelS, and SelW, among others. SelH is present in a variety of tissues and is relatively highly expressed during the early stages of embryonic development [25]. SelS is a transmembrane protein that is located in the endoplasmic reticulum (ER) and plasma membranes and is widely expressed in a variety of tissues [33]. SelW has been suggested to play an important role in muscle growth and differentiation by protecting the developing myoblasts from oxidative stress [20]. Se could regulate the expressions of these selenoproteins in different animals and organs. Hoffmann PR et al. [9] found that when Txnrds was significantly decreased, murine T cell sulfur thioredoxin reductase (TR) was lower in the livers and spleens of the Se-deficient group. Dio1 and Dio2 are expressed in the kidney, thyroid, liver, and pituitary of mammals. Lin SL et al. [17] found that the expression of Dios genes in the thyroids of chickens decreased in response to Se deficiency. SelI in the thymus and SelO and SelU in the bursa of Fabricius are highly expressed; these results are consistent with our prior studies [16, 17]. In this study, Se deficiency decreased the expression levels of 23 selenoproteins in broiler immune organs. Among the affected selenoproteins, Gpxs, Txnrds, SelW, SelI, SelH, and SelS were related to antioxidative functions or redox regulation functions [2, 6, 7]. Thus, in chicken immune organs, Se deficiency mainly influenced the expression of antioxidative selenoproteins. In recent year, groups of selenoproteins were classified into a new selenoprotein family, the Rdx family. This novel family includes the selenoproteins such as SelM, Sep15, Sepw1, and SelH, because these selenoproteins to preserve a similar thioredoxin (Trx)-like fold CxxC or CxxU (U is Sec) motif [5]. The high mRNA expression levels of these selenoproteins may be related to their similar fold motif. It is interesting that all 23 selenoproteins were decreased in over 50 % in the bursa of Fabricius tissues, but not in the other two immune tissues. Moreover, most of the members of Txnrds and Gpxs decreased by more than 85 %, and among them, Txnrd3 and Txnrd2 were the first- and second-most decreased Txnrds (96.8 and 96.4 %, respectively). As an underlying reason, it suggested that some selenoproteins may first use the Se with the price of other uncrucial selenoproteins, which indicated that Gpxs, Txnurds, Dios, and some other antioxidative selenoproteins may play crucial roles in broiler chicken immune organs.
In summary, 23 selenoproteins are expressed in the immune organs of broilers, and among these selenoproteins, antioxidative selenoproteins have the highest expression levels, especially Gpxs. Se deficiency decreased the expressions of 23 selenoproteins, 6 of which belong to antioxidative selenoproteins. Thus, Se deficiency mainly influences the expression of antioxidative selenoproteins in broiler immune organs. Additional work is required to understand the exact tissue distribution of the selenoproteins, the correlations between the 23 selenoproteins, and the potential functions of these proteins in broilers under Se deficiency.
References
Aachmann FL, Fomenko DE, Soragni A, Gladyshev VN, Dikiy A (2007) Solution structure of selenoprotein W and NMR analysis of its interaction with 14-3-3 proteins. The Journal of Biological chemistry 282(51):37036–37044. doi:10.1074/jbc.M705410200
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. The Biochemical Journal 422(1):11–22. doi:10.1042/BJ20090219
Burk RF, Hill KE, Motley AK, Austin LM, Norsworthy BK (2006) Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content. Biochim Biophys Acta 1760(12):1789–1793. doi:10.1016/j.bbagen.2006.08.010
Combs GF, Jr., Clark LC, Turnbull BW (2001) An analysis of cancer prevention by selenium. Biofactors 14 (1–4):153–159
Dikiy A, Novoselov SV, Fomenko DE, Sengupta A, Carlson BA, Cerny RL, Ginalski K, Grishin NV, Hatfield DL, Gladyshev VN (2007) SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry 46(23):6871–6882. doi:10.1021/bi602462q
Du SQ, Zhou J, Jia Y, Huang KX (2010a) SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys 502(2):137–143. doi:10.1016/j.abb.2010.08.001
Du S, Liu H, Huang K (2010b) Influence of SelS gene silence on beta-mercaptoethanol-mediated endoplasmic reticulum stress and cell apoptosis in HepG2 cells. Biochim Biophys Acta 1800(5):511–517. doi:10.1016/j.bbagen.2010.01.005
Hawkes WC, Kelley DS, Taylor PC (2001) The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res 81(3):189–213. doi:10.1385/BTER:81:3:189
Hoffmann PR (2008) Selenium and asthma: a complex relationship. Allergy 63(7):854–856. doi:10.1111/j.1398-9995.2008.01676.x
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280. doi:10.1002/mnfr.200700330
Holben DH, Smith AM (1999) The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc 99(7):836–843. doi:10.1016/S0002-8223(99)00198-4
Kaur P, Bansal MP (2005) Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition 21(3):351–357. doi:10.1016/j.nut.2004.05.028
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1994) Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biological trace element research 41(1–2):115–127
Lescure A, Rederstorff M, Krol A, Guicheney P, Allamand V (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790(11):1569–1574. doi:10.1016/j.bbagen.2009.03.002
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. BioMetals 23(4):695–705. doi:10.1007/s10534-010-9334-0
Liang Y, Lin SL, Wang CW, Yao HD, Zhang ZW, Xu SW (2014) Effect of Selenium on Selenoprotein Expression in the Adipose Tissue of Chickens. Biological trace element research 160(1):41–48. doi:10.1007/s12011-014-0024-6
Lin SL, Wang CW, Tan SR, Liang Y, Yao HD, Zhang ZW, Xu SW (2014) Selenium Deficiency Inhibits the Conversion of Thyroidal Thyroxine (T-4) to Triiodothyronine (T-3) in Chicken Thyroids. Biological trace element research 161(3):263–271. doi:10.1007/s12011-014-0083-8
Liu Y, Zhao H, Zhang Q, Tang J, Li K, Xia XJ, Wang KN, Li K, Lei XG (2012) Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. The Journal of Nutrition 142(8):1410–1416. doi:10.3945/jn.112.159020
Liu CP, Fu J, Lin SL, Wang XS, Li S (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159(1–3):192–198. doi:10.1007/s12011-014-0005-9
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100(10):1679–1684. doi:10.1016/j.jinorgbio.2006.05.018
Mariotti M, Ridge PG, Zhang Y, Lobanov AV, Pringle TH, Guigo R, Hatfield DL, Gladyshev VN (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7(3):e33066. doi:10.1371/journal.pone.0033066
Marsh JA, Combs GF, Jr., Whitacre ME, Dietert RR (1986) Effect of selenium and vitamin E dietary deficiencies on chick lymphoid organ development. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine 182 (4):425–436
Martin-Romero FJ, Kryukov GV, Lobanov AV, Carlson BA, Lee BJ, Gladyshev VN, Hatfield DL (2001) Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality. The Journal of Biological Chemistry 276(32):29798–29804. doi:10.1074/jbc.M100422200
McKenzie RC, Rafferty TS, Beckett GJ (1998) Selenium: an essential element for immune function. Immunol Today 19(8):342–345
Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, Gladyshev VN (2007) Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. Journal of Biological Chemistry 282(16):11960–11968. doi:10.1074/jbc.M701605200
Pappas AC, Zoidis E, Surai PF, Zervas G (2008) Selenoproteins and maternal nutrition. Comparative biochemistry and Physiology Part B, Biochemistry & Molecular Biology 151(4):361–372. doi:10.1016/j.cbpb.2008.08.009
Rasmussen LB, Hollenbach B, Laurberg P, Carle A, Hog A, Jorgensen T, Vejbjerg P, Ovesen L, Schomburg L (2009) Serum selenium and selenoprotein P status in adult Danes—8-year followup. Journal of Trace elements in Medicine and Biology : Organ of the Society for Minerals and Trace Elements 23(4):265–271. doi:10.1016/j.jtemb.2009.03.009
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241. doi:10.1016/S0140-6736(00)02490-9
Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. The Journal of Nutrition 134(4):707–710
Xu SW, Yao HD, Zhang J, Zhang ZW, Wang JT, Zhang JL, Jiang ZH (2013) The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res 151(2):225–233. doi:10.1007/s12011-012-9552-0
Yang Z, Liu C, Zheng W, Teng X, Li S (2015) The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res. doi:10.1007/s12011-015-0407-3
Yao HD, Zhao WC, Zhao X, Fan RF, Khoso PA, Zhang ZW, Liu W, Xu SW (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161(3):318–327. doi:10.1007/s12011-014-0125-2
Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429(6994):841–847. doi:10.1038/nature02656
Yu D, Li JL, Zhang JL, Gao XJ, Xu S (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res 144(1–3):678–687. doi:10.1007/s12011-011-9062-5
Zhao X, Yao H, Fan R, Zhang Z, Xu S (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161(3):341–349. doi:10.1007/s12011-014-0139-9
Acknowledgments
The authors thank the members of the Veterinary Internal Medicine Laboratory, College of Veterinary Medicine, and the Northeast Agriculture University for their help with sample collection.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All of the procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31472161).
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yang, Z., Liu, C., Liu, C. et al. Selenium Deficiency Mainly Influences Antioxidant Selenoproteins Expression in Broiler Immune Organs. Biol Trace Elem Res 172, 209–221 (2016). https://doi.org/10.1007/s12011-015-0578-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0578-y