Abstract
Selenium is an essential element with antioxidant roles in immune regulation, but there is little understanding of how Se acts in apoptosis in the immune organs of birds. The aim of study was to evaluate the influence of Se deficiency on oxygen free radicals, NO and apoptosis in immune organ of chickens. 160 1-day-old chickens were randomly assigned to two groups of 80 each and were fed on a low-Se diet (0.032 mg/kg Se) or a control diet (0.282 mg/kg Se), respectively. OFR production in blood was determined on days 30, 45, 60 and 75, respectively. The iNOS–NO system activity in immune organ (thymus, spleen, bursa of fabricius) was identified by NO content and NOS activity assay on days 30, 45, 60 and 75, respectively. Apoptosis was measured by DNA ladder analysis, ultrastructural observations, TdT-mediated dUTP nick end labeling TUNEL assay and flow cytometric analysis of apoptotic DNA. The transcription of factor-associated suicide, caspase-3 mRNA was tested by fluorescence quantitative PCR. The results showed that OFR production, NO and inducible NO synthases (iNOS) activity in the low-Se group were significantly increased (p < 0.05) than in the control group. In addition, apoptosis was observed in chicken immune organ in the low-Se group. The degree and the number of apoptotic cells rose in a time-dependent manner. The expression of Fas and caspase-3 mRNA increased (p < 0.05) than in the control group. It indicated that the oxidative stress and NO played a causative role in the apoptosis of immune tissues induced by selenium deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Se is an important nutritional trace element, which contributes significantly to host immune responses and antioxidant protection (Brigelius-Flohe and Flohe 2003). Se plays a role in protecting cells against free radicals and oxidative stress (Bansal and Kaur 2005). Deficiency in Se causes oxidative stress due to the diminished antioxidant property of this element as part of glutathione peroxidase (GSH-Px) (Chigurupati et al. 2008). Se deficiency has been associated with an increased expression of genes involved in oxidative stress, which can significantly impair immune defense parameters (Rao et al. 2001). In previous study in mice, low Se intake (0.02 ppm) decreased the number of B-cells in spleen, accompanied by lower glutathione content but unaltered lipid peroxidation (Vega et al. 2007). In addition, inadequate Se nutrition is associated with an increase in reactive nitrogen species such as NO production, which have been linked to increased risk of such diseases as cardiomyopathy (Tinkel et al. 2012). Many immunological cell types, including macrophages, synthesize NO, which regulates their cellular function (Chaturvedi et al. 2010). NO could induce biochemical characteristics of apoptosis in several cell types (Brookes et al. 2000; Messmer and Brune 1996). In particular, the caspase proteolytic enzymes, key mediators for apoptotic cell death, possess a redox-sensitive cysteine residue in the catalytic site. NO could modify this enzyme by S-nitrosylation in vitro and in vivo (Kim et al. 2002), indicating that biological and chemical NO generation regulates apoptotic cell death.
Se participates in the fundamental biological processes ranging from cellular antioxidant defense to the protection and repair of DNA and apoptosis (Fischer et al. 2006). Se deficiency causes cell cycle arrest and increased apoptotic activity by generating reactive oxygen species (Shen et al. 1999). Irmak et al’s. (2003) study showed that the apoptotic response to selenium deficiency was caused by oxidative stress. Also, at low concentration, selenite is known to induce apoptosis in cells via a free radical mechanism (Rao et al. 2001).
In a previous study, we observed that chickens fed deficient in Se diets exhibited lesions in immune organs, caused immune organs oxidative stress (a decrease in the activities of T-AOC, SOD, GSH-Px), decreased serum IL-1β, IL-2 content and serum TNF content, indicating that oxidative stress inhibited the development of immune organs and finally impaired the immune function of chickens (Zhang et al. 2012). However, little is known about how Se acts in apoptosis in the immune organs of chickens. The aim of study was to evaluate the influence of deficient levels of dietary Se on oxygen free radicals (OFR), NO, DNA damage and apoptosis in immune organ of chickens.
Materials and methods
Birds and diets
All procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. 160 chickens (1 day old, laying hens, Weiwei Co. Ltd., Harbin, China) were randomly divided into two groups (80 chickens per group). The chickens were maintained either on Se deficient granulated diet (L group, Se deficient granulated diet including corn, soybean meal and wheat bran from Longjiang County, Se deficiency region of Heilongjiang Province in China and blends material that did not add Se (Weiwei Co. Ltd., Harbin, China)) or the commercial granulated diet (Control group, Weiwei Co. Ltd., Harbin, China) for 30, 45, 60, 75 days. The Se contents of Se deficient granulated diet and basal commercial granulated diet were analysed by GB/T 13,883-2008 (PONY TEST Co., Beijing, China), which were contained 0.032 and 0.282 mg/kg Se (sodium selenite), respectively. The feed and water were supplied and libitum. Following euthanasia with sodium pentobarbital, the plasma and immune organ (including thymus, spleen, bursa of fabricius) were quickly collected at 30, 45, 60 and 75 days old.
Determination of OFR
Determination of OFR was based on the modified method of Driver et al. (2000). Bloods were prepared in ice-cold Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.0 mM CaCl2, 10 mM d-glucose and 5 mM HEPES, pH 7.4), which were allowed to warm at 21 °C for 5 min. The reaction mixture containing 10 μM DCFH-DA. After another 30 min of incubation, the conversion of DCFH to the fluorescent product DCF was measured with a fluorescence spectrophotometer, with excitation wavelength at 485 nm and emission wavelength at 530 nm. Background fluorescence (conversion of DCFH to DCF in the absence of homogenate) was corrected by the inclusion of parallel blanks.
NO and inducible NO synthases (iNOS) activity assay
The immune tissues’ homogenates and serum of each time point were used for NO and iNOS activity assay. The NO content and iNOS activity were determined using NO and iNOS activity assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions (Yang et al. 2006; Zhang et al. 2011).
DNA ladder analysis
The DNA fragmentation pattern (DNA ladder) was analyzed by agarose gel electrophoresis. DNA in immune tissue was purified according to the procedure of Hahnvajanawong et al. (2010). The DNA was dissolved in TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA). DNA was then loaded on 1.8 % agarose gel for electrophoresis analysis. The bands were visualized by EB staining under UV light.
Ultrastructural observations
For electron microscopy, immune tissue specimens were fixed with 2.5 % glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 3 h at 4 °C, washed in the same buffer for 1 h at 4 °C and postfixed with 1 % osmium tetroxide in sodium phosphate buffer for 1 h at 4 °C. The tissues were then dehydrated in graded series of ethanol, starting at 50 % each step for 10 min, after two changes in propylene oxide. The tissue specimens were embedded in araldite. Ultrathin sections were stained with Mg-uranyl acetate and lead citrate for transmission electron microscope evaluation.
TdT-mediated dUTP nick end labeling (TUNEL) Assay
We used in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany). The method distinguishes apoptotic cells from those undergoing necrosis, because damaged DNA in the former leads to a different distribution of staining and nuclear morphology. Paraffin wax-embedded tissue sections were treated with proteinase K and the endogenous peroxidase activity was blocked with hydrogen peroxide. The sections were incubated at 37 °C with the terminal TdT/nucleotide mixture for 1 h. Then, the reaction was stopped and the slides were rinsed with phosphate buffered saline. Nuclear labeling was developed with horseradish peroxidase and diaminobenzidine. Hematoxylin was used for counterstaining.
Flow cytometric analysis of apoptotic DNA
Immune tissues were dissected from each chick and immediately minced with surgical scissors. The cell suspensions was filtered through a 300-mesh nylon mesh, washed twice with cold phosphate buffered saline. The following assays were carried out at a concentration of 1.5 × 106 cells/mL. The cells with hypodiploid DNA were determined by cell cycle studies. After treatment, the cells were harvested and washed with PBS, fixed by adding ice-cold 70 % ethanol dropwise and stored at 4 °C overnight. The fixed cells were washed and suspended in 1 mL PBS. Following the addition of 200 μL phosphate citrate buffer (pH 7.8) to the cells, these were incubated for 60 min at room temperature. After centrifugation, the cells were resuspended in 0.5 mL propidium iodide (PI) stain (10 mg PI (Sigma-Aldrich, USA), 0.1 mL Triton-X 100 and 3.7 mg EDTA in 100 mL PBS) and 0.5 mL RNase A (50 μg/mL), incubated for 30 min in dark. The PI fluorescence was measured through a FL-2 filter (585 nm) and 10,000 events were acquired. The flow cytometric analysis was performed on BD-LSR flow cytometer (BD Biosciences, Canada). Cell debris characterized by a low FSC/SSC was excluded from analysis. The data analyzed by Cell Quest software (BD Biosciences, Canada) and mean fluorescence intensity was obtained by histogram statistics.
Quantification of factor-associated suicide (Fas), caspase-3 mRNA
Total RNA was isolated from tissue samples using Trizol reagent according to the manufacturer’s instructions (invitrogen). The RNA concentrations were determined using the GeneQuant 1300.
Reverse transcription reaction (40 μL) consisted of the following: 10 μg of total RNA, 1 μL of M-MLV reverse transcription, 1 μL of RNase inhibitor, 4 μL of dNTP, 2 μL of Oligo dT, 4 μL of dithiothreitol, and 8 μL of 5× buffer. The procedure of the reverse transcription was according to the manufacturer’s instructions (Invitrogen). The reverse transcription products (cDNA) were then stored at −20 °C for PCR.
To design primers, we used the chicken Fas, caspase-3 mRNA GenBank sequence with accession number of NM_001199487, NM_204725, respectively. Chicken β-actin (GenBank accession number L08165) as a housekeeping gene was used as an internal reference. Primers (Table 1) were designed using the Oligo 6.0 Software, and were synthesized by Invitrogen Biotechnology Co. Ltd. in Shanghai, China.
Real-time quantitative reverse transcription PCR was used to detect the expression of Fas, caspase-3 gene in thymus, spleen, bursa of fabricius by using SYBR Premix Ex TaqTM (Takara), and real time PCR work was done in the ABI PRISM 7,500 real-time PCR system (Applied Biosystems). The program was one cycle at 95 °C for 30 s and 40 cycles at 95 °C for 5 s and at 60 °C for 34 s. Dissociation curves were analyzed by Dissociation Curve 1.0 Software (Applied Biosystems) for each PCR reaction to detect and eliminate the possible primer-dimer and non-specific amplification. The mRNA relative abundance was calculated according to the method of Pfaffl (2001).
Statistical analysis
Statistical analysis of all data was performed using SPSS for Windows (version 13; SPSS Inc., Chicago, IL, USA). When a significant value (p < 0.05) was obtained by two-tailed paired Student’s t test. Data are expressed as mean ± SD.
Results
Mortality rate of chickens
At 30 days of age during the experiment (L group), there were disease and death in chickens. Disease chicken showed poor feathering, pancreatic degeneration, nutritional muscular dystrophy, and necrotic lesions in different tissues. The mortality rate in L group at 30–45 days was 3.75 %, 45–60 days was 6.67 % and 60–75 days had no death chicken.
Changes of the OFR
The effect of Se deficiency on the OFR production in blood is shown in (Fig. 1). The OFR production of blood was significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age. As the experiment progressed, a significant increase in the OFR production of blood was observed for chickens that were fed a Se-deficient basal diet.
Changes of NO production and iNOS activity
The NO production was measured by calculating the production of nitrite, a more stable product of NO in the culture medium. The effects of Se deficiency on the NO production in immune tissues and serum were shown in (Fig. 2). The NO productions of immune tissues and serum were significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age.
Effects of Se deficiency on the NO production in serum and immune tissue. a Result of effects of Se deficiency on NO production in thymus. b Result of effects of Se deficiency on NO production in spleen. c Result of effects of Se deficiency on NO production in bursa of fabricius. d Result of effects of Se deficiency on NO production in serum. Asterisk in a–d indicated that there were significant differences (p < 0.05) between the control group and the L group at the same time point. Each value represented the mean ± SD of five individuals
The effects of Se deficiency on the iNOS activity in immune tissues and serum were shown in (Fig. 3). The iNOS activities of immune tissues and serum were significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age. As the experiment progressed, a significant increase in the iNOS activity of immune tissues and serum was observed for chickens that were fed a Se-deficient basal diet.
Effects of Se deficiency on the iNOS activity in serum and immune tissue. a Result of effects of Se deficiency on iNOS activity in thymus. b Result of effects of Se deficiency on iNOS activity in spleen. c Result of effects of Se deficiency on iNOS activity in bursa of fabricius. d Result of effects of Se deficiency on iNOS activity in serum. Asterisk in a–d indicated that there were significant differences (p < 0.05) between the control group and the L group at the same time point. Each value represented the mean ± SD of five individuals
DNA ladder analysis
The biochemical hallmark of apoptosis, typical formation of 200 bp DNA fragments shown as ladders on agarose gels, was observed in immune tissues of Se deficiency chicken in a time-dependent manner (Fig. 4). Kinetic analysis of DNA fragmentation revealed that the formation of 200 bp DNA fragments in chicken immune tissues became apparent at 30 days under the Se deficiency conditions.
Effects of Se deficiency on DNA fragmentation by (1.8 %) agarose gel electrophoresis. a Result of Se deficiency on DNA fragmentation of thymus. b Result of Se deficiency on DNA fragmentation of spleen. c Result of Se deficiency on DNA fragmentation of bursa of fabricius. M: DNA Marker DL2000; 1–4: DNA in control group from 30, 45, 60, 75 days respectively; 5–8: DNA ladder patterns in L group from 30, 45, 60, 75 days respectively
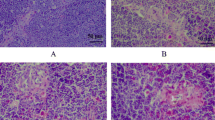
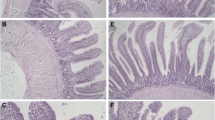
Ultrastructural changes
No ultrastructural change was observed in control group (Fig. 5a, c, e). Widened intercellular space between lymphocytes was observed in L groups. The mitochondria of lymphocytes from immune tissues of L group were enlarged and vacuolated with degenerating cristae. The apoptotic cells showed typical condensed nuclei with horseshoe-like or crescent-shaped cytoplasmic organelles with inconspicuous structure. Nuclei and organelles of some cells were unclear (Fig. 5b, d, f).
TUNEL assay
TUNEL assay revealed that apoptotic cells had brown-stain nuclei, which showed morphologic changes of condensed and irregular nuclei. There were increased frequencies of occurrence of apoptotic cells in L group when compared with that of control groups from 30 to 75 days of age (Fig. 6).
Induction of apoptotic cells
The effects of Se deficiency on the percentage of apoptotic cells in lymphocyte of immune tissues were shown in (Fig. 7). The percentage of apoptotic cells of immune tissues were significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age. As the experiment progressed, a significant increase in the percentage of apoptotic cells of immune tissues was observed for chickens that were fed a Se-deficient basal diet.
Effects of Se deficiency on the apoptotic rate in lymphocytes of immune tissue. a Result of effects of Se deficiency on apoptotic rate in thymic lymphocytes. b Result of effects of Se deficiency on apoptotic rate in splenic lymphocytes. c Result of effects of Se deficiency on apoptotic rate in bursal of fabricius lymphocytes. *a–c indicated that there were significant differences (p < 0.05) between the control group and the L group at the same time point. Each value represented the mean ± SD of five individuals
Effects of Se deficiency on the mRNA levels of Fas and caspase-3 in immune tissues of chickens
The effects of Se deficiency on the mRNA levels of Fas in immune tissues were shown in (Fig. 8). The mRNA levels of Fas gene in immune tissues were significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age.
Effects of Se deficiency on the mRNA levels of Fas in immune tissues. a Result of effects of Se deficiency on the mRNA levels of Fas in spleen. b Result of effects of Se deficiency on mRNA levels of Fas in spleen. c Result of effects of Se deficiency on mRNA levels of Fas in bursa of fabricius lymphocytes. *a–c indicated that there were significant differences (p < 0.05) between the control group and the L group at the same time point. Each value represented the mean ± SD of five individuals
The effects of Se deficiency on the mRNA levels of caspase-3 in immune tissues were shown in (Fig. 9). The mRNA levels of caspase-3 gene in immune tissues were significantly increased (p < 0.05) in L groups than in control groups from 30 to 75 days of age.
Effects of Se deficiency on the mRNA levels of caspase-3 in immune tissues. a Result of effects of Se deficiency on the mRNA levels of caspase-3 in spleen. b Result of effects of Se deficiency on mRNA levels of caspase-3 in spleen. c Result of effects of Se deficiency on mRNA levels of caspase-3 in bursa of fabricius lymphocytes. *a–c indicated that there were significant differences (p < 0.05) between the control group and the L group at the same time point. Each value represented the mean ± SD of five individuals
Discussion
Selenium is essential for optimum immune response and influences the innate and acquired immune systems. Se deficiency could cause various lesions, including encephalomalacia and exudative diathesis in chicken. Se concentrations affect oxidative status because of its known antioxidant nature in the tissues. Se-deficient animals show diminished antioxidant defense mechanism and increased oxidative stress (Griveau et al. 1995). Hoffmann et al. (2010) reported impaired activation and differentiation of T-cells in mice fed a Se-deficient (0.08 ppm Se) diet, while ROS levels in T-cells of the Se-deficient mice were not increased. On the other hand, Shrimali et al. (2008) detected increased ROS levels in T-cells from mice with genetically impaired biosynthesis of selenoproteins. It was found that the removal of selenium from the culture medium induced human T-leukemia ROS production and also lipid hydroperoxides (Saito et al. 2003). In a previous study (Zhang et al. 2012), we observed that the activities of T-AOC, SOD and GSH-Px decreased, while the XOD activity increased in serum and immune tissue of Se deficiency chicks, compared to the corresponding control ones. These results indicate the effects of oxidative stress on the immune functions of Se deficient chickens. In the present study, a statistically significant increase in the levels of OFR in the case of Se-deficient was observed, as compared to the chickens supplemented with the Se-adequate diet. A significant increase in ROS and peroxidation inside the cells in the case of Se deficiency has also been reported earlier (Kaushal and Bansal 2007; Saito et al. 2003).
NO is a small signaling molecule with important regulatory effects in many tissues. The NO reacts with molecular oxygen and other biological targets, which allow it to function as a rapidly reversible, specific, and local signal transduction molecule as well as a nonspecific mediator of tissue damage (Gonzalez et al. 2009; Inoue et al. 2003). It is catalyzed by one of the three isotypes of NO synthases (NOS)-endothelial NOS (eNOS), neuronal NOS (nNOS), or iNOS (Nathan 1992). Both nNOS and eNOS are constitutive isoforms. However, iNOS is generally not expressed under normal conditions and can be induced by various cytokine. The induction of iNOS expression may be a vital means of establishing a host defense, the overproduction of NO by iNOS has been implicated in various pathophysiologic processes (Zhang et al. 2011). NO can act as an antioxidant through diverse routes. At the cellular level, nitrosative stress has been linked to the inhibition of cell growth and apoptosis. In particular, the caspase proteolytic enzymes, key mediators for apoptotic cell death, possess a redox-sensitive cysteine residue in the catalytic site. NO could modify this enzyme by S-nitrosylation in vitro and in vivo, indicating that NO generation regulates apoptotic cell death (Kim et al. 2002). However, the effects of Se deficiency on NO production of immune tissues in poultry have been not studied so far. In present study, we investigated the change of NO and iNOS production caused by Se deficiency. The results showed an increase of NO production and iNOS activity in serum and immune tissue of Se deficiency chicks, compared to the corresponding control ones. It indicated that under Se deficiency condition, the antioxidant defense system would be undermined in vivo, and oxygen free radicals were accumulated, which would release a large number of inflammatory mediators, and the latter would stimulate the increase in the expression of iNOS and excessively release NO.
Se deficiency further enhances the severity of apoptosis by further increasing the ROS, caspase activity and down regulating anti-apoptosis genes (Verma et al. 2008). Reduction of O2 produces superoxide, peroxides, and hydroxyl free radicals, each of which have various essential cellular functions including the oxidation of fatty acids and alcohols, hydroxylation reactions, and the implementation of phagocytosis (Neve 1991). However, excessive cellular levels of these ROS may produce oxidative damage. ROS have been shown to play an important role in triggering apoptosis (Stewart et al. 1999). Apoptotic cells display a characteristic fragmentation pattern of DNA into distinct segments that can be visualized as a ladder of bands by gel electrophoresis. However, the DNA ladder formation is not ultimately required or causatively linked to the death process. Accordingly, Se deficiency was reported to induce DNA damage, particularly DNA strand breaks and base damage (Rikiishi 2007). Se deficiency in a majority of DNA damaging experiment, activates DNA damage signals and induces apoptosis in NIH 3T3 cells, suggesting that DNA damage plays a major role in selenite-induced apoptosis (Zhou et al. 2003). Saito et al. (2003) reported that the type of cell death caused by Se deficiency was necrotic rather than apoptotic. Some research showed that apoptosis or a syncretic process of apoptosis and necrosis was induced by Se deficiency (Cheng et al. 2003; Zeng 2002). Peng et al. (2011) suggested that low Se diet restrained the development of bursa of fabricius by cell cycle arrest and apoptosis. In the present study, the microscopic examination of chicken immune tissues in Se deficiency group revealed morphological changes typical of apoptosis such as membrane blebs and overall shrinkage of cells (Fig. 5b, d, f). TUNEL revealed that apoptotic cells with brown-stained nuclei were observed with increased frequency of occurrence in Se deficiency groups in comparison with that of control groups from 30 to 75 days of age (Fig. 6). In accordance with these morphological changes, apoptosis was further confirmed by the observation of Se deficiency inducing both DNA fragmentation (Fig. 4) and hypodiploid DNA (Fig. 7). The number of apoptotic chicken immune tissues’ lymphocytes induced by Se deficiency rose in time-dependent manner. It indicated that Se deficiency induced oxidative stress plays a major role in its potential to cause DNA damage and to induce apoptosis.
Likened to cellular suicide, apoptosis is an actively driven process with distinct morphological and biochemical features. Cell shrinkage, nuclear and cytoplasmic condensation, chromatin fragmentation, and caspase and tumour necrosis factor (TNF) activation are all hallmarks of apoptotic cell death (Kaiser et al. 2008). Fas, a 45 kDa type I membrane protein that belongs to the TNF/nerve growth factor (NGF) receptor superfamily, has an important role in apoptosis during development and in defence of multi-cellular organisms (Wu et al. 2008). It is the activation of caspases that ultimately results in apoptotic cellular degradation, while caspase-3 is the major effector protease involved in the apoptotic process (Nicotera et al. 2003). Se also alters cell survival genes, such as Bcl-2 (Cheng et al. 2003) and induces caspase activation (Nunes et al. 2003). Kaushal and Bansal (2007) found a significant increase in the expression of genes Bcl-2, caspase-3 and caspase-9, which were involved in different apoptotic pathways, thus directing towards the molecular mechanism of male germ cell apoptosis in response to Se deficiency-induced oxidative stress. Jaspers et al’s. (2007) study indicated that Se deficiency enhanced human bronchial epithelial cells apoptosis and caused a greater level of activated caspase-3. In our study, a statistically significant increase in the levels of Fas and caspased-3 in the case of Se-deficient was observed, as compared to the chickens supplemented with the Se-adequate diet.
In conclusion, the present study clearly showed that selenium deficiency increased the OFR production, NO production and the activity of iNOS, and eventually induced apoptosis of immune tissues. These results strongly indicate that the oxidative stress and NO play a causative role in the apoptosis of immune tissues induced by selenium deficiency.
References
Bansal MP, Kaur P (2005) Selenium, a versatile trace element: current research implications. Indian J Exp Biol 43:1119–1129
Brigelius-Flohe R, Flohe L (2003) Is there a role of glutathione peroxidases in signaling and differentiation. BioFactors 17:93–102
Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG (2000) Concentration-dependent effects of nitric oxide on mitochondrial permeabilitytransition and cytochrome C release. J Biol Chem 275:20474–20479
Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, de Sablet T, Piazuelo MB, Sarvaria AR, Cheng Y, Closs EI, Casero RA. Jr, Gobert AP, Wilson KT (2010) Polyamines impair immunity to helicobacter pylori by Inhibiting l-arginine uptake required for nitric oxide production. Gastroenterology 139:1686–1698, 1698.e1–1698.e6
Cheng WH, Quimby FW, Lei XG (2003) Impacts of glutathione peroxidase-1 knockout on the protection by injectedselenium against the pro-oxidant-induced liver aponecrosis and signaling inselenium-deficient mice. Free Radic Biol Med 34:918–927
Chigurupati S, Son TG, Hyun DH, Lathia JD, Mughal MR, Savell J, Li SC, Nagaraju GP, Chan SL, Arumugam TV, Mattson MP (2008) Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol 199:333–341
Driver AS, Kodavanti PR, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brainhomogenates. Neurotoxicol Teratol 22:175–181
Fischer JL, Lancia JK, Mathur A, Smith ML (2006) Selenium protection from DNA damage involves a ref1/p53/brca1 protein complex. Anticancer Res 26:899–904
Gonzalez DR, Treuer AV, Dulce RA (2009) Neuronal nitric oxide synthase in heart mitochondria: a matter of life or death. J Physiol 587:2719–2720
Griveau JF, Dumont E, Renard P, Callegari JP, Le LD (1995) Reactive oxygen species, lipid peroxidation and enzymatic defence systems inhuman spermatozoa. J Reprod Fertil 103:17–26
Hahnvajanawong C, Boonyanugomol W, Nasomyon T, Loilome W, Namwat N, Anantachoke N, Tassaneeyakul W, Sripa B, Namwat W, Reutrakul V (2010) Apoptotic activity of caged xanthones from garcinia hanburyi incholangiocarcinoma cell lines. World J Gastroenterol 16:2235–2243
Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR (2010) Dietary selenium modulates activation and differentiation of CD4 + T cells in mice through a mechanism involving cellular free thiols. J Nutr 140:1155–1161
Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K (2003) Cross talk of nitric oxide, oxygen radicals, and superoxide dismutase regulates the energy metabolism and cell death and determines the fates of aerobic life. Antioxid Redox Signal 5:475–484
Irmak MB, Ince G, Ozturk M, Cetin-Atalay R (2003) Acquired tolerance of hepatocellular carcinoma cells to selenium deficiency: aselective survival mechanism. Cancer Res 63:6707–6715
Jaspers I, Zhang W, Brighton LE, Carson JL, Styblo M, Beck MA (2007) Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med 42:1826–1837
Kaiser CL, Chapman BJ, Guidi JL, Terry CE, Mangiardi DA, Cotanche DA (2008) Comparison of activated caspase detection methods in the gentamicin-treated chickcochlea. Hear Res 240:1–11
Kaushal N, Bansal MP (2007) Dietary selenium variation-induced oxidative stress modulates CDC2/cyclin B1expression and apoptosis of germ cells in mice testis. J Nutr Biochem 18:553–564
Kim KM, Kim PK, Kwon YG, Bai SK, Nam WD, Kim YM (2002) Regulation of apoptosis by nitrosative stress. J Biochem Mol Biol 35:127–133
Messmer UK, Brune B (1996) Nitric oxide-induced apoptosis: p53-dependent and p53-independent signallingpathways. Biochem J 319(Pt 1):299–305
Nathan C (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6:3051–3064
Neve J (1991) Physiological and nutritional importance of selenium. Experientia 47:187–193
Nicotera TM, Hu BH, Henderson D (2003) The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol 4:466–477
Nunes VA, Gozzo AJ, Juliano MA, Cesar MC, Sampaio MU, Sampaio CA, Araujo MS (2003) Antioxidant dietary deficiency induces caspase activation in chick skeletalmuscle cells. Braz J Med Biol Res 36:1047–1053
Peng X, Cui Y, Cui W, Deng J, Cui H, Yang F (2011) The cell cycle arrest and apoptosis of bursa of fabricius induced by low seleniumin chickens. Biol Trace Elem Res 139:32–40
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Rao L, Puschner B, Prolla TA (2001) Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr 131:3175–3181
Rikiishi H (2007) Apoptotic cellular events for selenium compounds involved in cancer prevention. J Bioenerg Biomembr 39:91–98
Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E (2003) Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem 278:39428–39434
Shen HM, Yang CF, Ong CN (1999) Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2cells. Int J Cancer 81:820–828
Shrimali RK, Irons RD, Carlson BA, Sano Y, Gladyshev VN, Park JM, Hatfield DL (2008) Selenoproteins mediate T cell immunity through an antioxidant mechanism. J Biol Chem 283:20181–20185
Stewart MS, Spallholz JE, Neldner KH, Pence BC (1999) Selenium compounds have disparate abilities to impose oxidative stress and induceapoptosis. Free Radic Biol Med 26:42–48
Tinkel J, Hassanain H, Khouri SJ (2012) Cardiovascular antioxidant therapy: a review of supplements, pharmacotherapies, and mechanisms. Cardiol Rev 20:77–83
Vega L, Rodriguez-Sosa M, Garcia-Montalvo EA, Del RLM, Elizondo G (2007) Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food Chem Toxicol 45:1147–1153
Verma S, Molina Y, Lo YY, Cropp B, Nakano C, Yanagihara R, Nerurkar VR (2008) In vitro effects of selenium deficiency on West Nile virus replication and cytopathogenicity. Virol J 5:66
Wu JJ, Li WM, Zhao RX, Fang J, Wang C, Peng XL, Li X, Xiong JJ, Yang LG, Zhang SJ (2008) The effects of the polymorphism in exon 3 of the FAS gene on the death of chickenembryos during the incubation period. Anim Genet 39:558–560
Yang YJ, Zhao JL, You SJ, Wu YJ, Jing ZC, Yang WX, Meng L, Wang YW, Gao RL (2006) Different effects of tirofiban and aspirin plus clopidogrel on myocardial no-reflow in a mini-swine model of acute myocardial infarction and reperfusion. Heart 92:1131–1137
Zeng H (2002) Selenite and selenomethionine promote HL-60 cell cycle progression. J Nutr 132:674–679
Zhang ZW, Lv ZH, Li JL, Li S, Xu SW, Wang XL (2011) Effects of cold stress on nitric oxide in duodenum of chicks. Poult Sci 90:1555–1561
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149:352–361
Zhou N, Xiao H, Li TK, Nur-E-Kamal A, Liu LF (2003) DNA damage-mediated apoptosis induced by selenium compounds. J Biol Chem 278:29532–29537
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871902), the Science Foundation of the Education Department of Heilongjiang Province (11551030). The authors thank the members of the veterinary internal medicine laboratory in college of veterinary medicine, northeast agricultural university for help in feeding the chicks and analyzing the data.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Zw., Zhang, Jl., Zhang, Yh. et al. Effect of oxygen free radicals and nitric oxide on apoptosis of immune organ induced by selenium deficiency in chickens. Biometals 26, 355–365 (2013). https://doi.org/10.1007/s10534-013-9612-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9612-8