Abstract
Selenium (Se) is an essential trace element in many life forms due to its occurrence as selenocysteine (Sec) residue in selenoproteins. However, little is known about the expression pattern of selenoproteins in the liver of layer chicken. To investigate the effects of Se deficiency on the mRNA expressions of selenoproteins in the liver tissue of layer chickens, 1-day-old layer chickens were randomly allocated into two groups (n = 120/group). The Se-deficient group (−Se) was fed a Se-deficient corn–soy basal diet; the Se-adequate group as control (+Se) was fed the same basal diet supplemented with Se at 0.15 mg/kg (sodium selenite). The liver tissue was collected and examined for mRNA levels of 21 selenoprotein genes at 15, 25, 35, 45, 55, and 65 days old. The data indicated that the mRNA expressions of Gpx1, Gpx2, Gpx3, Gpx4, Sepn1, Sepp1, Selo, Sepx1, Selu, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, SPS2, Selm, SelPb, Sep15, and Sels were decreased (p < 0.05), but not the levels of Dio3 and Seli (p > 0.05). The results showed that the mRNA levels of 19 selenoprotein (except Seli and Dio3) genes in the layer chicken liver were regulated by diet Se level. The present study provided some compensated data about the roles of Se in the regulation of selenoproteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential micronutrient and plays an important role in biological functions in human and many other species [1]. It was indicated that Se plays important roles in chemoprevention, neurobiology, immune functions, muscle metabolism, reproduction, redox reactions, and many other aspects of health [2–4]. However, Se deficiency induces a number of diseases and injuries in chickens including skeletal myodegeneration, vascular lesions and hemorrhages in muscles, exudative diathesis (ED), pancreatic atrophy, reduced egg production, liver injury, decreased hatchability, and inhibited bursal and thymic growth [5, 6]. As indicated in previous studies, the liver is one target organ of Se deficiency, and the Se level could affect the function and structure of the liver.
The biological functions of Se are primarily implemented through its presence in selenoproteins [7, 8]. And 25 selenoprotein genes in human and chicken; 24 selenoprotein genes in mice, rat, and frog; and 38 selenoprotein genes in zebrafish have been identified [9]. It was indicated that these selenoproteins reserve kinds of functions [10] and are essential for the normal growth, development, and metabolism of many organisms [11, 12]. Selenoproteins are extensively expressed in animals. It was showed that there are 17 selenoproteins are all expressed in the liver, testis, thyroid, and pituitary of pigs [13]. Selenoproteins, such as GPxs, are found to express in the liver, kidney, and muscle in rats [14]; Selw and SelN are expressed in kinds of tissues in chicken, especially in muscles [15, 16]. In addition, the levels of several selenoproteins are modulated by the levels of dietary Se in chicken different tissues. Se-deficient could influence the mRNA levels of Selw in chicken pancreatic and gastrointestinal tract tissue (glandular stomach, gizzard, duodenum, small intestine, and rectum) [6, 11]. And dietary Se deficiency decreased the mRNA levels of seven common selenoprotein genes (Gpx1, Gpx4, Sepw1, Sepn1, Sepp1, Selo, and Selk) in broiler chicken muscle and liver [5]. These valuable data showed that the expression pattern of selenoproteins and the effect of Se on the level of these selenoproteins varied from different tissues and species. Although dietary Se could regulate the expression of several selenoproteins in chicken liver tissue, the expression pattern of complete selenoproteins (except SelW, SelN, and SelT) in layer chicken liver has not been reported yet, and the effect of dietary Se on the expression of selenoproteins genes is unclear. In the present study, we examined the expression levels of 21 selenoproteins and detected the effect of dietary Se deficiency on the mRNA levels of selenoproteins in layer chicken liver.
Materials and Methods
Birds and Diets
Two hundred and forty 1-day-old ISA layer chickens were purchased from Weiwei Co. Ltd. (Harbin, China) and were randomly allocated into two groups (Se deficiency group and the control group). Each treatment was replicated five times with 24 chickens each. The Se-deficient group (−Se) was fed a Se-deficient corn–soy basal diet (corn and soy were produced in the Se-deficient region of Heilongjiang Province, China, and the diet contained 0.03 mg of Se/kg). The Se-adequate group as control group (+Se) was fed the same basal diet supplemented with Se at 0.15 mg/kg (sodium selenite). The feeding experiment lasted for 65 days and the experimental animals were given free access to feed and water. All animals were checked daily and body weights of birds were recorded weekly. All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. On 15, 25, 35, 45, 55, and 65 days, chicken livers were collected from individual chickens after euthanized with sodium pentobarbital. The tissues were rinsed with ice-cold sterile deionized water, frozen immediately in liquid nitrogen, and stored at −80 °C until analysis.
Quantification of Selenoproteins mRNA
Total RNA of chickens tissues (50 mg tissue; n = 5/diet group) was isolated by using a Trizol reagent according to the manufacturer’s instructions (Invitrogen, China). The dried RNA pellets were resuspended in 50 μl of diethyl pyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 5 μg of total RNA using oligo dT primers and Superscript II reverse transcriptase according to the manufacturer’s instructions (Invitrogen, China). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C before use.
After quantification, the expression levels of selenoproteins genes were determined by the technology of Real-time quantitative reverse transcription PCR by using SYBR Premix ExTaq TM (Takara, China), and real time PCR system was ABI PRISM 7500 real-time PCR system (Applied Biosystems). The PCR primers (Table 1) were designed using Oligo Primer Analysis software (version6.0) and synthesized by Invitrogen (Shanghai, China).
Reactions were consisted of the following: 10 μl of 2× SYBR Green I PCR Master Mix (TaKaRa, China), 0.4 μl of 50× ROX reference Dye II, 0.4 μl of each primer (10 μM), 2 μl of either diluted cDNA, and 6.8 μl of PCR-grade water. The PCR procedure for selenoproteins and GAPDH consisted of 95 °C for 30 s followed by 40 cycles at 95 °C for 15 s and 60 °C for 30 s. Results (fold changes) were expressed as 2−ΔΔCt in which
where Ct selenoprotein and Ct GAPDH are the cycle thresholds for chicken selenoproteins and GAPDH genes in different treated groups, respectively, t is the Se-deficiency group, and c is the control group (Se-adequate group).
Statistical Analyses
Data were performed using SPSS for Windows system (SPSS, Chicago, IL, USA). Data are expressed as the mean ± standard deviation. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using two-tailed paired Student’s t test. p < 0.05 was considered statistically significant.
Results
The mRNA levels of 21 selenoprotein genes in layer chickens’ liver were detected in both Se-adequate group and Se-deficient group (Table 2). At day 15, the mRNA levels of 14 selenoprotein genes (Gpx2, Gpx3, Gpx4, Txnrd1, Txnrd3, Dio1, Sepp1, Selpb, Selh, Selm, Selo, Sels, Sepx1, and Selu) were lower in the −Se group than in the +Se group (p < 0.05); the −Se chickens had about 18.14–80 % lower (p < 0.05) mRNA levels of selenoproteins than those of +Se chickens. However, the mRNA level of Sep15 was increased (28.4 times) in the −Se group than that in the +Se group (p < 0.05), and there was no effect of Se deficiency on the expression of Gpx1, Txnrd2, Dio2, Dio3, SPS2, and Seli (p > 0.05). It was showed that 15 selenoprotein genes were regulated by Se deficiency.
At day 25, when compared with the control group, a significant decrease in the levels of 16 selenoprotein genes (Gpx1, Gpx2, Gpx3, Gpx4, Txnrd1, Txnrd3, Dio1, SPS2, Sepp1, Selpb, Selh, Selm, Sels, Selo, Sepx1, and Selu) was observed in Se deficiency group (p < 0.05). Among these decreased selenoproteins, Sels was reduced by 75.1 % compared with the control. And Sep15 was still increased (82.9 times) in the −Se group than in the +Se group (p < 0.05). And there was no significant difference between the −Se group and +Se group in the mRNA levels of Seli, Txnrd2, Dio2, and Dio3 (p > 0.05).
At day 35, the mRNA levels of Sep15 was still increased (5.4 times) in the −Se group than in +Se group (p < 0.05), and the mRNA levels of Seli, Dio2, and Dio3 were not influenced by Se deficiency (p > 0.05). In addition, the mRNA levels of other selenoprotein genes were decreased by 22.96–76.2 % (Sels) in the −Se group (p < 0.05). It showed that the number of selenoproteins that were influenced by Se deficiency was raised along with the extension of time.
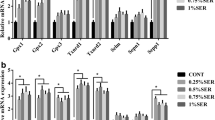
At day 45 (Fig. 1), the mRNA levels of 18 selenoprotein genes (Gpx1, Gpx2, Gpx3, Gpx4, Txnrd1, Txnrd2, Txnrd3, Dio1, Dio2, SPS2, Sepp1, Selpb, Selh, Selm, Selo, Sels, Sepx1, and Selu) were decreased by 40.8–88.78 % in the −Se group (p < 0.05), whereas the mRNA level of Sep15 was still increased in the −Se group (p < 0.05). And there was no significant difference between the −Se group and +Se group in the mRNA levels of Seli and Dio3 (p > 0.05).
At day 55 (Fig. 2), the mRNA levels of all selenoprotein genes (except Seli and Dio3) were decreased (34.33–87.52 %) in the −Se group than (p < 0.05). At this time point, the mRNA level of Sep15 was decreased by Se deficiency, which was different from the former time points. In addition, at day 65, the tendency of mRNA expressions of 21 selenoprotein genes was similar to that at 55 days. It indicated that the effect of Se deficiency on the expression of selenoproteins was affected by the extent of feeding time (data not shown) .
Discussion
The liver is primarily responsible for nutrient homeostasis by regulating protein, carbohydrate, and fat metabolism [17]. Se plays an important role in normal physiology in a wide range of species, including birds. Poultry Se deficiency results in slow growth and development, reduced egg production, decreased hatchability, pancreatic degeneration, nutritional muscular dystrophy, and morphology damage and change of GPx activity in the liver [18, 19]. In recent years, some studies showed that physiological functions of Se are considered to be mediated through selenoproteins [5, 20, 21]. However, there are only a few reports focusing on regulatory role of Se deficiency and the selenoproteins expression in chicken liver [5]. Previous research in our laboratory indicated that dietary Se affects the expression of four selenoprotein genes (Selw, Seln, Selk, and Selt) in the liver of layer chicken [20]. To further investigate the effects of dietary Se on selenoprotein expression, we examined the expression levels of 21 selenoprotein genes in chicken liver under deficient and adequate levels of dietary Se in the present study.
GPx, the first identified selenoprotein, is the most abundant in the liver. GPx family including GPxl, GPx2, GPx3, and GPx4 reserves kinds of enzymatic properties, and most are involved in the catabolism of peroxides [22]. Hill [23] suggested that Se deficiency caused the decrease of GPx mRNA level in HepG2 and H4IIE cells. The results of the present study showed a decreased expression of four GPx genes in chicken liver by Se deficiency. A similar observation was also made in rats [24] and turkey poults [25]. Interestingly, some studies indicated that the mRNA expressions of GPx1 and GPx2 were increased and the GPx4 mRNA expression was not influenced in mice by Se deficiency [26, 27]. The difference may be produced by the dietary Se level, treatment time, and animal species.
The thioredoxin reductase gene (TrxR) is one essential selenoprotein gene in different kinds of animals. Hill [28] reported that the mRNA expression and enzymatic activity of TrxR-1 decreased with increasing time feeding Se deficiency diet. A similar observation was also made in pig [29]. Our results showed that the gene expression of TrxR1, TrxR2, and TrxR3 was decreased in layer chicken liver by Se deficiency. In contrast, the TrxR1 mRNA expression was increased in broiler chicken liver [5]. The difference in TrxR1 mRNA expression may be due to breed of chickens.
Iodothyronine deiodinase (DIO) family plays an important role in thyroid metabolism [30]. Several studies have shown that the mRNA expression of Dio1 in Se-deficient mouse liver and kidney were significantly down-regulated [27], and the enzyme activity of Dio2 in Se-deficient rats brown adipose tissue was decreased [31]. There results suggested that dietary Se level can influence the mRNA expression and activity of Dio1 and Dio2 in vivo of animal. In the present study, we found that the mRNA expression of Dio1 and Dio2 was decreased in layer chicken liver by Se deficiency, and there was no significant change in the Dio3 expression, suggesting that the mRNA expression of Dio subtype may be independent. The Sep15 is located mainly in the endoplasmic reticulum and is believed to be involved in protein folding. It was showed that the mRNA expression of Sep15 in rodents was not regulated by dietary Se level [27]. Still, its precise function remains elusive [32]. The increase in the mRNA expression of Sep15 at 15, 25, 35, and 45 days in our study reflects the development of a compensatory mechanism in response to increased oxidative stress [33]. But due to little research, the effect of Se deficient on the mRNA levels of Dio and Sep15 gene needs to be further studied, especially in chicken.
Biosynthesis of selenoproteins depends on the production of monoselenophosphate (MSP), a Se donor compound from selenide and ATP, a reaction catalyzed by the enzyme, selenophosphate synthetase (SPS). Two isoforms of SPS were found in higher eukaryotes, SPS1 and SPS2 [34]. In in vitro experiments, SPS2 synthesized MSP from selenide and ATP, but SPS1 did not have this activity [35]. It indicated that SPS2 is involved in oxidative stress protection [36]. Sepp1 is the major transport form of Se. Selh was recently found to reside in nucleoli and predicted to possess a thioredoxin-like fold [37]. Selm may function as thiol disulfide oxidoreductase that participates in the formation of disulfide bonds, and can be implicated in calcium responses and redox regulation [38]. The functions for the proteins encoded by Selo, Sepx1, Sels, and Selu [12, 27] is less postulated in chicken. The effect of Se on the expressions of some of these selenoproteins was also reported. A decrease in Sepp1 mRNA level was observed in HepG2 and H4IIE cells by Se deficiency [39]. Messaoudi [40] suggested that the decrease of Sepp1 and GPx4 gene expression under Cd treatment was significantly restored in Cd +Se group in rat testicles. However, it was reported that the mRNA expressions of Sepx1, Selo, and Sels in rodents were not regulated by dietary Se [27]. The 3.0-mg Se/kg diet decreased Selh and Sepp1, but increased Sels mRNA levels in the liver of the rat offspring, compared with the 0.3-mg Se/kg diet [41]. In the present study, the gene expressions of SPS2, Sels, Selh, Selm, Selpb, Selu, Selo, Sepx1, and Sepp1 were significantly decreased in the liver of chicken at Se-deficient groups. Our results showed that the Sepx1 expression among these selenoprotein genes was more sensitive to dietary Se. After a Se-deficient diet was fed to weanling rats for 14.5 weeks, the Sepp1 mRNA in the liver remained higher at all time points compared with control [23]. These results suggested that the selenoprotein genes (SPS2, Sels, Selh, Selm, Selpb, Selu, Selo, Sepx1, and Sepp1) may play a role in oxidant defense and be involved in liver metabolism. Se deficiency diet influenced the mRNA expression of selenoprotein genes in chicken liver. However, the information remains limited about the physiology and function of SPS2, Sels, Selh, Selm, Selpb, Selu, Selo, Sepx1, and Sepp1 in birds, particularly in chicken.
In conclusion, 21 selenoprotein genes are expressed in the layer chicken liver. The transcription levels of 19 selenoprotein (except Seli and Dio3) genes in the layer chicken liver was regulated by diet Se level. The present study provides some compensated data about the roles of Se in the regulation of selenoproteins.
References
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100:1679–1684
Yu D, Li JL, Zhang JL, Gao XJ, Xu S (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res 144:678–687
Naziroglu M, Karaoglu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Naziroglu M, Yurekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589–599
Huang JQ, Li DL, Zhao H et al (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610
Gao X, Xing H, Li S et al (2012) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol Trace Elem Res 145:181–188
Behne D, Kyriakopoulos A (2001) Mammalian selenium-containing proteins. Annu Rev Nutr 21:453–473
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Mariotti M, Ridge PG, Zhang Y et al (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS One 7:e33066
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci CMLS 66:2457–2478
Wang R, Sun B, Zhang Z, Li S, Xu S (2011) Dietary selenium influences pancreatic tissue levels of selenoprotein W in chickens. J Inorg Biochem 105:1156–1160
Pappas AC, Zoidis E, Surai PF, Zervas G (2008) Selenoproteins and maternal nutrition. Comparative biochemistry and physiology. Part B, Biochem Mol Biol 151:361–372
Zhou JC, Zhao H, Li JG et al (2009) Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr 139:1061–1066
Barnes KM, Evenson JK, Raines AM, Sunde RA (2009) Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr 139:199–206
Li JL, Ruan HF, Li HX et al (2011) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian selenoprotein W from chicken. Mol Biol Rep 38:4015–4022
Zhang J, Li J, Zhang Z et al (2012) Ubiquitous expression of selenoprotein N transcripts in chicken tissues and early developmental expression pattern in skeletal muscles. Biol Trace Elem Res 146:187–191
Burk RF, Hill KE (2005) Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr 25:215–235
Thuluvath PJ, Triger DR (1992) Selenium in chronic liver disease. J Hepatol 14:176–182
Naziroglu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Sun B, Wang R, Li J, Jiang Z, Xu S (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143:1516–1523
Liu Y, Zhao H, Zhang Q et al (2012) Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr 142:1410–1416
Burk RF (1991) Molecular biology of selenium with implications for its metabolism. FASEB: Off Publ Fed Am Soc Exp Biol 5:2274–2279
Hill KE, Lyons PR, Burk RF (1992) Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem Biophys Res Commun 185:260–263
Bermano G, Nicol F, Dyer JA et al (1995) Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J 311(Pt 2):425–430
Sunde RA, Hadley KB (2010) Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood) 235:23–31
Hrdina J, Banning A, Kipp A et al (2009) The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J Nutr Biochem 20:638–648
Sunde RA, Raines AM, Barnes KM, Evenson JK (2009) Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep 29:329–338
Hill KE, McCollum GW, Boeglin ME, Burk RF (1997) Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun 234:293–295
Hostetler CE, Michal J, Robison M, Ott TL, Kincaid RL (2006) Effect of selenium intake and fetal age on mRNA levels of two selenoproteins in porcine fetal and maternal liver. J Anim Sci 84:2382–2390
Berry MJ, Banu L, Larsen PR (1991) Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349:438–440
Arthur JR, Nicol F, Beckett GJ, Trayhurn P (1991) Impairment of iodothyronine 5′-deiodinase activity in brown adipose tissue and its acute stimulation by cold in selenium deficiency. Can J Physiol Pharmacol 69:782–785
Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN (2001) Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem 276:15330–15336
Lin L, Zhou W, Dai H et al (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235–236:343–351
Yeh MS, Huang CJ, Guo CH et al (2012) Identification and cloning of a selenophosphate synthetase (SPS) from tiger shrimp, Penaeus monodon, and its transcription in relation to molt stages and following pathogen infection. Dev Comp Immunol 36:21–30
Xu XM, Carlson BA, Mix H et al (2007) Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol 5:e4
Costa FC, Oliva MA, de Jesus TC, Schenkman S, Thiemann OH (2011) Oxidative stress protection of Trypanosomes requires selenophosphate synthase. Mol Biochem Parasitol 180:47–50
Novoselov SV, Kryukov GV, Xu XM et al (2007) Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem 282:11960–11968
Guariniello S, Colonna G, Raucci R et al (2014) Structure–function relationship and evolutionary history of the human selenoprotein M (SelM) found over-expressed in hepatocellular carcinoma. Biochim Biophys Acta 1844:447–456
Hill KE, Chittum HS, Lyons PR, Boeglin ME, Burk RF (1996) Effect of selenium on selenoprotein P expression in cultured liver cells. Biochim Biophys Acta 1313:29–34
Messaoudi I, Banni M, Said L, Said K, Kerkeni A (2010) Involvement of selenoprotein P and GPx4 gene expression in cadmium-induced testicular pathophysiology in rat. Chem Biol Interact 188:94–101
Zeng MS, Li X, Liu Y et al (2012) A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med 52:1335–1342
Acknowledgments
This work was supported by the Graduate innovative research project in Heilongjiang Province China (No. YJSCX-005HLJ), China Postdoctoral Science Foundation (No. 2012 M520702), And Startup Foundation for Doctors of Northeast Agricultural University, China (No. 2012RCB92).
Author information
Authors and Affiliations
Corresponding author
Additional information
All other authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the journal.
Rights and permissions
About this article
Cite this article
Liu, C.P., Fu, J., Lin, S.L. et al. Effects of Dietary Selenium Deficiency on mRNA Levels of Twenty-One Selenoprotein Genes in the Liver of Layer Chicken. Biol Trace Elem Res 159, 192–198 (2014). https://doi.org/10.1007/s12011-014-0005-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0005-9