Opinion statement
Intracranial stereotactic radiosurgery (SRS) is an effective and convenient treatment for many brain conditions. Data regarding safety come mostly from retrospective single institutional studies and a small number of prospective studies. Variations in target delineation, treatment delivery, imaging follow-up protocols and dose prescription limit the interpretation of this data. There has been much clinical focus on radiation necrosis (RN) in particular, as it is being increasingly recognized on follow-up imaging. Symptomatic RN may be treated with medical therapy (such as corticosteroids and bevacizumab) with surgical resection being reserved for refractory patients. Nevertheless, RN remains a challenging condition to manage, and therefore upfront patient selection for SRS remains critical to provide complication-free control. Mitigation strategies need to be considered in situations where the baseline risk of RN is expected to be high—such as large target volume or re-irradiation. These may involve reduction in the prescribed dose or hypofractionated stereotactic radiation therapy (HSRT). Recently published guidelines and international meta-analysis report the benefit of HSRT in larger lesions, without compromising control rates. However, careful attention to planning parameters and SRS techniques still need to be adhered, even with HSRT. In cases where the risk is deemed to be high despite mitigation, a combination approach of surgery with or without post-operative radiation should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The delivery of conformal radiation in one (SRS) or 3–5 fractions (HSRT) has been used in brain lesions for > 50 years. It is proven to be effective and convenient and is being increasingly adopted worldwide. This is exemplified in the setting of brain metastases (BM), where the use of whole-brain radiotherapy (WBRT) is decreasing and the use of SRS is increasing [1]. Other conditions commonly treated with SRS include non-malignant tumours (meningioma, pituitary adenoma, vestibular schwannoma) and malignant (recurrent glioma) tumours, arteriovenous vascular malformations (AVM) and functional disorders (trigeminal neuralgia and movement disorders). The principles of SRS/HSRT involve accurate target localization, meticulous treatment planning with multiple beam angles allowing for conformal dose distribution around the target and image-guided treatment delivery. Multiple commercial platforms are currently available including Gamma Knife, CyberKnife or linear accelerator (LINAC), which are all able to produce a sharp dose fall-off outside the target. However, due to the physical properties of megavoltage radiation, the peri-lesional areas are invariably exposed to low-intermediate doses of radiation, resulting in treatment-related imaging changes which can be indistinguishable from tumour recurrence. These changes may represent radiation necrosis (inflammation from tissue death), which can lead symptomatic oedema and a decline in the patient’s quality of life.
In this focused review, we will elaborate on the pathophysiology, diagnosis and contributing factors for radiation necrosis. In addition, since established treatments are limited, we will outline mitigation strategies to avoid RN.
Search strategy
We performed a search on MEDLINE using the MESH headings “radiosurgery” and “necrosis” on September 10, 2020. Eighty four citations were retrieved, and relevant studies have been included in this narrative review.
Incidence of RN
The incidence of RN is probably between 5 and 26%, with roughly 1/3–1/2 being symptomatic [2,3,4,5,6,7]. This large variation exists, in part, due to the varying definitions of RN (some based on radiological changes while others requiring pathological confirmation). The incidence is likely higher amongst contemporary cohorts owing to improved quality of diagnostic imaging, increased awareness of RN and longer survival of patients given improved oncological treatment. The largest series from Cleveland Clinic, assessing 1650 patients with 2843 brain metastases, report a radiographic RN rate (per lesion) of 8% with half being symptomatic [7•].
Pathophysiology of RN
Early animal experiments, using single fractions ranging from 10 to 25 Gy, indicated that the radiation tolerance of brain parenchyma was dependent on radiation dose, irradiated volume and time elapsed post-radiation [8, 9]. Post-mortem analysis demonstrated reduction in vasculature and myelin in the irradiated areas. In addition, these pre-clinical experiments demonstrated that the necrosis free-interval shortens with higher doses.
There are two main theories regarding the pathophysiology of RN:
-
Vascular injury theory
Large fraction sizes (typically > 8 Gy) can activate acid sphingomyelinase and cause upregulation of ceramide, leading to endothelial apoptosis [10]. Kamiryo et al. have reported that endothelial changes precede frank necrosis [11]. These changes include reduction of capillary density, increase in capillary diameter and thickening of the capillary basement membrane. In addition, radiation can lead to a pro-inflammatory milieu (involving TNF-alpha, IL-1beta, VEGF, ICAM-1) eventually leading to fibrinoid necrosis of small vessels, resulting in ischemia and cell death of brain parenchyma [12,13,14,15,16].
-
Glial cell theory
Both oligodendrocytes and astrocytes can be affected by radiation, with the former being more sensitive [17]. Direct damage to oligodendrocytes and their precursors (oligodendrocyte type2 astrocytes) may lead to white matter demyelination and necrosis [18]. Radiation has been shown to cause activation of astrocytes (reactive astrogliosis), contributing to the inflammatory environment which is seen in RN [19].
Diagnosis of RN
The diagnosis of RN can be notoriously challenging, which is a major limitation when interpreting the literature. There lacks a current gold standard, although histopathological confirmation is preferred. In many situations, there can be a co-existence between radiation changes and residual tumour with the relative proportion influencing the final diagnosis [20, 21].
-
1.
Histopathological changes: Endothelial cells are highly susceptible, with morphological changes including fibrinoid necrosis, haemorrhage, hyalinisation and thrombosis of the blood vessels. The necrotic area has scant cellularity, surrounded by gliotic brain tissue containing GFAP-reactive astrocytes demonstrating prominent cytoplasmic ramification. Foamy macrophages and hemosiderophages can be seen, with occasional dystrophic calcification. Radiation-induced cytologic atypia may be encountered—with features of cytomegaly and bizarre-looking bubbly nuclei. In the setting of tumour recurrence with superimposed RN, immunohistochemical staining may be helpful (dependent on the primary pathology) to highlight viable tumour cells.
-
2.
Radiological investigations
-
Magnetic resonance imaging (MRI) is usually the initial investigation for patients with suspected RN. Unfortunately, both RN and tumour recurrence show contrast enhancement and peri-lesional oedema [22, 23]. Temporal changes (e.g. increase in lesion size) are not specific to either diagnosis. Characteristic enhancement patterns seen with RN, such as “swiss cheese” and “soap-bubble”, have a low positive predictive value [24]. Lesion-quotient (LQ) values below 0.3, referring to the ratio of nodule on T2 sequence to the total enhancing area on T1 sequence, have been suggested, but are not uniformly reproducible in other studies [24, 25]. The low predictive value of conventional MRI alone has prompted many groups to explore advanced imaging modalities to improve the diagnostic confidence of RN [26]. For example, serial radiomic changes and time-dependent contrast changes seen with conventional MRI imaging have shown potential to improve the diagnostic accuracy [27,28,29].
-
MR perfusion: Recurrent tumour has increased vasculature, resulting in higher perfusion and blood volume, compared to RN. An increased relative cerebral blood volume (rCBV) on dynamic susceptibility weighted MRI has been proposed to differentiate recurrent tumour from RN [30, 31]. However, the interpretation can be subjective. Hu et al. reported that rCBV < 0.71 of having a 92% sensitivity and 100% specificity for RN [31]. Other authors have suggested a higher rCBV cut-off of 2.1 (100% sensitivity and specificity) [32]. Acknowledging the significant overlap in rCBV cut-off values, Barajas et al. proposed using percentage of signal-intensity recovery as a metric for RN [22]. Intravoxel incoherent motion (IVIM) provides quantitative diffusion and perfusion readouts based on diffusion-weighted imaging and has been shown to be superior to rCBV for the diagnosis of RN [33].
-
MR spectroscopy: Intra-lesional metabolite composition may help with the diagnosis of tumour recurrence versus RN. For example, Zeng et al. have found that when both choline creatinine and choline-N-acetyl aspartate values were above 1.71, sensitivity, specificity and diagnostic accuracy were 94.1%, 100% and 96.2% respectively for tumour recurrence [34]. In contrast, an elevated lipid-lactate peak and low levels of metabolites would favour RN [35]. In addition, utilizing multi-voxel spectroscopy has been shown to be more accurate than single voxel [36]. However, MRS is limited by voxel size, requiring the lesion to be larger than 1 cm3, and is subject to sampling errors in heterogeneous tumours. Chemical exchange saturation transfer (CEST) imaging is a novel method looking at endogenous mobile peptides and has been shown to be differentiate RN from tumour recurrence with high accuracy in a small cohort [37].
-
Functional imaging: The main drawback of FDG-PET is the high physiological glucose metabolism in the normal brain parenchyma, which may mask tumour recurrences. Amino acid tracers such as carbon-11 methionine (MET), fluoro-1-thymidine (FLT) and fluroethyltyrosine (FET) have shown promise, as there is selective localization within the tumour, in contrast to normal brain parenchyma [38,39,40,41]. However, in a meta-analysis by Li et al., sub-group analysis comparing amino acid tracers and FDG had similar diagnostic accuracy in differentiating recurrent brain metastases from RN [42]. Apart from PET, thallium-201 SPECT has been shown to be useful to distinguish tumour recurrence from RN in brain metastases and high-grade gliomas [43].
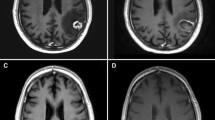
Figure 1 shows the various imaging modalities which can be used to diagnose RN.
a Tumour recurrence: (i) T2-weighted, (ii) post-contrast T1 and (iii) rCBV (relative cerebral blood volume) MR perfusion sequence of a lesion within the left temporal lobe. The lesion quotient of 0.71 and increased rCBV is suggestive of tumour recurrence. b Tumour recurrence: (i) rCBV and (ii) post-contrast T1 showing increased blood flow within the periphery of the lesion, which was histology proven to a tumour recurrence. Radiation necrosis: (iii) rCBV and (iv) post-contrast T1 showing no increase in blood flow. c Mixed picture of radiation necrosis and tumour recurrence: (i, ii) MR spectroscopy and (iii) post contrast T1 showing a growing pericallosal lesion post-WBRT. High lipid-lactate peak seen in radiation necrosis at the right cingulum while increased choline:creatine and choline:N-acetyl-aspartate ratios suggestive of tumour recurrence in the left cingulum. d Tumour recurrence: (i) F-18 FET PET showing amino acid tracer uptake within the enhancing lesion, with (ii) demonstrating the lesion on post-contrast T1 (adapted from Vellayappan B, Tan CL, Yong C, Khor LK, Koh WY, Yeo TT, Detsky J, Lo S, and Sahgal A. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front Oncol. 2018 Sep 28;8:395.doi: 10.3389/fonc.2018.00395. PMID: 30324090; PMCID: PMC6172328)
Contributory factors for RN
A direct cause effect relationship can be challenging, as the development of RN is likely multifactorial. The underlying condition for which SRS is being used should also be considered. Counter-intuitively, the risk of RN in patients with AVM undergoing SRS is lower than patients with BM, despite the shorter survival seen in patients with metastatic disease [44••].
-
1)
Dose-volume interplay: The radiation dose contributes to RN. SRS at doses above 24–25 Gy are at higher risk of causing RN [45]. Shehata et al. reported that in patients treated with prior WBRT, prescription doses exceeding 20 Gy (for brain metastases < 2 cm) increased the risk of Grade 3 or higher (G3+) toxicity [46]. In addition, the volume effect exists within the brain, where small targets can be safely treated to a higher dose, and a dose reduction is needed as the tumour volume increases. A stepwise reduction of safe SRS prescription doses, based on maximum tumour diameter, was determined by early RTOG trials [47]. In a multi-variable analysis from RTOG 90-05, tumour diameter was significantly associated with G3+ neuro-toxicity with tumours between 2 and 3 cm having a 7.3-fold higher risk and tumours 3 and 4 cm having a 16-fold higher risk (in comparison to tumours < 2 cm) [45].
-
2)
Volume of uninvolved brain parenchyma exposed to intermediate-high dose: This has been shown to be an independent risk factor in some studies [3, 5, 48, 49]. The V12 (volume of brain receiving 12 Gy or higher) metric has been commonly reported to correlate with RN [5, 48]. However, the cut-off values suggested by studies have varied, depending on the underlying condition and how the volumes were defined. It remains unclear if the gross target volume (GTV) should be subtracted from V12 when assessing this metric. This is an important consideration, especially with larger lesions. The recently published HyTEC report suggests a more conservative approach, where the tissue V12 is utilized (i.e. without subtracting GTV) [44••]. Based on their normal tissue complication probability (NTCP) modelling, the risk of RN is approximately 10% or less when V12 < 5 cc. SRS for AVM was associated with lower rates of RN, compared to brain metastases, for equivalent V12 values. With the increasing use of HSRT, many single institutional cohorts have reported the rates and dose-volume metrics associated with RN [50,51,52,53,54]. Inoue et al. and Peng et al. have suggested that the volume received by the single fraction equivalent dose of 14 Gy (SFED V14) is to be computed [51, 52, 54]. Assuming an alpha/beta value of 2, this translates to V23 (for 3 fractions) and V29 (for 5 fractions). The probability of G3+ toxicity (requiring surgical intervention) approximates 4% if these values exceed 20 cc [44••].
-
3)
Single versus multiple lesion SRS: Recent trials, and guidelines, have suggested SRS to be used in patients with multiple brain metastases [55,56,57]. Although there is no head-to-head comparison suggesting that the risk of RN is higher in patients with multiple BM undergoing SRS, the intermediate dose spillage to the brain parenchyma is expected to be higher than for a patient undergoing SRS to a single lesion. Previous studies have found the V12 metric to have a linear correlation with total tumour volume, rather than the number of targets [58]. With multiple targets, it remains unclear if the V12 metric should be assessed per lesion, or in totality for the patient (i.e. composite plan). Minniti et al. analysed that patients with >10 targets treated with LINAC-SRS report that the V12 per lesion predicts for post-treatment changes, rather than V12 from the composite plan [59].
-
4)
Prior radiation exposure: Re-irradiation, especially with previous SRS, increases the risk of RN significantly [60••]. The largest series from UCSF report a 1-year risk of 20% with re-SRS [4]. In situations where WBRT is used prior to or in conjunction with SRS, the risks of RN range between 4 and 8%, respectively [4]. These findings are not much higher than the rates seen with SRS alone.
-
5)
Use of concurrent systemic therapy (including immunotherapy, targeted therapy and chemotherapy): Controversy exists regarding the risk of RN with concurrent immunotherapy. Lehrer leads an individual patient data meta-analysis for patients with brain metastases treated with SRS and immunotherapy [61]. The overall incidence of RN was low at 5.3% (95% CI 0.3 to 15.7%), with ipilimumab being implicated with all cases [62,63,64]. This is much lower than single institutional studies, predominantly in patients with melanoma brain metastases, reporting RN to range from 14 to 37.5% when combining immunotherapy with SRS [65,66,67,68]. Martin et al. reviewed single institutional data and reported an increased risk of symptomatic RN with the use of ipilimumab and checkpoint inhibitors, for which only ipilimumab retained statistical significance [69]. Data regarding the safety of concurrent targeted therapy is sparse. In a study by Kim et al., the use of concurrent targeted therapy (defined as administration within five biological half-lives) increased the 12-month cumulative incidence of radiological RN (8.8 vs 5.3%, p < 0.01) [7•]. This was particularly pronounced with VEGFR tyrosine kinase inhibitors (TKI) and EGFR TKIs. Based on the ECOG consensus guidelines, the use of concurrent BRAF inhibitors do not seem to increase the risk of neuro-toxicity [70]. The use of concurrent chemotherapy, with SRS, has not been shown to increase the risk of RN, ranging from 4 to 17% [7, 71].
-
6)
Location of lesion: Certain brain locations may be more prone to developing RN (such frontal and occipital cortex, eloquent brain areas including thalamus, basal ganglia), whereas other locations (e.g. brainstem) are more resistant [72]. Superficial lesions may have a lower risk of RN, as the dose spillage happens within non-brain tissue (such as skull bone, skin) compared to deeper lesions [73].
-
7)
Planning target volume (PTV) margin: A larger gross-tumour-volume (GTV) to PTV margin would include a larger volume of normal brain parenchyma in the prescription isodose and therefore predispose to RN [74]. This has been investigated in a randomized controlled trial, comparing 1- and 3-mm GTV-PTV margins [75]. Local control was similar in both groups; however, the 3-mm PTV margin group had a higher incidence of RN.
-
8)
Intrinsic radiosensitivity of the patient: Studies from patients undergoing SRS for AVM suggest that the patients who developed RN were more sensitive to radiation [76]. This was shown using in vitro survival curves from skin fibroblasts obtained from patients who ultimately developed RN.
-
9)
SRS platform: SRS can be delivered by LINAC-based platforms (using dynamic conformal arcs or VMAT) or dedicated units including Gamma Knife or CyberKnife (using multiple non-coplanar beams). The risk of RN does not appear to vary between SRS platforms despite inherent differences in PTV margin and prescription practices. The frame-based Gamma Knife platform typically uses a 0-mm PTV margin and prescribes the dose to the 50% isodose line. This results in a higher maximum dose/prescription dose ratio (≥ 2), which has been associated with increased neuro-toxicity [47]. LINAC-based platforms, which often use relocatable masks, typically apply a 1–2-mm PTV margin. The dose is usually prescribed to the 60–80% isodose line, leading to less dose heterogeneity. For LINAC SRS, the dose gradient may be related to the prescription isodose line, with steeper gradients (less spillage to normal tissue) seen when the dose is prescribed to the 60–70% isodose line, in comparison to the 90% isodose line [77]. Dose heterogeneity (i.e. hot spots) has been suggested to contribute to RN, in particular if they occur within the GTV-PTV margin where normal brain parenchyma is located [78••]. Between platforms, the Gamma Knife appears to have less dose spillage, in comparison to LINAC, when treating multiple targets [79, 80].
-
10)
Lack of a quality assurance program: SRS is a technologically intensive treatment, and the potential for error is a major concern in view of the highly conformal nature, steep dose gradients and large dose per fraction. The lack of a robust end-to-end quality assurance program (i.e. simulation, target delineation, planning, delivery) does increase the risks of treatment-related complications, including RN [81].
Management of RN
In general, asymptomatic RN can be observed closely. Unlike tumour recurrence, RN may resolve spontaneously, with up to 76% of lesions resolving by 18 months [82]. First-line medical therapy for symptomatic patients involves corticosteroids (e.g. dexamethasone). Corticosteroids provide an anti-inflammatory effect and reduce the leakiness of the blood-tumour barrier [83]. Commonly used is a start dose of 4–8 mg/day, with a gradual reduction in dose. Patients may require steroids for prolonged periods of time being subject to steroid toxicities including myopathy, gastritis and immune suppression. Bevacizumab (anti-VEGF monoclonal antibody) can be used as a steroid-sparing agent. A pooled analysis has shown that bevacizumab causes radiographic and clinical improvement in majority of patients [84]. A small double-blinded randomized controlled trial and a multicentre prospective trial have both shown majority of patients having radiographic and clinical improvement with bevacizumab [85, 86]. The duration of effect was durable (median 10 months). Although bevacizumab is promising, the drug-related toxicities including haemorrhage, thrombosis and impairing wound healing must be considered [87]. Other therapies such as anti-coagulants and vitamin E/pentoxifylline have been explored but are yet to be considered mainstream [88, 89]. Hyperbaric oxygen therapy (HBOT) has shown to have potential in the prophylaxis of RN [90]. The use of HBOT to reverse established RN is equivocal with most studies being limited to case reports [91, 92].
Patients who remain symptomatic despite conservative management, or patients with neurological deterioration from mass effect, should be considered for surgical resection [93].
Surgical resection aids in confirming the diagnosis and allows rapid relief of mass effect and a quicker reduction of steroids [94]. However, surgical resection may only be possible for medically fit patients and for readily accessible cortical lesions.
For lesions which are less surgically accessible, a novel intervention using a laser-emitting diode placed within the centre of the lesion called laser interstitial thermal ablation (LITT) has been increasingly used [95]. It is considerably less invasive than an open craniotomy and manages to obtain a tissue diagnosis. Interestingly, LITT has been shown to be effective for both recurrent metastatic lesions and RN [96, 97]. A prospective multicentre study investigated the utility of LITT for lesions which are progressing after SRS. Patients who underwent total ablation for RN (n = 4) and recurrence (n = 4) had a 3-month control rate of 100% and 75% respectively [98••]. Approximately one in three patients is able to reduce, or stop, steroid use within 3 months of the procedure. Although the sample size is small, these results appear promising.
Mitigation strategies for RN
The diagnosis and management of RN are challenging. Strategies to prevent and mitigate RN should be considered upfront in the clinical management of patients. Our proposed mitigation strategies have been outlined in Table 1. Notably, multifraction SRS (or HSRT) is being increasingly used. Multiple authors have reported the use of HSRT in large lesions to reduce the risk of RN while maintaining control rates [51,52,53, 99, 100]. Lehrer published an international meta-analysis of 24 trials studying the effects of fractionation and tumour volume [101••]. Fractionation reduced the risk of RN significantly for lesions > 3 cm diameter (7.3% vs 23.1%, p = 0.003). There was no significant difference, with fractionation, for lesions < 3 cm (6.5 vs 11.7%, p = 0.29). The recently published HyTEC paper confirms the use of HSRT to reduce the risk of RN in larger lesions [44••]. However, the exact cut-off to be used for what is considered large varies from > 2 to > 3 cm in diameter.
Clearly, large brain lesions being treated with single fraction SRS place patients at higher risk of RN. As such, HSRT should be considered. If acceptable dose constraints are not attainable, upfront surgical resection followed by cavity radiation should be considered. Regarding the dose constraints, the HyTEC paper has summarized the available evidence, reporting that if tissue V12 (i.e. including target volume) exceeds 5, 10 and >15 cc, the risk of symptomatic RN approximates 10, 15 and 20% respectively [44••]. This corroborates with QUANTEC recommendation of V12 to be < 5–10 cc and the UK SABR consortium guidelines of V12 < 10 cc [102, 103]. In the setting of HSRT, the HyTEC paper recommends that SFED 14 Gy to be < 20 cc (which corresponds to V23 in 3 fractions and V29Gy in 5 fractions) to limit G3+ toxicity to < 4% [44••]. Clearly, caution should be exercised in patients who have undergone prior SRS [4]. The recovery of brain parenchyma from prior radiation, or the preferred time interval, is still unclear. Using the smallest possible PTV margin, by optimizing the patient setup and the use of image guidance, reduces the volume of normal brain parenchyma exposed to high doses of radiation [75].
Novel approaches, which are currently investigational, such as angiotensin receptor blockers (single institutional retrospective cohort) and ultra-high dose rate (FLASH) radiation (pre-clinical), have shown some signals in reducing the radiation-induced inflammatory responses [104, 105].
Summary
Symptomatic RN tends to be permanent and continues to be challenging to manage—as such the premise lies in mitigation and, if possible, prevention. Clearly, rigorous SRS techniques (including immobilization, target visualization, treatment planning and delivery) and adherence to known dose limits are important. Additional mitigation strategies will need to be considered in situations where the baseline risk of RN is expected to be high—such as large target volume or re-irradiation. These may involve reduction in the prescribed dose or employing strategies such as surgery with or without radiotherapy or fractionated approach. Notably, the vast majority of reported RN is retrospective in nature, typically from single institutional series. Multi-institutional prospective registries with clear, consensus-based, endpoints would be useful to guide clinical practice.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Barbour AB, Jacobs CD, Williamson H, Floyd SR, Suneja G, Torok JA, et al. Radiation therapy practice patterns for brain metastases in the United States in the stereotactic radiosurgery era. Adv Radiat Oncol. 2020;5(1):43–52. https://doi.org/10.1016/j.adro.2019.07.012.
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neuro-Oncol. 2015;125(1):149–56. https://doi.org/10.1007/s11060-015-1881-3.
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. https://doi.org/10.1186/1748-717X-6-48.
Sneed PK, Mendez J. Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–86. https://doi.org/10.3171/2014.10.JNS141610.
• Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006 One of the early studies to suggest irradiated volume to predict for RN.
Schuttrumpf LH, Niyazi M, Nachbichler SB, Manapov F, Jansen N, Siefert A, et al. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1-3 cerebral metastases. Radiat Oncol. 2014;9:105. https://doi.org/10.1186/1748-717X-9-105.
Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro-Oncol. 2017;133(2):357–68. https://doi.org/10.1007/s11060-017-2442-8 Large retrospective study from Clevland Clinic looking at the association between RN and the use of various systemic therapeuatic agents.
Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61(731):1043–52. https://doi.org/10.1259/0007-1285-61-731-1043.
Benczik J, Tenhunen M, Snellman M, Joensuu H, Farkkila M, Joensuu R, et al. Late radiation effects in the dog brain: correlation of MRI and histological changes. Radiother Oncol. 2002;63(1):107–20. https://doi.org/10.1016/s0167-8140(02)00028-2.
Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. https://doi.org/10.1016/j.ccr.2005.07.014.
Kamiryo T, Lopes MB, Kassell NF, Steiner L, Lee KS. Radiosurgery-induced microvascular alterations precede necrosis of the brain neuropil. Neurosurgery. 2001;49(2):409–14; discussion 14-5. https://doi.org/10.1097/00006123-200108000-00026.
Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61(24):8859–65.
Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–53. https://doi.org/10.1158/1078-0432.CCR-03-0426.
Nordal RA, Wong CS. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys. 2005;62(1):279–87. https://doi.org/10.1016/j.ijrobp.2005.01.039.
Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neuro-Oncol. 2011;105(2):423–31. https://doi.org/10.1007/s11060-011-0610-9.
Burger PC, Mahley MS Jr, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44(4):1256–72. https://doi.org/10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t.
Panagiotakos G, Alshamy G, Chan B, Abrams R, Greenberg E, Saxena A, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2007;2(7):e588. https://doi.org/10.1371/journal.pone.0000588.
Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity--molecular and cellular mechanisms. Br J Cancer. 2001;85(9):1233–9. https://doi.org/10.1054/bjoc.2001.2100.
Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21(3):457–67. https://doi.org/10.1016/j.nbd.2005.08.006.
Truong MT, St Clair EG, Donahue BR, Rush SC, Miller DC, Formenti SC, et al. Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery. 2006;59(1):86–97; discussion 86-97. https://doi.org/10.1227/01.NEU.0000219858.80351.38.
Jagannathan J, Bourne TD, Schlesinger D, Yen CP, Shaffrey ME, Laws ER Jr, et al. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery. 2010;66(1):208–17. https://doi.org/10.1227/01.NEU.0000359318.90478.69.
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30(2):367–72. https://doi.org/10.3174/ajnr.A1362.
Forsyth PA, Kelly PJ, Cascino TL, Scheithauer BW, Shaw EG, Dinapoli RP, et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg. 1995;82(3):436–44. https://doi.org/10.3171/jns.1995.82.3.0436.
Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63(5):898–903; discussion 4. https://doi.org/10.1227/01.NEU.0000333263.31870.31.
Chao ST, Ahluwalia MS, Barnett GH, Stevens GH, Murphy ES, Stockham AL, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–57. https://doi.org/10.1016/j.ijrobp.2013.05.015 Concise opinion paper on RN.
Stockham AL, Tievsky AL, Koyfman SA, Reddy CA, Suh JH, Vogelbaum MA, et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neuro-Oncol. 2012;109(1):149–58. https://doi.org/10.1007/s11060-012-0881-9.
Zhang Z, Yang J, Ho A, Jiang W, Logan J, Wang X, et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur Radiol. 2018;28(6):2255–63. https://doi.org/10.1007/s00330-017-5154-8.
Zach L, Guez D, Last D, Daniels D, Grober Y, Nissim O, et al. Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro-Oncology. 2015;17(3):457–65. https://doi.org/10.1093/neuonc/nou230.
Wagner S, Lanfermann H, Eichner G, Gufler H. Radiation injury versus malignancy after stereotactic radiosurgery for brain metastases: impact of time-dependent changes in lesion morphology on MRI. Neuro-Oncology. 2017;19(4):586–94. https://doi.org/10.1093/neuonc/now193.
Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD. Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neuro-Oncol. 2009;95(1):1–11. https://doi.org/10.1007/s11060-009-9897-1.
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30(3):552–8. https://doi.org/10.3174/ajnr.A1377.
Muto M, Frauenfelder G, Senese R, Zeccolini F, Schena E, Giurazza F, et al. Dynamic susceptibility contrast (DSC) perfusion MRI in differential diagnosis between radionecrosis and neoangiogenesis in cerebral metastases using rCBV, rCBF and K2. Radiol Med. 2018;123(7):545–52. https://doi.org/10.1007/s11547-018-0866-7.
Detsky JS, Keith J, Conklin J, Symons S, Myrehaug S, Sahgal A, et al. Differentiating radiation necrosis from tumor progression in brain metastases treated with stereotactic radiotherapy: utility of intravoxel incoherent motion perfusion MRI and correlation with histopathology. J Neuro-Oncol. 2017;134(2):433–41. https://doi.org/10.1007/s11060-017-2545-2.
Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neuro-Oncol. 2007;84(1):63–9. https://doi.org/10.1007/s11060-007-9341-3.
Shah R, Vattoth S, Jacob R, Manzil FF, O'Malley JP, Borghei P, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–59. https://doi.org/10.1148/rg.325125002.
Chernov M, Hayashi M, Izawa M, Ochiai T, Usukura M, Abe K, et al. Differentiation of the radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg. 2005;48(4):228–34. https://doi.org/10.1055/s-2005-870952.
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ. Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. 2017;23(14):3667–75. https://doi.org/10.1158/1078-0432.CCR-16-2265.
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98(5):1056–64. https://doi.org/10.3171/jns.2003.98.5.1056.
Tomura N, Kokubun M, Saginoya T, Mizuno Y, Kikuchi Y. Differentiation between treatment-induced necrosis and recurrent tumors in patients with metastatic brain tumors: comparison among (11)C-methionine-PET, FDG-PET, MR permeability imaging, and MRI-ADC-preliminary results. AJNR Am J Neuroradiol. 2017;38(8):1520–7. https://doi.org/10.3174/ajnr.A5252.
Enslow MS, Zollinger LV, Morton KA, Butterfield RI, Kadrmas DJ, Christian PE, et al. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med. 2012;37(9):854–61. https://doi.org/10.1097/RLU.0b013e318262c76a.
Heinzel A, Muller D, Yekta-Michael SS, Ceccon G, Langen KJ, Mottaghy FM, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET for evaluation of brain metastasis recurrence after radiotherapy: an effectiveness and cost-effectiveness analysis. Neuro-Oncology. 2017;19(9):1271–8. https://doi.org/10.1093/neuonc/now310.
Li H, Deng L, Bai HX, Sun J, Cao Y, Tao Y, et al. Diagnostic accuracy of amino acid and FDG-PET in differentiating brain metastasis recurrence from radionecrosis after radiotherapy: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39(2):280–8. https://doi.org/10.3174/ajnr.A5472.
Matsunaga S, Shuto T, Takase H, Ohtake M, Tomura N, Tanaka T, et al. Semiquantitative analysis using thallium-201 SPECT for differential diagnosis between tumor recurrence and radiation necrosis after gamma knife surgery for malignant brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(1):47–52. https://doi.org/10.1016/j.ijrobp.2012.03.008.
Milano MT, Grimm J, Niemierko A, Soltys SG, Moiseenko V, Redmond KJ et al. 2020. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2020.08.013. Important publication from the HyTEC group summarising the available evidence of tolerances
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–8. https://doi.org/10.1016/s0360-3016(99)00507-6.
Shehata MK, Young B, Reid B, Patchell RA, St Clair W, Sims J, et al. Stereotatic radiosurgery of 468 brain metastases < or = 2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59(1):87–93. https://doi.org/10.1016/j.ijrobp.2003.10.009.
Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34(3):647–54. https://doi.org/10.1016/0360-3016(95)02106-x.
Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006, 64(2):419–24. https://doi.org/10.1016/j.ijrobp.2005.07.980 Pioneering manuscript suggesting the use of the V12 metric for gamma knife treated patients.
Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94(6):899–904. https://doi.org/10.3171/jns.2001.94.6.0899.
Faruqi S, Ruschin M, Soliman H, Myrehaug S, Zeng KL, Husain Z et al. Adverse radiation effect after hypofractionated stereotactic radiosurgery in 5 daily fractions for surgical cavities and intact brain metastases. International Journal of Radiation Oncology • Biology • Physics. doi:10.1016/j.ijrobp.2019.12.002. Recent publication from Sunnybrooke reporting the outcome of patients treated with 30Gy in 5 fractions (cavity and intact). This paper also suggests tolerances to adhere to during HSRT.
Inoue HK, Seto K, Nozaki A, Torikai K, Suzuki Y, Saitoh J, et al. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res. 2013;54(4):727–35. https://doi.org/10.1093/jrr/rrt006.
Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J, et al. Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res. 2014;55(2):334–42. https://doi.org/10.1093/jrr/rrt127.
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81(1):18–24. https://doi.org/10.1016/j.radonc.2006.08.024.
Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys. 2018;102(4):1236–43. https://doi.org/10.1016/j.ijrobp.2018.05.041.
Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–25. https://doi.org/10.1016/j.prro.2011.12.004.
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95. https://doi.org/10.1016/S1470-2045(14)70061-0.
Hughes RT, Masters AH, McTyre ER, Farris MK, Chung C, Page BR, et al. Initial SRS for patients with 5 to 15 brain metastases: results of a multi-institutional experience. Int J Radiat Oncol Biol Phys. 2019;104(5):1091–8. https://doi.org/10.1016/j.ijrobp.2019.03.052.
Hatiboglu MA, Akdur K. Evaluating critical brain radiation doses in the treatment of multiple brain lesions with gamma knife radiosurgery. Stereotact Funct Neurosurg. 2017;95(4):268–78. https://doi.org/10.1159/000478272.
Minniti G, Capone L, Nardiello B, El Gawhary R, Raza G, Scaringi C, et al. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neuro-Oncol. 2020;148(1):47–55. https://doi.org/10.1007/s11060-020-03442-7.
•• Siddiqui ZA, Squires BS, Johnson MD, Baschnagel AM, Chen PY, Krauss DJ, et al. Predictors of radiation necrosis in long-term survivors after gamma knife stereotactic radiosurgery for brain metastases. Neurooncol Pract. 2020;7(4):400–8. https://doi.org/10.1093/nop/npz067 Recent publication from Beaumont, Michigan suggesting that the risk of RN continues to be present for approximately 4 years post SRS, and the risks with re-SRS can be as high as 33%.
Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quinones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol. 2019;130:104–12. https://doi.org/10.1016/j.radonc.2018.08.025.
Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127(5):1007–14. https://doi.org/10.3171/2016.9.JNS161585.
Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6(3):e1283461. https://doi.org/10.1080/2162402X.2017.1283461.
Yusuf MB, Amsbaugh MJ, Burton E, Chesney J, Woo S. Peri-SRS Administration of immune checkpoint therapy for melanoma metastatic to the brain: investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg. 2017;100:632–40 e4. https://doi.org/10.1016/j.wneu.2017.01.101.
Fang P, Jiang W, Allen P, Glitza I, Guha N, Hwu P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neuro-Oncol. 2017;133(3):595–602. https://doi.org/10.1007/s11060-017-2470-4.
Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125(1):17–23. https://doi.org/10.3171/2015.6.JNS142763.
Kaidar-Person O, Zagar TM, Deal A, Moschos SJ, Ewend MG, Sasaki-Adams D, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anti-Cancer Drugs. 2017;28(6):669–75. https://doi.org/10.1097/CAD.0000000000000497.
Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer. 2019;7(1):102. https://doi.org/10.1186/s40425-019-0588-y.
Martin AM, Cagney DN, Catalano PJ, Alexander BM, Redig AJ, Schoenfeld JD, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018;4(8):1123–4. https://doi.org/10.1001/jamaoncol.2017.3993.
Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95(2):632–46. https://doi.org/10.1016/j.ijrobp.2016.01.038.
Shen CJ, Kummerlowe MN, Redmond KJ, Rigamonti D, Lim MK, Kleinberg LR. Stereotactic radiosurgery: treatment of brain metastasis without interruption of systemic therapy. Int J Radiat Oncol Biol Phys. 2016;95(2):735–42. https://doi.org/10.1016/j.ijrobp.2016.01.054.
Flickinger JC, Kondziolka D, Lunsford LD, Kassam A, Phuong LK, Liscak R, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000;46(5):1143–8. https://doi.org/10.1016/s0360-3016(99)00513-1.
Ohtakara K, Hayashi S, Nakayama N, Ohe N, Yano H, Iwama T, et al. Significance of target location relative to the depth from the brain surface and high-dose irradiated volume in the development of brain radionecrosis after micromultileaf collimator-based stereotactic radiosurgery for brain metastases. J Neuro-Oncol. 2012;108(1):201–9. https://doi.org/10.1007/s11060-012-0834-3.
Jhaveri J, Chowdhary M, Zhang X, Press RH, Switchenko JM, Ferris MJ, et al. Does size matter? Investigating the optimal planning target volume margin for postoperative stereotactic radiosurgery to resected brain metastases. J Neurosurg. 2018;130(3):797–803. https://doi.org/10.3171/2017.9.JNS171735.
Kirkpatrick JP, Wang Z, Sampson JH, McSherry F, Herndon JE 2nd, Allen KJ, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–8. https://doi.org/10.1016/j.ijrobp.2014.09.004.
Raaphorst GP, Malone S, Alsbeih G, Souhani L, Szumacher E, Girard A. Skin fibroblasts in vitro radiosensitivity can predict for late complications following AVM radiosurgery. Radiother Oncol. 2002;64(2):153–6. https://doi.org/10.1016/s0167-8140(02)00076-2.
Zhang Q, Zheng D, Lei Y, Morgan B, Driewer J, Zhang M, et al. A new variable for SRS plan quality evaluation based on normal tissue sparing: the effect of prescription isodose levels. Br J Radiol. 2014;87(1043):20140362. https://doi.org/10.1259/bjr.20140362.
•• Tanenbaum DG, Buchwald ZS, Jhaveri J, Schreibmann E, Switchenko JM, Prabhu RS, et al. Dosimetric factors related to radiation necrosis after 5-fraction radiosurgery for patients with resected brain metastases. Pract Radiat Oncol. 2020;10(1):36–43. https://doi.org/10.1016/j.prro.2019.09.014 Another recent publication reporting the outcome of HSRT and providing suggestions for dose tolerances.
Liu H, Andrews DW, Evans JJ, Werner-Wasik M, Yu Y. Dicker AP et al. Plan quality and treatment efficiency for radiosurgery to multiple brain metastases: non-coplanar RapidArc vs gamma knife Front Oncol. 2016;6:26. https://doi.org/10.3389/fonc.2016.00026.
Ma L, Nichol A, Hossain S, Wang B, Petti P, Vellani R, et al. Variable dose interplay effects across radiosurgical apparatus in treating multiple brain metastases. Int J Comput Assist Radiol Surg. 2014;9(6):1079–86. https://doi.org/10.1007/s11548-014-1001-4.
Solberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: executive summary. Pract Radiat Oncol. 2012;2(1):2–9. https://doi.org/10.1016/j.prro.2011.06.014.
Wang YX, King AD, Zhou H, Leung SF, Abrigo J, Chan YL, et al. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;254(1):210–8. https://doi.org/10.1148/radiol.09090428.
Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67(6):1799–815; discussion 815. https://doi.org/10.1227/NEU.0b013e3181fa775b.
Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J Neuro-Oncol. 2014;117(2):321–7. https://doi.org/10.1007/s11060-014-1391-8.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95. https://doi.org/10.1016/j.ijrobp.2009.12.061.
Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis(dagger). Neurooncol Pract. 2016;3(4):272–80. https://doi.org/10.1093/nop/npv064.
Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 2015;20(2):166–75. https://doi.org/10.1634/theoncologist.2014-0330.
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–7. https://doi.org/10.1212/wnl.44.11.2020.
Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86(6):359–66. https://doi.org/10.1159/000163557.
Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007;67(1):248–55. https://doi.org/10.1016/j.ijrobp.2006.08.009.
Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209(1-2):115–7. https://doi.org/10.1016/s0022-510x(03)00007-8.
Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70(Suppl 1):229–36. https://doi.org/10.1159/000056426.
McPherson CM, Warnick RE. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neuro-Oncol. 2004;68(1):41–7. https://doi.org/10.1023/b:neon.0000024744.16031.e9.
Nath SK, Sheridan AD, Rauch PJ, Yu JB, Minja FJ, Vortmeyer AO, et al. Significance of histology in determining management of lesions regrowing after radiosurgery. J Neuro-Oncol. 2014;117(2):303–10. https://doi.org/10.1007/s11060-014-1389-2.
Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: An overview. Expert Rev Neurother. 2016;16(2):223–32. https://doi.org/10.1586/14737175.2016.1135736.
Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neuro-Oncol. 2019;142(2):309–17. https://doi.org/10.1007/s11060-019-03097-z.
Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH, et al. Laser thermal ablation for metastases failing radiosurgery: a multicentered retrospective study. Neurosurgery. 2018;82(1):56–63. https://doi.org/10.1093/neuros/nyx142.
•• Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130(3):804–11. https://doi.org/10.3171/2017.11.JNS171273 Important prospective study investigating the use of LITT to treat both RN and brain tumors.
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–8. https://doi.org/10.1016/j.ijrobp.2016.03.013.
Dore M, Martin S, Delpon G, Clement K, Campion L, Thillays F. Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer Radiother. 2017;21(1):4–9. https://doi.org/10.1016/j.canrad.2016.06.010.
•• Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, et al. Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys. 2019;103(3):618–30. https://doi.org/10.1016/j.ijrobp.2018.10.038 International MA suggesting that HSRT is safer, and just as effective, for larger lesions.
UK Consortium: Stereotactic ablative body radiation therapy (SABR): a resource. https://www.sabr.org.uk/wp-content/uploads/2019/04/SABRconsortium-guidelines-2019-v6.1.0.pdf. Accessed 12 November 2020
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–7. https://doi.org/10.1016/j.ijrobp.2009.02.091.
Chowdhary M, Okwan-Duodu D, Switchenko JM, Press RH, Jhaveri J, Buchwald ZS, et al. Angiotensin receptor blockade: a novel approach for symptomatic radiation necrosis after stereotactic radiosurgery. J Neuro-Oncol. 2018;136(2):289–98. https://doi.org/10.1007/s11060-017-2652-0.
Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, et al. Ultra-high-dose-rate FLASH irradiation limits reactive gliosis in the brain. Radiat Res. 2020. https://doi.org/10.1667/RADE-20-00067.1.
Navarria P, Pessina F, Clerici E, Franceschini D, Gay LG, De Rose F, et al. Surgery followed by hypofractionated radiosurgery on the tumor bed in oligometastatic patients with large brain metastases. Results of a Phase 2 Study. Int J Radiat Oncol Biol Phys. 2019;105(5):1095–105. https://doi.org/10.1016/j.ijrobp.2019.08.054.
Prabhu RS, Patel KR, Press RH, Soltys SG, Brown PD, Mehta MP, et al. Preoperative vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery. 2019;84(1):19–29. https://doi.org/10.1093/neuros/nyy146.
Ruschin M, Sahgal A, Soliman H, Myrehaug S, Tsao M, Yeboah C, et al. Investigation of irradiated volume in linac-based brain hypo-fractionated stereotactic radiotherapy. Radiat Oncol. 2017;12(1):117. https://doi.org/10.1186/s13014-017-0853-5.
Nedzi LA, Kooy. H, Alexander E, 3rd, Gelman RS, Loeffler JS. Variables associated with the development of complications from radiosurgery of intracranial tumors. Int J Radiat Oncol Biol Phys. 1991;21(3):591–9. https://doi.org/10.1016/0360-3016(91)90675-t.
McDonald D, Schuler J, Takacs I, Peng J, Jenrette J, Vanek K. Comparison of radiation dose spillage from the gamma knife perfexion with that from volumetric modulated arc radiosurgery during treatment of multiple brain metastases in a single fraction. J Neurosurg. 2014;121(Suppl):51–9. https://doi.org/10.3171/2014.7.GKS141358.
Sahgal A, Barani IJ, Novotny J Jr, Zhang B, Petti P, Larson DA, et al. Prescription dose guideline based on physical criterion for multiple metastatic brain tumors treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78(2):605–8. https://doi.org/10.1016/j.ijrobp.2009.11.055.
Tallet AV, Dhermain F, Le Rhun E, Noel G, Kirova YM. Combined irradiation and targeted therapy or immune checkpoint blockade in brain metastases: toxicities and efficacy. Ann Oncol. 2017;28(12):2962–76. https://doi.org/10.1093/annonc/mdx408.
Schoonbeek A, Monshouwer R, Hanssens P, Raaijmakers E, Nowak P, Marijnissen JP, et al. Intracranial radiosurgery in the Netherlands. A planning comparison of available systems with regard to physical aspects and workload. Technol Cancer Res Treat. 2010;9(3):279–90. https://doi.org/10.1177/153303461000900307.
Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27(5):1231–9. https://doi.org/10.1016/0360-3016(93)90548-a.
Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105(Suppl):194–201. https://doi.org/10.3171/sup.2006.105.7.194.
Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219–22. https://doi.org/10.3171/jns.2000.93.supplement.
Wagner TH, Bova FJ, Friedman WA, Buatti JM, Bouchet LG, Meeks SL. A simple and reliable index for scoring rival stereotactic radiosurgery plans. Int J Radiat Oncol Biol Phys. 2003;57(4):1141–9. https://doi.org/10.1016/s0360-3016(03)01563-3.
Reynolds TA, Jensen AR, Bellairs EE, Ozer M. Dose gradient index for stereotactic radiosurgery/radiation therapy. Int J Radiat Oncol Biol Phys. 2020;106(3):604–11. https://doi.org/10.1016/j.ijrobp.2019.11.408.
Seung SK, Larson DA, Galvin JM, Mehta MP, Potters L, Schultz CJ, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS). Am J Clin Oncol. 2013;36(3):310–5. https://doi.org/10.1097/COC.0b013e31826e053d.
Sahgal A, Kellett S, Ruschin M, Greenspoon J, Follwell M, Sinclair J, et al. A cancer care Ontario organizational guideline for the delivery of stereotactic radiosurgery for brain metastasis in Ontario. Canada Pract Radiat Oncol. 2020;10(4):243–54. https://doi.org/10.1016/j.prro.2019.11.002.
Hartgerink D, Swinnen A, Roberge D, Nichol A, Zygmanski P, Yin FF, et al. LINAC based stereotactic radiosurgery for multiple brain metastases: guidance for clinical implementation. Acta Oncol. 2019;58(9):1275–82. https://doi.org/10.1080/0284186X.2019.1633016.
Growcott S, Dembrey T, Patel R, Eaton D, Cameron A. Inter-observer variability in target volume delineations of benign and metastatic brain tumours for stereotactic radiosurgery: results of a National Quality Assurance Programme. Clin Oncol (R Coll Radiol). 2020;32(1):13–25. https://doi.org/10.1016/j.clon.2019.06.015.
Vellayappan BA, Doody J, Vandervoort E, Szanto J, Sinclair J, Caudrelier JM, et al. Pre-operative versus post-operative radiosurgery for brain metastasis: effects on treatment volume and inter-observer variability. J Radiosurg SBRT. 2018;5(2):89–97.
Rick JW, Shahin M, Chandra A, Dalle Ore C, Yue JK, Nguyen A, et al. Systemic therapy for brain metastases. Crit Rev Oncol Hematol. 2019;142:44–50. https://doi.org/10.1016/j.critrevonc.2019.07.012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matthew Foote has received travel and honoraria from Elekta, consulting fee from Varian. Kristin Redmond has received research funding and travel expenses from Accuray, has received research funding from Elekta, has received honorarium from AstraZeneca and acts as a consultant for Medtronic. Simon Lo has received research support from Elekta AB for Gamma Knife ICON Expert Group. Balamurugan A. Vellayappan declares that there is no conflict of interest. Tresa McGranahan declares that there is no conflict of interest. Jerome Graber declares that there is no conflict of interest. Lynne Taylor declares that there is no conflict of interest. Vyshak Venur declares that there is no conflict of interest. Richard Ellenbogen declares that there is no conflict of interest. Andrew E. Sloan declares that there is no conflict of interest. Samuel T. Chao declares that there is no conflict of interest. John H. Suh declares that there is no conflict of interest. Eric L. Chang declares that there is no conflict of interest. Arjun Sahgal declares that there is no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Vellayappan, B.A., McGranahan, T., Graber, J. et al. Radiation Necrosis from Stereotactic Radiosurgery—How Do We Mitigate?. Curr. Treat. Options in Oncol. 22, 57 (2021). https://doi.org/10.1007/s11864-021-00854-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00854-z