Abstract
Purpose
Following surgical resection of brain metastases (BMs), adjuvant stereotactic radiosurgery (SRS) has become the standard of care post-operative cavity irradiation. Recent studies, however, have demonstrated that with the current sequence of surgery and radiation, risk of leptomeningeal disease (LMD) and radiation necrosis (RN) remains high. Pre-operative, or neoadjuvant, SRS (nSRS) has been proposed as an alternative treatment strategy which not only minimizes local recurrence (LR) but also LMD and RN. It is thought that nSRS sterilizes the tumor, allowing for minimal spillage of viable tumor cells during resection, creating less favorable conditions for LMD. Furthermore, nSRS allows for easier contouring and decreased margin irradiation during planning and treatment, respectively, diminishing the risk of symptomatic RN. While nSRS has already been adopted for treating other extra-cranial tumors, its role in treating BMs is yet to be defined. We aim to summarize recent studies in nSRS usage for BMs and the rationale of this treatment strategy.
Methods
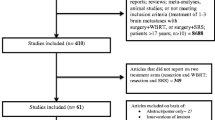
We performed a search for articles regarding nSRS for BMs published in PubMed from 2018 to 2022 using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) method. We summarized a total of 14 retrospective reviews, case series, dose/timing studies, and ongoing Phase II & III clinical trials.
Conclusion
In this review, we describe the findings of current studies and identify prospective clinical trials with the aim of understanding the efficacy of nSRS over current treatment standards. Herein, we also discuss the theoretical advantages and limitations of nSRS (both biologic and clinical) to help guide future clinical investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BM) are a common occurrence in patients with cancer, affecting 20–40% of cancer patients overall (~ 200,000 patients per year) [1, 2]. Neurosurgical resection of BMs remains an important treatment option for patients who have limited intracranial disease burden, have large tumors causing significant symptoms, or where diagnosis is warranted. With surgery alone, however, the likelihood of recurrence within the resection cavity (local recurrence (LR)) can be as high as 50% at 1–2 years following surgery [3].
Following maximal safe resection, employment of post-operative radiotherapy has been shown to significantly reduce this risk of LR and improve surgical outcomes. Over the past decade, adjuvant stereotactic radiosurgery (SRS) has been increasingly adopted to the surgical bed over the traditional use of adjuvant whole brain radiation therapy (WBRT) or combined SRS and WBRT treatments. A randomized phase III trial done in 2017 demonstrated that SRS had greater local control (LC) when compared with observation alone [3]. Another randomized phase III trial showed that when compared to WBRT, SRS had better cognitive preservation while maintaining similar survival rates (OS) [4]. Recent advances in neuroimaging and therapies have improved systemic disease management, consequently improving OS [5]. As patients continue to live longer, cognitive preservation remains important, and current clinical guidelines reflect this by recommending SRS instead of WBRT [6, 7].
The risks of other concerning central nervous system (CNS) outcomes, such as leptomeningeal disease (LMD) and radiation necrosis (RN), remain high with the current sequence of surgery and SRS. The median crude risk of LMD after craniotomy (alone or with post-operative radiation) is reported to be 16.1% by a recent meta-analysis [8], with rates as high as 28% [3] and 35% [9] reported in the literature specifically following adjuvant SRS. LMD is thought to occur secondary to tumor spillage during resection with the risk especially increased in patients undergoing intralesional debulking of the metastases (i.e. piecemeal resections (PR) [10, 11]). An LMD diagnosis carries significant adverse consequences, with a single-institution study reporting a neurologic death rate of 72% following confirmed diagnosis of nodular LMD [12]. RN rates at one-year for treated BMs were reported at 24% by one study, although only 10% presented with neurologic symptoms with the other 14% remaining asymptomatic [13]. Another study reported one-year RN rates of 17.2% for treated BMs [14].
Preoperative, or neoadjuvant SRS (nSRS), has emerged as an alternative treatment strategy of interest. While nSRS is already a popular option for the treatment of other disease sites (sarcoma, rectal, esophageal, and pancreatic cancers) [15,16,17,18], expanding evidence evaluating the theoretical risks and benefits of nSRS in BM treatment has been favorable. In this topic review, we summarize recent studies in nSRS usage for BMs and the rationale behind this treatment strategy.
Methods
PubMed was searched for articles related to nSRS for BMs with MeSH terms “stereotactic radiosurgery”, “Gamma Knife”, or “fractionated stereotactic radiosurgery” and “preoperative” or “neoadjuvant” and “brain metastases” from January, 2017 to August, 2022 using the Preferred Reporting Items for Systematic Reviews & Meta-analyses (PRISMA) method. We summarized retrospective reviews, primary articles, case reports, and clinical trials to provide a systemic overview of this subject (Fig. 1).
Results
Outcomes of nSRS studies
In the past 5 years, there has been active research into nSRS for BMs that undergo surgical excision. Early data suggests that radiation before resection may reduce the risk of RN and LMD, while preserving high rates of LC [19, 20]. Since 2017, several studies, including three multi-center cohort studies and one large single-institution study, have demonstrated comparable rates of LC and survival with nSRS when compared to cohorts in other studies treated with SRS (Table 1). These studies also generally show reduced rates of LMD and RN in nSRS cohorts compared to rates reported in literature for typical post-operative SRS [20,21,22,23,24,25].
The PROPS-BM multi-center study done by Prabhu et al. in 2021 represents the largest retrospective nSRS study performed to date. Prabhu et al. analyzed the outcomes of 242 nSRS patients with 253 BMs. The majority of patients (98.8%) were treated with single fraction nSRS prior to undergoing resection (93.7% gross total resection (GTR)) after a median of 1 day (interquartile range (IQR): 1–3 days). A median dose of 15 Gy (IQR: 14–16 Gy) was applied to a median gross tumor volume (GTV) of 9.9 cc. This study reported LC, distant control (DC), and OS rates of 85%, 63.7%, and 57.7% at one-year, respectively. The study also reported one-year LMD and RN rates of 6.1% and 7.4% with only 1.2% of the entire population experiencing grade 3 or higher toxicity [21].
[Paragraph & Citation removed—Data from Prabhu et al. 2018 is included in their 2021 study].
Palmer et al. recently (2022) published a multi-center analysis, representing the largest report studying neoadjuvant fractionated stereotactic radiotherapy (nFSRT). They hypothesized that fractionated treatment may allow for delivery of a higher biological dose to minimize LR, RN, and LMD. They reported on 53 patients with 55 BMs treated with nFSRT followed by surgery after a median of 2 days (IQR: 1–4.5). A median dose of 24 Gy over 3 fractions or 25 Gy over 5 fractions was applied to a median GTV of 12 cc. This study reported LC and OS rates of 100 and 70%, respectively, at one-year. Only 1.9% of patients experienced LMD, however, 12% had RN (6% symptomatic), with 3.8% reported to have grade 3 or higher toxicity. The high RN rates are hypothesized by the authors to be due to small sample size [22].
Several limited case series also highlight the potential advantages of nSRS. Udovicich et al. analyzed 28 patients that received nFSRT with a dose of either 20 or 24 Gy over 1 and 3 fractions, respectively, prior to surgery after a median of 1 (1–5) days. They reported LC and OS rates of 91.3% and 60.1%, respectively, at one-year, with 4.0% of patients experiencing LMD and 5.0% experiencing RN [23]. Deguchi et al. analyzed 20 patients that received nFSRT with a dose of 30 or 35 Gy over 5 fractions prior to surgery after a median of 4 (1–7) days. They reported LC and OS rates of 95.0% (2 months) and 60.0% (7 months), respectively, with 5.0% of patients experiencing LMD (3 months) and 0.0% experiencing RN [24]. Patel et al. reported on 12 patients receiving neoadjuvant SRS with a dose of 16 Gy (12–21 Gy) in a single fraction prior to surgery after a median of 1 (0–12) day. They reported one-year LC and OS rates of 49.1 and 74.1%, respectively [25].
nSRS vs. SRS
Currently, only one study (Patel et al.) comparing nSRS to post-op SRS exists, in which the authors compared 66 nSRS patients to 114 post-op SRS patients. They observed a two-year LMD rate of 3.2 and 16.6% for nSRS and post-op SRS, respectively (p = 0.01). They also observed a two-year symptomatic RN rate of 4.9 and 16.4% for nSRS and post-op SRS, respectively (p = 0.01). Multivariate analyses depicted no significant differences between groups for LC, OS, and DC [20]. Another study compares nSRS to post-op WBRT (Patel et al.), but the authors report similar rates between both cohorts for LC, OS, LMD, and RN, with no significance in multivariate analyses assessing survival and recurrence [19].
Guidelines for nSRS
The optimal timing of nSRS is unclear without any conclusive suggestions from the current literature. A limited case series (22 patients) by Kotecha et al. analyzing the biological effects of nSRS on BMs observed that tumor necrosis occurred around 24 h after treatment and persisted for several days [26]. Another small series (10 patients) by Steverink et al. examined patients receiving SBRT for spinal metastases and observed that within a 6-h window following treatment, 0.0% of biopsy specimens demonstrated necrosis. Comparatively, 83% of specimens collected at least 21 h post-SBRT demonstrated necrosis [27]. Both studies support that sufficient tumor necrosis occurs after a day, at which point surgery may be performed. Waiting until histopathologic necrosis has not been proven to be beneficial in the nSRS literature and it is plausible that performing surgery within the first 24 h of SRS may prove effective as irradiated tumor cells can harbor irreversible DNA damage which occurs prior to histopathologic correlation. Future studies are needed to examine this further. Waiting longer than 48-h, however, may increase the risk of acute or sub-acute radiogenic tissue reactions such as fibrosis or inflammation [28,29,30]. Most patients in the previously mentioned nSRS studies had surgery within several days of radiation. It seems that the general trend amongst clinicians using nSRS is to avoid delaying resection for longer than a week, although current clinical trials range from a few days up to 4 weeks post-SRS.
In most of the studies mentioned in this review, medians of 15 Gy, 24 Gy, and 25 Gy were used in single, three, and five-fraction nSRS cases, respectively, while Deguchi et al. observed patients treated with 30 or 35 Gy in five-fractions. In the comparative study mentioned earlier by Patel et al., nSRS patients received 14.5 Gy compared to 18 Gy for post-op SRS patients (both in single fractions). Although there is a lack of Radiation Therapy Oncology Group (RTOG) or American Society for Radiation Oncology (ASTRO) guidelines, a dose reduction trend has been established for nSRS; an ~ 80% isodose post-op SRS is the experimental standard. A recent Phase I dose escalation trial, however, reported low rates of toxicity with dose escalation in nSRS for BMs > 2 cm with great LC and low risk of LMD [31]. Kotecha et al. also did not find a relationship between radiotoxic events and the use of a standard schedule in their study (18 Gy in 1 fraction) [26].
Discussion
The studies identified in this review support that nSRS may be as safe and effective as post-operative radiosurgery due to similar LC and OS rates. Furthermore, nSRS may even be recommended over current treatment standards for patients with a higher risk of LMD due to individual tumor characteristics. No authors made definitive claims of decreased RN, although some studies depicted lower rates of RN compared to post-op SRS. Finally, nSRS dose and fractionation is still undefined by guidelines and should continue to be clinically decided based on tumor characteristics and the risks/benefit to patient outcomes. The decision to adjust dose when using nSRS for a case will be guided by current and future clinical trials.
There are limited controls and inherent bias due to the retrospective nature of the studies just mentioned. Additional limitations include variable sample sizes, and multi-institutional datasets. Several Phase II trials (Table 2) controlling for patient characteristics and nSRS treatment factors have been designed and are currently recruiting to assess the efficacy of nSRS as related to adverse outcomes. Future retrospective cohort studies should also be designed with sufficient power to explicitly compare outcomes of nSRS with post-operative SRS. Several Phase III clinical trials (Table 3) comparing neoadjuvant and adjuvant SRS have also been designed and are currently recruiting patients.
Hypothetical advantages
Recent reviews and studies alternatively suggest that nSRS to tumor site(s) potentially improves LC while reducing rates of toxicity and LMD when compared to postoperative SRS [19, 20, 32, 33]. This section highlights some of the hypothetical advantages mentioned in the current body of literature.
LMD, the spread of tumor cells into the leptomeninges or cerebrospinal fluid (CSF) (presenting in either classical or nodular form), is a clinically significant adverse event due to its high probability of resulting in CNS death. In addition to certain disease characteristics, such as tumor size and location, neurosurgical resection itself has been identified as a causative factor of LMD in a recent meta-analysis [8]. One study identified that resection carried a hazard ratio of 3.70 (p = 0.02) in predicting LMD [34]. Particularly in the cases of sub-total resections (STR), PR, posterior fossa resections, and/or ventricular violations, there is a significantly increased risk of intra-operative seeding into the CSF, leading to LMD [35,36,37]. Although the employment of en bloc resection has been shown to significantly reduce the development of post-operative LMD, en bloc resection is not always feasible for large tumors or tumors in eloquent, or deep seated, regions of the brain [10, 11]. Radiation can help mitigate LMD risk following surgery; however, local radiation performs significantly worse than WBRT in this effort [38, 39].
By sterilizing the tumor, nSRS may hypothetically reduce the spillage of viable, malignant cells into the subarachnoid space. Restricting viable tumor cell dissemination during surgery may improve LC rates and reduce the downstream risk of LMD [19, 32]. Furthermore, resection cavities are expected to be hypoxic due to cellular injury and thus, theoretically less radiosensitive than pre-operative tumor cells [40]. Administering nSRS to more radiosensitive cells may allow for less fractions and a lower dose [41, 42].
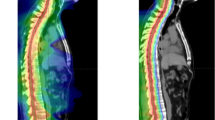
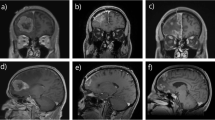
RN is a complication of high dose radiation (used in SRS), which results in the permanent death of irradiated brain tissue. Aside from dose, RN has also been significantly correlated with larger target volumes by several studies [43,44,45]. Resection cavities often remodel unpredictably during the time intervals between surgery, SRS planning, and SRS administration, making it challenging to derive an accurate clinical target volume (CTV) and leaving much variability in clinician practice [46,47,48]. Current consensus contouring guidelines for a clinical target volume include contouring the entire surgical tract (regardless of tumor location), extending the CTV 5–10 mm along the dura overlying the bone flap, and adding 5 mm margin into the adjacent sinus when preoperative venous sinus contact was present [48]. Such a large volume of irradiation leaves parenchymal tissue easily vulnerable to radiotoxicity. The use of hypofractionated post-op SRS (HSRT) appears to be one strategy used recently to reduce the rates of RN for large surgical cavities. nSRS may reduce morbidity associated with RN because the target volume is easily interpretable in scans and therefore, contoured without much interpretation, leaving less tissue irradiated. Furthermore, during surgery, any irradiated rim of healthy tissue is usually removed, resulting in less injured tissue and lower cytokine concentrations required to catalyze RN [14, 19, 33, 49]. Figure 2 shows a treatment planned target volume contour for a patient treated with nSRS and Fig. 3 overlays a mock planned target volume contour (with an additional 1 mm. margin) over the post-operative cavity; these figures illustrate the advantages of nSRS in planning and treatment.
Left temporal post-operative cavity (blue) with an added 1 mm. margin (pink) and mock Gamma Knife plan (yellow; 27 Gy prescription dose, 3 fractions) based on the T1 post contrast MRI images in the coronal a, transverse b, and sagittal c views. This patient was treated with neo-adjuvant SRS prior to surgical resection and is the same patient in Fig. 2
Theoretical limitations
Orchestrating OR schedules for resection, and planning SRS are individually challenging tasks for providers and administrators. Due to the recommended timing between nSRS and resection, however, both procedures would ideally be performed within a single hospitalization in consideration of patient cost and comfort. Combining the two treatments within one hospital stay requires more communication and coordination between healthcare providers, presenting a likely barrier to pre-operative SRS, especially for large and/or symptomatic metastases. Patients admitted to hospitals without SRS or even RT capabilities would have to be treated at an outpatient center or transferred between hospitals after nSRS which may be impractical. Furthermore, a lack of reimbursement neutrality between inpatient and outpatient centers may lead to decreased reimbursement for nSRS which is typically performed in the inpatient setting. While it is not a great reason, this may lead to the reduced adoption of nSRS (an issue specific to the United States). Finally, nSRS may not be feasible for patients admitted with certain tumors that are causing altered mental status, hydrocephalus, or other focal neurologic deficits. In these instances, performing nSRS may not be practical, secondary to patient tolerance and/or the need for urgent surgical intervention.
A pathological diagnosis of BMs prior to radiation therapy (RT) generally ensures that radiation is being used appropriately and not in cases where SRS may be contraindicated (autoimmune conditions, lymphomas, primary CNS malignancies). In current standard cases, biopsy and pathological analysis is usually performed intraoperatively, prior to adjuvant SRS. Due to risk of tumor spread within the BM-biopsy tract, it is difficult to recommend CNS biopsy prior to nSRS, especially when invasive surgery is planned to follow as part of the course of treatment [50].
Further studies and analysis of tumor resection technique in the context of nSRS are needed to fully understand whether the decreased rates of LMD may be applicable to tumors that are resected in a piecemeal fashion or in instances of STR. Several studies have found significant correlations between resection technique/extent and LMD, independent of RT treatment factors [11, 51]. Suki et al. observed that only 5.7% of en bloc resection cases developed LMD versus 13.9% of PR cases (p = 0.006) [10].
Interestingly, one of the case series reported in this review concluded that nSRT appeared to be safe and effective for patients undergoing PR. Deguchi et al. observed a 5% LMD rate (1 patient) in their cohort of 20 patients that received either 30 or 35 Gy of nFSRT in 5 fractions followed by surgery [24]. It is possible that the higher total dose may have contributed to increased tumor necrosis through the entire BM capsule, preventing the spillage associated with PR and STR. Further evidence comparing adverse event rates between nSRS for en bloc/PR and STR is needed to substantiate the efficacy of nSRS for different resection techniques.
Ultimately, non-surgical strategies carry the lowest risk of LMD. Targeted RT such as SRS and fractionated stereotactic radiotherapy (FSRT) or HSRT as well as immunotherapies have been shown in limited studies, to carry the lowest risk of LMD. Marcrom et al. published a retrospective analysis including 125 patients with 125 large BMs (≥ 3 cm.). Of these patients, 45% developed LMD after treatment with surgical resection and post-op SRS. In contrast, only 19% of patients treated with FSRT alone developed LMD (p = 0.048). Another study by Minniti et al. reported that patients receiving HSRT for resected BMs had a higher odds of developing LMD than those receiving HSRT for intact BMs (odds ratio (OR): 2.30, p = 0.008). The same study also reported that patients receiving targeted therapy or immunotherapy (nivolumab/pembrolizumab) following HSRT of intact lesions had a lower odds of developing LMD (OR: 0.178, p = 0.023) when compared to surgery and HSRT [52].
Conclusion
Neoadjuvant SRS is an emerging radiosurgical technique that may decrease post-operative rates of LMD while potentially reducing RN rates. Prospective studies are currently enrolling patients to further define the role of nSRS. Despite the promise of nSRS, certain logistic barriers may remain, thereby, potentially limiting the generalization of this technique to all patients who require surgery for large and/or symptomatic BMs.
Data availability
Not applicable.
References
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol 22(14):2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Arvold ND, Lee EQ, Mehta MP et al (2016) Updates in the management of brain metastases. Neuro Oncol 18(8):1043–1065. https://doi.org/10.1093/neuonc/now127
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18(8):1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18(8):1049–1060. https://doi.org/10.1016/S1470-2045(17)30441-2
Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL (2020) Current approaches to the management of brain metastases. Nat Rev Clin Oncol 17(5):279–299. https://doi.org/10.1038/s41571-019-0320-3
Nabors LB, Portnow J, Ahluwalia M et al (2020) Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 18(11):1537–1570. https://doi.org/10.6004/jnccn.2020.0052
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol 12(4):265–282. https://doi.org/10.1016/j.prro.2022.02.003
Tewarie IA, Jessurun CAC, Hulsbergen AFC, Smith TR, Mekary RA, Broekman MLD (2021) Leptomeningeal disease in neurosurgical brain metastases patients: a systematic review and meta-analysis. Neuro-Oncol Adv 3(1):162. https://doi.org/10.1093/noajnl/vdab162
Foreman PM, Jackson BE, Singh KP et al (2018) Postoperative radiosurgery for the treatment of metastatic brain tumor: evaluation of local failure and leptomeningeal disease. J Clin Neurosci 49:48–55. https://doi.org/10.1016/j.jocn.2017.12.009
Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD (2008) Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 108(2):248–257. https://doi.org/10.3171/JNS/2008/108/2/0248
Suki D, Hatiboglu MA, Patel AJ et al (2009) Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 64(4):664–674. https://doi.org/10.1227/01.NEU.0000341535.53720.3E
Cagney DN, Lamba N, Sinha S et al (2019) Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol 5(5):703–709. https://doi.org/10.1001/jamaoncol.2018.7204
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48. https://doi.org/10.1186/1748-717X-6-48
Kohutek ZA, Yamada Y, Chan TA et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156. https://doi.org/10.1007/s11060-015-1881-3
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740. https://doi.org/10.1056/NEJMoa040694
Lazarev S, McGee H, Moshier E, Ru M, Demicco EG, Gupta V (2017) Preoperative vs postoperative radiation therapy in localized soft tissue sarcoma: nationwide patterns of care and trends in utilization. Pract Radiat Oncol 7(6):e507–e516. https://doi.org/10.1016/j.prro.2017.04.010
van Hagen P, Hulshof MCCM, van Lanschot JJB et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084. https://doi.org/10.1056/NEJMoa1112088
Versteijne E, Suker M, Groothuis K et al (2020) Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 38(16):1763–1773. https://doi.org/10.1200/JCO.19.02274
Patel KR, Burri SH, Boselli D et al (2017) Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: a multi-institutional analysis. J Neurooncol 131(3):611–618. https://doi.org/10.1007/s11060-016-2334-3
Patel KR, Burri SH, Asher AL et al (2016) Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery 79(2):279–285. https://doi.org/10.1227/NEU.0000000000001096
Prabhu RS, Dhakal R, Vaslow ZK et al (2021) Preoperative radiosurgery for resected brain metastases: the PROPS-BM multicenter cohort study. Int J Radiat Oncol Biol Phys 111(3):764–772. https://doi.org/10.1016/j.ijrobp.2021.05.124
Palmer JD, Perlow HK, Matsui JK et al (2022) Fractionated pre-operative stereotactic radiotherapy for patients with brain metastases: a multi-institutional analysis. J Neuro-Oncol. https://doi.org/10.1007/s11060-022-04073-w
Udovicich C, Ng SP, Tange D, Bailey N, Haghighi N (2022) From postoperative to preoperative: a case series of hypofractionated and single-fraction neoadjuvant stereotactic radiosurgery for brain metastases. Oper Neurosurg 22(4):208–214. https://doi.org/10.1227/ONS.0000000000000101
Deguchi S, Mitsuya K, Yasui K et al (2022) Neoadjuvant fractionated stereotactic radiotherapy followed by piecemeal resection of brain metastasis: a case series of 20 patients. Int J Clin Oncol 27(3):481–487. https://doi.org/10.1007/s10147-021-02083-8
Patel AR, Nedzi L, Lau S et al (2018) Neoadjuvant stereotactic radiosurgery before surgical resection of cerebral metastases. World Neurosurg 120:e480–e487. https://doi.org/10.1016/j.wneu.2018.08.107
Kotecha R, Tonse R, Menendez MAR et al (2022) Evaluation of the impact of pre-operative stereotactic radiotherapy on the acute changes in histopathologic and immune marker profiles of brain metastases. Sci Rep 12(1):4567. https://doi.org/10.1038/s41598-022-08507-3
Steverink JG, Willems SM, Philippens MEP et al (2018) Early tissue effects of stereotactic body radiation therapy for spinal metastases. Int J Radiat Oncol Biol Phys 100(5):1254–1258. https://doi.org/10.1016/j.ijrobp.2018.01.005
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15(4):387–395. https://doi.org/10.1016/S1470-2045(14)70061-0
El Shafie RA, Tonndorf-Martini E, Schmitt D et al (2019) Pre-operative versus post-operative radiosurgery of brain metastases—volumetric and dosimetric impact of treatment sequence and margin concept. Cancers (Basel) 11(3):294. https://doi.org/10.3390/cancers11030294
Walker AJ, Ruzevick J, Malayeri AA et al (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 10(7):1277–1297. https://doi.org/10.2217/fon.13.271
Murphy ES, Yang K, Suh JH et al (2019) Prospective phase I dose escalation study for neoadjuvant radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 105(1):S10–S11. https://doi.org/10.1016/j.ijrobp.2019.06.399
Routman DM, Yan E, Vora S et al (2018) Preoperative stereotactic radiosurgery for brain metastases. Front Neurol. https://doi.org/10.3389/fneur.2018.00959
Asher AL, Burri SH, Wiggins WF et al (2014) A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys 88(4):899–906. https://doi.org/10.1016/j.ijrobp.2013.12.013
Marcrom SR, Foreman PM, Colvin TB et al (2020) Focal management of large brain metastases and risk of leptomeningeal disease. Adv Radiat Oncol 5(1):34–42. https://doi.org/10.1016/j.adro.2019.07.016
Ahn JH, Lee SH, Kim S et al (2012) Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection: clinical article. J Neurosurg 116(5):984–993. https://doi.org/10.3171/2012.1.JNS111560
Soliman H, Myrehaug S, Tseng CL et al (2019) Image-guided, linac-based, surgical cavity-hypofractionated stereotactic radiotherapy in 5 daily fractions for brain metastases. Neurosurgery 85(5):E860–E869. https://doi.org/10.1093/neuros/nyz162
DePaoli B, Gozal YM, Pater LE et al (2019) Ventricular violation increases the risk of leptomeningeal disease in cavity-directed radiosurgery treated patients. J Radiat Oncol 8(1):23–29. https://doi.org/10.1007/s13566-018-0368-1
Prabhu RS, Turner BE, Asher AL et al (2021) Leptomeningeal disease and neurologic death after surgical resection and radiosurgery for brain metastases: a multi-institutional analysis. Adv Radiat Oncol 6(2):100644. https://doi.org/10.1016/j.adro.2021.100644
Patchell RA, Tibbs PA, Regine WF et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. J Am Med Assoc 280(17):1485–1489. https://doi.org/10.1001/jama.280.17.1485
Scharl S, Kirstein A, Kessel KA et al (2019) Stereotactic irradiation of the resection cavity after surgical resection of brain metastases—when is the right timing? Acta Oncol 58(12):1714–1719. https://doi.org/10.1080/0284186X.2019.1643917
Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. BJR 26(312):638–648. https://doi.org/10.1259/0007-1285-26-312-638
Udovicich C, Phillips C, Kok DL et al (2019) Neoadjuvant stereotactic radiosurgery: a further evolution in the management of brain metastases. Curr Oncol Rep 21(8):73. https://doi.org/10.1007/s11912-019-0817-z
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103(3):618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Akanda ZZ, Hong W, Nahavandi S, Haghighi N, Phillips C, Kok DL (2020) Post-operative stereotactic radiosurgery following excision of brain metastases: a systematic review and meta-analysis. Radiother Oncol 142:27–35. https://doi.org/10.1016/j.radonc.2019.08.024
Jhaveri J, Chowdhary M, Zhang X et al (2018) Does size matter? Investigating the optimal planning target volume margin for postoperative stereotactic radiosurgery to resected brain metastases. J Neurosurg 130(3):797–803. https://doi.org/10.3171/2017.9.JNS171735
Patel RA, Lock D, Helenowski IB et al (2018) Postsurgical cavity evolution after brain metastasis resection: how soon should postoperative radiosurgery follow? World Neurosurg 110:e310–e314. https://doi.org/10.1016/j.wneu.2017.10.159
Shah JK, Potts MB, Sneed PK, Aghi MK, McDermott MW (2016) Surgical cavity constriction and local progression between resection and adjuvant radiosurgery for brain metastases. Cureus 8(4):e575. https://doi.org/10.7759/cureus.575
Soliman H, Ruschin M, Angelov L et al (2018) Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 100(2):436–442. https://doi.org/10.1016/j.ijrobp.2017.09.047
Yang R, Duan C, Yuan L et al (2018) Inhibitors of HIF-1α and CXCR4 mitigate the development of radiation necrosis in mouse brain. Int J Radiat Oncol Biol Phys 100(4):1016–1025. https://doi.org/10.1016/j.ijrobp.2017.12.257
Carnevale JA, Imber BS, Winston GM et al (2021) Risk of tract recurrence with stereotactic biopsy of brain metastases: an 18-year cancer center experience. J Neurosurg 136(4):1045–1051. https://doi.org/10.3171/2021.3.JNS204347
Schödel P, Jünger ST, Wittersheim M et al (2020) Surgical resection of symptomatic brain metastases improves the clinical status and facilitates further treatment. Cancer Med 9(20):7503–7510. https://doi.org/10.1002/cam4.3402
Minniti G, Lanzetta G, Capone L et al (2021) Leptomeningeal disease and brain control after postoperative stereotactic radiosurgery with or without immunotherapy for resected brain metastases. J Immunother Cancer 9(12):e003730. https://doi.org/10.1136/jitc-2021-003730
Wegner R. A phase II study of pre-op SRS followed by surgical resection for brain metastases. ClinicalTrials.gov identifier: NCT05341739. Updated April 22, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT05341739
Yu M. Preop fSRS for resectable brain metastases. ClinicalTrials.gov identifier: NCT05267587. Updated March 4, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT05267587
Brun L. Preoperative stereotactic radiosurgery for brain metastases (STEP). ClinicalTrials.gov identifier: NCT04503772. Updated April 8, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT04503772
Agarwal N. Pre-operative stereotactic radiosurgery followed by resection for patients with brain metastases. ClinicalTrials.gov identifier: NCT03398694. Updated June 9, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT03398694
Clump D. Preoperative stereotactic radiosurgery followed by resection for brain metastases. ClinicalTrials.gov identifier: NCT02514195. Updated April 13, 2021. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT02514195
Yeboa D. Pre-operative SRS or post-operative SRS in treating cancer patients with brain metastases. ClinicalTrials.gov identifier: NCT03741673. Updated June 13, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT03741673
Huhammud F. Pre-versus post-operative SRS for resectable brain metastases. ClinicalTrials.gov identifier: NCT04474925. Updated September 29, 2021. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT04474925
Yan E. Pre-operative or post-operative stereotactic radiosurgery in treating patients with operative metastatic brain tumors. ClinicalTrials.gov identifier: NCT03750227. Updated February 14, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT03750227
Rogers S. Trial of preoperative radiosurgery versus postoperative stereotactic radiotherapy for resectable brain metastases (PREOP-2). ClinicalTrials.gov identifier: NCT05124236. Updated September 10, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT05124236
Burri S. Comparing the addition of radiation either before or after surgery for patients with brain metastases. ClinicalTrials.gov identifier: NCT05438212. Updated August 29, 2022. Accessed September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT05438212
Acknowledgements
The authors thank Sarah Carey, MS, Jade Chang, and Jacalyn Newman, PhD, of Allegheny Health Network’s Health System Publication Support Office (HSPSO) for their assistance in editing and formatting the manuscript. The HSPSO is funded by Highmark Health (Pittsburgh, PA, United States of America) and all work was done in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the content of the review. Material preparation and literature review was performed by all authors. The first draft of the manuscript was written by SR, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
Rodney Wegner, MD has received grant funding from Elekta (not in support of this study). No other authors have declared financial interests.
Ethical approval
This is a literature and topic review. The Allegheny Health Network ethics committee has verified that no ethics approval is required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajkumar, S., Liang, Y., Wegner, R.E. et al. Utilization of neoadjuvant stereotactic radiosurgery for the treatment of brain metastases requiring surgical resection: a topic review. J Neurooncol 160, 691–705 (2022). https://doi.org/10.1007/s11060-022-04190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04190-6