Abstract
Distinguishing radiation necrosis (RN) from tumor recurrence after stereotactic radiosurgery (SRS) for brain metastases is challenging. This study assesses the sensitivity (SN) and specificity (SP) of an MRI-based parameter, the “lesion quotient” (LQ), in characterizing tumor progression from RN. Records of patients treated with SRS for brain metastases between 01/01/1999 and 12/31/2009 and with histopathologic analysis of a subsequent contrast enhancing enlarging lesion at the treated site at a single institution were examined. The LQ, the ratio of maximal nodular cross sectional area on T2-weighted imaging to the corresponding maximal cross sectional area of T1-contrast enhancement, was calculated by a neuroradiologist blinded to the histopathological outcome. Cutoffs of <0.3, 0.3–0.6, and >0.6 have been previously suggested to have correlated with RN, mixed findings and tumor recurrence, respectively. These cutoff values were evaluated for SN, SP, positive predictive value (PPV) and negative predictive value (NPV). Logistic regression analysis evaluated for associated clinical factors. For the 51 patients evaluated, the SN, SP, PPV and NPV for identifying RN (LQ < 0.3) were 8, 91, 25 and 73 %, respectively. For the combination of recurrent tumor and RN (LQ 0.3–0.6) the SN, SP, PPV and NPV were 0, 64, 0 and 83 %. The SN, SP, PPV and NPV of the LQ for recurrent tumor (LQ > 0.6) were 59, 41, 62 and 39 %, respectively. Standard MRI techniques do not reliably discriminate between tumor progression and RN after treatment with SRS for brain metastases. Additional imaging modalities are warranted to aid in distinguishing between these diagnoses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stereotactic radiosurgery (SRS) for the treatment of a small number of well-circumscribed intracranial metastases has been tested and shown to be useful with a local control rate of 72.5 % when used as a monotherapy and 88.7 % when used in combination with whole brain radiotherapy [1]. Major advantages of SRS are the minimally invasive nature of the treatment and the patient-friendly schedule of a single fraction outpatient treatment. However, when patients are followed over time and the treated lesion demonstrates radiographic enlargement and/or the patient develops additional symptoms attributable to a lesion at the site of prior SRS, tumor progression must be distinguished from radiation necrosis. Historically, surgical resection of lesions thought to represent tumor progression demonstrated that 10 % of specimens contained radiation necrosis alone (Fig. 1) [2]. The correct diagnosis is paramount as the treatment for tumor progression versus radiation necrosis differs markedly. Treatment for tumor progression may include additional radiotherapy, chemotherapy, and/or surgical resection/ablation, whereas radiation necrosis is often a self-limited entity managed symptomatically with a short course of steroids, anti-VEGF agents, and only occasionally surgical intervention.
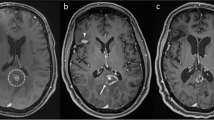
57 year old right-handed female with history of melanoma, diagnosed with left frontal brain and lung metastases 8 years after initial diagnosis of an arm lesion treated with resection. Treated with Gamma Knife® radiosurgery to the left frontal lobe to 2400 cGy prescribed to the 76 % isodose line, which covered 100 % of the target. The plan utilized 1 shot using 16 mm sectors. Target volume = 1.2 cc. Maximum dose = 3160.0 cGy. Maximum diameter = 1.5 cm. MD/PD = 1.317. PIV/TV = 1.917 (a). At 3 months post-Gamma Knife®, left frontal metastasis demonstrated progression of the left frontal lesion with surrounding vasogenic edema and hemorrhage (b). The patient was treated with whole brain radiotherapy to a dose of 3750 cGy in 15 fractions of 250 cGy/fraction with 6MV photons prescribed to the mid-plane. The patient did well until 7 months post-Gamma Knife®, when she experienced return of left lower extremity weakness. MRI demonstrated an enlarging mass with rim enhancement compatible with cystic tumor and recurrence metastasis (c). She again underwent excisional biopsy of the lesion, which demonstrated radiation necrosis and hemorrhage without evidence of tumor recurrence
In an effort to avoid invasive diagnostic approaches such as biopsy or surgical resection, great interest exists in identifying reliable clinical and/or imaging features that would help differentiate radiation necrosis from tumor progression. Some clinical factors that have been associated with a higher likelihood of radiation necrosis include the time from treatment to radiographic enlargement of the lesion and the volume of tissue treated with SRS [3, 4]. Because these lesions appear similarly on contrast enhanced T1-weighted MRI images, more sophisticated imaging modalities have also been studied and used clinically to aid in making this elusive diagnosis in a non-invasive fashion [5–11]. However, because of its availability some investigators have sought to identify a reliable tool to help discern between radiation necrosis and tumor progression using the available data from standard MRI sequences. Dequesada et al. [12] studied a novel parameter, the “Lesion Quotient” (LQ), defined as “the ratio of maximal cross sectional area of a definable nodule on T2-weighted imaging to the corresponding maximal cross sectional area of T1-contrast enhancement,” and found a LQ of <0.3, between 0.3–0.6, and >0.6 correlated well with radiation necrosis, mixed findings of necrosis and tumor, and tumor progression/recurrence, respectively. Others have studied a ratio known as a “T1/T2 mismatch,” defined as “lack of a clear and defined lesion margin on T2-weighted images compared to the margin of contrast uptake on T1-weighted images,” and found mismatches correlated with radiation necrosis [4].
Because this latter ratio is less quantitative and more subjective, we chose to examine the more consistently quantifiable LQ in our unique and fairly large patient sample and validate their findings. We also sought to identify whether a variety of technical and clinical parameters were specifically associated with either radiation necrosis or tumor progression.
Patients and methods
Between January 1, 1999 and December 31, 2009, 1202 patients with 3288 brain metastases were treated with Gamma Knife® radiosurgery at a single institution, utilizing the Elekta Model B®, Elekta Model C®, and Elekta Perfexion® systems. Of these 1202, all patients who underwent histopathologic analysis via biopsy and/or resection of the treated lesion(s) in the setting of radiation necrosis versus tumor progression, had preoperative MRI imaging within 60 days of surgery, and who had a definitive histopathologic diagnosis made by a dedicated neuropathologist were included in this study. Clinical information, such as demographic characteristics, oncologic history, timeline of events, tumor type, SRS characteristics including lesion size, prescribed dose, conformality index (prescribed isodose volume divided by the target volume), homogeneity index (maximum dose divided by the prescribed dose), administration of whole-brain radiotherapy (WBRT), type of surgical intervention, and Karnofsky Performance Score (KPS) at the time of surgery were retrospectively collected for each patient. Dosing for SRS was per RTOG 90-05 (24 Gy for tumors ≤2.0 cm, 18 Gy for tumors 2.1–3.0 cm, 15 Gy for tumors 3.1–4.0 cm) [13].
Preoperative clinical MRI scans were performed on commercial 1.0, 1.5 and 3.0 Tesla scanners (Siemens, Erlangen, Germany) utilizing standard RARE T2-weighted, Inversion Recovery FLAIR, and post contrast T1-weighted pulse sequences. Gadolinium chelate contrast agents were administered at standard dose of 0.1 mmol/kg for all patients. Lesion evaluation was performed on a Siemens PACS viewing station, or using eFilm (Merge Healthcare) or Siemens Syngo Imaging on a PC, utilizing standard measurement tools by an experienced neuroradiologist (ALT) who was blinded as to the tumor type, radiosurgery dose and final histopathology. Orthogonal measurements of maximal cross-sectional dimensions and perimeter tracings or elliptical area measurements were generated for each case (see Fig. 2). The maximal cross-sectional area on each imaging modality was utilized to evaluate the lesion quotient.
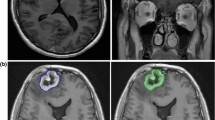
43 year old woman with metastatic melanoma status-post SRS to the right frontal lobe 2 years prior to imaging to a dose of 18 Gy. Axial MRI demonstrating the two techniques utilized to obtain measurements. On the left, orthogonal diameters are demonstrated on the T2 axial MRI of the lesion at the right frontal lobe (1.7 cm in anteroposterior direction and 1.8 cm in transverse orientation) as well as an ellipsoid measurement of the lesion area, 2.5 cm2. On the right, the same lesion on T1-contrast enhanced axial MRI is assessed with same measurements. In the case of irregular shapes, perimeter tracings were performed by hand in addition to maximal diameter measurements. LQ was 0.63 which predicted radiation necrosis. Histopathology revealed radiation necrosis without evidence of tumor progression
The sensitivity (SN), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) using the previously reported cut-points of <0.3, 0.3–0.6, and >0.6, were calculated for the endpoints of radiation necrosis, mixed findings of necrosis and tumor, and tumor progression only when compared with histopathology. Logistic regression was performed to identify other imaging characteristics, patient characteristics or treatment related factors significantly associated with the development of radiation necrosis versus tumor progression after SRS.
Results
Fifty-seven patients of the initial 1202 treated patients underwent surgical intervention when diagnosis of tumor progression versus radiation necrosis was in question. Indications for surgical intervention included for focal neurological deficit in 22 patients (43 %), for treatment decision-making in 16 patients (31 %), for intractable seizures in 8 patients (16 %), and for altered cognition in 5 patients (10 %). Thirty-four patients were noted to have tumor progression, 16 were noted to have necrosis without evidence of tumor, 6 were noted to have mixed tumor progression and radiation necrosis, and 1 patient had indeterminate histology. When the time from MRI to resection was limited to <60 days, 51 patients remained.
Thus, 51 patients, comprised of 28 females (55 %) and 23 males (45 %), met our inclusion criteria and were included in the analysis. Eleven histologies were represented, with non-small cell lung cancer and breast cancer being the two most common histologies (Table 1). Median age at the time of SRS was 57 years (range 28–73). Median target volume was 3.2 cm3 (range 0.025–20.3) and median diameter was 2.19 cm (range 0.46–4.5). Median conformality index was 1.65 (range 1.35–5.92), median homogeneity index was 1.96 (range 1.05–2.02), and median maximum dose was 35.5 Gy (range 23.1–48.1 Gy). WBRT was administered in 73 % of all patients, including 74 % of patients without necrosis and 69 % of patients in whom only necrosis was identified.
Surgical intervention for the 51 patients included in the analysis consisted of stereotactic biopsy in 4 patients (8 %) and surgical resection in 47 patients (92 %). Histopathologic examination of the 51 patients demonstrated 13 (25 %) cases of radiation necrosis, 5 (10 %) cases of mixed radiation necrosis and tumor, and 33 (55 %) cases of tumor progression. The SN, SP, PPV and NPV for identifying radiation necrosis (LQ < 0.3) were 8, 91, 25 and 73 %, respectively. For the combination of recurrent tumor and radiation necrosis (LQ 0.3–0.6) the SN, SP, PPV and NPV were 0, 64, 0 and 83 %. The SN, SP, PPV and NPV of the lesion quotient for distinguishing recurrent tumor (LQ > 0.6) were 59, 41, 62 and 39 %, respectively. Additional analysis was performed overall, as well as with different LQ cut points, but did not significantly improve the predictive ability of the test (data not shown). For all additional analyses, the 5 cases or mixed radiation necrosis and tumor progression were folded into the tumor progression category as these patients were felt to have symptoms most likely related to tumor progression rather than radiation necrosis alone and were managed as having tumor progression/recurrence.
Logistic regression analysis was used to identify whether any of the following variables were significantly associated with necrosis or tumor progression: KPS at the time of surgical resection, type of resection (biopsy versus tumor resection), the use and timing of WBRT, SRS dose, target volume, T1 maximum cross-sectional area, T2 maximum cross-sectional area, T2 maximum cross-sectional area divided by T1 maximum cross-sectional, the product of dose by volume, the time from SRS to MRI suspicious for radiation necrosis, time from MRI to surgery, time from SRS to surgery, T2 area, T2 cystic area, T2 anteroposterior dimension, T2 mediolateral dimension, T1 contrast-enhanced area, area of the T1 contrast-enhanced cyst, T1 contrast-enhanced anteroposterior measurement, T1 contrast-enhanced mediolateral measurement, ratio of T2 area to T1 contrast-enhanced area, ratio of T2 cyst area to T1 contrast-enhanced cyst area, and T2 divided by T1 contrast-enhanced anteroposterior and mediolateral measurements (Table 2). None of these values were significantly correlated with either RN or tumor progression on univariate analysis (Table 3).
Discussion
SRS has been established as a safe and effective treatment option for brain metastases. Its most clinically significant adverse effect is radiation necrosis, which occurs in approximately 7 % of lesions treated with SRS [14]. At our institution, approximately 12 % of patients experience RN based on radiographic findings [unpublished data], less than 5 % of whom required surgical intervention for question of RN versus tumor progression. Given the invasive nature of biopsy and/or resection for diagnosis of radiation necrosis and the widely differing treatments for RN versus tumor progression/recurrence, a reliable, non-invasive method for the diagnosis of radiation necrosis versus tumor progression for an enlarging mass at the site of previous SRS is appealing.
While MRI is routinely utilized for follow-up of patients treated with SRS, its ability to distinguish between radiation necrosis and progressive tumor has been problematic. Physiologic imaging modalities, including PET, MR-SPECT, and perfusion MRI have been used in conjunction with standard series MRI to distinguish tumor progression from radiation necrosis, with promising results (Table 4) [5, 7, 10, 11]. While not providing a definitive diagnosis, these modalities have been shown to be reliable enough that they are now used at select institutions to help establish a diagnosis and guide treatment without the need for surgical intervention. However, these additional imaging modalities are costly and not as widely available as conventional MRI.
As such, recent attempts to use the more cost-effective and widely available standard sequence MRI to help distinguish between radiation necrosis and tumor progression are both intriguing and potentially clinically meaningful. Dequesada et al. reviewed a number of different MRI morphologic findings and enhancement patterns, none of which reliably distinguished between the two entities when compared with histopathologic examination. However, a novel parameter, the LQ, was found to have a NPV of 96 % for radiation necrosis if the LQ was >0.3, a NPV of 100 % for tumor progression if the LQ was >0.6, and a PPV of 100 % for combined necrosis and tumor progression with a LQ of 0.3–0.6 [12]. While the sensitivity for recurrent tumor was 100 %, the specificity was only 32 %. For necrosis, sensitivity was 80 % and specificity was 96 %. For combination recurrent tumor and radiation necrosis, sensitivity was 15 % and specificity was 100 %.
Our data, which included more accurate parameters as well as orthogonal diameter measurements by an experienced neuroradiologist and a larger number of patients, did not confirm these findings. Our study reveals that the LQ is inadequately sensitive (59 %) and specific (41 %) for determining recurrent tumor only. The tool in this study had an acceptable specificity (91 %), but poor sensitivity (8 %) for determining RN. The test was neither sensitive (0 %) nor specific (64 %) for the combination of recurrent tumor and radiation necrosis. The predictive values were unacceptably low for all outcomes (see Table 3).
It is unclear why our data differs from their findings. Some factors that differed between our cohorts include potentially different treatment time periods, changes in practice, patient demographics and technological differences including conformality index, homogeneity index, and dosing. SRS also differed in our study as it was not performed with a linear accelerator, but with Gamma Knife®. This may offer one explanation for the differences in outcomes as dosimetry between linear accelerator delivered SRS and Gamma Knife® varies, with the central hotspot receiving a higher dose with Gamma Knife® SRS than linear accelerator SRS, potentially affecting the biologic and radiographic sequelae of SRS. There is, however, no evidence of superiority of radiosurgery delivered with a linear accelerator versus Gamma Knife® system versus cyclotron [15].
Another conventional MRI parameter that has been studied is the “T1/T2 mismatch” defined as “enhancement on T1-weighted MRI scans [not matching] any corresponding and similar low-intensity mass on T2-weighted images” [4]. It was found to be significantly correlated with necrosis (p < .0001). The converse was also true; if a T1/T2 match was identified, the lesion was significantly correlated with tumor progression (p < .0001). The T1/T2 mismatch for the identification of radiation necrosis was determined to be 83.3 % sensitive and 91.1 % specific. While these results are intriguing, this lack of objective methodology is limited in its qualitative rather than quantitative nature. Its subjective nature makes it difficult to implement in a consistent fashion, which is why we chose not to repeat this approach on our patient cohort.
Of the many clinical and treatment related factors that we analyzed, none consistently correlated with either radiation necrosis or tumor progression. Others have found that a longer time from SRS to surgery was associated with radiation necrosis, while those patients requiring surgical intervention at a shorter time after SRS were more likely to have residual tumor at the time of resection [4]. In this study, the median time from SRS to resection for suspicion of tumor progression versus radiation necrosis was 9.6 months for all patients, 7.1 months for those with tumor progression, and 11.7 months for those in whom necrosis only was identified. While this is consistent with published findings, it did not reach statistical significance.
Emerging data promoting advanced imaging as a non-invasive, though imperfect, method for diagnosis of RN versus tumor progression/recurrence has been published (Table 4). Patients may also be empirically treated with steroids and monitored for radiographic response. Overall, the treatment of radiation necrosis, tumor progression, or a combination of the two is controversial and is a topic of current study [16–20]. However, before the issue of treatment is considered, confidence in the diagnosis of radiation necrosis versus tumor progression is required. Our findings suggest that metrics derived from standard T1 post-contrast and T2-weighted sequences are unreliable to make this diagnosis, and that advanced imaging techniques may be requisite.
In fact, the results of our study are consistent with already well known MRI principles and extensive clinical experience with this modality for the evaluation of treated brain tumors [21]. The underlying physiological mechanisms of T1 shortening in gadolinium contrast enhanced MRI occur secondary to blood–brain barrier disruption with accumulation of paramagnetic agents in the extracellular space and are not histology-specific. Accordingly, peripheral enhancement surrounding an SRS treated tumor may be due to either RN or tumor progression/recurrence regardless of relative area. Similarly, hypointensity on T2-weighted images (T2 shortening) is equally non-specific, and may be secondary to dense cellularity from active tumor or the presence of dephasing secondary to hemoglobin degradation products in regions treated with radiosurgery, as an example. Those changes in the relative size of the areas of central and peripheral T1 and T2 shortening lesions was non predictive of histology in our study, which is consistent with extensive clinical MRI experience over that past 20 years and has provided the impetus behind the intense interest in advanced physiology and biochemistry-based imaging techniques in recent years, which include DSC perfusion MRI (“CBV”), DSC T1 weighted (“permeability”) perfusion MRI, FDG PET, MR Spectroscopy, and others [22–24].
Conclusions
Distinguishing between RN and tumor progression/recurrence after SRS for brain metastases is challenging. Standard MRI sequences are neither suitably sensitive nor specific to reliably make this diagnosis. More sophisticated imaging modalities which reflect tumor physiology and biochemistry are indicated to aid in this diagnosis. Histopathologic confirmation remains the gold standard.
References
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Truong MT, St Clair EG, Donahue BR, Rush SC, Miller DC, Formenti SC et al (2006) Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery 59:86–97; discussion 86–97
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491; discussion 491–482
Chao ST, Suh JH, Raja S, Lee SY, Barnett G (2001) The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer 96:191–197
Huang CF, Chiou SY, Wu MF, Tu HT, Liu WS, Chuang JC (2010) Apparent diffusion coefficients for evaluation of the response of brain tumors treated by Gamma Knife surgery. J Neurosurg 113(Suppl):97–104
Jain R, Narang J, Sundgren PM, Hearshen D, Saksena S, Rock JP et al (2010) Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol 100:17–29
Mitsuya K, Nakasu Y, Horiguchi S, Harada H, Nishimura T, Bando E et al (2010) Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 99:81–88
Serizawa T, Saeki N, Higuchi Y, Ono J, Matsuda S, Sato M et al (2005) Diagnostic value of thallium-201 chloride single-photon emission computerized tomography in differentiating tumor recurrence from radiation injury after gamma knife surgery for metastatic brain tumors. J Neurosurg 102(Suppl):266–271
Kimura T, Sako K, Tohyama Y, Aizawa S, Yoshida H, Aburano T, et al. (2003) Diagnosis and treatment of progressive space-occupying radiation necrosis following stereotactic radiosurgery for brain metastasis: value of proton magnetic resonance spectroscopy. Acta Neurochir (Wien) 145:557–564; discussion 564
Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ et al (2009) Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 256:878–887
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63:898–903; discussion 904
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298
Chin LS, Ma L, DiBiase S (2001) Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg 94:899–904
Suh JH (2010) Stereotactic radiosurgery for the management of brain metastases. N Engl J Med 362:1119–1127
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495
Matuschek C, Bolke E, Nawatny J, Hoffmann TK, Peiper M, Orth K et al (2011) Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol 187:135–139
Perez-Espejo MA, Garcia-Fernandez R, Tobarra-Gonzalez BM, Palma-Copete JD, Gonzalez-Lopez A, De la Fuente-Munoz I et al (2009) Usefulness of hyperbaric oxygen in the treatment of radionecrosis and symptomatic brain edema after LINAC radiosurgery. Neurocirugia (Astur) 20:449–453
Vecil GG, Suki D, Maldaun MV, Lang FF, Sawaya R (2005) Resection of brain metastases previously treated with stereotactic radiosurgery. J Neurosurg 102:209–215
Yamanaka K, Iwai Y, Yasui T, Nakajima H, Komiyama M, Nishikawa M et al (1999) Gamma Knife radiosurgery for metastatic brain tumor: the usefulness of repeated Gamma Knife radiosurgery for recurrent cases. Stereotact Funct Neurosurg 72(Suppl 1):73–80
Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, Norman D, Newton TH (1986) Brain radiation lesions: MR imaging. Radiology 158:149–155
Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV (2003) Assessment of brain metastases with dynamic susceptibility-weighted contrast-enhanced MR imaging: initial results. Radiology 228:193–199
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S (2009) Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30:367–372
Narang J, Jain R, Arbab AS, Mikkelsen T, Scarpace L, Rosenblum ML et al (2011) Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro-oncology 13:1037–1046
Acknowledgments
Thank you to Dr. David Chooljian for his review of the manuscript. Dr. Tievsky, Research grant from Bayer; Dr. Suh, Abbott Oncology; Dr. Vogelbaum, Honoraria from Merck.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stockham, A.L., Tievsky, A.L., Koyfman, S.A. et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol 109, 149–158 (2012). https://doi.org/10.1007/s11060-012-0881-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0881-9